Introduction
Identifying patterns in host–parasite relationships can help unveil what shapes such interactions, enabling an understanding of how parasites diversify and circulate among different hosts (Fountain-Jones et al., Reference Fountain-Jones, Pearse, Escobar, Alba-Casals, Carver, Davies, Kraberger, Papes, Vandegrift, Worsley-Tonks and Craft2017). The use of different host species by parasites, i.e. their niche breadth, is an intrinsic property described as host specificity (Poulin, Reference Poulin2006). Parasite host specificity is not inflexible and can vary according to the composition of host assemblage and the environment (Fountain-Jones et al., Reference Fountain-Jones, Pearse, Escobar, Alba-Casals, Carver, Davies, Kraberger, Papes, Vandegrift, Worsley-Tonks and Craft2017; Saldaña-Vázquez et al., Reference Saldaña-Vázquez, Sandoval-Ruiz, Veloz-Maldonado, Durán and Ramírez-Martínez2019). Thus, some parasite species may be specialists or generalists according to ecological context. The success of parasite association with different hosts within a community or population is generally measured through parameters such as infection prevalence and intensity. Similar, to host specificity, such extrinsic infection parameters may vary according to ecological context and the observed scale.
Despite there being numerous studies reporting on how host and environment affect infection prevalence, intensity and host specificity (Poulin, Reference Poulin1996, Reference Poulin2007; Poulin and Guegan, Reference Poulin and Guegan2000), little is known about how such parasite infection properties are related to each other. This knowledge is elementary to the identification of patterns in host–parasite interactions (Cooper et al., Reference Cooper, Griffin, Franz, Omotayo and Nunn2012) and to shedding new light on how they may be affected by host characteristics. Considering that host evolutionary history and functional traits may be related to variation in the expression of extrinsic characteristics of parasites (Fountain-Jones et al., Reference Fountain-Jones, Pearse, Escobar, Alba-Casals, Carver, Davies, Kraberger, Papes, Vandegrift, Worsley-Tonks and Craft2017; Saldaña-Vázquez et al., Reference Saldaña-Vázquez, Sandoval-Ruiz, Veloz-Maldonado, Durán and Ramírez-Martínez2019), assessing parasite host specificity and its relationship with infection parameters is crucial to understanding parasite establishment in host communities and to identifying potential host shifts (Fountain-Jones et al., Reference Fountain-Jones, Pearse, Escobar, Alba-Casals, Carver, Davies, Kraberger, Papes, Vandegrift, Worsley-Tonks and Craft2017; Saldaña-Vázquez et al., Reference Saldaña-Vázquez, Sandoval-Ruiz, Veloz-Maldonado, Durán and Ramírez-Martínez2019).
The evolutionary history of hosts can influence the dynamics of parasite communities and populations (Barrett et al., Reference Barrett, Thrall, Burdon and Linde2008; Lutz et al., Reference Lutz, Hochachka, Engel, Bell, Tkach, Bates, Hackett and Weckstein2015), and can explain the infection of multiple hosts by parasites, i.e. their specificity, through the sharing of phenotypic similarities and phylogenetically conserved resources among hosts (de Oliveira et al., Reference de Oliveira, Ávila and Morais2019; Fecchio et al., Reference Fecchio, Wells, Bell, Tkach, Lutz, Weckstein, Clegg and Clark2019). Thus, phylogenetic relationships among hosts can reflect parasite specificity, and the success of parasite colonization and establishment will be reflected in infection metrics. Similarly, host functional traits also affect their interactions with parasites (Dobson et al., Reference Dobson, Lafferty, Kuris, Hechinger and Jetz2008; Kamiya et al., Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014). Host body size is a known predictor of host importance to the success of host–parasite associations, with several studies reporting that larger hosts have a positive relationship with parasite infection parameters (Poulin, Reference Poulin1996; Kamiya et al., Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014; Campião et al., Reference Campião, Ribas, Morais, da Silva and Tavares2015; Johnson et al., Reference Johnson, Calhoun, Riepe and Koprivnikar2019). Experimental studies have shown that host body size is positively related to their attractiveness to parasites, thus influencing parasite choice (i.e. realized infection) of certain hosts when multiple host species are available (Johnson et al., Reference Johnson, Calhoun, Riepe and Koprivnikar2019). Host attractiveness to parasites is also mediated by chemical signs and behaviour (Haas, Reference Haas2003). Moreover, host habitat use is another factor that can potentially influence the diversity and composition of parasite communities, as it directly influences the scale of exposure to infective stages (Koprivnikar et al., Reference Koprivnikar, Urichuk and Szuroczki2017; Leung and Koprivnikar, Reference Leung and Koprivnikar2019; Euclydes et al., Reference Euclydes, Dudczak and Campião2021). Although both body size and habitat may be evolutionarily determined, such traits may not present a phylogenetic signal when analysed from a community perspective (Blomberg et al., Reference Blomberg, Garland and Ives2003; Pavoine et al., Reference Pavoine, Baguette, Stevens, Leibold, Turlure and Bonsall2014), because the assembling of local communities may result in a heterogeneous pool of sympatric species. In this context, assessing the host specificity of parasite species from an ecological or functional perspective can provide new information about the role of non-phylogenetically related filters in the establishment of parasites in a host community (Clark and Clegg, Reference Clark and Clegg2017).
Anuran species represent a good model to assess patterns of host use by parasites (Brooks et al., Reference Brooks, León-Règagnon, McLennan and Zelmer2006; Hamann et al., Reference Hamann, Kehr and González2013; Johnson et al., Reference Johnson, Preston, Hoverman and Richgels2013) since they are a species-rich group comprising high evolutionary distinctiveness and a great diversity of ecological traits (Jetz and Pyron, Reference Jetz and Pyron2018; Womack and Bell, Reference Womack and Bell2020). Their parasite communities result from evolutionary and/or ecological aspects (Poulin and Morand, Reference Poulin and Morand2000; D'Bastiani and Campião, Reference D'Bastiani and Campião2021), and analysing the relationship between parasite infection and evolutionary and ecological characteristics can contribute to disentangling how these two factors influence the distribution and abundance of parasites in different hosts (Bongers and Ferris, Reference Bongers and Ferris1999; Hechinger et al., Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007). In this context, the Atlantic Forest is a heterogeneous environment with a great diversity of anuran species (Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009) that provides variable ecological and phylogenetic opportunities for anuran–parasite interaction. Here, we assess parasite infection of anuran species from the Atlantic Forest, and the relationship between parasite host specificity and infection prevalence and mean intensity. We propose two measures of parasite specificity based on the phylogenetic and ecological relatedness of infected host species and analysed how such indexes influence parasite infection prevalence and intensity at both community (all hosts) and population (infected host species) scales.
Materials and methods
Host collection and parasite identification
Anurans were collected in Marumbi State Park (Mananciais da Serra), state of Paraná, southern Brazil (25°29′31.9″S; 48°59′36.8″W). The area has a subtropical climate and is composed of rainforests of typical Atlantic Forest formations, such as ombrophilous forest, which presents trees and shrubs in association with ferns and terrestrial bamboos (Scheer and Blum, Reference Scheer, Blum, Grillo and Venora2011), in addition to Araucaria angustifolia, the dominant tree that distinguishes this type of forest (Reginato and Goldenberg, Reference Reginato and Goldenberg2007; Scheer and Blum, Reference Scheer, Blum, Grillo and Venora2011). Anuran collections, employed visual and auditory active search techniques to find the target species (Crump and Scott Jr., Reference Crump, Scott, Heyer, Donnelly, McDiarmid, Donnelly Heyek and Foster1994). A total of 213 individual anurans (135 males and 78 females, all adults)were captured by hand. Field sampling occurred in the warm and rainy seasons from October 2018 to February 2019. Captured specimens were transported to the laboratory where they were measured for snout-vent length and classified according to habitat use as arboreal and/or terrestrial and/or semi-aquatic (Supplementary Table 1), based on Moen et al. (Reference Moen, Morlon and Wiens2016) and Haddad et al. (Reference Haddad, Toledo, Prado, Loebmann, Gasparini and Sazima2013). A total of 11 anuran species of six families of anurans (Brachycephalidae, Hylodidae, Hylidae, Leptodactylidae, Odontophrynidae and Bufonidae) were analysed. The sampled anurans varied in body size and occupied arboreal, semi-aquatic and terrestrial habitats (Supplementary Table 1).
Table 1. Diversity, specificity and infection parameters of parasites associated with 11 anuran species of the Atlantic Forest. We report the number of associated hosts (No. host), net relatedness index (NRI) values for the phylogenetic and ecological specificity of the parasites, and parasite prevalence and mean intensity of infection (MII) at the community (all hosts) and population (infected host species) scales

The anurans were euthanized with 4% Lidocaine, following the Federal Council of Biology (CFBIO – Resolution 308), and then necropsied by longitudinal incision along the antero-posterior axis for the collection of parasites. All organs of the gastrointestinal tract, plus lungs, kidneys, bladder and abdominal cavity, of the hosts were examined. Anuran nomenclature was updated according to the American Museum of Natural History (Frost, Reference Frost2021). The collected specimens were deposited at the Museum of Natural History Capão da Imbuia in Curitiba, Paraná, Brazil.
Following anuran dissections, all parasites were collected and fixed in 70% ethyl alcohol. For identification, emporary slides were mounted for all specimens. Nematodes were clarified with Aman's lactophenol and acanthocephalans with lactic acid, while platyhelminths were subjected to hydrochloric-carmine staining (described by Amato and Amato, Reference Amato, Amato, Matter, Straube, Accordi, Piacentini and Candido2010). The specimens were preserved in 70% ethyl alcohol and deposited in the Invertebrate Collection of the Federal University of Paraná. Parasite nomenclature follows Anderson et al. (Reference Anderson, Chabaud and Willmott2009) for Nematoda, Amin (Reference Amin, Crompton and Nickol1985) for Acanthocephala and Khalil et al. (Reference Khalil, Jones and Bray1994) for Cestoda.
Infection parameters and host specificity metrics
We used two infection parameters to describe the populations of parasite taxa: parasite prevalence and mean infection intensity (MII). Each of these metrics was calculated for two different scales: within the anuran community and in the population of infected host species. For prevalence at the community scale, the number of hosts infected by a species of parasite was divided by the total anuran sample (213 anurans). For the population scale, we considered all individuals of the species of hosts infected divided by the total number of hosts, that latter combining individuals of the populations of the different infected species. MII at the community scale is the mean number of parasite specimens found in infected hosts, regardless of host species, whereas MII at the population scale is the mean number of parasites within the total number of individuals of an infected host species (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997).
The calculation of host phylogenetic specificity used the anuran phylogenetic tree provided by Jetz and Pyron (Reference Jetz and Pyron2018), pruned based on the anuran species sampled using the ‘match.phylo.data’ function (picante package, Kembel et al., Reference Kembel, Cowan, Helmus, Cornwell, Morlon, Ackerly, Blomberg and Webb2010). A distance matrix among the sampled anuran species was created based on phylogenetic relatedness, which was used to calculate the phylogenetic specificity measure for the host species infected by each parasite taxa with the ‘ses.mpd’ function(picante package, Kembel et al., Reference Kembel, Cowan, Helmus, Cornwell, Morlon, Ackerly, Blomberg and Webb2010).
Anuran functional traits, namely snout-vent length and habitat, were used to determine ecological specificity. The first is related to body size, a trait that directly affects parasite establishment (Kamiya et al., Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014), while the latter reflects infection opportunity due to exposure to parasite species, since different habitats can restrict or facilitate parasite–host encounters (Anderson, Reference Anderson2000; D'Bastiani et al., Reference D'Bastiani, Campião, Boeger and Araújo2020). Snout-vent length is a continuous variable, whereas habitat comprises three binomial variables (presence/absence for arboreal, terrestrial and semi-aquatic), with anuran species being able to be present in more than one habitat. For the calculation of distance of variables of different statistical types, an ecological dataset was first prepared, based on mixed-variables coefficient of distance (Pavoine et al., Reference Pavoine, Vallet, Dufour, Gachet and Daniel2009), and then a distance matrix was generated using modified Gower distance (Dray and Dufour, Reference Dray and Dufour2007) to represent ecological dissimilarity among anuran hosts. The same procedure used to calculate phylogenetic host specificity was then employed but using the host ecological distance matrix instead of the host phylogenetic distance matrix (Pavoine and Bonsall, Reference Pavoine and Bonsall2011).
It is worth noting that anuran functional traits were tested for phylogenetic signal using the “phylosignal” function (picante package; Kembel et al., Reference Kembel, Cowan, Helmus, Cornwell, Morlon, Ackerly, Blomberg and Webb2010). Based on these analyses, we found no phylogenetic clustering for any anuran functional traits, with the exception of arboreal habitat (because all hylids are arboreal). Nonetheless, we also did not find the host phylogenetic distance matrix and the host ecological distance matrix to be correlated (Supplementary Script), so we considered both host specificity indexes as suitable to perform statistical analysis.
We used three metrics to calculate host specificity for each parasite taxa: number of host species, host phylogenetic specificity and host ecological specificity. The first metric is simply the number of host species infected by a given parasite taxa, whereas the latter two were calculated based on the net relatedness index (NRI) among the infected host species (Webb, Reference Webb2000). The NRI approach is commonly used in ecological studies to assess the amount of phylogenetic and ecological information in a given community (Webb, Reference Webb2000; Webb et al., Reference Webb, Ackerly and Kembel2008). These analyses determine whether a community is formed by phylogenetically closely or distantly related species (high or low phylogenetic NRI; Webb et al., Reference Webb, Ackerly and Kembel2008). Similarly, NRI also determines whether a community has ecologically redundant or divergent species (high or low ecological NRI; Pavoine and Bonsall, Reference Pavoine and Bonsall2011). Phylogenetic and ecological specificity metrics were not calculated for 11 parasite species (out of the 25) because they were found infecting only one host species, and these values are based on the distance between host species.
The NRI varies from 1.96 to −1.96, which represents more specialist or more generalist than expected by chance, respectively. Thus, higher phylogenetic NRI values correspond to parasites infecting closely related host species, which are thus considered phylogenetic specialists. In contrast, lower phylogenetic NRI values correspond to parasites infecting distantly related host species, which are thus considered as phylogenetic generalists. We opted to use NRI to calculate both host phylogenetic specificity and host ecological specificity. This approach avoids any spurious correlation with the number of host species, and overcomes bias related to differences in sample size among anuran species. The NRI is based on a null model comparison (Miller et al., Reference Miller, Farine and Trisos2017), thus null models were generated through 1000 randomizations of host species names in both the ecological distance matrix and the phylogenetic distance matrix, the values of which were then compared to observed values of the respective host specificity metric. Comparisons were made by subtracting the random mean from the observed values and dividing the result by the random standard deviation, with random values being represented by zero. Values below zero indicate low host specificity (phylogenetically distantly related and/or ecologically distinct hosts), whereas values above zero indicate high host specificity (the parasite tends to infect phylogenetically closely related and/or ecologically similar hosts).
Statistical analysis
To test if host specificity affects the parasite infection metrics at both community (all host species) and population (infected host species) scales, we considered all analysed parasite taxa and only multi-host parasite species. To do so, we created different datasets: (i) two based on the infection metrics (prevalence and MII) at the community scale, with columns presenting these parameters for each parasite species, and (ii) two based on the infection metrics at the scale of populations of infected hosts, with rows presenting the values of prevalence and MII for a parasite species in the infected host populations.
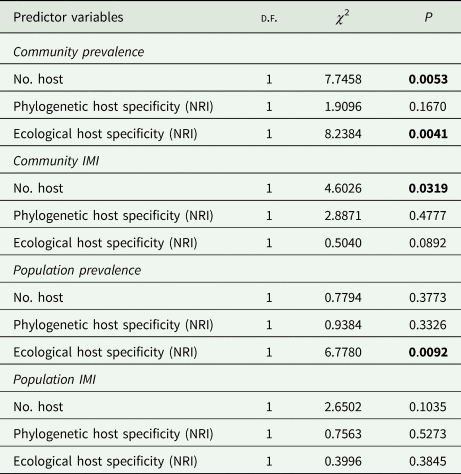
We then tested the effects of taxonomic, ecological and phylogenetic host specificity on the infection metrics. For taxonomic specificity, we tested the effect of the number of host species on prevalence and MII at community scale and at the scale of populations of infected hosts using the complete dataset for both. We then created a data subset to analyse parasite species that infect two or more host species (multi-host parasites), and analysed whether the number of host species, phylogenetic host specificity and ecological host specificity determine the prevalence and MII of multi-host parasites. We used generalized linear models (GLM) for the analyses at the community scale and generalized linear mixed models (GLMM) at the scale of populations of infected host. Different error distribution families were applied for each model to respect the statistical assumptions (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). For the GLM, we used beta regression for the models of prevalence (Ferrari and Cribari-Neto, Reference Ferrari and Cribari-Neto2004) and linear regression (Gaussian family) for the models of MII. For the GLMM, we used a binomial family error distribution in the models analysing prevalence and a Gaussian family error distribution to analyse parasite MII. We used this distribution family for both the total dataset and the multi-host species dataset, and also considered host species and parasite species as random variables in all population scale analyses. For each model, we assessed the significance of each variable using analysis of variance (ANOVA) considering α < 0.05.
Prior to running the models, we calculated the log10 of MII values for better model fitting. We also validated all models based on residual distribution, leverage and Cook-distance inflation factor (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). We report the estimated coefficients and 95% confidence intervals (CI), and consider significant those variables with a 95% CI that does not encompass zero. We used R software version 4.0.0 for all analyses (R Core Team, 2020), the scripts of which can be found in Supplementary material.
Results
The parasite community comprised 27 parasite taxa belonging to three taxonomic groups: Acanthocephala, Nematoda and Platyhelminthes. Acanthocephala was represented by one taxon of Centrorhynchidae and Platyhelminthes by one taxon of Cestoda. Nematoda was the most representative taxonomic group with 25 taxa.
Host phylogenetic specificity ranged between −1.582 and 1.621, with parasite species that occurred in phylogenetically close hosts (e.g. Oxyascaris cf. caudacutus) and parasites species that occurred in phylogenetically distant hosts (e.g. Schrankiana formosula). The same was observed for host ecological specificity, as some species occurred in ecologically close hosts (such as Rhabdias fuelleborni with ecological NRI = 2348) and others occurred in ecologically distant hosts (such as Cosmocerca parva with ecological NRI = −0.841, Oswaldocruzia sp.1 with ecological NRI = −0.819 and S. formosula with ecological NRI = −0.791) (Table 1).
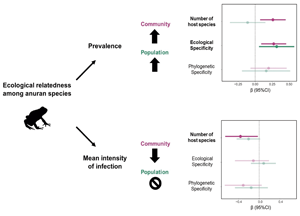
The analysis of all parasite taxa, both single and multi-host, indicated a relationship between the number of infected hosts and infection parameters. Parasite prevalence was affected by the number of hosts at both community (all hosts) and population (infected host species) scales. However, the effect of this predictor variable presented opposite patterns: parasite prevalence was positively related to the number of host species in the community (β = 0.22, 95% CI = 0.07–0.38), whereas this relationship was negative in the populations of infected hosts (β = −0.31, 95% CI = −0.58 to −0.03). This means that when using the number of associated host species as a specificity measure, the prevalences of generalist parasites tends to be higher in the host community but lower in the populations of infected hosts. The number of host species did not affect MII at the community scale but was also negatively related to the populations of infected hosts (β = −0.21, 95% CI = −0.40 to −0.02) (Fig. 1).

Fig. 1. Relationship between the number of host species and infection metrics for the community (left) and infected host populations (right): (A) infection prevalence; (B) mean intensity of infection (MII). The β estimate and its respective 95% confidence interval are shown in the upper-right of each scatter plot. The fitted linear curve (blue line) and the 95% confidence interval (grey area) are also presented.
When considering only multi-host parasite species, the parasite infection parameters were not related to parasite phylogenetic specificity (host phylogenetic NRI) neither at community (all hosts) or population (infected hosts) scales (Fig. 2). However, parasite prevalence was positively related to ecological specificity at both community and population scales (host ecological NRI, community: β = 0.27, 95% CI = 0.08–0.45; infected host populations: β = 0.31, 95% CI = −0.57 to −0.06). The prevalence of parasite taxa at the community scale was also positively related to the number of host species (β = 0.26, 95% CI = 0.07–0.44). Host specificity was less relevant for MII, since this infection parameter was negatively related only to the number of host species (Fig. 2), and this relationship was observed only at the scale of host community (β = −0.32, 95% CI = −0.66 to 0.03) (Table 2).

Fig. 2. The effect of parasite host specificity on (A) infection prevalence and (B) mean intensity of infection MII. The β estimate (circle) and 95% confidence interval (line) for both the community (pink) and infected host populations (dark green) are presented. Non-significant estimates are shown transparently.
Table 2. Factors related to the prevalence of anuran parasites of the Atlantic Forest area. Analysis of variance (ANOVA) considering the correlation of parasite prevalence with the number of infected hosts, and the net phylogenetic and ecological relatedness indexes (NRI) at community (all hosts) and population (infected host species) scales.

Discussion
The use of multiple hosts by parasites, as well as their variable competence among the infected hosts, is directly related to the emergence of infectious diseases. The comprehension of every factor associated with parasite specificity and infection success is important to improve the predictive power and application of knowledge in disease ecology. In this study, we described how the range of use of host species by parasites, host phylogeny and host functional attributes influenced parasite infection parameters. Parasite species that occur in ecologically close hosts are more prevalent, which shows that both the specificity and infection parameters of parasites are associated with the ecological characteristics of the hosts, such as habitat use, and it is related to the opportunities for parasites to exchange among hosts.
We found that the sampled parasite community is assembled by species that are notphylogenetic specialists, indicating host functional traits to be more relevant than host evolutionary history in this parasite community. Analysis of both functional and phylogenetic host specificity of multi-host parasite taxa allowed us to identify whether the parasite community analysed here may be tracking hosts that offer specific resources, which may or may not be phylogenetically conserved. The concepts of false specialist and false generalist parasites define a false specialist as a generalist limited to one or a few host species due to ecological, spatial or environmental factors (Brooks and McLennan, Reference Brooks and McLennan2002). On the other hand, false generalists are specialists on a particular phylogenetically diffused resource (Brooks and McLennan, Reference Brooks and McLennan2002; Agosta et al., Reference Agosta, Janz and Brooks2010; Nyman, Reference Nyman2010; Roy and Handley, Reference Roy and Handley2012). The species we found on multiple hosts may be resource-specialist parasites (false generalists). Good examples of this pattern are the host functional specialist species Aplectana. macintochi and Rhabdias fuelleborni, which were found only in hosts that share the same habitat (arboreal and terrestrial respectively). Thus, ecological opportunity, such as habitat use by phylogenetically distant hosts, may be mediating shifts between hosts (Brooks et al., Reference Brooks, León-Règagnon, McLennan and Zelmer2006; Agosta et al., Reference Agosta, Janz and Brooks2010).
Since hosts are resource patches for parasite colonization, the number of host species available in an environment can influence the probability of encountering parasites, which, in association with parasite specificity, can influence prevalence and MII (Hellgren et al., Reference Hellgren, Pérez-Tris and Bensch2009; Ellis et al., Reference Ellis, Huang, Westerdahl, Jönsson, Hasselquist, Neto, Nilsson, Nilsson, Hegemann, Hellgren and Bensch2020). When we analysed the entire sampled community, we found a positive correlation between the prevalence of parasite infection and the number of infected host species. This result corroborates the resource breadth hypothesis (Krasnov et al., Reference Krasnov, Poulin, Shenbrot, Mouillot and Khokhlova2004), which states that species with greater niche breadths tend to have better local performance (high prevalence in the present study). According to this hypothesis, the same attributes of parasites that enable the association with different hosts will also allow the parasites to infect and exploit the hosts more efficiently, resulting in higher values of infection parameters (Krasnov et al., Reference Krasnov, Poulin, Shenbrot, Mouillot and Khokhlova2004; Pinheiro et al., Reference Pinheiro, Félix, Chaves, Lacorte, Santos, Braga and Mello2016; Garcia-Longoria et al., Reference Garcia-Longoria, Marzal, de Lope and Garamszegi2019).
Interestingly, when we changed the scale and considered only the populations infected by each parasite taxa, we observed a negative relationship between prevalence and the number of host species. This result diverges from the resource breadth hypothesis and fits with the trade-off hypothesis. The trade-off hypothesis can also help to understand the patterns observed here, as it assumes a negative relationship between the range of hosts and parasite performance (Poulin, Reference Poulin1998; Krasnov et al., Reference Krasnov, Poulin, Shenbrot, Mouillot and Khokhlova2004). According to the trade-off hypothesis, parasites that associate with multiple hosts would have higher energy costs to reach physiological compatibilities and overcome immune defences of different species, and this would reduce transmission (or reproduction) capacity and, consequently, prevalence (Robalinho Lima and Bensch, Reference Robalinho Lima and Bensch2014).
The occurrence of single-host parasite taxa (such as most species of Rhabdias) is another relevant factor for the contrasting results in the relationship between prevalence and number of infected hosts at community (all hosts) and population (infected host species) scales. These lung-worm parasites had high prevalence values for the populations of infected hosts (≥0.5), despite the population size (assumed here as the sampling effort). Such single-host parasites had low prevalence at the community scale, since they occur in only one species and, consequently, in a low number of hosts when compared to the total number of anuran individuals in the community analysed here. Thus, our results emphasize the importance of exploring infection metrics at different scales and that presumably contrasting hypotheses (such as niche breadth and trade-off) are not necessarily mutually exclusive since one may have more influence than the other depending on the scale analysed (Pinheiro et al., Reference Pinheiro, Félix, Chaves, Lacorte, Santos, Braga and Mello2016).
Analyses with multi-host parasite taxa revealed that infection metrics at community and population scales did not correlate with host phylogeny, suggesting that a shared evolutionary history between hosts does not necessarily affect their chances of being infected by the same species of parasites (Brooks et al., Reference Brooks, León-Règagnon, McLennan and Zelmer2006; Agosta et al., Reference Agosta, Janz and Brooks2010). Parasite species can, potentially, use available resources, such as a different environment or host, without necessarily having to undergo changes in genotype, but because they use existing characteristics, i.e. via ecological fitting (Janzen, Reference Janzen1985; Brooks et al., Reference Brooks, León-Règagnon, McLennan and Zelmer2006; Agosta and Klemens, Reference Agosta and Klemens2008; Araujo et al., Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015). The prevalence of parasites in the community and populations of anurans analysed here was positively correlated with parasite ecological specificity, so that parasites associated with ecologically close hosts were more prevalent. Similar results were observed in other studies (see Johnson et al., Reference Johnson, Calhoun, Riepe and Koprivnikar2019 for a good example), inferring that host attributes, such as body size and habitat, are relevant to infection, regardless of host species identity (Kamiya et al., Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014; D'Bastiani et al., Reference D'Bastiani, Campião, Boeger and Araújo2020; Euclydes et al., Reference Euclydes, Dudczak and Campião2021). These results, together with those observed here, point to the importance of functional characteristics of hosts in the metrics of infection by parasites. Thus, ecological characteristics of hosts can promote host–parasite interactions and associations due to the exaptation capacity of parasites, and consequently influence the prevalence of these parasites (Agosta, Reference Agosta2006; Agosta et al., Reference Agosta, Janz and Brooks2010).
We conclude that parasite infection parameters are related to host specificity, and the use of different scales can provide complimentary information about this relationship. The effects of the ecological specificity of parasites on their respective infection metrics, both at community and population scales, indicates the importance of host attributes in the assembly of the parasite community. The influence of host functional traits and the absence of a phylogenetic effect indicate the importance of ecological similarities among hosts as a driver for potential host shifts, which resulted from increased opportunity of contact for the exchange of hosts by the parasites. Expanding studies related to the aspects of parasite specificity allows a better understanding of the characteristics of hosts and parasites that are related to higher infection among individuals within host populations and communities.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000087
Acknowledgements
We thank Sanepar for the authorization and support for carrying out the collections. We are also grateful to Mauricio O. Moura for his suggestions on the first version of this manuscript, and the Biological Interactions group for the constructive comments and discussions of the theoretical framework that were essential to maturing our hypotheses.
Author contributions
K.M.C., L.E. and G.M.D.T. originally formulated the idea. K,M.C., L.E., A.C.D., G.M.D.T. and F.T.V.M. performed data collection and generated the data analysis. K.M.C., L.E. and G.M.D.T. interpreted the results and wrote the manuscript.
Financial support
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflict of interest
None.
Ethical standards
The collections and observations of this study were carried out in accordance with the license 62552-1 (Sistema de Autorização e Informação em Biodiversidade – Instituto Chico Mendes de Conservação da Biodiversidade – SISBIO). The Comitê de Ética para Uso de Animais da Seção de Ciências Biológicas da Universidade Federal do Paraná (Opinion no. 1167) certified that the procedures with the use of animals in this work are approved.