Clostridioides difficile is a spore-forming, anaerobic, gram-positive bacillus; it is the leading cause of infectious healthcare-associated diarrhea. Reference Magill, Edwards and Bamberg1 The economic burden of this infection in North America is estimated at $5.4 billion in the healthcare setting and $725 million in the community setting. Reference Desai, Gupta and Dubberke2 Clostridium difficile infection (CDI) has increased in frequency and severity since 2002 due to the emergence of a hypervirulent strain (known as North American pulsed-field gel electrophoresis type-1 (NAP-1), or polymerase chain reaction (PCR) ribotype 027) Reference Pepin, Alary and Valiquette3 that is associated with increased toxin production and decreased susceptibility to antibiotic therapy. Reference Ofosu4-Reference Bartlett6 Moreover, ~20%–28% of patients infected with the mentioned strain develop recurrent infection. Reference Pepin, Alary and Valiquette3,Reference Musher, Aslam and Logan7 In 2011, 83,000 recurrences of CDI were estimated in the United States with an annual healthcare cost of US$2.8 billion. Reference Lessa, Mu and Bamberg8,Reference Rodrigues, Barber and Ananthakrishnan9 Multiple preventive efforts to reduce CDI recurrence have been implemented using fidaxomicin, Reference Butler, Olson and Drekonja10 fecal transplant, or bezlotoxumab. Reference Chapin, Lee and McCoy11 Recently, several studies assessed the use of oral vancomycin prophylaxis (OVP) in high-risk populations including elderly patients, immunosuppressed patients, and patients exposed to systemic antibiotics. Reference Pepin, Alary and Valiquette12-Reference Abou Chakra, Pepin and Sirard15 We identified multiple studies published in the past 3 years examining the use of OVP for the primary and secondary prevention of CDI, and we performed a comprehensive systematic review and meta-analysis exploring available evidence to evaluate the benefit of using OVP for the primary and secondary prevention of CDI.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Reference Liberati, Altman and Tetzlaff16 and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Reference Stroup, Berlin and Morton17 guidelines were followed in this systematic review and meta-analysis.
Eligibility criteria
Studies were included if they reported on the efficacy of oral vancomycin prophylaxis for CDI prevention. Adequate description of diagnostic methodology for CDI and recurrent CDI (rCDI) as well as prophylaxis regimen and systemic antibiotics used were required. No limitations were applied based on study design, definition of CDI, recurrence, or prophylaxis regimen used. Only studies published in English language were considered eligible.
Search technique
We performed a computerized search of the MEDLINE, EMBASE and Cochrane databases from inception to March 2019. We used the following search terms: “Clostridium difficile,” “Clostridium difficile recurrence,” “oral vancomycin prophylaxis,” “Clostridium difficile prophylaxis,” “oral vancomycin,” “prophylaxis.” References were reviewed independently by 2 authors (S.B. and B.E.), and case reports, comments, review articles, systematic reviews, practice guidelines, conference abstracts and duplicate publications were excluded. The abstracts of remaining articles were reviewed, and unrelated articles were excluded. The remaining articles were reviewed in detail for eligibility criteria (by B.E. and S.B. independently).
Data extraction and quality assessment
For each study, data were extracted on year published, study design, sample size, population characteristics, OVP regimen, definition of CDI, rCDI as well as vancomycin-resistant enterococci (VRE) infection rates in both OVP and control groups. Quality was assessed using the Newcastle-Ottawa scale (NOS) for observational studies 18 ; studies rated 7 or higher were considered high-quality studies, and studies with scores <6 were considered poor-quality studies.
Statistical analysis
Odds ratios (OR) for CDI, rCDI, and VRE infection with 95% confidence intervals (CI) were calculated based on event/total ratios using a random-effects model. Heterogeneity was assessed using the I2 measure and the Cochran Q statistic. The following stratified analyses were conducted to address sources of heterogeneity: (1) mean age, (2) immune status of studied population, (3) metronidazole use, (4) type of systemic antibiotics used, (5) type of prevention (primary vs secondary), (6) total daily dose of OVP used, and (7) study quality (per NOS). Meta regression analysis was performed to assess covariates, which might explain interstudy variation and help establish sources of heterogeneity in CDI rates among included studies. This analysis also identified factors associated with increased risk of CDI. Publication bias was assessed using the Egger test. Statistical analysis was performed using Comprehensive Meta-Analysis (CMA) version 3.3.070 software (Biostat, Englewood, NJ). P < .05 was considered significant.
Results
Search results
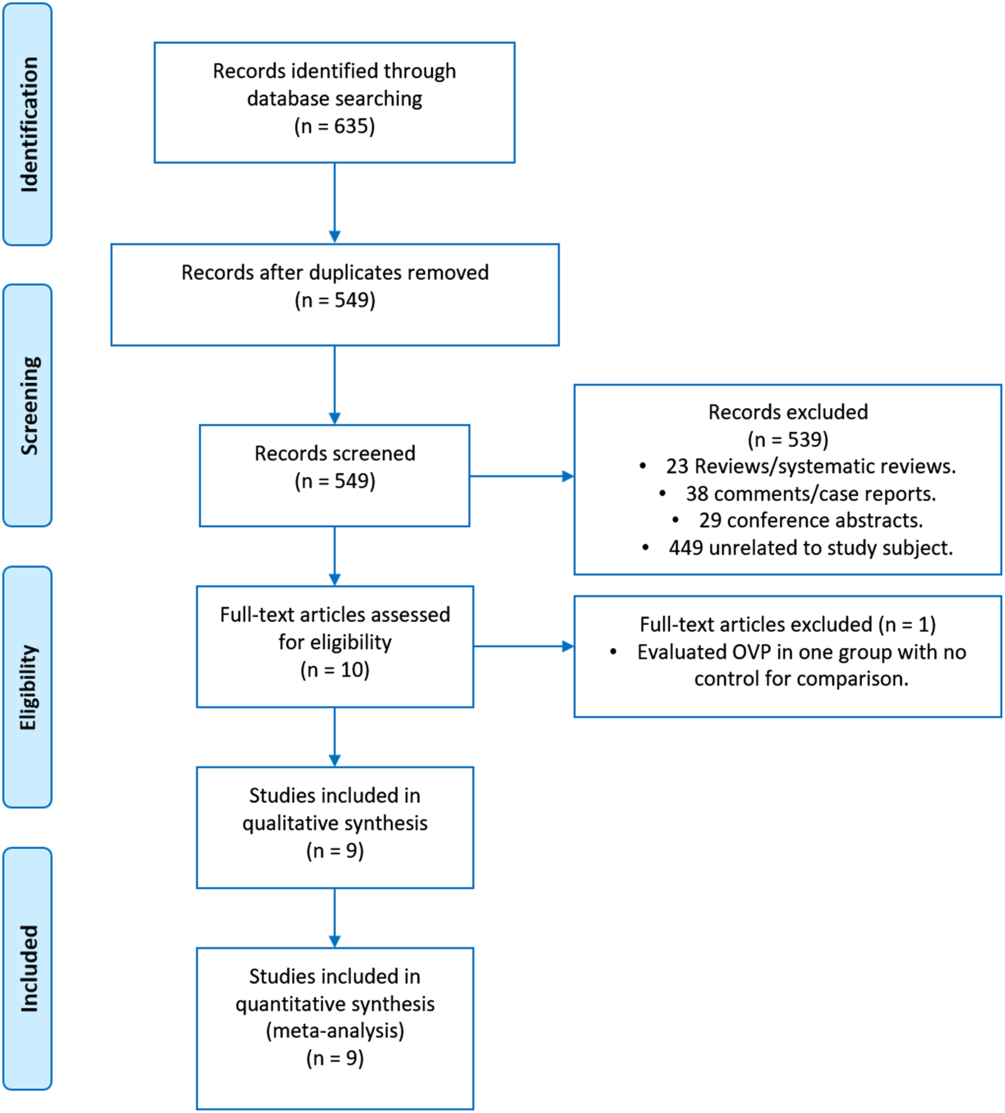
The search identified 635 articles; 86 were excluded as duplicates. The remaining 549 articles were screened, and 539 case reports, comments, reviews or systematic reviews, practice guidelines and unrelated articles were excluded. Thus, 10 studies were reviewed in detail, and 9 met all of the inclusion criteria (Fig. 1).
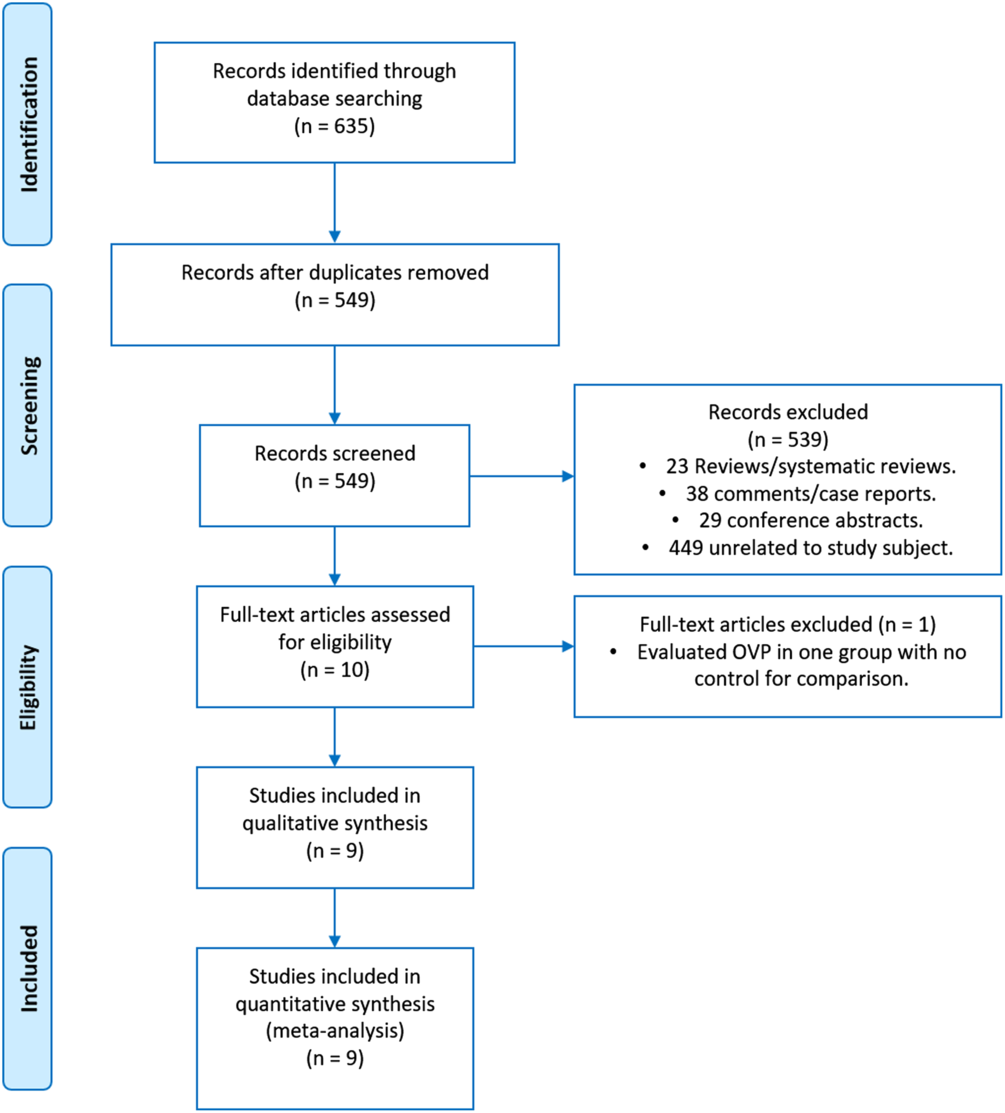
Fig. 1. Flow diagram of record allocation.
Study characteristics
The final review included 8 retrospective cohort studies and 1 randomized prospective study published between 2016 and 2019 that examined a total of 2,174 patients. Of these studies, 7 were conducted in the United States, 1 was conducted in Canada, and 1 in Croatia. Of the 8 cohort studies, 4 evaluated patients with a previous CDI episode requiring systemic antibiotics for a different indication. Reference Van Hise, Bryant and Hennessey19-Reference Caroff, Menchaca and Zhang22 The remaining studies evaluated CDI recurrence in renal transplant patients Reference Splinter, Kerstenetzky and Jorgenson23 and CDI occurrence in hematopoietic stem cell transplant recipients Reference Ganetsky, Han and Hughes24,Reference Morrisette, Van Matre and Miller25 or in elderly patients. Reference Papic, Maric and Vince26,Reference Johnson, Brown and Priest27 The end points of CDI or rCDI were defined as polymerase chain reaction (PCR) assay or toxin-proven CDI within 4 weeks of systemic antibiotic use, Reference Van Hise, Bryant and Hennessey19,Reference Splinter, Kerstenetzky and Jorgenson23 within 60 days of resolution of previous CDI episode, Reference Morrisette, Van Matre and Miller25 within 90 days of systemic antibiotic exposure, Reference Caroff, Menchaca and Zhang22 within 12 months of subsequent hospitalization requiring systemic antibiotics, Reference Knight, Schiller and Fulman21 within 6 months of previous diagnosis, Reference Carignan, Poulin and Martin20 or diarrhea (>3 loose stools in 24 hours) in patients with positive stool PCR for C. difficile >72 hours into hospitalization, Reference Johnson, Brown and Priest27 or when the attending physician ordered OVP empirically. Reference Carignan, Poulin and Martin20 The most common dose used for OVP was 125 mg twice daily. Reference Van Hise, Bryant and Hennessey19,Reference Carignan, Poulin and Martin20,Reference Splinter, Kerstenetzky and Jorgenson23,Reference Ganetsky, Han and Hughes24 Only 3 studies Reference Morrisette, Van Matre and Miller25,Reference Carignan, Poulin and Martin28,Reference Knight, Schiller and Fulman29 included patients who received metronidazole as part of their systemic antibiotic regimen. Study characteristics are listed in Table 1.
Table 1. Study Characteristics

Note. CDI, Clostridium difficile infection; OVP, oral vancomycin prophylaxis; Abx, antibiotics; QD, once daily; BID, twice daily; QID, four times daily; C.diff, Clostridium difficile; PCR, polymerase chain reaction; NOS, Newcastle-Ottawa scale.
Quality assessment
All 9 of the included studies scored 7 or higher on the NOS for retrospective cohort studies and were considered high-quality studies (Supplementary Table 1 online).
Meta-analysis
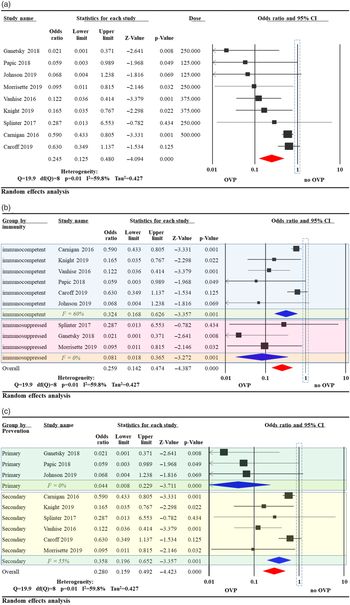
Overall, CDI recurrence was less likely in patients who received OVP compared to controls (odds ratio [OR], 0.245; 95% confidence interval [CI], 0.13–0.48) with significant heterogeneity (I2 = 60%) (Fig. 2A). The studies were further stratified based on immune status of the study population. In 6 studies evaluating immunocompetent patients, OVP was associated with reduced CDI (OR, 0.32; 95% CI, 0.17–0.63; I2 = 60%) Reference Van Hise, Bryant and Hennessey19-Reference Caroff, Menchaca and Zhang22,Reference Papic, Maric and Vince26 compared to (OR, 0.08; 95% CI, 0.02–0.37; I2 = 0%) Reference Splinter, Kerstenetzky and Jorgenson23–Reference Morrisette, Van Matre and Miller25 in 3 studies examining immunosuppressed patients (Fig. 2B). The studies were further stratified based on type of prevention intended (primary vs secondary). In 3 studies evaluating the efficacy of OVP for primary CDI prevention, CDI was less likely to occur in patients receiving OVP (OR, 0.04; 95% CI, 0.01–0.23; I2 = 0%). In 6 studies evaluating the efficacy of OVP for secondary CDI prevention, CDI recurrence was less likely in patients receiving OVP (OR, 0.36; 95% CI, 0.20–0.65; I2 = 55%) (Fig. 2C).

Fig. 2. Odds ratio (OR) of Clostridioides difficile infection (CDI) after oral vancomycin prophylaxis (OVP). (A) Overall OR of CDI after OVP. (B) OR of CDI after OVP subgrouped by patient immune status. (C) OR of CDI after OVP subgrouped by type of prevention intended.
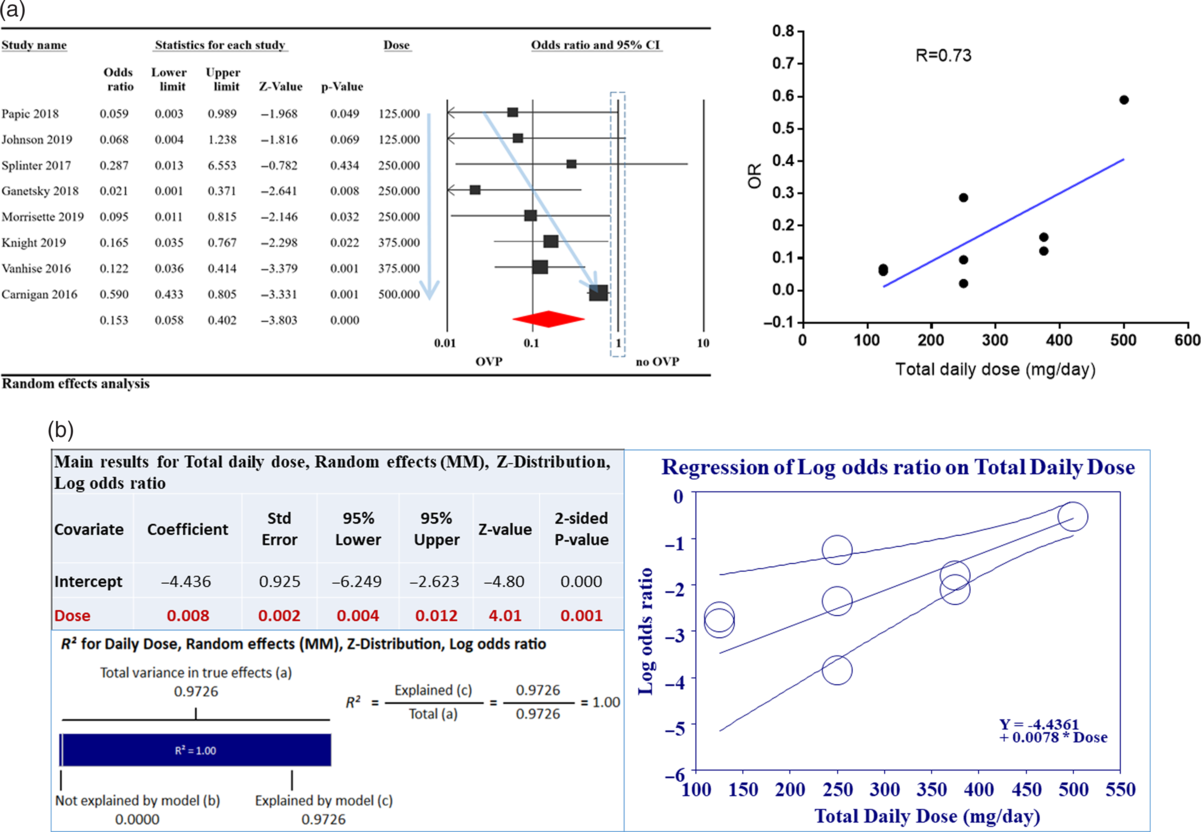
To further address heterogeneity between studies, we performed a meta-regression analysis based on covariates such as (1) mean age, (2) immune status of studied population, (3) metronidazole use, (4) type of systemic antibiotics used, (5) type of prevention (primary vs secondary), (6) total daily dose of OVP used, and (7) study quality per (NOS). Only total daily dose of OVP used showed a significant correlation with odds for CDI (Fig. 3A). This correlation was able to explain 100% of the statistical heterogeneity between included studies (Fig. 3B).
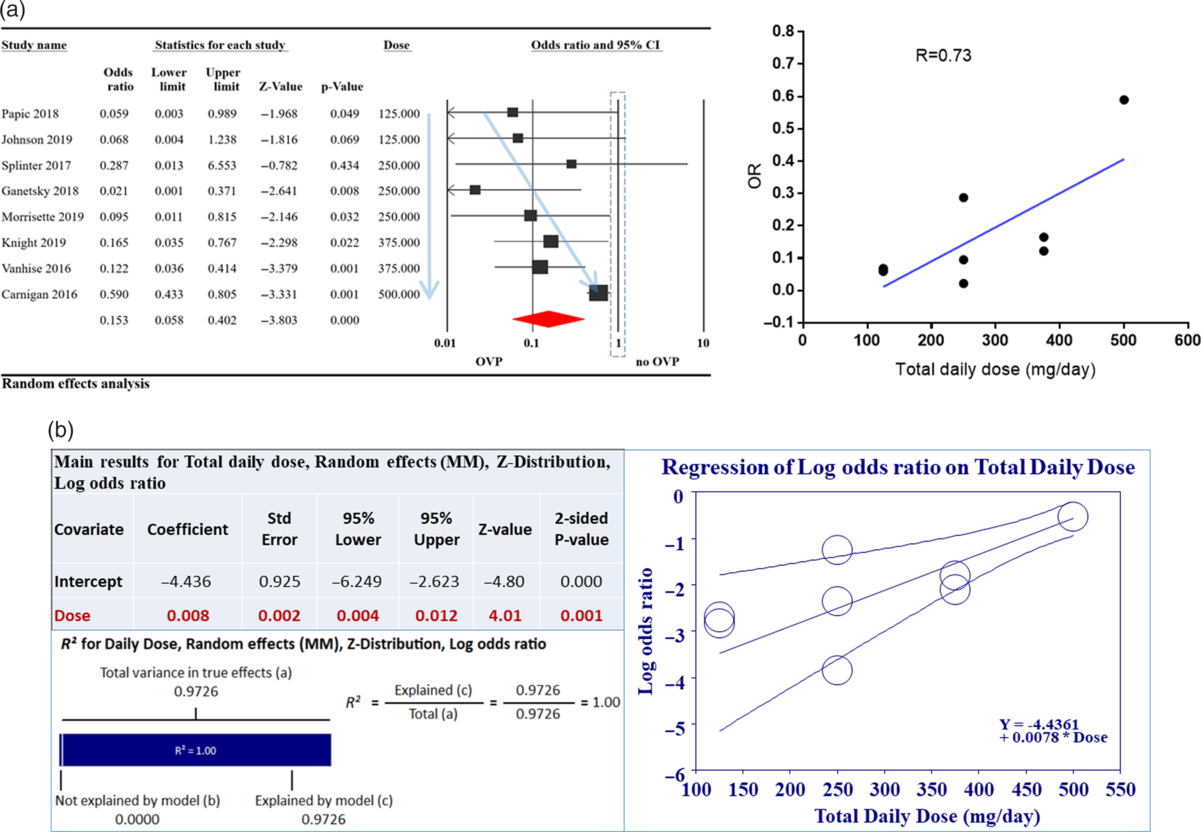
Fig. 3. Relationship of oral vancomycin prophylaxis (OVP) total daily dose to odds ratio (OR) for Clostridioides difficile infection (CDI). (A) Total daily dose of OVP was correlated to OR for CDI with R = 0.73 (right-hand side). This is also depicted on the left with faint blue arrows; as OVP total dose increased, CDI OR increased. (B) Meta regression exploring the relationship between total daily dose of OVP and CDI OR. As indicated in the accompanying table, the correlation was statistically significant and able to explain 100% (R2) of heterogeneity noted between studies. For R2 calculation, to compute the total variance (of all studies about the grand mean), we ran the regression with no covariates. To compute the variance not explained by the model (of all studies about the regression line), we ran the regression with the covariates. (3) The difference between these values gives us the variance explained by the model.
Three studies evaluated the risk of VRE infection after OVP, a pooled analysis of data provided by these studies showed no significant increase in VRE infection rate in the OVP group compared to the control group (Fig. 4). Only Johnson et al Reference Johnson, Brown and Priest27 assessed the risk of VRE colonization after OVP and found no increase in colonization; however, only 64% of patients were tested for VRE after treatment due to patient refusal of perirectal swab. No publication bias was found using the Egger test.

Fig. 4. Odds ratio for vancomycin-resistant enterococci (VRE) infection after oral vancomycin prophylaxis (OVP).
Discussion
This is the first systematic review and meta-analysis to evaluate the efficacy of OVP for the primary and secondary prevention of CDI. Notably, OVP was associated with a significant reduction in CDI. This reduction was seen in both immunocompetent as well as immunosuppressed patients. OVP was associated with reduced primary CDI as well as rCDI. The total daily dose of OVP used was correlated with the OR of CDI. These results emphasize the role of OVP in CDI prevention in hospitalized patients and may suggest that a lower OVP dose could be more effective for CDI prevention.
Despite known risk factors for CDI (including old age, prolonged hospitalization, immunodeficiency, use of proton pump inhibitors and exposure to systemic antibiotics Reference Pepin, Alary and Valiquette12-Reference Abou Chakra, Pepin and Sirard15,Reference Carignan, Poulin and Martin28 ), there are currently no recommendations for CDI prophylaxis in these populations. Although the 2017 IDSA/SHEA C. difficile guidelines suggest that it might be prudent to use low doses of oral vancomycin for prevention of CDI recurrence based on individual institutional policy, Reference Knight, Schiller and Fulman29 there is no mention of OVP use for primary CDI prevention. Moreover, there is no current consensus on the dose of OVP, duration of treatment or long-term outcomes. In this review, we evaluated 8 studies that examined patients with different risk factors for CDI, including previous CDI followed by systemic antibiotic therapy, Reference Van Hise, Bryant and Hennessey19,Reference Caroff, Menchaca and Zhang22,Reference Carignan, Poulin and Martin28,Reference Knight, Schiller and Fulman29 immunosuppression due to solid organ transplant, Reference Splinter, Kerstenetzky and Jorgenson23 or hematopoietic cell transplant, Reference Ganetsky, Han and Hughes24,Reference Morrisette, Van Matre and Miller25 and elderly patients. Reference Papic, Maric and Vince26,Reference Johnson, Brown and Priest27 Despite clear differences between the populations included in the studies, 7 of 9 studies found OVP use to be associated with lower CDI. Splinter et al Reference Splinter, Kerstenetzky and Jorgenson23 and Caroff et al Reference Caroff, Menchaca and Zhang22 found this reduction to be statistically insignificant. However, the first study was underpowered, including only 29 patients, while in the latter the mean duration of OVP treatment was 2.29 days, which might have been insufficient exposure.
OVP use was associated with an overall reduction in CDI (OR, 0.245; 95% CI, 0.13–0.48). However, this result showed significant heterogeneity (I2 = 60%). To address this heterogeneity, we stratified the studies based on the immune status of evaluated patients and type of prevention attempted. Although this stratification showed significant reduction in CDI with OVP use in all subgroups, it was unable to address the overall heterogeneity between studies. This prompted us to perform a meta regression analysis accounting for multiple covariates. Only total daily dose of OVP showed significant correlation with OR of CDI and was able to statistically explain 100% of the heterogeneity between included studies. These results indicate that OVP might be effective in CDI primary and secondary prevention in at-risk patients regardless of their immune status. It also suggests that a lower OVP dose might be associated with lower CDI rates. The mechanism behind this observation is not fully understood; however, oral vancomycin has been shown to significantly affect the intestinal microbial composition, leaving hosts susceptible to pathogenic intestinal colonization Reference Isaac, Scher and Djukovic30 including by Clostridium difficile. Reference Ajami, Cope and Wong31,Reference Johnson, Homann and Bettin32 The effect of oral vancomycin on intestinal flora has also been shown to correlate to vancomycin fecal concentration. Reference Edlund, Barkholt and Olsson-Liljequist33 Therefore, higher OVP doses could result in unintended disruption of the intestinal microbiome.
Duration of treatment varied among studies. Carignan et al Reference Carignan, Poulin and Martin28 showed that OVP was more effective when given for a duration longer than 50% of the duration of systemic antibiotic treatment. However, the remaining studies did not examine the effect of duration of treatment of OVP on treatment success. This issue would be best addressed by prospective randomized controlled trials designed to explore the outcomes of different OVP regimens for CDI prevention.
Concerns over the risk of VRE emergence as a result of long-term or recurrent exposure to oral vancomycin have been reported. Reference Shin, Yong and Kim34 Colonization resistance to VRE does appear to be prolonged by vancomycin tapering regimens in a murine study. Reference Tomas, Mana and Wilson35 Our analysis of 3 studies Reference Ganetsky, Han and Hughes24,Reference Morrisette, Van Matre and Miller25,Reference Knight, Schiller and Fulman29 that evaluated the risk of VRE infection showed no significant increase after OVP; however, data on VRE colonization risks in the included studies were severely limited. This concern will need to be addressed by larger prospective trials examining the long-term outcomes of OVP for CDI prevention.
Our systematic review and meta-analysis has several limitations. In terms of study design, conference proceedings were not reviewed for evaluation and neither were other systematic reviews or review articles individually reviewed, potentially resulting in missed citations. All but 1 of the included studies were retrospective in nature and hence were susceptible to multiple biases, including selection biases and presence of confounders unaccounted for considering the absence of randomization. None of the studies employed propensity matching analysis, which could have improved the reliability of results by controlling for some confounders. The decision of utilizing prophylaxis being deferred to the treating physician creates room for allocation bias. The variability in treatment duration, follow-up time, interstudy heterogeneity in OVP dose, lack of stratification by antibiotic type in terms of CDI risks, and the inability to account for important factors including attrition, and patient comorbidities, are all potential bias sources. The small sample sizes limited our assessment of OVP efficacy in CDI prevention and safety concerns about persistent VRE colonization. Despite the ability of the meta regression analysis to statistically explain heterogeneity, the underreporting of other important variables, including extensive comorbidity evaluation, functional status, living arrangements, and setting of treatment, make this analysis incomplete. All of these biases and limitations can likely be successfully addressed by rigorous prospective randomized controlled trials with long-term follow-up and evaluation of epidemiological metrics for VRE colonization.
In summary, our results suggest that OVP may be associated with reduced rates of primary and secondary CDI. Higher doses of OVP might be associated with higher rates of CDI. However, caution must be exercised interpreting these results while awaiting confirmation by larger prospective, randomized, blinded controlled trials that include uniform dosing and duration of OVP, uniform diagnostic strategies of CDI with algorithm-based testing, and standardized follow up for both efficacy and safety outcomes including VRE colonization and infection.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.277
Acknowledgments
We acknowledge Dr Robert Orenstein of the Department of Infectious diseases at Mayo Clinic-AZ and Dr. Amy Foxx-Orenstein of the Division of Gastroenterology at Mayo Clinic-AZ for reviewing the manuscript and providing expert opinion.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.