1. Introduction
Biological membranes are often modelled as being homogeneous in composition, a simplification which has resulted in a trove of understanding of their shapes, dynamics in flows, fission and beyond. But real biological membranes contain a vast array of proteins which can form domains resulting in spatial variations in material properties, leading to changes in vesicle shapes (Seifert Reference Seifert1997; Hu, Weikl & Lipowsky Reference Hu, Weikl and Lipowsky2011). Simpler systems of synthetic multicomponent vesicles, whose membranes can be composed of different lipid species, have been used to study the rich patterns and accompanying morphologies which emerge from elastic heterogeneity (Baumgart, Hess & Webb Reference Baumgart, Hess and Webb2003; Veatch & Keller Reference Veatch and Keller2003). These findings have been corroborated and expanded upon using both numerical and analytical techniques (Elliott & Stinner Reference Elliott and Stinner2013; Barrett, Garcke & Nürnberg Reference Barrett, Garcke and Nürnberg2017), which in turn are of use when attempting to infer membrane properties experimentally (Engelhardt, Duwe & Sackmann Reference Engelhardt, Duwe and Sackmann1985; Baumgart et al. Reference Baumgart, Das, Webb and Jenkins2005; Tian et al. Reference Tian, Johnson, Wang and Baumgart2007).
The analytical study of vesicles with single-component membranes in flow has a long history. Keller & Skalak (Reference Keller and Skalak1982) demonstrated with a two-dimensional elliptical membrane a transition from tank-treading (in which the membrane shape and orientation are fixed but the membrane material slides along the surface) to tumbling (the long axis rotates in a periodic fashion) beyond a critical interior/exterior viscosity contrast. But in general a vesicle is not ellipsoidal and its shape must be determined through a balance of interfacial forces, for instance, by describing the shape using a series expansion about small parameters such as excess area (Misbah Reference Misbah2006; Lebedev, Turitsyn & Vergeles Reference Lebedev, Turitsyn and Vergeles2007; Vlahovska & Gracia Reference Vlahovska and Gracia2007). Detailed reviews on the small-deformation analyses for both vesicles and capsules are provided by Vlahovska (Reference Vlahovska2015) and Vlahovska & Misbah (Reference Vlahovska and Misbah2019). Barthes-Biesel (Reference Barthes-Biesel1980), Barthes-Biesel & Rallison (Reference Barthes-Biesel and Rallison1981) and Barthes-Biesel (Reference Barthes-Biesel1991) also considered the impact of the internal/external viscosity ratio for nearly spherical capsules assuming zero membrane bending stiffness. The leading-order solution demonstrates that in addition to tank-treading and tumbling behaviour as predicted by the Keller–Skalak model, there is another mode called ‘swinging’ (Noguchi & Gompper Reference Noguchi and Gompper2007), ‘vacillating breathing’ (Misbah Reference Misbah2006) or ‘trembling’ (Kantsler & Steinberg Reference Kantsler and Steinberg2006; Lebedev, Turitsyn & Vergeles Reference Lebedev, Turitsyn and Vergeles2008) in which the orientation of the major axis oscillates while the vesicle undergoes significant deformations – see also the review by Lebedev et al. (Reference Lebedev, Turitsyn and Vergeles2008). Some of the above terms are used interchangeably by various authors, a semantic issue also noted by Misbah (Reference Misbah2012).
Phase diagrams for the shapes and dynamics of vesicles in linear flows have been mapped out by numerous authors (Lebedev et al. Reference Lebedev, Turitsyn and Vergeles2008; Deschamps et al. Reference Deschamps, Kantsler, Segre and Steinberg2009a; Deschamps, Kantsler & Steinberg Reference Deschamps, Kantsler and Steinberg2009b; Zhao & Shaqfeh Reference Zhao and Shaqfeh2011; Zabusky et al. Reference Zabusky, Segre, Deschamps, Kantsler and Steinberg2011; Abreu et al. Reference Abreu, Levant, Steinberg and Seifert2014); see also Barthes-Biesel (Reference Barthes-Biesel2016). The roles of nearby boundaries (Zhao, Spann & Shaqfeh Reference Zhao, Spann and Shaqfeh2011), inertia (Salac & Miksis Reference Salac and Miksis2012), semi-permeability (Quaife, Gannon & Young Reference Quaife, Gannon and Young2021), enclosed particles (Veerapaneni et al. Reference Veerapaneni, Young, Vlahovska and Bławzdziewicz2011b), fluid viscoelasticity (Mushenheim et al. Reference Mushenheim, Pendery, Weibel, Spagnolie and Abbott2016; Seol et al. Reference Seol, Tseng, Kim and Lai2019), thermal fluctuations (Wortis, Jarić & Seifert Reference Wortis, Jarić and Seifert1997; Schneider, Jenkins & Webb Reference Schneider, Jenkins and Webb1984; Morse & Milner Reference Morse and Milner1994; Michalet, Bensimon & Fourcade Reference Michalet, Bensimon and Fourcade1994; Seifert Reference Seifert1999; Finken et al. Reference Finken, Lamura, Seifert and Gompper2008; Ahmadpoor & Sharma Reference Ahmadpoor and Sharma2016) and active internal stresses (Gao & Li Reference Gao and Li2017; Young, Shelley & Stein Reference Young, Shelley and Stein2021) are among the many additional physical and biological features that have been considered, and a large body of literature is devoted to suspensions of many deformable particles such as cells and vesicles in flows (Kantsler, Segre & Steinberg Reference Kantsler, Segre and Steinberg2008; Vlahovska, Podgorski & Misbah Reference Vlahovska, Podgorski and Misbah2009; Veerapaneni et al. Reference Veerapaneni, Rahimian, Biros and Zorin2011a; Zhao, Shaqfeh & Narsimhan Reference Zhao, Shaqfeh and Narsimhan2012; Freund Reference Freund2014; Kumar & Graham Reference Kumar and Graham2015; Raffiee, Dabiri & Ardekani Reference Raffiee, Dabiri and Ardekani2019).
The behaviours of multicomponent vesicles in flows, meanwhile, has only just begun to attract attention. Analytical results are scarce but numerical simulations have offered substantial insight. Simulations in a stationary environment have revealed wrinkling and budding deformations (Li, Lowengrub & Voigt Reference Li, Lowengrub and Voigt2012) and the formation of multicomponent vesicles by adhesion and fusion (Zhao & Du Reference Zhao and Du2011). Sohn et al. (Reference Sohn, Tseng, Li, Voigt and Lowengrub2010) studied two-dimensional multicomponent vesicles in a background shear flow, along with the evolution of distinct surface phases, finding highly complex morphologies and dynamics for highly deformed vesicles. Smith & Uspal (Reference Smith and Uspal2007) showed using dissipative particle dynamics simulations that a shear flow can be used to separate buds from a multicomponent vesicle. The influence of both bending rigidity and spontaneous curvature variation on the equilibrium shape of a vesicle has also been investigated (Cox & Lowengrub Reference Cox and Lowengrub2015). Subsequent boundary integral simulations by Liu et al. (Reference Liu, Marple, Allard, Li, Veerapaneni and Lowengrub2017) showed a transition from tumbling to tank-treading to ‘phase-treading’ of the constituents along the surface upon increasing the shear rate. Analytic results have also shown that a variation of bending rigidity along a surface can induce migration in tank-treading vesicles (Olla Reference Olla2011). Synthetic systems have also been fruitful for testing theoretical predictions. Experiments using a two-phase lipid vesicle in such a flow as a simplified model of red blood cell dynamics showed similarly complex features (Tusch et al. Reference Tusch, Loiseau, Al-Halifa, Khelloufi, Helfer and Viallat2018). Gera & Salac (Reference Gera and Salac2018b) then used simulations to probe a wide array of morphological changes due to spatially varying bending stiffness and line tension between two lipid phases. The phase separation process itself is naturally of great interest, and experiments have been used to study spinodal decomposition and viscous fingering along membrane surfaces (Veatch & Keller Reference Veatch and Keller2003; Lowengrub, Rätz & Voigt Reference Lowengrub, Rätz and Voigt2009; Marenduzzo & Orlandini Reference Marenduzzo and Orlandini2013; Stanich et al. Reference Stanich, Honerkamp-Smith, Putzel, Warth, Lamprecht, Mandal, Mann, Hua and Keller2013).
In this article we derive analytical predictions for a two-dimensional, multicomponent vesicle in a shear flow under the assumption of small deformations and already-formed domains. Among the fruits of the reduced-order model so produced is a single equation describing the inclination angle dynamics when the distribution of material properties varies in the second spatial mode, the frequency in which they interact most strongly with the extensional part of the background flow. In this most dynamic case, a change in behaviour from swinging with tank-treading to tumbling is identified, passing through a transition regime with periodic phase-lagging of the material relative to the vesicle's elongated axis. The method of matched asymptotics is used to produce an approximate solution to the inclination angle equation through this sharp transition, as well as the critical value of the bifurcation parameter signalling the transition from swinging to tumbling which depends on the material property gradient, shear rate and internal/external viscosity contrast. The asymptotic predictions are shown to compare favourably to the results of full numerical simulations, even for highly deformed vesicles.
The paper is organized as follows. After presenting the mathematical framework in § 2 to describe the coupling of the fluid flow and elastic membrane stresses at zero Reynolds number (Stokes flow), an expansion is performed around a nearly circular vesicle to reduce the system down to time-dependent shape equations. The classical case of constant membrane material properties is presented in § 3, in which the results of asymptotic predictions are compared to full numerical simulations. In § 4 attention is turned to the case of interest, that of spatially varying material properties, in which the resulting dynamics is shown to depend strongly on the spectrum of the material properties, and in particular the parity of the number of domains. Concluding remarks are provided in § 5.
2. Mathematical model
2.1. Membrane shape and small deformations
The membrane, or vesicle surface, ![]() $S$, is described by a surface parameterization
$S$, is described by a surface parameterization ![]() ${{\boldsymbol {r}}} (s,t)$, where
${{\boldsymbol {r}}} (s,t)$, where ![]() $s$ is the arclength and
$s$ is the arclength and ![]() $t$ is time. The unit tangent and outward-pointing normal vectors on the surface are written as
$t$ is time. The unit tangent and outward-pointing normal vectors on the surface are written as ![]() ${{\hat {\boldsymbol {s}}}}={{\boldsymbol {r}}}_s$ and
${{\hat {\boldsymbol {s}}}}={{\boldsymbol {r}}}_s$ and ![]() ${{\hat {\boldsymbol {n}}}} = {{\hat {\boldsymbol {s}}}}^{\perp }$. The membrane is assumed area-preserving with area
${{\hat {\boldsymbol {n}}}} = {{\hat {\boldsymbol {s}}}}^{\perp }$. The membrane is assumed area-preserving with area ![]() $A$ and inextensible with length
$A$ and inextensible with length ![]() $L=2{\rm \pi} a$ (so that
$L=2{\rm \pi} a$ (so that ![]() $s\in [0,L)$), where a is the characteristic radius.
$s\in [0,L)$), where a is the characteristic radius.
In the event that the membrane area is not far removed from that of a circle of length ![]() $L$, it becomes convenient to work in polar coordinates
$L$, it becomes convenient to work in polar coordinates ![]() $(r,\theta )$, with unit vectors
$(r,\theta )$, with unit vectors ![]() ${{\hat {\boldsymbol {r}}}}$ and
${{\hat {\boldsymbol {r}}}}$ and ![]() $\hat {\boldsymbol {\theta }}$, and we represent the surface
$\hat {\boldsymbol {\theta }}$, and we represent the surface ![]() $S$ as
$S$ as ![]() ${{\boldsymbol {r}}} (s(\theta,t),t)= r(\theta,t) {{\hat {\boldsymbol {r}}}}(\theta ) = a(1+\varepsilon \rho (\theta,t)+\varepsilon ^{2}\rho ^{(2)}(\theta,t) ) {{\hat {\boldsymbol {r}}}}(\theta )$, where
${{\boldsymbol {r}}} (s(\theta,t),t)= r(\theta,t) {{\hat {\boldsymbol {r}}}}(\theta ) = a(1+\varepsilon \rho (\theta,t)+\varepsilon ^{2}\rho ^{(2)}(\theta,t) ) {{\hat {\boldsymbol {r}}}}(\theta )$, where ![]() $\varepsilon$ is a small non-negative constant. For small
$\varepsilon$ is a small non-negative constant. For small ![]() $\varepsilon$ we have
$\varepsilon$ we have ![]() ${{\hat {\boldsymbol {s}}}}= \hat {\boldsymbol {\theta }}+\varepsilon \rho _\theta {{\hat {\boldsymbol {r}}}} +O(\varepsilon ^{2})$ and
${{\hat {\boldsymbol {s}}}}= \hat {\boldsymbol {\theta }}+\varepsilon \rho _\theta {{\hat {\boldsymbol {r}}}} +O(\varepsilon ^{2})$ and ![]() ${{\hat {\boldsymbol {n}}}}={{\hat {\boldsymbol {r}}}}-\varepsilon \rho _\theta \hat {\boldsymbol {\theta }}+O(\varepsilon ^{2})$. A schematic is provided in figure 1. Fourier series representations of the shape functions
${{\hat {\boldsymbol {n}}}}={{\hat {\boldsymbol {r}}}}-\varepsilon \rho _\theta \hat {\boldsymbol {\theta }}+O(\varepsilon ^{2})$. A schematic is provided in figure 1. Fourier series representations of the shape functions ![]() $\rho$ and
$\rho$ and ![]() $\rho ^{(2)}$ are given by
$\rho ^{(2)}$ are given by
 \begin{equation} \rho(\theta,t)= \sum_{n=0}^{\infty}{a_{n}} (t)\cos(n \theta)+{b_{n}} (t)\sin(n \theta),\end{equation}
\begin{equation} \rho(\theta,t)= \sum_{n=0}^{\infty}{a_{n}} (t)\cos(n \theta)+{b_{n}} (t)\sin(n \theta),\end{equation}
where ![]() $a_n(t)$ and
$a_n(t)$ and ![]() $b_n(t)$ are the time-varying Fourier coefficients, with a similar expression for
$b_n(t)$ are the time-varying Fourier coefficients, with a similar expression for ![]() $\rho ^{(2)}(\theta,t)$ with coefficients
$\rho ^{(2)}(\theta,t)$ with coefficients ![]() ${a^{(2)}_{n}}(t)$ and
${a^{(2)}_{n}}(t)$ and ![]() ${b^{(2)}_{n}}(t)$. The length of the membrane may then be written (suppressing the time dependence for the sake of presentation), for
${b^{(2)}_{n}}(t)$. The length of the membrane may then be written (suppressing the time dependence for the sake of presentation), for ![]() $\varepsilon \ll 1$ as
$\varepsilon \ll 1$ as
 \begin{equation} L = \int_0^{2{\rm \pi}} |{{\boldsymbol{r}}}_\theta|\,{\rm d}\theta = 2 {\rm \pi}a\left(1+\varepsilon a_0 + \varepsilon^{2}a_0^{(2)}\right)+\frac{{\rm \pi} a \varepsilon^{2}}{2}\sum_{n=1}^{\infty} n^{2}\left(a_n^{2}+b_n^{2}\right)+O(\varepsilon^{3}). \end{equation}
\begin{equation} L = \int_0^{2{\rm \pi}} |{{\boldsymbol{r}}}_\theta|\,{\rm d}\theta = 2 {\rm \pi}a\left(1+\varepsilon a_0 + \varepsilon^{2}a_0^{(2)}\right)+\frac{{\rm \pi} a \varepsilon^{2}}{2}\sum_{n=1}^{\infty} n^{2}\left(a_n^{2}+b_n^{2}\right)+O(\varepsilon^{3}). \end{equation}
Fixing the membrane length to ![]() $2{\rm \pi} a$ thus requires that
$2{\rm \pi} a$ thus requires that ![]() ${a_0}=0$ and
${a_0}=0$ and
 \begin{equation} a_0^{(2)} ={-}\sum_{n=1}^{\infty} \frac{n^{2}}{4}\left(a_n^{2}+b_n^{2}\right), \end{equation}
\begin{equation} a_0^{(2)} ={-}\sum_{n=1}^{\infty} \frac{n^{2}}{4}\left(a_n^{2}+b_n^{2}\right), \end{equation}and the enclosed area may in that case be written as
 \begin{equation} A=\int^{2{\rm \pi}}_{0} \frac{r^{2}}{2}{\rm d}\theta ={\rm \pi} a^{2}\left(1-\frac{\varepsilon^{2}}{2}\sum_{n=1}^{\infty}\left(n^{2}-1\right)\left({a_{n}} ^{2}+{b_{n}} ^{2} \right)\right)+O(\varepsilon^{3}).\end{equation}
\begin{equation} A=\int^{2{\rm \pi}}_{0} \frac{r^{2}}{2}{\rm d}\theta ={\rm \pi} a^{2}\left(1-\frac{\varepsilon^{2}}{2}\sum_{n=1}^{\infty}\left(n^{2}-1\right)\left({a_{n}} ^{2}+{b_{n}} ^{2} \right)\right)+O(\varepsilon^{3}).\end{equation}
The constant ![]() $\varepsilon$ may be written in terms of the area enclosed by the membrane if desired as
$\varepsilon$ may be written in terms of the area enclosed by the membrane if desired as ![]() $\varepsilon =(2/3)^{1/2}(1-R_A)^{1/2}/Q$, where
$\varepsilon =(2/3)^{1/2}(1-R_A)^{1/2}/Q$, where ![]() $R_A = A/({\rm \pi} a^{2})$ is the ‘reduced area’ (equal to unity when the membrane is circular) and
$R_A = A/({\rm \pi} a^{2})$ is the ‘reduced area’ (equal to unity when the membrane is circular) and
 \begin{equation} Q=\left(\tfrac 13\sum_{n=1}^{\infty}(n^{2}-1)({a_{n}} ^{2}+{b_{n}} ^{2})\right)^{1/2}. \end{equation}
\begin{equation} Q=\left(\tfrac 13\sum_{n=1}^{\infty}(n^{2}-1)({a_{n}} ^{2}+{b_{n}} ^{2})\right)^{1/2}. \end{equation}
The value of ![]() $Q$ must be constant in time if the dynamics is area-preserving. The Fourier contributions at mode
$Q$ must be constant in time if the dynamics is area-preserving. The Fourier contributions at mode ![]() $n=1$ correspond to translation of the vesicle without shape change up to
$n=1$ correspond to translation of the vesicle without shape change up to ![]() $O(\varepsilon ^{3})$, and hence do not contribute in the expression above.
$O(\varepsilon ^{3})$, and hence do not contribute in the expression above.

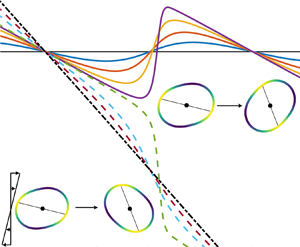
Figure 1. (a) Schematic of the two-dimensional inextensible membrane with spatially varying bending stiffness (or spontaneous curvature) in a shear flow, ![]() $\dot {\gamma } y{{\hat {\boldsymbol {x}}}}$;
$\dot {\gamma } y{{\hat {\boldsymbol {x}}}}$; ![]() $\kappa$ denotes the spatial variation in bending stiffness in (2.14a,b). Softer regions are lighter in colour than the darker, stiffer domains. Here the material properties vary in the second spatial mode (e.g. the membrane has two stiffer domains). (b) The stream function for the external and internal flows are denoted by
$\kappa$ denotes the spatial variation in bending stiffness in (2.14a,b). Softer regions are lighter in colour than the darker, stiffer domains. Here the material properties vary in the second spatial mode (e.g. the membrane has two stiffer domains). (b) The stream function for the external and internal flows are denoted by ![]() $\psi ^{+}$ and
$\psi ^{+}$ and ![]() $\psi ^{-}$, respectively; the viscous traction,
$\psi ^{-}$, respectively; the viscous traction, ![]() ${{\boldsymbol {f}}}$, in (2.7), instantaneously balances the elastic traction in (2.12).
${{\boldsymbol {f}}}$, in (2.7), instantaneously balances the elastic traction in (2.12).
2.2. Stokes equations and viscous traction
The incompressible Stokes equations describing viscous flow both outside (![]() $+$) and inside (
$+$) and inside (![]() $-$) the vesicle are given by
$-$) the vesicle are given by
where ![]() ${{\boldsymbol {u}}}({{\boldsymbol {x}}},t)$ is the fluid velocity a point
${{\boldsymbol {u}}}({{\boldsymbol {x}}},t)$ is the fluid velocity a point ![]() ${{\boldsymbol {x}}}=(x,y)$ at time
${{\boldsymbol {x}}}=(x,y)$ at time ![]() $t$ and
$t$ and ![]() $\boldsymbol {\sigma }^{\pm } = -p^{\pm }{{\boldsymbol {I}}}+\mu ^{\pm }(\boldsymbol {\nabla }{{\boldsymbol {u}}} ^{\pm }+\boldsymbol {\nabla }^{T}{{\boldsymbol {u}}} ^{\pm })$ are the Newtonian stress-tensors for each fluid domain, with
$\boldsymbol {\sigma }^{\pm } = -p^{\pm }{{\boldsymbol {I}}}+\mu ^{\pm }(\boldsymbol {\nabla }{{\boldsymbol {u}}} ^{\pm }+\boldsymbol {\nabla }^{T}{{\boldsymbol {u}}} ^{\pm })$ are the Newtonian stress-tensors for each fluid domain, with ![]() $p^{\pm }$ and
$p^{\pm }$ and ![]() $\mu ^{\pm }$ the pressures and fluid viscosities external and internal to the membrane. The undisturbed background flow is a linear, horizontal shear flow with shear rate
$\mu ^{\pm }$ the pressures and fluid viscosities external and internal to the membrane. The undisturbed background flow is a linear, horizontal shear flow with shear rate ![]() $\dot {\gamma }$,
$\dot {\gamma }$, ![]() ${{\boldsymbol {u}}} = \dot {\gamma } y {{\hat {\boldsymbol {x}}}}$, with constant pressure
${{\boldsymbol {u}}} = \dot {\gamma } y {{\hat {\boldsymbol {x}}}}$, with constant pressure ![]() $p_\infty$. A no-slip boundary condition is assumed between the fluid and membrane velocities on both sides of the membrane (there is no relative slipping between the inner and outer membrane surfaces). The local viscous tractions,
$p_\infty$. A no-slip boundary condition is assumed between the fluid and membrane velocities on both sides of the membrane (there is no relative slipping between the inner and outer membrane surfaces). The local viscous tractions, ![]() ${{\boldsymbol {f}}} ^{\pm } = \pm {{\hat {\boldsymbol {n}}}} \boldsymbol {\cdot } \boldsymbol {\sigma }^{\pm }$, acting on the membrane from the exterior and interior interfaces result in the combined local viscous traction
${{\boldsymbol {f}}} ^{\pm } = \pm {{\hat {\boldsymbol {n}}}} \boldsymbol {\cdot } \boldsymbol {\sigma }^{\pm }$, acting on the membrane from the exterior and interior interfaces result in the combined local viscous traction
with ![]() $[f]_S=(f^{+}-f^{-})|_S$ defined to be the jump in
$[f]_S=(f^{+}-f^{-})|_S$ defined to be the jump in ![]() $f$ across the boundary
$f$ across the boundary ![]() $S$.
$S$.
The continuity equation in the bulk fluid is immediately satisfied with the introduction of a stream function, ![]() $\psi$, defined such that
$\psi$, defined such that ![]() ${{\boldsymbol {u}}} = \boldsymbol {\nabla }^{\perp } \psi = \psi _y {{\hat {\boldsymbol {x}}}}-\psi _x {{\hat {\boldsymbol {y}}}}$. The Stokes equations then reduce to biharmonic equations interior and exterior to the membrane:
${{\boldsymbol {u}}} = \boldsymbol {\nabla }^{\perp } \psi = \psi _y {{\hat {\boldsymbol {x}}}}-\psi _x {{\hat {\boldsymbol {y}}}}$. The Stokes equations then reduce to biharmonic equations interior and exterior to the membrane:
with ![]() $\psi ^{+} \to \dot {\gamma } y^{2}/2$ as
$\psi ^{+} \to \dot {\gamma } y^{2}/2$ as ![]() $|{{\boldsymbol {x}}}|\to \infty$, the background shear flow. The general form of the
$|{{\boldsymbol {x}}}|\to \infty$, the background shear flow. The general form of the ![]() $\theta$-periodic solution to the biharmonic equation is given in Appendix A. Continuity of velocity across the membrane boundary demands that
$\theta$-periodic solution to the biharmonic equation is given in Appendix A. Continuity of velocity across the membrane boundary demands that
and surface inextensibility along the membrane demands that
where ![]() $\boldsymbol {\nabla }_s$ is the surface del operator.
$\boldsymbol {\nabla }_s$ is the surface del operator.
2.3. Force and moment balance
The membrane is modelled as a thin linearly elastic shell. The bending moment is approximated by ![]() ${{\boldsymbol {M}}}=B(s)(H-\tilde {H}(s)) {{\hat {\boldsymbol {x}}}} \times {{\hat {\boldsymbol {y}}}}$, where
${{\boldsymbol {M}}}=B(s)(H-\tilde {H}(s)) {{\hat {\boldsymbol {x}}}} \times {{\hat {\boldsymbol {y}}}}$, where ![]() $B(s)$ and
$B(s)$ and ![]() $\tilde {H}(s)$ are the spatially varying bending stiffness and spontaneous curvature, and
$\tilde {H}(s)$ are the spatially varying bending stiffness and spontaneous curvature, and ![]() $H = {{\hat {\boldsymbol {s}}}} \boldsymbol {\cdot } \partial _s{{\hat {\boldsymbol {n}}}}$ is the mean curvature. Force and moment balance along the membrane surface at arclength
$H = {{\hat {\boldsymbol {s}}}} \boldsymbol {\cdot } \partial _s{{\hat {\boldsymbol {n}}}}$ is the mean curvature. Force and moment balance along the membrane surface at arclength ![]() $s$ are given by
$s$ are given by ![]() ${\rm d}{{\boldsymbol {M}}}/{\rm d}s+{{\hat {\boldsymbol {s}}}} \times {{\boldsymbol {F}}} =\boldsymbol {0}$ and
${\rm d}{{\boldsymbol {M}}}/{\rm d}s+{{\hat {\boldsymbol {s}}}} \times {{\boldsymbol {F}}} =\boldsymbol {0}$ and ![]() ${{\boldsymbol {f}}} _{elastic}+{{\boldsymbol {f}}} =\boldsymbol {0}$, where
${{\boldsymbol {f}}} _{elastic}+{{\boldsymbol {f}}} =\boldsymbol {0}$, where ![]() ${{\boldsymbol {M}}}$ is a thickness-averaged first moment of the elastic stress with units of force,
${{\boldsymbol {M}}}$ is a thickness-averaged first moment of the elastic stress with units of force, ![]() ${{\boldsymbol {f}}} _{elastic}$ is the elastic force per area of the membrane on a surrounding medium, with
${{\boldsymbol {f}}} _{elastic}$ is the elastic force per area of the membrane on a surrounding medium, with ![]() ${{\boldsymbol {f}}} _{elastic}={\rm d}{{\boldsymbol {F}}} /{\rm d}s$, and
${{\boldsymbol {f}}} _{elastic}={\rm d}{{\boldsymbol {F}}} /{\rm d}s$, and ![]() ${{\boldsymbol {f}}}$ is the viscous traction acting on the membrane, (2.7). Defining the tangential component of
${{\boldsymbol {f}}}$ is the viscous traction acting on the membrane, (2.7). Defining the tangential component of ![]() ${{\boldsymbol {F}}}$ as
${{\boldsymbol {F}}}$ as ![]() $T(s)$ (the tension per unit length), we then have
$T(s)$ (the tension per unit length), we then have
and the elastic force acting on the surrounding medium is then given by
where subscripts indicate partial derivatives. A different expression for the traction appears in the literature starting with the Helfrich free energy which amounts to adding a term ![]() $B(s)(H-\tilde {H}(s))^{2}/2$ to
$B(s)(H-\tilde {H}(s))^{2}/2$ to ![]() $T$ above (Guckenberger & Gekle Reference Guckenberger and Gekle2017); see also the review articles by Powers (Reference Powers2010) and Deserno (Reference Deserno2015). In either case
$T$ above (Guckenberger & Gekle Reference Guckenberger and Gekle2017); see also the review articles by Powers (Reference Powers2010) and Deserno (Reference Deserno2015). In either case ![]() $T$ plays the mathematical role of a Lagrange multiplier which assures instantaneous membrane inextensibility, at every moment taking whatever form is necessary to enforce this constraint.
$T$ plays the mathematical role of a Lagrange multiplier which assures instantaneous membrane inextensibility, at every moment taking whatever form is necessary to enforce this constraint.
2.4. Non-dimensionalization
A competition of viscous and elastic effects emerges when the stresses associated with the flow, the material property variations and the shape deformations are all on the same scale. In order to see this more clearly, the system is made dimensionless by scaling lengths by ![]() $a$, velocities by
$a$, velocities by ![]() $a \dot {\gamma }$, forces by
$a \dot {\gamma }$, forces by ![]() $\mu ^{+} a^{2} \dot {\gamma }$, stresses by
$\mu ^{+} a^{2} \dot {\gamma }$, stresses by ![]() $\mu ^{+} \dot {\gamma }$ and energies by
$\mu ^{+} \dot {\gamma }$ and energies by ![]() $\mu ^{+} a^{3} \dot {\gamma }$, while time is scaled upon
$\mu ^{+} a^{3} \dot {\gamma }$, while time is scaled upon ![]() $\varepsilon /\dot {\gamma }$. The remaining dimensionless scalar parameters governing the system are
$\varepsilon /\dot {\gamma }$. The remaining dimensionless scalar parameters governing the system are
where ![]() $R_A$ is the reduced area,
$R_A$ is the reduced area, ![]() $\lambda$ is the inner/outer viscosity ratio,
$\lambda$ is the inner/outer viscosity ratio, ![]() $\tilde {H}_0$ is the mean spontaneous curvature (with
$\tilde {H}_0$ is the mean spontaneous curvature (with ![]() $\langle \cdot \rangle$ an average over the membrane perimeter),
$\langle \cdot \rangle$ an average over the membrane perimeter), ![]() $\textit {Ca}$ is the bending capillary number and
$\textit {Ca}$ is the bending capillary number and ![]() $\mathcal {C}$ is a parameter which is
$\mathcal {C}$ is a parameter which is ![]() $O(1)$ as
$O(1)$ as ![]() $\varepsilon \to 0$. In addition to these scalar parameters, and with variations away from their mean values assumed to be small, we have the dimensionless distributions of the bending stiffness and spontaneous curvature along the membrane surface as
$\varepsilon \to 0$. In addition to these scalar parameters, and with variations away from their mean values assumed to be small, we have the dimensionless distributions of the bending stiffness and spontaneous curvature along the membrane surface as
respectively. Like the membrane shape we represent ![]() $\kappa(\theta,t)$ by its Fourier series,
$\kappa(\theta,t)$ by its Fourier series, ![]() $\kappa (\theta,t) = \sum _{n=1}^{\infty } c_{n}(t)\cos (n\theta )+d_{n}(t)\sin (n\theta )$, and
$\kappa (\theta,t) = \sum _{n=1}^{\infty } c_{n}(t)\cos (n\theta )+d_{n}(t)\sin (n\theta )$, and ![]() $\zeta (\theta,t)$ similarly with coefficients
$\zeta (\theta,t)$ similarly with coefficients ![]() $e_{n}(t)$ and
$e_{n}(t)$ and ![]() $f_{n}(t)$. Henceforth all variables are understood to be dimensionless.
$f_{n}(t)$. Henceforth all variables are understood to be dimensionless.
For a membrane of length ![]() $2{\rm \pi} a\approx 120\ \mathrm {\mu }{\rm m}$ and bending rigidity
$2{\rm \pi} a\approx 120\ \mathrm {\mu }{\rm m}$ and bending rigidity ![]() $B\approx 20 k_b T$, with k b the Boltzmann constant, as measured for a vesicle composed of dioleoylphosphatidylcholine (DOPC) lipids (Dahl et al. Reference Dahl, Narsimhan, Gouveia, Kumar, Shaqfeh and Muller2016; Faizi et al. Reference Faizi, Reeves, Georgiev, Vlahovska and Dimova2020), and using the viscosity of water,
$B\approx 20 k_b T$, with k b the Boltzmann constant, as measured for a vesicle composed of dioleoylphosphatidylcholine (DOPC) lipids (Dahl et al. Reference Dahl, Narsimhan, Gouveia, Kumar, Shaqfeh and Muller2016; Faizi et al. Reference Faizi, Reeves, Georgiev, Vlahovska and Dimova2020), and using the viscosity of water, ![]() $\mu ^{+} \approx 10^{-3}\ {\rm Pa}\ {\rm s}$, the bending capillary number
$\mu ^{+} \approx 10^{-3}\ {\rm Pa}\ {\rm s}$, the bending capillary number ![]() $\textit {Ca}$ is roughly
$\textit {Ca}$ is roughly ![]() $100\,\dot {\gamma } [1\ \text {s}]$ (e.g. if
$100\,\dot {\gamma } [1\ \text {s}]$ (e.g. if ![]() $\dot {\gamma }=10^{-1}\ {\rm s}^{-1}$ then
$\dot {\gamma }=10^{-1}\ {\rm s}^{-1}$ then ![]() $\textit {Ca}\approx 10$). The experimental work of Baumgart et al. (Reference Baumgart, Das, Webb and Jenkins2005), where the bending rigidity ratio is approximately
$\textit {Ca}\approx 10$). The experimental work of Baumgart et al. (Reference Baumgart, Das, Webb and Jenkins2005), where the bending rigidity ratio is approximately ![]() $1.25$, corresponds here to
$1.25$, corresponds here to ![]() $\|\varepsilon \kappa \|_\infty \approx 0.1$. The capillary number is highly sensitive to the size; for instance, using a length more appropriate to modelling a red blood cell,
$\|\varepsilon \kappa \|_\infty \approx 0.1$. The capillary number is highly sensitive to the size; for instance, using a length more appropriate to modelling a red blood cell, ![]() $2{\rm \pi} a\approx 20\ \mathrm {\mu }{\rm m}$, and with
$2{\rm \pi} a\approx 20\ \mathrm {\mu }{\rm m}$, and with ![]() $B\approx 50 k_b T$ (Evans Reference Evans1983), then
$B\approx 50 k_b T$ (Evans Reference Evans1983), then ![]() $\textit {Ca}\approx \dot {\gamma } /4$. We proceed with the understanding that all variables are now dimensionless. The dimensionless background flow, for instance, is given by
$\textit {Ca}\approx \dot {\gamma } /4$. We proceed with the understanding that all variables are now dimensionless. The dimensionless background flow, for instance, is given by ![]() ${{\boldsymbol {u}}} = y {{\hat {\boldsymbol {x}}}}$, and the dimensionless membrane perimeter is
${{\boldsymbol {u}}} = y {{\hat {\boldsymbol {x}}}}$, and the dimensionless membrane perimeter is ![]() $L=2{\rm \pi}$.
$L=2{\rm \pi}$.
2.5. Membrane shape dynamics
In order to compute the dynamics of the membrane shape, the traction balance is carried out order by order in ![]() $\varepsilon$, included as Appendix B, and the tension so found is used instantaneously to solve for the stream function, included as Appendix C. To summarize the results, expansions are written for the pressure,
$\varepsilon$, included as Appendix B, and the tension so found is used instantaneously to solve for the stream function, included as Appendix C. To summarize the results, expansions are written for the pressure, ![]() $p=p^{(0)}+\varepsilon p^{(1)}+\dots$, tension
$p=p^{(0)}+\varepsilon p^{(1)}+\dots$, tension ![]() $T=T^{(0)}+\varepsilon T^{(1)}+\dots$ and velocity
$T=T^{(0)}+\varepsilon T^{(1)}+\dots$ and velocity ![]() ${{\boldsymbol {u}}}= u_n {{\hat {\boldsymbol {n}}}}+ u_s {{\hat {\boldsymbol {s}}}}=(u_n^{(1)}+\varepsilon u_n^{(2)}+\dots ){{\hat {\boldsymbol {n}}}}+(u_s^{(1)}+\varepsilon u_s^{(2)}+\dots ){{\hat {\boldsymbol {s}}}}$. The normal and tangential components of the velocity are given at leading order by
${{\boldsymbol {u}}}= u_n {{\hat {\boldsymbol {n}}}}+ u_s {{\hat {\boldsymbol {s}}}}=(u_n^{(1)}+\varepsilon u_n^{(2)}+\dots ){{\hat {\boldsymbol {n}}}}+(u_s^{(1)}+\varepsilon u_s^{(2)}+\dots ){{\hat {\boldsymbol {s}}}}$. The normal and tangential components of the velocity are given at leading order by
 \begin{gather} \left.u_n\right|_{S}=\frac{1}{(1+\lambda)}\sin(2 \theta)- 2 \sum_{n=2}^{\infty} n\left(A_n \cos(n\theta)+B_n \sin(n\theta) \right)+O(\varepsilon), \end{gather}
\begin{gather} \left.u_n\right|_{S}=\frac{1}{(1+\lambda)}\sin(2 \theta)- 2 \sum_{n=2}^{\infty} n\left(A_n \cos(n\theta)+B_n \sin(n\theta) \right)+O(\varepsilon), \end{gather} \begin{gather}\left.u_s\right|_{S}={-}\frac{1}{2}+\frac{1}{2(1+\lambda)}\cos(2 \theta)-2 \sum_{n=2}^{\infty}\left( B_n \cos(n\theta)- A_n \sin(n\theta) \right)+O(\varepsilon), \end{gather}
\begin{gather}\left.u_s\right|_{S}={-}\frac{1}{2}+\frac{1}{2(1+\lambda)}\cos(2 \theta)-2 \sum_{n=2}^{\infty}\left( B_n \cos(n\theta)- A_n \sin(n\theta) \right)+O(\varepsilon), \end{gather}where
 \begin{equation} A_n =\frac{\mathcal{C}^{{-}1} \left[\alpha_n(t) a_n+(1-\tilde{H}_0 )c_n-e_n\right]}{4(1+\lambda)},\quad B_n =\frac{\mathcal{C}^{{-}1} \left[\alpha_n(t) b_n+(1-\tilde{H}_0 )d_n-f_n\right]}{4(1+\lambda)}. \end{equation}
\begin{equation} A_n =\frac{\mathcal{C}^{{-}1} \left[\alpha_n(t) a_n+(1-\tilde{H}_0 )c_n-e_n\right]}{4(1+\lambda)},\quad B_n =\frac{\mathcal{C}^{{-}1} \left[\alpha_n(t) b_n+(1-\tilde{H}_0 )d_n-f_n\right]}{4(1+\lambda)}. \end{equation}
Here we have used the Fourier coefficients for the variations in shape given by ![]() $a_n, b_n$, in bending stiffness by
$a_n, b_n$, in bending stiffness by ![]() $c_n, d_n$, and in spontaneous curvature by
$c_n, d_n$, and in spontaneous curvature by ![]() $e_n, f_n$, and that
$e_n, f_n$, and that ![]() $\mathcal {C}=\textit {Ca}/\varepsilon =O(1)$ as
$\mathcal {C}=\textit {Ca}/\varepsilon =O(1)$ as ![]() $\varepsilon \to 0$, and have defined
$\varepsilon \to 0$, and have defined
The function ![]() $P_0(t)$ is the leading-order mean pressure jump across the membrane, or equivalently the scaled mean tension, (the two are bound together by an elastic analogue of the Young–Laplace law) and is given by
$P_0(t)$ is the leading-order mean pressure jump across the membrane, or equivalently the scaled mean tension, (the two are bound together by an elastic analogue of the Young–Laplace law) and is given by
 \begin{equation} P_0(t)=\frac{14b_2-\mathcal{C}^{{-}1}\displaystyle\sum _{n=2}^{\infty}n(2n^{2}-1)C_n}{\displaystyle\sum _{n=2}^{\infty}n(2n^{2}-1)({a_{n}} ^{2}+{b_{n}} ^{2})},\end{equation}
\begin{equation} P_0(t)=\frac{14b_2-\mathcal{C}^{{-}1}\displaystyle\sum _{n=2}^{\infty}n(2n^{2}-1)C_n}{\displaystyle\sum _{n=2}^{\infty}n(2n^{2}-1)({a_{n}} ^{2}+{b_{n}} ^{2})},\end{equation}where
Note that ![]() $n=2$ terms are present inside the summations in (2.15), (2.16) and (2.19).
$n=2$ terms are present inside the summations in (2.15), (2.16) and (2.19).
Finally, the dynamics of the membrane shape is found using the normal component of the velocity field along the surface. As derived in Appendix D, the shape functions satisfy
with no ambiguity about the stream function (internal or external) owing to the continuity of velocity, (2.9). The end result is that the Fourier modes describing the membrane shape at first order in ![]() $\varepsilon$ evolve according to
$\varepsilon$ evolve according to
where ![]() $\alpha _n(t)$ is given in (2.18), and
$\alpha _n(t)$ is given in (2.18), and ![]() $\delta _{n2}$ is unity when
$\delta _{n2}$ is unity when ![]() $n=2$ and is zero otherwise. In addition,
$n=2$ and is zero otherwise. In addition, ![]() $a_1(t) = a_1(0)$ and
$a_1(t) = a_1(0)$ and ![]() $b_1(t) = b_1(0)$, which represents that the system is insensitive to translations of the membrane in either direction at first order in
$b_1(t) = b_1(0)$, which represents that the system is insensitive to translations of the membrane in either direction at first order in ![]() $\varepsilon$ (the body translates along with the background flow with any vertical perturbation but the shape dynamics is unchanged). The
$\varepsilon$ (the body translates along with the background flow with any vertical perturbation but the shape dynamics is unchanged). The ![]() $n=2$ mode is special since this corresponds to elongation along the principal direction of the background shear flow, at an angle
$n=2$ mode is special since this corresponds to elongation along the principal direction of the background shear flow, at an angle ![]() ${\rm \pi} /4$ relative to the
${\rm \pi} /4$ relative to the ![]() $x$-axis.
$x$-axis.
Since ![]() $P_0(t)$ depends on the membrane shape, the expressions above are immediately nonlinear, even when only considering the leading-order shape dynamics in small
$P_0(t)$ depends on the membrane shape, the expressions above are immediately nonlinear, even when only considering the leading-order shape dynamics in small ![]() $\varepsilon$. If the mean spontaneous curvature is unity (
$\varepsilon$. If the mean spontaneous curvature is unity (![]() $\tilde {H}_0 =1$) the membrane remains close enough to its preferred state at first order in
$\tilde {H}_0 =1$) the membrane remains close enough to its preferred state at first order in ![]() $\varepsilon$ so that no additional forces are induced by bending, and only spontaneous curvature variations affect the shape dynamics. For any other mean spontaneous curvature (
$\varepsilon$ so that no additional forces are induced by bending, and only spontaneous curvature variations affect the shape dynamics. For any other mean spontaneous curvature (![]() $\tilde {H}_0 \neq 1$), however, the effects of spontaneous curvature are mathematically indistinguishable from bending stiffness at leading order via (2.23)–(2.24). For the remainder of the paper, we will assume zero spontaneous curvature (
$\tilde {H}_0 \neq 1$), however, the effects of spontaneous curvature are mathematically indistinguishable from bending stiffness at leading order via (2.23)–(2.24). For the remainder of the paper, we will assume zero spontaneous curvature (![]() $e_n=f_n=0$ for all
$e_n=f_n=0$ for all ![]() $n$, and
$n$, and ![]() $\tilde {H}_0 =0$), but all of the results to come can be viewed as owing to variations to spontaneous curvature rather than bending stiffness, or any combination thereof.
$\tilde {H}_0 =0$), but all of the results to come can be viewed as owing to variations to spontaneous curvature rather than bending stiffness, or any combination thereof.
3. Dynamics of a membrane with uniform material properties
We begin by studying the dynamics of a membrane with uniform bending stiffness (![]() $c_n =d_n=0$ for all
$c_n =d_n=0$ for all ![]() $n$, and zero spontaneous curvature). In the steady (moving) state, since
$n$, and zero spontaneous curvature). In the steady (moving) state, since ![]() ${a_{n}}$ and
${a_{n}}$ and ![]() ${b_{n}}$ are constant in time, the pressure jump
${b_{n}}$ are constant in time, the pressure jump ![]() $P_0(t)$ in (2.19) is also constant in time. The dynamics in (2.23)–(2.24) then reveals that all Fourier components vanish exponentially fast with the exception of
$P_0(t)$ in (2.19) is also constant in time. The dynamics in (2.23)–(2.24) then reveals that all Fourier components vanish exponentially fast with the exception of ![]() $b_2$, leaving the steady shape function
$b_2$, leaving the steady shape function ![]() $\rho (\theta,t) = \tilde {b}_{2} \sin (2\theta )$, with
$\rho (\theta,t) = \tilde {b}_{2} \sin (2\theta )$, with ![]() $\tilde {b}_{2}$ easily determined using area conservation alone:
$\tilde {b}_{2}$ easily determined using area conservation alone:
Here ![]() $R_A$ is the reduced area, having referenced (2.4) when only
$R_A$ is the reduced area, having referenced (2.4) when only ![]() $b_2$ is non-zero.
$b_2$ is non-zero.
In particular, a membrane with an initial shape of the form ![]() $\rho (\theta,0)=b\sin (2\theta )$ is instantly in a steady state for any
$\rho (\theta,0)=b\sin (2\theta )$ is instantly in a steady state for any ![]() $b$ at first order in
$b$ at first order in ![]() $\varepsilon$. This corresponds to a tilt angle of
$\varepsilon$. This corresponds to a tilt angle of ![]() ${\rm \pi} /4$ between the vesicle's elongated axis and the direction of flow. Although the shape is stationary, material is still moving along the tangential direction in a so-called tank-treading motion. In this configuration, the steady-state pressure jump is given by
${\rm \pi} /4$ between the vesicle's elongated axis and the direction of flow. Although the shape is stationary, material is still moving along the tangential direction in a so-called tank-treading motion. In this configuration, the steady-state pressure jump is given by ![]() $P_0 = -3\mathcal {C}^{-1}+\tilde {b}_{2}^{-1}$.
$P_0 = -3\mathcal {C}^{-1}+\tilde {b}_{2}^{-1}$.
Since the bending stiffness is uniform, we are able to examine the steady shape and orientation to higher order in ![]() $\varepsilon$. Assuming that the membrane shape has already relaxed to the point that
$\varepsilon$. Assuming that the membrane shape has already relaxed to the point that ![]() $u_n^{(1)}=0$, and hence
$u_n^{(1)}=0$, and hence ![]() $\rho _t=0$ from (2.21), a straight-forward continuation of the regular asymptotic expansion yields equations describing the fluid flow at second order resulting in the normal velocity on the membrane surface
$\rho _t=0$ from (2.21), a straight-forward continuation of the regular asymptotic expansion yields equations describing the fluid flow at second order resulting in the normal velocity on the membrane surface
 \begin{align} u_n^{(2)}&= \tilde{b}_{2} \cos(2\theta)-\frac{3\tilde{b}_{2}^{2}}{2(1+\lambda)}\left(7\mathcal{C}^{{-}1}+\tilde{b}_{2}^{{-}1} \right) \cos(4 \theta)\nonumber\\ &\quad -\frac{1}{2(1+\lambda)}\sum_{n=2}^{\infty} n\left((n^{2}-4)\mathcal{C}^{{-}1}+ \tilde{b}_{2}^{{-}1}\right)\left( {a^{(2)}_{n}} \cos(n\theta)+{b^{(2)}_{n}} \sin(n\theta)\right). \end{align}
\begin{align} u_n^{(2)}&= \tilde{b}_{2} \cos(2\theta)-\frac{3\tilde{b}_{2}^{2}}{2(1+\lambda)}\left(7\mathcal{C}^{{-}1}+\tilde{b}_{2}^{{-}1} \right) \cos(4 \theta)\nonumber\\ &\quad -\frac{1}{2(1+\lambda)}\sum_{n=2}^{\infty} n\left((n^{2}-4)\mathcal{C}^{{-}1}+ \tilde{b}_{2}^{{-}1}\right)\left( {a^{(2)}_{n}} \cos(n\theta)+{b^{(2)}_{n}} \sin(n\theta)\right). \end{align}
(Note that there are ![]() $\cos (2\theta )$ and
$\cos (2\theta )$ and ![]() $\cos (4\theta )$ terms inside the infinite sum.) The steady state at second order is reached once
$\cos (4\theta )$ terms inside the infinite sum.) The steady state at second order is reached once ![]() $u_n^{(2)}=0$:
$u_n^{(2)}=0$:
where
with ![]() $\tilde {b}_{2}$ given in (3.1). Although we assumed above that
$\tilde {b}_{2}$ given in (3.1). Although we assumed above that ![]() $\mathcal {C} = O(1)$ as
$\mathcal {C} = O(1)$ as ![]() $\varepsilon \to 0$, the limit of infinite capillary number matches the results of Zahalak, Rao & Sutera (Reference Zahalak, Rao and Sutera1987) who assumed zero bending stiffness. That the zero-bending-stiffness limit is recovered as
$\varepsilon \to 0$, the limit of infinite capillary number matches the results of Zahalak, Rao & Sutera (Reference Zahalak, Rao and Sutera1987) who assumed zero bending stiffness. That the zero-bending-stiffness limit is recovered as ![]() $\mathcal {C} \to \infty$ likely identifies this as a regular limit and not a singular one, though a more general analysis for arbitrary
$\mathcal {C} \to \infty$ likely identifies this as a regular limit and not a singular one, though a more general analysis for arbitrary ![]() $\mathcal {C}$ would be needed to make this result rigorous.
$\mathcal {C}$ would be needed to make this result rigorous.
3.1. Steady-state deformation and inclination angle
The deformation parameter and orientation angle are two common metrics used to characterize the dynamics of a membrane in flow. The Taylor deformation parameter is defined as ![]() $D=(L_1-L_2)/(L_1+L_2)$, where
$D=(L_1-L_2)/(L_1+L_2)$, where ![]() $2L_1$ and
$2L_1$ and ![]() $2L_2$ are the major and minor axis lengths of an ellipse which shares the same inertia tensor, derived in Appendix E, resulting as
$2L_2$ are the major and minor axis lengths of an ellipse which shares the same inertia tensor, derived in Appendix E, resulting as ![]() $\varepsilon \to 0$ in the representation
$\varepsilon \to 0$ in the representation
For the case of uniform bending stiffness in the tank-treading steady state,
which is notably independent of any other physics in the problem. The eigenvectors of the inertia tensor, meanwhile, are used to define an inclination angle, ![]() $\phi$, the angle between the elongated axis of the membrane and the direction of flow, which has representation (see Appendix E)
$\phi$, the angle between the elongated axis of the membrane and the direction of flow, which has representation (see Appendix E)
 \begin{equation} \phi(t)=\arctan{\left( \frac{-a_{2} +\sqrt{a_{2}^{2} + b_{2}^{2}} }{b_{2}} \right) } +\varepsilon \frac{\left(b^{(2)}_{2}a_{2} -a^{(2)}_{2}b_{2} \right)}{2 \left(a_{2}^{2} + b_{2}^{2} \right)}+O(\varepsilon^{2}). \end{equation}
\begin{equation} \phi(t)=\arctan{\left( \frac{-a_{2} +\sqrt{a_{2}^{2} + b_{2}^{2}} }{b_{2}} \right) } +\varepsilon \frac{\left(b^{(2)}_{2}a_{2} -a^{(2)}_{2}b_{2} \right)}{2 \left(a_{2}^{2} + b_{2}^{2} \right)}+O(\varepsilon^{2}). \end{equation}In the case of uniform bending stiffness, in the steady state we find the angle
consistent with the theory of Finken et al. (Reference Finken, Lamura, Seifert and Gompper2008) for ![]() $\varepsilon \ll 1$. The predictions above are plotted in figure 2 as lines for a range of reduced areas
$\varepsilon \ll 1$. The predictions above are plotted in figure 2 as lines for a range of reduced areas ![]() $R_A$.
$R_A$.

Figure 2. The steady-state deformation parameter (a) and inclination angle (b) in the case of constant bending stiffness with viscosity ratio ![]() $\lambda =1$ and
$\lambda =1$ and ![]() $\varepsilon$ varying from
$\varepsilon$ varying from ![]() $0$ to
$0$ to ![]() $0.15$. Simulations (symbols) and analysis (lines) are in close agreement even for highly deformed membranes.
$0.15$. Simulations (symbols) and analysis (lines) are in close agreement even for highly deformed membranes.
For nearly circular membranes, the inclination angle approaches ![]() ${\rm \pi} /4$. When fluid is removed from the interior of the membrane, the inclination angle decreases and the membrane tilts forward towards the direction of flow. An increase in the viscosity ratio
${\rm \pi} /4$. When fluid is removed from the interior of the membrane, the inclination angle decreases and the membrane tilts forward towards the direction of flow. An increase in the viscosity ratio ![]() $\lambda =\mu ^{-}/\mu ^{+}$ further tilts the membrane down towards the direction of flow. From (3.9) the critical value of the viscosity ratio for which the steady inclination angle is equal to zero scales as
$\lambda =\mu ^{-}/\mu ^{+}$ further tilts the membrane down towards the direction of flow. From (3.9) the critical value of the viscosity ratio for which the steady inclination angle is equal to zero scales as ![]() $1/(1-R_A)^{1/2}$ as
$1/(1-R_A)^{1/2}$ as ![]() $R_A \to 1$. Beyond this critical viscosity ratio the membrane shape is no longer fixed in space and instead undergoes periodic tumbling. The result is independent of the capillary number, so the same result has been observed in previous work that assumes zero bending rigidity (Zahalak et al. Reference Zahalak, Rao and Sutera1987) and elsewhere with constant bending stiffness (Finken et al. Reference Finken, Lamura, Seifert and Gompper2008). The result also qualitatively matches the dynamics of a membrane in three dimensions studied by Vlahovska & Gracia (Reference Vlahovska and Gracia2007), where the inclination angle was also found to be independent of bending rigidity in the small-deformation regime.
$R_A \to 1$. Beyond this critical viscosity ratio the membrane shape is no longer fixed in space and instead undergoes periodic tumbling. The result is independent of the capillary number, so the same result has been observed in previous work that assumes zero bending rigidity (Zahalak et al. Reference Zahalak, Rao and Sutera1987) and elsewhere with constant bending stiffness (Finken et al. Reference Finken, Lamura, Seifert and Gompper2008). The result also qualitatively matches the dynamics of a membrane in three dimensions studied by Vlahovska & Gracia (Reference Vlahovska and Gracia2007), where the inclination angle was also found to be independent of bending rigidity in the small-deformation regime.
To assess the validity of the asymptotic approximations derived above, we solve the complete fluid-structure interaction problem numerically. The incompressible Navier–Stokes equations (which limit to the Stokes equations in (2.8) as the Reynolds number tends to zero) are solved at Reynolds number ![]() $10^{-3}$ on a regular grid using a projection method (Kolahdouz & Salac Reference Kolahdouz and Salac2015) and the vesicle is represented using a semi-implicit level set scheme (Osher & Fedkiw Reference Osher and Fedkiw2002). A generalized minimal residual algorithm (GMRES) with algebraic multigrid as provided by the Portable, Extensible Toolkit for Scientific Computation (PETSc) library (Balay et al. Reference Balay, Brown, Buschelman, Gropp, Kaushik, Knepley, McInnes, Smith and Zhang2012, Reference Balay2018, Reference Balay, Gropp, McInnes and Smith1997) is used for the level-set solver. Derivatives of the level sets are also tracked, in a so-called ‘jet’-scheme, to improve the accuracy of interpolants needed to communicate information from the membrane to the fluid and vice versa (Nave, Rosales & Seibold Reference Nave, Rosales and Seibold2010; Seibold, Rosales & Nave Reference Seibold, Rosales and Nave2012). More details on the numerical methods used and a convergence study for the code are available in the literature (Velmurugan, Kolahdouz & Salac Reference Velmurugan, Kolahdouz and Salac2016; Gera & Salac Reference Gera and Salac2018a).
$10^{-3}$ on a regular grid using a projection method (Kolahdouz & Salac Reference Kolahdouz and Salac2015) and the vesicle is represented using a semi-implicit level set scheme (Osher & Fedkiw Reference Osher and Fedkiw2002). A generalized minimal residual algorithm (GMRES) with algebraic multigrid as provided by the Portable, Extensible Toolkit for Scientific Computation (PETSc) library (Balay et al. Reference Balay, Brown, Buschelman, Gropp, Kaushik, Knepley, McInnes, Smith and Zhang2012, Reference Balay2018, Reference Balay, Gropp, McInnes and Smith1997) is used for the level-set solver. Derivatives of the level sets are also tracked, in a so-called ‘jet’-scheme, to improve the accuracy of interpolants needed to communicate information from the membrane to the fluid and vice versa (Nave, Rosales & Seibold Reference Nave, Rosales and Seibold2010; Seibold, Rosales & Nave Reference Seibold, Rosales and Nave2012). More details on the numerical methods used and a convergence study for the code are available in the literature (Velmurugan, Kolahdouz & Salac Reference Velmurugan, Kolahdouz and Salac2016; Gera & Salac Reference Gera and Salac2018a).
Figure 2 includes the results of the full simulations (symbols). The steady-state deformation parameter and inclination angle both show excellent agreement with the numerical simulations (and the predicted order of accuracy as ![]() $\varepsilon \to 0$, not shown), providing fortuitous accuracy even for substantial membrane deformations where the asymptotic approximations are not immediately expected to hold. The slight overestimate of the deformation parameter for general
$\varepsilon \to 0$, not shown), providing fortuitous accuracy even for substantial membrane deformations where the asymptotic approximations are not immediately expected to hold. The slight overestimate of the deformation parameter for general ![]() $\varepsilon$ is accompanied by a slight underestimate of the inclination angle, owing to the higher velocities sampled by a more elongated vesicle. In general, the transition between tank-treading and tumbling can depend weakly on the bending capillary number, which the above analysis suggests enters at the next order in
$\varepsilon$ is accompanied by a slight underestimate of the inclination angle, owing to the higher velocities sampled by a more elongated vesicle. In general, the transition between tank-treading and tumbling can depend weakly on the bending capillary number, which the above analysis suggests enters at the next order in ![]() $\varepsilon$ (Lebedev et al. Reference Lebedev, Turitsyn and Vergeles2007; Noguchi Reference Noguchi2010; Zhao & Shaqfeh Reference Zhao and Shaqfeh2011).
$\varepsilon$ (Lebedev et al. Reference Lebedev, Turitsyn and Vergeles2007; Noguchi Reference Noguchi2010; Zhao & Shaqfeh Reference Zhao and Shaqfeh2011).
4. Dynamics of a membrane with variable material properties
If the membrane composition is not uniform, the advection of material around the surface can contribute substantially to the membrane dynamics. Again owing to the mathematically similar contributions of bending stiffness and spontaneous curvature variation we focus our attention on bending stiffness variations. Since the bending stiffness and its spatial variation, ![]() $\kappa$, are assumed to be material quantities, they evolve in time according to a surface advection equation which is coupled to the shape equations, introducing a serious analytical challenge. For the sake of tractability, however, we assume that mode mixing is small and treat
$\kappa$, are assumed to be material quantities, they evolve in time according to a surface advection equation which is coupled to the shape equations, introducing a serious analytical challenge. For the sake of tractability, however, we assume that mode mixing is small and treat ![]() $\kappa$ as simply advecting by the mean tangential velocity
$\kappa$ as simply advecting by the mean tangential velocity ![]() $-1/2$ in (2.16). We will see that this approximation leads to predictions that match very well with the results of full numerical simulations. With the bending stiffness variation confined to a single mode
$-1/2$ in (2.16). We will see that this approximation leads to predictions that match very well with the results of full numerical simulations. With the bending stiffness variation confined to a single mode ![]() $M$ with amplitude
$M$ with amplitude ![]() $\bar {\kappa }$ (assumed positive), we thus write
$\bar {\kappa }$ (assumed positive), we thus write
where ![]() $c_M(t)=\bar {\kappa }\cos (\varepsilon M t/2)$ and
$c_M(t)=\bar {\kappa }\cos (\varepsilon M t/2)$ and ![]() $d_M(t)=-\bar {\kappa }\sin (\varepsilon Mt/2)$. The shape equations are still those in (2.23)–(2.24), with
$d_M(t)=-\bar {\kappa }\sin (\varepsilon Mt/2)$. The shape equations are still those in (2.23)–(2.24), with ![]() $c_m$ and
$c_m$ and ![]() $d_m$ also now appearing in (2.19). The cases
$d_m$ also now appearing in (2.19). The cases ![]() $M=2$ and
$M=2$ and ![]() $M \neq 2$ are of distinctly different character, and we now proceed to consider them independently.
$M \neq 2$ are of distinctly different character, and we now proceed to consider them independently.
4.1. A bifurcation in the dynamic case,  $M= 2$
$M= 2$
The situation in which the bending stiffness variation is present in the second spatial mode (i.e. two stiff domains, as in figure 1) is a special case, as this is where the distribution of material properties most strongly interacts with the elongating deformation induced by the flow. From (2.23)–(2.24) the shape dynamics in the second mode evolve according to
where ![]() $\alpha _2(t)=3+\mathcal {C} P_0(t)$. Inserting
$\alpha _2(t)=3+\mathcal {C} P_0(t)$. Inserting ![]() $P_0(t)$, or equivalently solving for
$P_0(t)$, or equivalently solving for ![]() $\alpha _2(t)$ so that
$\alpha _2(t)$ so that ![]() ${\rm d}(a_2^{2}+b_2^{2})/{\rm d}t={\rm d}(Q^{2})/{\rm d}t=0$, the above simplify to
${\rm d}(a_2^{2}+b_2^{2})/{\rm d}t={\rm d}(Q^{2})/{\rm d}t=0$, the above simplify to
where
Recall that ![]() $Q$ is a constant which is set at
$Q$ is a constant which is set at ![]() $t=0$; if the initial shape deformation resides only in the second Fourier mode, for instance, then
$t=0$; if the initial shape deformation resides only in the second Fourier mode, for instance, then ![]() $Q=(a_2(0)^{2}+b_2(0)^{2})^{1/2}$. At first order in
$Q=(a_2(0)^{2}+b_2(0)^{2})^{1/2}$. At first order in ![]() $\varepsilon$ there is no change in the deformation parameter:
$\varepsilon$ there is no change in the deformation parameter: ![]() $D(t) = (2/3)^{1/2}(1-R_A)^{1/2}+O(\varepsilon ^{2})$, so the observed shape does not exhibit large variations in time. The inclination angle, however, reveals something striking. Writing
$D(t) = (2/3)^{1/2}(1-R_A)^{1/2}+O(\varepsilon ^{2})$, so the observed shape does not exhibit large variations in time. The inclination angle, however, reveals something striking. Writing ![]() $(a_2,b_2)=Q(\cos 2\phi ^{(0)},\sin 2\phi ^{(0)})$ (the inclination angle is given by
$(a_2,b_2)=Q(\cos 2\phi ^{(0)},\sin 2\phi ^{(0)})$ (the inclination angle is given by ![]() $\phi = \phi ^{(0)}+O(\varepsilon )$) and inserting into (4.4a,b), an equation for
$\phi = \phi ^{(0)}+O(\varepsilon )$) and inserting into (4.4a,b), an equation for ![]() $\phi ^{(0)}$ arises:
$\phi ^{(0)}$ arises:
with ![]() $\beta =(1+\lambda )^{-1}Q^{-1}$. This equation is more constructively analysed by defining the slower time scale
$\beta =(1+\lambda )^{-1}Q^{-1}$. This equation is more constructively analysed by defining the slower time scale ![]() $\tau =\varepsilon t$, so that
$\tau =\varepsilon t$, so that
Numerical solutions of (4.7) for ![]() $\bar {\kappa }\mathcal {C}^{-1}=0.8$ and
$\bar {\kappa }\mathcal {C}^{-1}=0.8$ and ![]() $\bar {\kappa }\mathcal {C}^{-1}=1.2$ are shown as lines in figure 3(a), for
$\bar {\kappa }\mathcal {C}^{-1}=1.2$ are shown as lines in figure 3(a), for ![]() $\varepsilon =10^{-2}$,
$\varepsilon =10^{-2}$, ![]() $\lambda =1$ and
$\lambda =1$ and ![]() $Q=3$. The dynamics alternate between a slow linear drift of
$Q=3$. The dynamics alternate between a slow linear drift of ![]() $\phi ^{(0)}(\tau )$ where
$\phi ^{(0)}(\tau )$ where ![]() $\phi ^{(0)}_\tau =O(1)$ and a rapid departure when
$\phi ^{(0)}_\tau =O(1)$ and a rapid departure when ![]() $\phi ^{(0)}$ is near zero. Figure 4, along with supplementary movies M1–M3 available at https://doi.org/10.1017/jfm.2022.40, show the complex dynamics associated with these plots. When
$\phi ^{(0)}$ is near zero. Figure 4, along with supplementary movies M1–M3 available at https://doi.org/10.1017/jfm.2022.40, show the complex dynamics associated with these plots. When ![]() $\bar {\kappa }\mathcal {C}^{-1} =0.8$, the elongated axis swings back and forth relative to the direction of flow; when
$\bar {\kappa }\mathcal {C}^{-1} =0.8$, the elongated axis swings back and forth relative to the direction of flow; when ![]() $\bar {\kappa }\mathcal {C}^{-1}=1.2$, the shape slowly nears a zero inclination angle, then undergoes a rapid tumble. Also shown in figure 3(a) as symbols are the results found using the full numerical simulations, as in § 3, showing close agreement with the solutions generated by (4.7).
$\bar {\kappa }\mathcal {C}^{-1}=1.2$, the shape slowly nears a zero inclination angle, then undergoes a rapid tumble. Also shown in figure 3(a) as symbols are the results found using the full numerical simulations, as in § 3, showing close agreement with the solutions generated by (4.7).

Figure 3. (a) The inclination angle as a function of time from simulations (symbols) and theory (lines), with ![]() $\varepsilon =10^{-2}$,
$\varepsilon =10^{-2}$, ![]() $Q=3$, or
$Q=3$, or ![]() $R_A=0.998$ and
$R_A=0.998$ and ![]() $\lambda =1$. Swinging is observed for
$\lambda =1$. Swinging is observed for ![]() $\bar {\kappa }\mathcal {C}^{-1} =0.8$, tumbling for
$\bar {\kappa }\mathcal {C}^{-1} =0.8$, tumbling for ![]() $\kappa =1.2$. Both include additional tank-treading motion, indicated by lines in the snapshots – see also figure 4 and supplementary movies M1–M3. (b) Contours of the minimum inclination angle during periodic orbits,
$\kappa =1.2$. Both include additional tank-treading motion, indicated by lines in the snapshots – see also figure 4 and supplementary movies M1–M3. (b) Contours of the minimum inclination angle during periodic orbits, ![]() $\min _t \phi (t)$, computed using the full numerical simulations. Note that
$\min _t \phi (t)$, computed using the full numerical simulations. Note that ![]() $\bar {\kappa }\mathcal {C}^{-1}=\varepsilon \bar {\kappa }\textit {Ca}^{-1}$ so that the vertical axis also depends on
$\bar {\kappa }\mathcal {C}^{-1}=\varepsilon \bar {\kappa }\textit {Ca}^{-1}$ so that the vertical axis also depends on ![]() $\varepsilon$.
$\varepsilon$.

Figure 4. Snapshots of the dynamics associated with figure 3(a): swinging with tank-treading (![]() $\bar {\kappa }\mathcal {C}^{-1}=0.8$, a) and tumbling with phase-lagging (
$\bar {\kappa }\mathcal {C}^{-1}=0.8$, a) and tumbling with phase-lagging (![]() $\bar {\kappa }\mathcal {C}^{-1}=1.2$, b) with variable bending stiffness in the
$\bar {\kappa }\mathcal {C}^{-1}=1.2$, b) with variable bending stiffness in the ![]() $M=2$ mode. A line in each snapshot connects the two softer regions, which are lighter in colour than the darker, stiffer regions. The vesicle elongates so that the softer regions tend to sit in large curvature regions, while the principal direction of the background flow stretches the vesicle towards inclination angle
$M=2$ mode. A line in each snapshot connects the two softer regions, which are lighter in colour than the darker, stiffer regions. The vesicle elongates so that the softer regions tend to sit in large curvature regions, while the principal direction of the background flow stretches the vesicle towards inclination angle ![]() ${\rm \pi} /4$. See also supplementary movies M1–M3.
${\rm \pi} /4$. See also supplementary movies M1–M3.
When ![]() $\bar {\kappa }\mathcal {C}^{-1}$ is small, the bending stiffness variation only introduces a periodic perturbation of the constant bending stiffness dynamics. Linearizing (4.7) about
$\bar {\kappa }\mathcal {C}^{-1}$ is small, the bending stiffness variation only introduces a periodic perturbation of the constant bending stiffness dynamics. Linearizing (4.7) about ![]() $\phi^{(0)}={\rm \pi}/4$, for small
$\phi^{(0)}={\rm \pi}/4$, for small ![]() $\bar{\kappa}\mathcal{C}^{-1}$ we arrive at the periodic solution
$\bar{\kappa}\mathcal{C}^{-1}$ we arrive at the periodic solution
whose period, ![]() $\Delta \tau =2{\rm \pi}$ (or
$\Delta \tau =2{\rm \pi}$ (or ![]() $\Delta t = 2{\rm \pi} /\varepsilon$), is twice that of the material's tangential motion along the surface (since the mean surface tangential velocity is
$\Delta t = 2{\rm \pi} /\varepsilon$), is twice that of the material's tangential motion along the surface (since the mean surface tangential velocity is ![]() $-\varepsilon /2$), owing naturally to the number (two) of stiffer domains. Material tank-treads tangentially along the membrane while the shape swings back and forth. The rapid slipping in figure 3(a) for the smaller
$-\varepsilon /2$), owing naturally to the number (two) of stiffer domains. Material tank-treads tangentially along the membrane while the shape swings back and forth. The rapid slipping in figure 3(a) for the smaller ![]() $\bar {\kappa }\mathcal {C}^{-1}$ value occurs when the stiffer material passes quickly over the region of highest curvature.
$\bar {\kappa }\mathcal {C}^{-1}$ value occurs when the stiffer material passes quickly over the region of highest curvature.
For very large values of ![]() $\bar {\kappa }\mathcal {C}^{-1}$, the dynamics limit to a pure (rigid-body) tumbling motion. Assuming a regular perturbation expansion in small
$\bar {\kappa }\mathcal {C}^{-1}$, the dynamics limit to a pure (rigid-body) tumbling motion. Assuming a regular perturbation expansion in small ![]() $\mathcal {C}/\bar {\kappa }$, the inclination angle has the asymptotic behaviour
$\mathcal {C}/\bar {\kappa }$, the inclination angle has the asymptotic behaviour
with all other parameters assumed ![]() $O(1)$. When
$O(1)$. When ![]() $\bar {\kappa }\mathcal {C}^{-1}$ is finite, the tumbling motion is joined by a small relative tangential material oscillation. This periodic phase-lag of the material becomes more pronounced as
$\bar {\kappa }\mathcal {C}^{-1}$ is finite, the tumbling motion is joined by a small relative tangential material oscillation. This periodic phase-lag of the material becomes more pronounced as ![]() $\bar {\kappa }\mathcal {C}^{-1}$ is reduced closer to unity, and vanishes as
$\bar {\kappa }\mathcal {C}^{-1}$ is reduced closer to unity, and vanishes as ![]() $\bar {\kappa }\mathcal {C}^{-1} \to \infty$, leading ultimately to pure (rigid-body) tumbling motion.
$\bar {\kappa }\mathcal {C}^{-1} \to \infty$, leading ultimately to pure (rigid-body) tumbling motion.
For a given material property contrast, we have now seen that decreasing the shear rate below a critical value produces, perhaps counter-intuitively, a tumbling motion, while increasing it above this value invites the vesicle to swing. With a very slow background flow, the vesicle elongates in the directions of its softest components (or higher spontaneous curvature regions) in a quasi-steady manner – the material is nearly matched to the shape as it rotates like a rigid body. But in a flow with a large shear rate, material is driven around the surface with larger viscous stresses relative to the elastic stresses, and the stiffer material may be driven past the high curvature regions. High curvature regions can rapidly align with the softer regions via a rapid swing.
Similar transitions from swinging to tumbling have been observed in red blood cells (Noguchi Reference Noguchi2009), capsules (Kessler, Finken & Seifert Reference Kessler, Finken and Seifert2008; Barthes-Biesel Reference Barthes-Biesel1991, Reference Barthes-Biesel2016) and vesicles even with uniform bending rigidity (Kantsler & Steinberg Reference Kantsler and Steinberg2006; Lebedev et al. Reference Lebedev, Turitsyn and Vergeles2007; Deschamps et al. Reference Deschamps, Kantsler, Segre and Steinberg2009a,Reference Deschamps, Kantsler and Steinbergb) but at smaller reduced areas. In addition, the variation in spontaneous curvature along the surface of a red blood cell has previously been modelled through a simple energy barrier – there too the contrast in material properties revealed a transition from tumbling to swinging (Skotheim & Secomb Reference Skotheim and Secomb2007). More directly, Vlahovska et al. (Reference Vlahovska, Young, Danker and Misbah2011) investigated the dynamics of microcapsules of non-spherical reference shape in shear flow, much like the specification of a non-uniform spontaneous curvature. In their fully three-dimensional treatment they too observed tumbling to swinging behaviour upon increasing the shear rate beyond a critical value.
4.1.1. Matched asymptotic analysis
Between these two extremes lies a critical value of ![]() $\bar {\kappa }\mathcal {C}^{-1}$ which signals a bifurcation from swinging to tumbling. Figure 3(b) shows the minimum inclination angle achieved during the periodic dynamics for a range of
$\bar {\kappa }\mathcal {C}^{-1}$ which signals a bifurcation from swinging to tumbling. Figure 3(b) shows the minimum inclination angle achieved during the periodic dynamics for a range of ![]() $\varepsilon$ and
$\varepsilon$ and ![]() $\bar {\kappa }\mathcal {C}^{-1}$ found using the full numerical simulations. The bending stiffness variation needed to set off a tumbling dynamics is near unity as
$\bar {\kappa }\mathcal {C}^{-1}$ found using the full numerical simulations. The bending stiffness variation needed to set off a tumbling dynamics is near unity as ![]() $\varepsilon \to 0$ (consistent with the much simpler numerical solutions of (4.7)) and is a decreasing function of
$\varepsilon \to 0$ (consistent with the much simpler numerical solutions of (4.7)) and is a decreasing function of ![]() $\varepsilon$. Note that
$\varepsilon$. Note that ![]() $\bar {\kappa }\mathcal {C}^{-1}=\varepsilon \bar {\kappa }\textit {Ca}^{-1}$ depends on
$\bar {\kappa }\mathcal {C}^{-1}=\varepsilon \bar {\kappa }\textit {Ca}^{-1}$ depends on ![]() $\varepsilon$ in figure 3(b). A thin band near this bifurcation ridge shows an unexpected result: the membrane's inclination angle can decrease to values less than zero even during a (rather dramatic) swinging motion. Common intuition from single-component membrane dynamics suggests that once the elongated axis has dipped below the
$\varepsilon$ in figure 3(b). A thin band near this bifurcation ridge shows an unexpected result: the membrane's inclination angle can decrease to values less than zero even during a (rather dramatic) swinging motion. Common intuition from single-component membrane dynamics suggests that once the elongated axis has dipped below the ![]() $x$-axis the membrane will surely tumble; this intuition is thus not always correct.
$x$-axis the membrane will surely tumble; this intuition is thus not always correct.
We are therefore led to investigate the regime where ![]() $\bar {\kappa }\mathcal {C}^{-1}=1+O(\varepsilon )$ and we define
$\bar {\kappa }\mathcal {C}^{-1}=1+O(\varepsilon )$ and we define ![]() $\alpha =(\bar {\kappa }\mathcal {C}^{-1}-1)/\varepsilon$ with
$\alpha =(\bar {\kappa }\mathcal {C}^{-1}-1)/\varepsilon$ with ![]() $\alpha =O(1)$ as
$\alpha =O(1)$ as ![]() $\varepsilon \to 0$. For values of
$\varepsilon \to 0$. For values of ![]() $\tau$ where
$\tau$ where ![]() $\phi ^{(0)}_\tau =O(1)$ as
$\phi ^{(0)}_\tau =O(1)$ as ![]() $\varepsilon \to 0$, the inclination angle is drifting slowly and an outer solution is derived assuming a regular expansion in
$\varepsilon \to 0$, the inclination angle is drifting slowly and an outer solution is derived assuming a regular expansion in ![]() $\varepsilon$,
$\varepsilon$, ![]() $\phi ^{(0)}=\phi ^{(0)}_{outer}+O(\varepsilon )$, resulting in
$\phi ^{(0)}=\phi ^{(0)}_{outer}+O(\varepsilon )$, resulting in ![]() $\phi ^{(0)}_{outer}(\tau ) = 3{\rm \pi} /8-\tau /4+O(\varepsilon )$. The initial value of
$\phi ^{(0)}_{outer}(\tau ) = 3{\rm \pi} /8-\tau /4+O(\varepsilon )$. The initial value of ![]() $\phi ^{(0)}$ does not appear in the outer solution because the distribution of bending stiffness begins entirely in the
$\phi ^{(0)}$ does not appear in the outer solution because the distribution of bending stiffness begins entirely in the ![]() $\cos (2\theta )$ mode at
$\cos (2\theta )$ mode at ![]() $\tau =0$ from (4.1); there is a rapid correction on a time scale
$\tau =0$ from (4.1); there is a rapid correction on a time scale ![]() $O(\varepsilon )$ (just visible near
$O(\varepsilon )$ (just visible near ![]() $\tau =0$ in figure 3a) before the outer solution becomes dominant, and memory of the initial state is almost immediately lost.
$\tau =0$ in figure 3a) before the outer solution becomes dominant, and memory of the initial state is almost immediately lost.
An inner region of rapid variation in ![]() $\phi ^{(0)}$ emerges when
$\phi ^{(0)}$ emerges when ![]() $\phi ^{(0)}\approx 0$, or when
$\phi ^{(0)}\approx 0$, or when ![]() $\tau \approx 3{\rm \pi} /2$. The scaling of the inner region in
$\tau \approx 3{\rm \pi} /2$. The scaling of the inner region in ![]() $\tau$ and the solution there are found by appealing to dominant balance as
$\tau$ and the solution there are found by appealing to dominant balance as ![]() $\varepsilon \to 0$ (Bender & Orszag Reference Bender and Orszag2013), leading to the definition of an inner variable
$\varepsilon \to 0$ (Bender & Orszag Reference Bender and Orszag2013), leading to the definition of an inner variable ![]() $\sigma = (\tau -3{\rm \pi} /2)/\varepsilon ^{1/2}$, so that (4.7) reads as
$\sigma = (\tau -3{\rm \pi} /2)/\varepsilon ^{1/2}$, so that (4.7) reads as
where ![]() $\beta =(1+\lambda )^{-1}Q^{-1}$, and an ansatz
$\beta =(1+\lambda )^{-1}Q^{-1}$, and an ansatz ![]() $\phi ^{(0)}_{inner}=p_{inner}^{(0)}(\sigma )+\varepsilon ^{1/2}p_{inner}^{(1)}(\sigma )+O(\varepsilon )$. At leading order we find
$\phi ^{(0)}_{inner}=p_{inner}^{(0)}(\sigma )+\varepsilon ^{1/2}p_{inner}^{(1)}(\sigma )+O(\varepsilon )$. At leading order we find
which has solution
with ![]() $C_0$ an integration constant. However, in order for this inner solution to merge with the outer solution, or
$C_0$ an integration constant. However, in order for this inner solution to merge with the outer solution, or
we must have that ![]() $C_0=0$. At the next order (4.10) then produces
$C_0=0$. At the next order (4.10) then produces
and the solution
where ![]() $C_1$ is an integration constant and
$C_1$ is an integration constant and
 \begin{equation} \varUpsilon = \frac{1}{8}\left(\frac{\rm \pi}{ \beta}\right)^{1/2}(1-4\beta \alpha). \end{equation}
\begin{equation} \varUpsilon = \frac{1}{8}\left(\frac{\rm \pi}{ \beta}\right)^{1/2}(1-4\beta \alpha). \end{equation}
The error function, ![]() $\mbox {erf}(\beta ^{1/2}\sigma )=(2/\sqrt {{\rm \pi} })\int _0^{\beta ^{1/2}\sigma } \,{\rm e}^{-t^{2}}\,{\rm d}t$, is an odd function which tends towards
$\mbox {erf}(\beta ^{1/2}\sigma )=(2/\sqrt {{\rm \pi} })\int _0^{\beta ^{1/2}\sigma } \,{\rm e}^{-t^{2}}\,{\rm d}t$, is an odd function which tends towards ![]() $-1$ as
$-1$ as ![]() $\sigma \to -\infty$ and to
$\sigma \to -\infty$ and to ![]() $1$ as
$1$ as ![]() $\sigma \to \infty$. Again the requirement of matching to the outer solution demands that terms which are unbounded as
$\sigma \to \infty$. Again the requirement of matching to the outer solution demands that terms which are unbounded as ![]() $\sigma \to -\infty$ vanish, leading to
$\sigma \to -\infty$ vanish, leading to ![]() $C_1 = 1$. The inner solution alone then represents a composite approximation for
$C_1 = 1$. The inner solution alone then represents a composite approximation for ![]() $\tau \in (O(\varepsilon ),3{\rm \pi} /2]$:
$\tau \in (O(\varepsilon ),3{\rm \pi} /2]$:
 \begin{equation} \phi^{(0)}(\tau)\sim\frac{3{\rm \pi}}{8}-\frac{\tau}{4}+\varepsilon^{1/2}\varUpsilon \left[\mbox{erf}\left(\sqrt{\frac{\beta}{\varepsilon}} \left(\tau -\frac{3 {\rm \pi}}{2}\right)\right)+1\right]\exp({\beta(\tau-3{\rm \pi}/2)^{2}/\varepsilon}). \end{equation}
\begin{equation} \phi^{(0)}(\tau)\sim\frac{3{\rm \pi}}{8}-\frac{\tau}{4}+\varepsilon^{1/2}\varUpsilon \left[\mbox{erf}\left(\sqrt{\frac{\beta}{\varepsilon}} \left(\tau -\frac{3 {\rm \pi}}{2}\right)\right)+1\right]\exp({\beta(\tau-3{\rm \pi}/2)^{2}/\varepsilon}). \end{equation}
This solution becomes unbounded when ![]() $\tau$ increases beyond
$\tau$ increases beyond ![]() $3{\rm \pi} /2$, so is incapable of merging with the possible outer solutions to the right of
$3{\rm \pi} /2$, so is incapable of merging with the possible outer solutions to the right of ![]() $\tau =3{\rm \pi} /2$, either
$\tau =3{\rm \pi} /2$, either ![]() $7{\rm \pi} /8-\tau /4$ if
$7{\rm \pi} /8-\tau /4$ if ![]() $(1-4\beta \alpha )>0$ (a swing) or
$(1-4\beta \alpha )>0$ (a swing) or ![]() $-{\rm \pi} /8-\tau /4$ if
$-{\rm \pi} /8-\tau /4$ if ![]() $(1-4\beta \alpha )<0$ (a tumble). Instead we continue the solution by solving (4.7) on
$(1-4\beta \alpha )<0$ (a tumble). Instead we continue the solution by solving (4.7) on ![]() $\tau \geqslant 3{\rm \pi} /2$ using the initial data from the inner solution above,
$\tau \geqslant 3{\rm \pi} /2$ using the initial data from the inner solution above, ![]() $\phi ^{(0)}(3{\rm \pi} /2)=\varepsilon ^{1/2}\varUpsilon +O(\varepsilon )$.
$\phi ^{(0)}(3{\rm \pi} /2)=\varepsilon ^{1/2}\varUpsilon +O(\varepsilon )$.
The solution at leading order is again that in (4.12) but this time ![]() $C_0\neq 0$. After finding the solution at the next order (included as Appendix F), however, in order to match both the data at
$C_0\neq 0$. After finding the solution at the next order (included as Appendix F), however, in order to match both the data at ![]() $\tau =3{\rm \pi} /2$ and to merge with an outer solution it becomes clear that
$\tau =3{\rm \pi} /2$ and to merge with an outer solution it becomes clear that ![]() $C_0 = O(\varepsilon ^{1/2})$, and then the equation for
$C_0 = O(\varepsilon ^{1/2})$, and then the equation for ![]() $p_{inner}^{(1)}$ in (4.14) and its general solution in (4.15) go unchanged. Removing unbounded terms as
$p_{inner}^{(1)}$ in (4.14) and its general solution in (4.15) go unchanged. Removing unbounded terms as ![]() $\sigma \to \infty$ selects
$\sigma \to \infty$ selects ![]() $C_1=-1$, and matching the data at
$C_1=-1$, and matching the data at ![]() $\tau =3{\rm \pi} /2$ selects
$\tau =3{\rm \pi} /2$ selects ![]() $C_0=\tan (2\varepsilon ^{1/2}\varUpsilon )$, resulting in the following composite solution for
$C_0=\tan (2\varepsilon ^{1/2}\varUpsilon )$, resulting in the following composite solution for ![]() $\tau \in [3{\rm \pi} /2,3{\rm \pi} /2+2{\rm \pi} -O(\varepsilon ^{1/2}))$:
$\tau \in [3{\rm \pi} /2,3{\rm \pi} /2+2{\rm \pi} -O(\varepsilon ^{1/2}))$:
 \begin{align} \phi^{(0)}(\tau)&\sim \frac{3{\rm \pi}}{8}-\frac{\tau}{4}+\tan^{{-}1}\left[\tan\left(2\varepsilon^{1/2}\varUpsilon\right) \exp({\beta(\tau-3{\rm \pi}/2)/\varepsilon})\right]\nonumber\\ &\quad +\varepsilon^{1/2}\varUpsilon \left[\mbox{erf}\left(\sqrt{\frac{\beta}{\varepsilon}} \left(\tau - \frac{3 {\rm \pi}}{2}\right)\right)-1\right]\exp({\beta(\tau-3{\rm \pi}/2)^{2}/\varepsilon}). \end{align}
\begin{align} \phi^{(0)}(\tau)&\sim \frac{3{\rm \pi}}{8}-\frac{\tau}{4}+\tan^{{-}1}\left[\tan\left(2\varepsilon^{1/2}\varUpsilon\right) \exp({\beta(\tau-3{\rm \pi}/2)/\varepsilon})\right]\nonumber\\ &\quad +\varepsilon^{1/2}\varUpsilon \left[\mbox{erf}\left(\sqrt{\frac{\beta}{\varepsilon}} \left(\tau - \frac{3 {\rm \pi}}{2}\right)\right)-1\right]\exp({\beta(\tau-3{\rm \pi}/2)^{2}/\varepsilon}). \end{align} The critical dependence of the dynamics on the sign of ![]() $\bar {\kappa }\mathcal {C}^{-1}-1$ as
$\bar {\kappa }\mathcal {C}^{-1}-1$ as ![]() $\varepsilon \to 0$ is thus established, most clearly through the dependence of the argument of
$\varepsilon \to 0$ is thus established, most clearly through the dependence of the argument of ![]() $\tan ^{-1}$ on the sign of
$\tan ^{-1}$ on the sign of ![]() $\varUpsilon$, and thus on the sign of
$\varUpsilon$, and thus on the sign of ![]() $(1-4\beta \alpha )$ (and recalling that
$(1-4\beta \alpha )$ (and recalling that ![]() $\alpha = (\bar {\kappa }\mathcal {C}^{-1}-1)/\varepsilon$).
$\alpha = (\bar {\kappa }\mathcal {C}^{-1}-1)/\varepsilon$).
Since ![]() $\beta >0$, if
$\beta >0$, if ![]() $\bar {\kappa }\mathcal {C}^{-1}<1$ then
$\bar {\kappa }\mathcal {C}^{-1}<1$ then ![]() $(1-4\beta \alpha )>0$ and the solution above shows a rapid return to a positive inclination angle just less than
$(1-4\beta \alpha )>0$ and the solution above shows a rapid return to a positive inclination angle just less than ![]() ${\rm \pi} /2$, representing a dramatic swinging motion. If
${\rm \pi} /2$, representing a dramatic swinging motion. If ![]() $\bar {\kappa }\mathcal {C}^{-1}>1$, however, then the dynamics depend on
$\bar {\kappa }\mathcal {C}^{-1}>1$, however, then the dynamics depend on ![]() $\beta =[(1+\lambda )Q]^{-1}$. If
$\beta =[(1+\lambda )Q]^{-1}$. If ![]() $\beta >1/(4\alpha )$, then
$\beta >1/(4\alpha )$, then ![]() $(1-4\beta \alpha )<0$ and as
$(1-4\beta \alpha )<0$ and as ![]() $\tau$ increases beyond
$\tau$ increases beyond ![]() $3{\rm \pi} /2$ the inclination angle dips rapidly towards negative values and below
$3{\rm \pi} /2$ the inclination angle dips rapidly towards negative values and below ![]() $-{\rm \pi} /2$, representing a tumble. If
$-{\rm \pi} /2$, representing a tumble. If ![]() $\beta <1/(4\alpha )$, however, the inclination angle becomes negative as
$\beta <1/(4\alpha )$, however, the inclination angle becomes negative as ![]() $\tau$ increases away from
$\tau$ increases away from ![]() $3{\rm \pi} /2$ for a short while, but then for longer times it launches back towards positive values: in this case the membrane's long axis dips below the horizontal, hinting at a tumble, but then rapidly pulls back up into positive inclination angles in a high-amplitude swing. Inclination angles from numerical solution of (4.6) with
$3{\rm \pi} /2$ for a short while, but then for longer times it launches back towards positive values: in this case the membrane's long axis dips below the horizontal, hinting at a tumble, but then rapidly pulls back up into positive inclination angles in a high-amplitude swing. Inclination angles from numerical solution of (4.6) with ![]() $\varepsilon =10^{-2}$ are plotted for a range of
$\varepsilon =10^{-2}$ are plotted for a range of ![]() $\bar {\kappa }\mathcal {C}^{-1}$ in figure 5. The approximations in (4.17)–(4.18) are visibly indistinguishable (and not shown) from numerical solution of (4.6) in this parameter regime.
$\bar {\kappa }\mathcal {C}^{-1}$ in figure 5. The approximations in (4.17)–(4.18) are visibly indistinguishable (and not shown) from numerical solution of (4.6) in this parameter regime.

Figure 5. Theoretical inclination angle (solutions of (4.6)) with spatial period ![]() $M=2$ of material property variation, viscosity ratio
$M=2$ of material property variation, viscosity ratio ![]() $\lambda =\mu ^{-}/\mu ^{+}=1$,
$\lambda =\mu ^{-}/\mu ^{+}=1$, ![]() $\varepsilon =10^{-2}$ or
$\varepsilon =10^{-2}$ or ![]() $R_A=0.998$, and
$R_A=0.998$, and ![]() $Q=3$ for a range of
$Q=3$ for a range of ![]() $\bar {\kappa }\mathcal {C}^{-1}$.
$\bar {\kappa }\mathcal {C}^{-1}$.
The inclination angle equation, (4.6), only provides a solution for the ![]() $O(1)$ behaviour of the inclination angle,
$O(1)$ behaviour of the inclination angle, ![]() $\phi ^{(0)}(t)$, so while the expressions above are accurate asymptotic solutions to (4.6), the equation itself is only representing the
$\phi ^{(0)}(t)$, so while the expressions above are accurate asymptotic solutions to (4.6), the equation itself is only representing the ![]() $O(1)$ behaviour of the inclination angle
$O(1)$ behaviour of the inclination angle ![]() $\phi (t)$. While these analytical representations show remarkable accuracy when compared to the full numerical simulations, seen in figure 3(a), certain aspects of the full system are delicate. For instance, the analysis above suggests that the critical
$\phi (t)$. While these analytical representations show remarkable accuracy when compared to the full numerical simulations, seen in figure 3(a), certain aspects of the full system are delicate. For instance, the analysis above suggests that the critical ![]() $\bar {\kappa }\mathcal {C}^{-1}$ beyond which tumbling occurs is an increasing function of
$\bar {\kappa }\mathcal {C}^{-1}$ beyond which tumbling occurs is an increasing function of ![]() $\varepsilon$, but this lies in stark contrast to the results of the full numerical simulations shown in figure 3(b). The analysis above shows, however, that the critical value for the onset of tumbling is indeed
$\varepsilon$, but this lies in stark contrast to the results of the full numerical simulations shown in figure 3(b). The analysis above shows, however, that the critical value for the onset of tumbling is indeed ![]() $\bar {\kappa }\mathcal {C}^{-1}=1+O(\varepsilon )$ as
$\bar {\kappa }\mathcal {C}^{-1}=1+O(\varepsilon )$ as ![]() $\varepsilon \to 0$, and generally provides accurate dynamics in a very wide variety of settings.
$\varepsilon \to 0$, and generally provides accurate dynamics in a very wide variety of settings.
4.2. The case  $M\neq 2$
$M\neq 2$
Turning now to the case where ![]() $M\neq 2$, the daunting system is rendered harmless upon observation of a periodic steady state in which
$M\neq 2$, the daunting system is rendered harmless upon observation of a periodic steady state in which ![]() $P_0(t)$ is constant. According to (2.23)–(2.24) with
$P_0(t)$ is constant. According to (2.23)–(2.24) with ![]() $P_0$ assumed constant, as
$P_0$ assumed constant, as ![]() $t\to \infty$ we find
$t\to \infty$ we find ![]() $a_n=0$ and
$a_n=0$ and ![]() $b_n=0$ for all
$b_n=0$ for all ![]() $n\notin \{2,M\}$. Meanwhile, as in the constant bending stiffness case,
$n\notin \{2,M\}$. Meanwhile, as in the constant bending stiffness case, ![]() $b_2$ relaxes to an equilibrium value
$b_2$ relaxes to an equilibrium value ![]() $\tilde {b}_{2}$, where
$\tilde {b}_{2}$, where ![]() $\tilde {b}_{2}=\mathcal {C}/\alpha _2=\mathcal {C} (3 +\mathcal {C}\,P_0 )^{-1}$.
$\tilde {b}_{2}=\mathcal {C}/\alpha _2=\mathcal {C} (3 +\mathcal {C}\,P_0 )^{-1}$.
Shape deformations continue periodically in the ![]() $M{\rm th}$ Fourier mode, however, according to (2.23)–(2.24) (upon inserting
$M{\rm th}$ Fourier mode, however, according to (2.23)–(2.24) (upon inserting ![]() $c_M(t)$ and
$c_M(t)$ and ![]() $d_M(t)$ from (4.1)). At leading order in
$d_M(t)$ from (4.1)). At leading order in ![]() $\varepsilon$ the system is quasi-steady; with
$\varepsilon$ the system is quasi-steady; with ![]() $\tau =\varepsilon t$ again, we write
$\tau =\varepsilon t$ again, we write ![]() $\partial _t a_M = \varepsilon \partial _\tau a_M$ (similarly for
$\partial _t a_M = \varepsilon \partial _\tau a_M$ (similarly for ![]() $b_M$). Neglecting a transient relaxation from initial data, to leading order in small
$b_M$). Neglecting a transient relaxation from initial data, to leading order in small ![]() $\varepsilon$ we find
$\varepsilon$ we find
Simply, then, in the periodic steady state we have ![]() $a_M^{2}+b_M^{2} = \bar {\kappa }^{2}/\alpha _M^{2}$ and
$a_M^{2}+b_M^{2} = \bar {\kappa }^{2}/\alpha _M^{2}$ and ![]() $a_M c_M+ b_M d_M =-\bar {\kappa }^{2}/\alpha _M$. As both are constant, along with the constant value of
$a_M c_M+ b_M d_M =-\bar {\kappa }^{2}/\alpha _M$. As both are constant, along with the constant value of ![]() $b_2$ in the limit as
$b_2$ in the limit as ![]() $t\to \infty$, upon inspection of
$t\to \infty$, upon inspection of ![]() $P_0$ in (2.19) we verify the consistency of this result:
$P_0$ in (2.19) we verify the consistency of this result: ![]() $P_0$ is indeed constant in this periodic steady state. Since
$P_0$ is indeed constant in this periodic steady state. Since ![]() $\tilde {b}_{2}$ is determined purely by the constraint of constant area, from (3.1), the pressure jump
$\tilde {b}_{2}$ is determined purely by the constraint of constant area, from (3.1), the pressure jump ![]() $P_0$ associated with these dynamics is the same as that in the constant bending case. Moreover, the deformation parameter and inclination angle in the
$P_0$ associated with these dynamics is the same as that in the constant bending case. Moreover, the deformation parameter and inclination angle in the ![]() $M\neq 2$ case are also unchanged. The membrane simply elongates in the direction of the principle axis of the straining flow while shape oscillations in the
$M\neq 2$ case are also unchanged. The membrane simply elongates in the direction of the principle axis of the straining flow while shape oscillations in the ![]() $M{\rm th}$ mode traverse along this constant background geometry in a trembling dynamics.
$M{\rm th}$ mode traverse along this constant background geometry in a trembling dynamics.
When ![]() $\bar {\kappa }\mathcal {C}^{-1}$ is sufficiently large, interactions between the modes of bending stiffness can no longer be neglected (i.e. our simple specification of
$\bar {\kappa }\mathcal {C}^{-1}$ is sufficiently large, interactions between the modes of bending stiffness can no longer be neglected (i.e. our simple specification of ![]() $\kappa (\theta )$ in (4.1) becomes inaccurate). Full numerical simulations are used to explore this challenging region of parameter space. Figure 6 shows the inclination angles computed using the full numerical simulations for
$\kappa (\theta )$ in (4.1) becomes inaccurate). Full numerical simulations are used to explore this challenging region of parameter space. Figure 6 shows the inclination angles computed using the full numerical simulations for ![]() $M\in \{1, 2,\ldots, 8\}$, with
$M\in \{1, 2,\ldots, 8\}$, with ![]() $\varepsilon =0.1$ and
$\varepsilon =0.1$ and ![]() $\lambda =1$ fixed, for three different bending stiffness variations:
$\lambda =1$ fixed, for three different bending stiffness variations: ![]() $\bar {\kappa }\mathcal {C}^{-1}=0.75$,
$\bar {\kappa }\mathcal {C}^{-1}=0.75$, ![]() $\bar {\kappa }\mathcal {C}^{-1}=2$ and
$\bar {\kappa }\mathcal {C}^{-1}=2$ and ![]() $\bar {\kappa }\mathcal {C}^{-1}=4$. The
$\bar {\kappa }\mathcal {C}^{-1}=4$. The ![]() $M=2$ mode results in tumbling in all three cases, consistent with figure 3(b). The swinging amplitude with even
$M=2$ mode results in tumbling in all three cases, consistent with figure 3(b). The swinging amplitude with even ![]() $M$ values increases with increasing
$M$ values increases with increasing ![]() $\bar {\kappa }\mathcal {C}^{-1}$, however, and the
$\bar {\kappa }\mathcal {C}^{-1}$, however, and the ![]() $M=4$ case transitions from swinging to tumbling for some
$M=4$ case transitions from swinging to tumbling for some ![]() $\bar {\kappa }\mathcal {C}^{-1}\in (2,4)$. Supplementary movies M4–M6 show the dynamics of vesicles with
$\bar {\kappa }\mathcal {C}^{-1}\in (2,4)$. Supplementary movies M4–M6 show the dynamics of vesicles with ![]() $M\in \{1, 2,\ldots, 8\}$ represented in figure 6(a–c).
$M\in \{1, 2,\ldots, 8\}$ represented in figure 6(a–c).

Figure 6. Inclination angle dynamics with bending stiffness variations in the ![]() $M{\rm th}$ spatial mode for
$M{\rm th}$ spatial mode for ![]() $M\in \{1, 2,\ldots, 8\}$ with
$M\in \{1, 2,\ldots, 8\}$ with ![]() $\varepsilon =0.1$ or
$\varepsilon =0.1$ or ![]() $R_A=0.865$ and
$R_A=0.865$ and ![]() $\lambda =1$ for three variation amplitudes, from the full numerical simulations. (a)
$\lambda =1$ for three variation amplitudes, from the full numerical simulations. (a) ![]() $\bar {\kappa }\mathcal {C}^{-1}=0.75$, (b)
$\bar {\kappa }\mathcal {C}^{-1}=0.75$, (b) ![]() $\bar {\kappa }\mathcal {C}^{-1}=2$ and (c)
$\bar {\kappa }\mathcal {C}^{-1}=2$ and (c) ![]() $\bar {\kappa }\mathcal {C}^{-1}=4$. Tumbling is observed in small, even modes. (See also supplementary movies M4–M6.)
$\bar {\kappa }\mathcal {C}^{-1}=4$. Tumbling is observed in small, even modes. (See also supplementary movies M4–M6.)
In the simulated dynamics, we observe membrane swinging for small, even ![]() $M$, but not odd
$M$, but not odd ![]() $M$, or large even
$M$, or large even ![]() $M$ with an insufficiently large value of
$M$ with an insufficiently large value of ![]() $\bar {\kappa }\mathcal {C}^{-1}$. When
$\bar {\kappa }\mathcal {C}^{-1}$. When ![]() $M$ is even, the two regions of largest curvature have a symmetric interaction with the membrane, and elongation in the direction of the softer material reduces the energy at both ends. Bending stiffness information in the
$M$ is even, the two regions of largest curvature have a symmetric interaction with the membrane, and elongation in the direction of the softer material reduces the energy at both ends. Bending stiffness information in the ![]() $M=4$, mode, for instance, bleeds into the
$M=4$, mode, for instance, bleeds into the ![]() $M=2$ mode, which interacts directly with the extensional part of the background flow and can lead to tumbling, as discussed in the previous section. When
$M=2$ mode, which interacts directly with the extensional part of the background flow and can lead to tumbling, as discussed in the previous section. When ![]() $M$ is odd, however, the large curvature regions have an asymmetric interaction with the membrane; reorientation of the elongated axis which would reduce the bending energy on one end would increase it on the other end. Finally, when
$M$ is odd, however, the large curvature regions have an asymmetric interaction with the membrane; reorientation of the elongated axis which would reduce the bending energy on one end would increase it on the other end. Finally, when ![]() $M$ is large, either even or odd, averaging results in convergence to the case of constant bending stiffness, and departures from the inclination angle chosen by the principal axis of the background flow,
$M$ is large, either even or odd, averaging results in convergence to the case of constant bending stiffness, and departures from the inclination angle chosen by the principal axis of the background flow, ![]() ${\rm \pi} /4$ as
${\rm \pi} /4$ as ![]() $\varepsilon \to 0$, become negligible. It remains to be seen whether a sufficiently large
$\varepsilon \to 0$, become negligible. It remains to be seen whether a sufficiently large ![]() $\bar {\kappa }\mathcal {C}^{-1}$ can result in tumbling for any even
$\bar {\kappa }\mathcal {C}^{-1}$ can result in tumbling for any even ![]() $M$; extremely stiff regions do not pass easily across high curvature regions, suggesting that tumbling might ensue for very large values of
$M$; extremely stiff regions do not pass easily across high curvature regions, suggesting that tumbling might ensue for very large values of ![]() $\bar {\kappa }\mathcal {C}^{-1}$, but high spatial frequency averaging suggests convergence to pure tank-treading as in § 3. The answer may well depend on the reduced area and viscosity ratio. We leave this intriguing question for future inquiry.
$\bar {\kappa }\mathcal {C}^{-1}$, but high spatial frequency averaging suggests convergence to pure tank-treading as in § 3. The answer may well depend on the reduced area and viscosity ratio. We leave this intriguing question for future inquiry.
5. Discussion
The material property variations along the surface of a multicomponent vesicle can impact the vesicle dynamics in a background flow differently depending on the spatial modes of its distribution, the magnitude of those variations and even the parity of the number of domains. Small amplitude variations in material properties lead to periodic oscillations of a pure tank-treading steady state about an inclination angle of ![]() ${\rm \pi} /4$; large variations can result in a rigid-body tumbling mode with a constant rotation rate; and an intermediate regime shows a bifurcation from swinging with tank-treading to tumbling with periodic material phase-lag. As the membrane becomes more deflated, the critical value of
${\rm \pi} /4$; large variations can result in a rigid-body tumbling mode with a constant rotation rate; and an intermediate regime shows a bifurcation from swinging with tank-treading to tumbling with periodic material phase-lag. As the membrane becomes more deflated, the critical value of ![]() $\bar {\kappa }\mathcal {C}^{-1}$ required for the vesicle to tumble is found to decrease, with
$\bar {\kappa }\mathcal {C}^{-1}$ required for the vesicle to tumble is found to decrease, with ![]() $\bar {\kappa }\mathcal {C}^{-1}$ approaching 1 as
$\bar {\kappa }\mathcal {C}^{-1}$ approaching 1 as ![]() $\varepsilon$ approaches 0. As a general principle, the vesicle has a tendency to elongate so that the softer parts of the membrane sit in the regions of largest curvature, while the background flow tends to elongate the vesicle along the principal axis with a fixed inclination angle of
$\varepsilon$ approaches 0. As a general principle, the vesicle has a tendency to elongate so that the softer parts of the membrane sit in the regions of largest curvature, while the background flow tends to elongate the vesicle along the principal axis with a fixed inclination angle of ![]() ${\rm \pi} /4$. When these two directions are not aligned, swinging, or even tumbling, ensues. That the capillary number is highly sensitive to the vesicle size may be of use to experimental realizations of the results described in this paper.
${\rm \pi} /4$. When these two directions are not aligned, swinging, or even tumbling, ensues. That the capillary number is highly sensitive to the vesicle size may be of use to experimental realizations of the results described in this paper.
Although we have focused on variations in bending stiffness, at leading order we find the same shapes, dynamics and bifurcation from swinging to tumbling when considering variations in spontaneous curvature instead. Equations (2.23)–(2.24) indicate that when the preferred mean curvature along the membrane, ![]() $\tilde {H}_0$, is not unity, the effects of bending stiffness variations and spontaneous curvature variations are indistinguishable for each Fourier mode. A model linking the two (e.g. if bending stiffness is proportional to spontaneous curvature for a given lipid species) may then be necessary to make claims about material properties in full using this passive means of probing membrane composition. If
$\tilde {H}_0$, is not unity, the effects of bending stiffness variations and spontaneous curvature variations are indistinguishable for each Fourier mode. A model linking the two (e.g. if bending stiffness is proportional to spontaneous curvature for a given lipid species) may then be necessary to make claims about material properties in full using this passive means of probing membrane composition. If ![]() $\tilde {H}_0=1$, however, then only spontaneous curvature variations enter at first order in
$\tilde {H}_0=1$, however, then only spontaneous curvature variations enter at first order in ![]() $\varepsilon$.
$\varepsilon$.
Replacing bending stiffness by spontaneous curvature, if ![]() $\tilde {H}_0 =0$, the transition between tumbling and swinging for the
$\tilde {H}_0 =0$, the transition between tumbling and swinging for the ![]() $M=2$ spatial mode is predicted at the critical value
$M=2$ spatial mode is predicted at the critical value ![]() $a[\tilde {H}]\mathcal {C}^{-1}=1$ as
$a[\tilde {H}]\mathcal {C}^{-1}=1$ as ![]() $\varepsilon \to 0$, with
$\varepsilon \to 0$, with ![]() $[\tilde {H}]$ the curvature contrast. Estimating the spontaneous curvature variations of a red blood cell to be roughly
$[\tilde {H}]$ the curvature contrast. Estimating the spontaneous curvature variations of a red blood cell to be roughly ![]() $[\tilde {H}]=0.5\ \mathrm {\mu }{\rm m}^{-1}$ with
$[\tilde {H}]=0.5\ \mathrm {\mu }{\rm m}^{-1}$ with ![]() $a\approx 3\ \mathrm {\mu }{\rm m}$, and with
$a\approx 3\ \mathrm {\mu }{\rm m}$, and with ![]() $\textit {Ca}\approx \dot {\gamma } /4$ from § 2.4, the theoretical prediction is that the bifurcation should appear near
$\textit {Ca}\approx \dot {\gamma } /4$ from § 2.4, the theoretical prediction is that the bifurcation should appear near ![]() $\dot {\gamma } \approx 2\ {\rm s}^{-1}$. This is very close to the shear rates used in experiments showing the onset of this transition (Abkarian, Faivre & Viallat Reference Abkarian, Faivre and Viallat2007; Abkarian & Viallat Reference Abkarian and Viallat2008).
$\dot {\gamma } \approx 2\ {\rm s}^{-1}$. This is very close to the shear rates used in experiments showing the onset of this transition (Abkarian, Faivre & Viallat Reference Abkarian, Faivre and Viallat2007; Abkarian & Viallat Reference Abkarian and Viallat2008).
In the fully three-dimensional system, material domains are not confined to motion in the flow direction only and this may result in a substantial departure from the results described herein in certain regimes. Particularly when slow motions yield to sudden reorganization, as in a rapid swing or tumbling event, the addition of such an escape direction may prove critical. But some material properties cannot so easily be disturbed, for instance, the spontaneous curvature of a red blood cell provided by the scaffolding of its spectrin network (Dao, Lim & Suresh Reference Dao, Lim and Suresh2003; Hatami-Marbini & Mofrad Reference Hatami-Marbini and Mofrad2015). That the transition from tumbling to swinging in red blood cells appears to be predicted already using this two-dimensional analysis, however, is intriguing.
The distribution of membrane domains is of substantial biological importance. Membrane heterogeneity can impact fundamental cellular functions such as signal transduction and membrane trafficking (Simons & Toomre Reference Simons and Toomre2000; Maxfield Reference Maxfield2002; Edidin Reference Edidin2003), and improper composition can cause diseases such as Alzheimer's (Vetrivel & Thinakaran Reference Vetrivel and Thinakaran2010; Rajendran & Annaert Reference Rajendran and Annaert2012). The predictions of this work suggests a means of determining not only the constant material properties of a membrane or vesicle using a background flow, which has been an experimentally viable method for decades, but now also of determining material property variations by linking time-series dynamics to spatial material variations, and even the possibility of using a simple pressure probe near such a swinging, tumbling and trembling membrane. With good fortune, these predictions will be of use for measuring heterogeneous membrane properties using only viscous stresses in the near future.
Supplementary movies
Supplementary movies are available at https://doi.org/10.1017/jfm.2022.40.
Funding
S.E.S. acknowledges the support of the NSF/NIH (DMS-1661900, DMR-2003819).
Declaration of interests
The authors report no conflict of interest.
Appendix A. Stream function and incompressibility in polar coordinates
The (dimensional) biharmonic equation in polar coordinates ![]() $(r,\theta )$ has a general solution known as the Michell solution (Michell Reference Michell1899). Neglecting terms which are non-periodic in
$(r,\theta )$ has a general solution known as the Michell solution (Michell Reference Michell1899). Neglecting terms which are non-periodic in ![]() $\theta$, the biharmonic equations inside (
$\theta$, the biharmonic equations inside (![]() $-$) and outside (
$-$) and outside (![]() $+$) the vesicle are solved by
$+$) the vesicle are solved by
 \begin{align} \psi^{{\pm}} &= A_0^{{\pm}} r^{2}+B_0^{{\pm}} r^{2}\ln(r)+C^{{\pm}}_0\ln(r)+\left(A^{{\pm}}_1 r+B^{{\pm}}_1 r^{{-}1}+C^{{\pm}}_1 r^{3}+D^{{\pm}}_1 r\ln(r)\right)\cos(\theta)\nonumber\\ &\quad +\left(E^{{\pm}}_1 r+F^{{\pm}}_1 r^{{-}1}+G^{{\pm}}_1 r^{3}+H^{{\pm}}_1 r\ln(r)\right)\sin(\theta)\nonumber\\ &\quad +\sum_{n=2}^{\infty} \left(A^{{\pm}}_n r^{n}+B^{{\pm}}_n r^{{-}n}+C^{{\pm}}_n r^{n+2}+D^{{\pm}}_n r^{2-n}\right)\cos(n \theta)\nonumber\\ &\quad+\sum_{n=2}^{\infty}\left(E^{{\pm}}_n r^{n}+F^{{\pm}}_n r^{{-}n}+G^{{\pm}}_n r^{n+2}+H^{{\pm}}_n r^{2-n}\right)\sin(n \theta). \end{align}
\begin{align} \psi^{{\pm}} &= A_0^{{\pm}} r^{2}+B_0^{{\pm}} r^{2}\ln(r)+C^{{\pm}}_0\ln(r)+\left(A^{{\pm}}_1 r+B^{{\pm}}_1 r^{{-}1}+C^{{\pm}}_1 r^{3}+D^{{\pm}}_1 r\ln(r)\right)\cos(\theta)\nonumber\\ &\quad +\left(E^{{\pm}}_1 r+F^{{\pm}}_1 r^{{-}1}+G^{{\pm}}_1 r^{3}+H^{{\pm}}_1 r\ln(r)\right)\sin(\theta)\nonumber\\ &\quad +\sum_{n=2}^{\infty} \left(A^{{\pm}}_n r^{n}+B^{{\pm}}_n r^{{-}n}+C^{{\pm}}_n r^{n+2}+D^{{\pm}}_n r^{2-n}\right)\cos(n \theta)\nonumber\\ &\quad+\sum_{n=2}^{\infty}\left(E^{{\pm}}_n r^{n}+F^{{\pm}}_n r^{{-}n}+G^{{\pm}}_n r^{n+2}+H^{{\pm}}_n r^{2-n}\right)\sin(n \theta). \end{align}
The coefficients above are determined instantaneously in time by demanding that ![]() $\psi ^{-}$ and its derivatives are bounded at the origin, convergence to the far-field limit (
$\psi ^{-}$ and its derivatives are bounded at the origin, convergence to the far-field limit (![]() $\psi ^{+} \to \dot {\gamma } r^{2}\sin ^{2}(\theta )/2$ as
$\psi ^{+} \to \dot {\gamma } r^{2}\sin ^{2}(\theta )/2$ as ![]() $r \to \infty$), continuity of velocity across the membrane boundary,
$r \to \infty$), continuity of velocity across the membrane boundary, ![]() $[\boldsymbol {\nabla } \psi ]_S=\boldsymbol {0}$, traction balance (see Appendix B), and surface inextensibility along the membrane,
$[\boldsymbol {\nabla } \psi ]_S=\boldsymbol {0}$, traction balance (see Appendix B), and surface inextensibility along the membrane, ![]() $\boldsymbol {\nabla }_s \boldsymbol {\cdot } {{\boldsymbol {u}}}|_S=0$, where
$\boldsymbol {\nabla }_s \boldsymbol {\cdot } {{\boldsymbol {u}}}|_S=0$, where ![]() $\boldsymbol {\nabla }_s$ is the surface del operator,
$\boldsymbol {\nabla }_s$ is the surface del operator,
 \begin{align} \boldsymbol{\nabla}_s &= {{\hat{\boldsymbol{s}}}}\left({{\hat{\boldsymbol{s}}}}\boldsymbol{\cdot}\boldsymbol{\nabla} \right) =\left(\hat{\boldsymbol{\theta}}+\varepsilon \rho_\theta{{\hat{\boldsymbol{r}}}}+O(\varepsilon^{2}) \right)\left(\hat{\boldsymbol{\theta}}+\varepsilon \rho_\theta{{\hat{\boldsymbol{r}}}} +O(\varepsilon^{2}) \right) \boldsymbol{\cdot} \left({{\hat{\boldsymbol{r}}}} \partial_r +\hat{\boldsymbol{\theta}}\frac{1}{r}\partial_\theta \right)\nonumber\\ &=\hat{\boldsymbol{\theta}}\frac{1}{r} \partial_\theta +\varepsilon \left(\hat{\boldsymbol{\theta}} \rho_\theta \partial_r +{{\hat{\boldsymbol{r}}}} \frac{\rho_\theta}{r} \partial_\theta \right)+O(\varepsilon^{2}). \end{align}
\begin{align} \boldsymbol{\nabla}_s &= {{\hat{\boldsymbol{s}}}}\left({{\hat{\boldsymbol{s}}}}\boldsymbol{\cdot}\boldsymbol{\nabla} \right) =\left(\hat{\boldsymbol{\theta}}+\varepsilon \rho_\theta{{\hat{\boldsymbol{r}}}}+O(\varepsilon^{2}) \right)\left(\hat{\boldsymbol{\theta}}+\varepsilon \rho_\theta{{\hat{\boldsymbol{r}}}} +O(\varepsilon^{2}) \right) \boldsymbol{\cdot} \left({{\hat{\boldsymbol{r}}}} \partial_r +\hat{\boldsymbol{\theta}}\frac{1}{r}\partial_\theta \right)\nonumber\\ &=\hat{\boldsymbol{\theta}}\frac{1}{r} \partial_\theta +\varepsilon \left(\hat{\boldsymbol{\theta}} \rho_\theta \partial_r +{{\hat{\boldsymbol{r}}}} \frac{\rho_\theta}{r} \partial_\theta \right)+O(\varepsilon^{2}). \end{align}
Inextensibility is given in terms of the radial and azimuthal velocity components ![]() $u_r$ and
$u_r$ and ![]() $u_\theta$ by
$u_\theta$ by
In terms of the stream function, the relations ![]() $u_r = \psi _\theta /r$ and
$u_r = \psi _\theta /r$ and ![]() $u_\theta =-\psi _r$ are inserted into the above to give
$u_\theta =-\psi _r$ are inserted into the above to give
More terms above are kept to extend the approximation to higher order.
Appendix B. Traction balance asymptotics
Traction balance is demanded order by order in the small parameter ![]() $\varepsilon$. Regular perturbation expansions for the (dimensionless) stream function,
$\varepsilon$. Regular perturbation expansions for the (dimensionless) stream function, ![]() $\psi =\psi ^{(1)}+\varepsilon \psi ^{(2)}+\dots$, pressure
$\psi =\psi ^{(1)}+\varepsilon \psi ^{(2)}+\dots$, pressure ![]() $p=p^{(0)}+\varepsilon p^{(1)}+\varepsilon ^{2} p^{(2)}+\dots$ and viscous traction
$p=p^{(0)}+\varepsilon p^{(1)}+\varepsilon ^{2} p^{(2)}+\dots$ and viscous traction ![]() ${{\boldsymbol {f}}} ={{\boldsymbol {f}}}^{(0)}+ \varepsilon {{\boldsymbol {f}}}^{(1)}+\dots$ are assumed. The dimensionless viscous traction is given at leading order (from (2.7)) by
${{\boldsymbol {f}}} ={{\boldsymbol {f}}}^{(0)}+ \varepsilon {{\boldsymbol {f}}}^{(1)}+\dots$ are assumed. The dimensionless viscous traction is given at leading order (from (2.7)) by ![]() ${{\boldsymbol {f}}}^{(0)}=-[p^{(0)}]{{\hat {\boldsymbol {n}}}}$, and the contribution at first order in
${{\boldsymbol {f}}}^{(0)}=-[p^{(0)}]{{\hat {\boldsymbol {n}}}}$, and the contribution at first order in ![]() $\varepsilon$ is given by
$\varepsilon$ is given by
 \begin{align} {{\boldsymbol{f}}}^{(1)} &={-}\left([p^{(1)}+\rho(\theta,t) \partial_r p^{(0)}]+2 [\partial_\theta\psi^{(1)}- \partial_{r\theta}\psi^{(1)}]^{(\lambda)} \right) {{\hat{\boldsymbol{n}}}}\nonumber\\ &\quad +[ \partial_{\theta\theta}\psi^{(1)}+\partial_{r}\psi^{(1)}-\partial_{rr}\psi^{(1)} ]^{(\lambda)} {{\hat{\boldsymbol{s}}}}, \end{align}
\begin{align} {{\boldsymbol{f}}}^{(1)} &={-}\left([p^{(1)}+\rho(\theta,t) \partial_r p^{(0)}]+2 [\partial_\theta\psi^{(1)}- \partial_{r\theta}\psi^{(1)}]^{(\lambda)} \right) {{\hat{\boldsymbol{n}}}}\nonumber\\ &\quad +[ \partial_{\theta\theta}\psi^{(1)}+\partial_{r}\psi^{(1)}-\partial_{rr}\psi^{(1)} ]^{(\lambda)} {{\hat{\boldsymbol{s}}}}, \end{align}where we have defined a jump operator which incorporates the viscosity ratio,
This viscous traction must balance with the elastic traction. At leading order, traction balance in the tangential and normal directions returns
Hence, ![]() $T^{(0)}=-[p^{(0)}] =:P_0(t)$; the leading-order isotropic tension is balanced with the leading-order pressure jump across the interface, a dimensionless statement of an elastic Young–Laplace law. At the next order in
$T^{(0)}=-[p^{(0)}] =:P_0(t)$; the leading-order isotropic tension is balanced with the leading-order pressure jump across the interface, a dimensionless statement of an elastic Young–Laplace law. At the next order in ![]() $\varepsilon$, traction balance in the tangential and normal directions are given by
$\varepsilon$, traction balance in the tangential and normal directions are given by
with ![]() $\mathcal {C} = \textit {Ca}/\varepsilon$ defined in (2.13a–e),
$\mathcal {C} = \textit {Ca}/\varepsilon$ defined in (2.13a–e), ![]() $\kappa$ and
$\kappa$ and ![]() $\zeta$ the first-order material property variations defined in (2.14a,b), and
$\zeta$ the first-order material property variations defined in (2.14a,b), and
 \begin{align} &-T^{(1)}+\left(\rho+\partial^{2}_{\theta}\rho\right)P_0-\mathcal{C}^{{-}1} \left((\tilde{H}\tilde{H}_0 -1)\partial^{2}_{\theta} \kappa+\partial^{2}_{\theta}\zeta+\partial^{4}_{\theta}\rho+\partial^{2}_{\theta}\rho\right)\nonumber\\ &\quad -\left([p^{(1)}+\rho(\theta,t) \partial_r p^{(0)}]+2 [\partial_\theta\psi^{(1)}- \partial_{r\theta}\psi^{(1)}]^{(\lambda)} \right)=0. \end{align}
\begin{align} &-T^{(1)}+\left(\rho+\partial^{2}_{\theta}\rho\right)P_0-\mathcal{C}^{{-}1} \left((\tilde{H}\tilde{H}_0 -1)\partial^{2}_{\theta} \kappa+\partial^{2}_{\theta}\zeta+\partial^{4}_{\theta}\rho+\partial^{2}_{\theta}\rho\right)\nonumber\\ &\quad -\left([p^{(1)}+\rho(\theta,t) \partial_r p^{(0)}]+2 [\partial_\theta\psi^{(1)}- \partial_{r\theta}\psi^{(1)}]^{(\lambda)} \right)=0. \end{align}
In the limit of infinite bending capillary number (i.e. zero bending stiffness) these expressions are consistent with those provided by Zahalak et al. (Reference Zahalak, Rao and Sutera1987). The membrane length and area constraints, enforced out to second order in ![]() $\varepsilon$ as
$\varepsilon$ as ![]() $\varepsilon \to 0$, are used to determine pressure jump at the interface
$\varepsilon \to 0$, are used to determine pressure jump at the interface ![]() $P_0$ at leading order (or the isotropic tension
$P_0$ at leading order (or the isotropic tension ![]() $T^{(0)}$), leading to the expression in (2.19).
$T^{(0)}$), leading to the expression in (2.19).
Appendix C. First-order solution
Equations (2.8), (2.9), (2.10), (B5) and (B6) are solved simultaneously for the dimensionless first-order stream function ![]() $\psi ^{(1)}$ (via (A1), properly scaled) and pressure
$\psi ^{(1)}$ (via (A1), properly scaled) and pressure ![]() $p^{(1)}$ both inside and outside the vesicle, and for the first-order membrane tension,
$p^{(1)}$ both inside and outside the vesicle, and for the first-order membrane tension, ![]() $T^{(1)}$. The resulting stream functions are given by
$T^{(1)}$. The resulting stream functions are given by
 \begin{align} \psi^{(1)-}&=\frac{r^{2}}{4}-\frac{r^{2}(3-r^{2})}{4(1+\lambda)}\cos(2\theta)\nonumber\\ &\quad +\sum_{n=2}^{\infty} r^{n}\left((n+1)-(n-1)r^{2}\right)\left(B_n \cos(n\theta) -A_n\sin(n\theta)\right) \end{align}
\begin{align} \psi^{(1)-}&=\frac{r^{2}}{4}-\frac{r^{2}(3-r^{2})}{4(1+\lambda)}\cos(2\theta)\nonumber\\ &\quad +\sum_{n=2}^{\infty} r^{n}\left((n+1)-(n-1)r^{2}\right)\left(B_n \cos(n\theta) -A_n\sin(n\theta)\right) \end{align}and
 \begin{align} \psi^{(1)+}&=\frac{r^{2}}{4}-\left(\frac{\lambda+r^{2}(1-2\lambda)+r^{4}(1+\lambda) }{4r^{2}(1+\lambda) } \right)\cos(2\theta) \nonumber\\ &\quad +\sum_{n=2}^{\infty}\left((n+1)r^{2-n}-(n-1)r^{{-}n}\right)\left(B_n\cos(n\theta)-A_n\sin(n\theta)\right) \end{align}
\begin{align} \psi^{(1)+}&=\frac{r^{2}}{4}-\left(\frac{\lambda+r^{2}(1-2\lambda)+r^{4}(1+\lambda) }{4r^{2}(1+\lambda) } \right)\cos(2\theta) \nonumber\\ &\quad +\sum_{n=2}^{\infty}\left((n+1)r^{2-n}-(n-1)r^{{-}n}\right)\left(B_n\cos(n\theta)-A_n\sin(n\theta)\right) \end{align}
(note that ![]() $n=2$ terms are also in the summation), where
$n=2$ terms are also in the summation), where ![]() $A_n$,
$A_n$, ![]() $B_n$ are given in (2.17a,b). With
$B_n$ are given in (2.17a,b). With ![]() $p^{(0)+}=p_\infty$ and
$p^{(0)+}=p_\infty$ and ![]() $p^{(0)-}=p_\infty +P_0$ the (spatially constant) leading-order pressure fields, with
$p^{(0)-}=p_\infty +P_0$ the (spatially constant) leading-order pressure fields, with ![]() $P_0$ given in (2.19), the first-order pressure fields are
$P_0$ given in (2.19), the first-order pressure fields are
 \begin{equation} p^{(1)-}=\varPi-\frac{3\lambda r^{2}}{1+\lambda}\sin(2\theta) + 4\lambda \sum^{\infty}_{n=2}(n^{2}-1)r^{n} \left( B_n \sin(n\theta)+ A_n \cos(n\theta)\right) \end{equation}
\begin{equation} p^{(1)-}=\varPi-\frac{3\lambda r^{2}}{1+\lambda}\sin(2\theta) + 4\lambda \sum^{\infty}_{n=2}(n^{2}-1)r^{n} \left( B_n \sin(n\theta)+ A_n \cos(n\theta)\right) \end{equation}and
 \begin{equation} p^{(1)+}=\frac{(2\lambda-1)}{r^{2}(1+\lambda)}\sin(2\theta) +4 \sum^{\infty}_{n=2}r^{{-}n}(n^{2}-1)\left(B_n \sin(n\theta) -A_n \cos(n\theta)\right), \end{equation}
\begin{equation} p^{(1)+}=\frac{(2\lambda-1)}{r^{2}(1+\lambda)}\sin(2\theta) +4 \sum^{\infty}_{n=2}r^{{-}n}(n^{2}-1)\left(B_n \sin(n\theta) -A_n \cos(n\theta)\right), \end{equation}
where ![]() $\varPi$ is a constant. Finally, the membrane tension at first order is given by
$\varPi$ is a constant. Finally, the membrane tension at first order is given by
 \begin{equation} T^{(1)}=\varPi -\sin(2 \theta)-\sum^{\infty}_{n=1} \left(4(1+\lambda)B_n-P_0b_n\right)\sin(n\theta)+ \left(4(1+\lambda)A_n-P_0a_n\right)\cos(n\theta). \end{equation}
\begin{equation} T^{(1)}=\varPi -\sin(2 \theta)-\sum^{\infty}_{n=1} \left(4(1+\lambda)B_n-P_0b_n\right)\sin(n\theta)+ \left(4(1+\lambda)A_n-P_0a_n\right)\cos(n\theta). \end{equation}
The free constant ![]() $\varPi$ appears in both
$\varPi$ appears in both ![]() $p^{(1)-}$ and
$p^{(1)-}$ and ![]() $T^{(1)}$, indicating an ambiguity which is understood upon interpretation of the pressure and tension fields as Lagrange multipliers which enforce fluid and membrane incompressibility and inextensibility, respectively, and recalling that the two are linked by the Young–Laplace law. The value of
$T^{(1)}$, indicating an ambiguity which is understood upon interpretation of the pressure and tension fields as Lagrange multipliers which enforce fluid and membrane incompressibility and inextensibility, respectively, and recalling that the two are linked by the Young–Laplace law. The value of ![]() $\varPi$ has no bearing on the dynamics.
$\varPi$ has no bearing on the dynamics.
Appendix D. From the stream function to the surface velocity
For a given station in arclength ![]() $s$, the no-slip condition is written as
$s$, the no-slip condition is written as ![]() $\partial _t {{\boldsymbol {r}}}(s,t) = {{\boldsymbol {u}}}({{\boldsymbol {r}}}(s,t),t)$; to focus on fixed values of
$\partial _t {{\boldsymbol {r}}}(s,t) = {{\boldsymbol {u}}}({{\boldsymbol {r}}}(s,t),t)$; to focus on fixed values of ![]() $\theta$ we write
$\theta$ we write ![]() ${{\boldsymbol {r}}}(s,t)={{\boldsymbol {r}}}(s(\theta,t),t)=r(\theta,t){{\hat {\boldsymbol {r}}}}(\theta )$. Then noting that
${{\boldsymbol {r}}}(s,t)={{\boldsymbol {r}}}(s(\theta,t),t)=r(\theta,t){{\hat {\boldsymbol {r}}}}(\theta )$. Then noting that
dotting with the normal vector removes the need to determine ![]() $\partial _t s$ for fixed
$\partial _t s$ for fixed ![]() $\theta$:
$\theta$:
and thus ![]() $\partial _t (r(\theta,t) {{\hat {\boldsymbol {r}}}})\boldsymbol {\cdot } {{\hat {\boldsymbol {n}}}}= {{\hat {\boldsymbol {n}}}} \boldsymbol {\cdot } {{\boldsymbol {u}}}|_S$. Then with
$\partial _t (r(\theta,t) {{\hat {\boldsymbol {r}}}})\boldsymbol {\cdot } {{\hat {\boldsymbol {n}}}}= {{\hat {\boldsymbol {n}}}} \boldsymbol {\cdot } {{\boldsymbol {u}}}|_S$. Then with ![]() ${{\boldsymbol {u}}} = u_n {{\hat {\boldsymbol {n}}}} + u_s {{\hat {\boldsymbol {s}}}} =u_r {{\hat {\boldsymbol {r}}}} + u_\theta \hat {\boldsymbol {\theta }}$, and all dimensionless velocities expanded as
${{\boldsymbol {u}}} = u_n {{\hat {\boldsymbol {n}}}} + u_s {{\hat {\boldsymbol {s}}}} =u_r {{\hat {\boldsymbol {r}}}} + u_\theta \hat {\boldsymbol {\theta }}$, and all dimensionless velocities expanded as ![]() ${{\boldsymbol {u}}} = {{\boldsymbol {u}}}^{(1)}+\varepsilon {{\boldsymbol {u}}}^{(2)}+\dots$,
${{\boldsymbol {u}}} = {{\boldsymbol {u}}}^{(1)}+\varepsilon {{\boldsymbol {u}}}^{(2)}+\dots$,
and
Recall that the dimensional velocity is scaled by ![]() $\dot {\gamma }$, so that a dimensionless velocity
$\dot {\gamma }$, so that a dimensionless velocity ![]() ${{\boldsymbol {u}}}^{(1)}$ which is
${{\boldsymbol {u}}}^{(1)}$ which is ![]() $O(1)$ as
$O(1)$ as ![]() $\varepsilon \to 0$ corresponds to a dimensional velocity
$\varepsilon \to 0$ corresponds to a dimensional velocity ![]() $\dot {\gamma } {{\boldsymbol {u}}}^{(1)}$ which is
$\dot {\gamma } {{\boldsymbol {u}}}^{(1)}$ which is ![]() $O(\varepsilon )$ as
$O(\varepsilon )$ as ![]() $\varepsilon \to 0$. Since the velocities in the radial and azimuthal directions are given by
$\varepsilon \to 0$. Since the velocities in the radial and azimuthal directions are given by
 \begin{gather} \left.u_r \right|_S= \left.\frac{1}{r}\psi_\theta(r,\theta) \right|_{r=1+\varepsilon\rho+O(\varepsilon^{2})}= \left.\psi_\theta^{(1)}\right|_{r=1}+\left.\varepsilon\left(\psi_\theta^{(2)}+\rho\left( \psi_{r\theta}^{(1)}- \psi_\theta^{(1)}\right)\right)\right|_{r=1}+O(\varepsilon^{2}), \end{gather}
\begin{gather} \left.u_r \right|_S= \left.\frac{1}{r}\psi_\theta(r,\theta) \right|_{r=1+\varepsilon\rho+O(\varepsilon^{2})}= \left.\psi_\theta^{(1)}\right|_{r=1}+\left.\varepsilon\left(\psi_\theta^{(2)}+\rho\left( \psi_{r\theta}^{(1)}- \psi_\theta^{(1)}\right)\right)\right|_{r=1}+O(\varepsilon^{2}), \end{gather}the velocity in the surface normal direction may be written as
Hence, since the dimensional time is scaled by ![]() $\varepsilon /\dot {\gamma }$,
$\varepsilon /\dot {\gamma }$,
and
Since the gradient of the stream function is continuous across the membrane boundary, either ![]() $\psi ^{+}$ or
$\psi ^{+}$ or ![]() $\psi ^{-}$ may be inserted into the above without ambiguity. Using the results of Appendix C, the normal and tangential components of the velocity are then given by (2.15)–(2.16).
$\psi ^{-}$ may be inserted into the above without ambiguity. Using the results of Appendix C, the normal and tangential components of the velocity are then given by (2.15)–(2.16).
Appendix E. Inertia tensor, deformation parameter and inclination angle
The deformation parameter, ![]() $D=(L_1-L_2)/(L_1+L_2)$, is defined using the axis lengths
$D=(L_1-L_2)/(L_1+L_2)$, is defined using the axis lengths ![]() $2L_1$ and
$2L_1$ and ![]() $2L_2$ of the ellipse which shares the same inertia tensor. With
$2L_2$ of the ellipse which shares the same inertia tensor. With ![]() $\varOmega$ denoting the vesicle's interior, the inertia tensor is defined as
$\varOmega$ denoting the vesicle's interior, the inertia tensor is defined as
When ![]() $\varOmega$ is the interior of an ellipse with major and minor axis lengths
$\varOmega$ is the interior of an ellipse with major and minor axis lengths ![]() $2L_1$ and
$2L_1$ and ![]() $2L_2$, respectively, oriented with its major axis at an angle
$2L_2$, respectively, oriented with its major axis at an angle ![]() $\theta$ relative to the
$\theta$ relative to the ![]() $x$-axis, this tensor has eigenvalues
$x$-axis, this tensor has eigenvalues ![]() $\lambda _1 = {\rm \pi}L_1^{3} L_2/4$ and
$\lambda _1 = {\rm \pi}L_1^{3} L_2/4$ and ![]() $\lambda _2 = {\rm \pi}L_1 L_2^{3}/4$, with associated eigenvectors
$\lambda _2 = {\rm \pi}L_1 L_2^{3}/4$, with associated eigenvectors ![]() $\boldsymbol {v}_1 = (\sin ^{2}\theta,-\sin (2\theta )/2)$ and
$\boldsymbol {v}_1 = (\sin ^{2}\theta,-\sin (2\theta )/2)$ and ![]() $\boldsymbol {v}_2 = (\cos ^{2}\theta,\sin (2\theta )/2)$. In terms of the eigenvalues of the inertia tensor, then,
$\boldsymbol {v}_2 = (\cos ^{2}\theta,\sin (2\theta )/2)$. In terms of the eigenvalues of the inertia tensor, then, ![]() $L_1 = (4/{\rm \pi} )^{1/4}(\lambda _1^{3}/\lambda _2)^{1/8}$ and
$L_1 = (4/{\rm \pi} )^{1/4}(\lambda _1^{3}/\lambda _2)^{1/8}$ and ![]() $L_2 = (4/{\rm \pi} )^{1/4}(\lambda _2^{3}/\lambda _1)^{1/8}$, the deformation parameter is given by
$L_2 = (4/{\rm \pi} )^{1/4}(\lambda _2^{3}/\lambda _1)^{1/8}$, the deformation parameter is given by
 \begin{equation} D = \frac{L_1-L_2}{L_1+L_2} = \frac{\lambda_1^{1/2}-\lambda_2^{1/2}}{\lambda_1^{1/2}+\lambda_2^{1/2}}, \end{equation}
\begin{equation} D = \frac{L_1-L_2}{L_1+L_2} = \frac{\lambda_1^{1/2}-\lambda_2^{1/2}}{\lambda_1^{1/2}+\lambda_2^{1/2}}, \end{equation}
and the inclination angle may be recovered from ![]() $\boldsymbol {v}_2$ via
$\boldsymbol {v}_2$ via ![]() $\theta = \tan ^{-1}({{\hat {\boldsymbol {y}}}}\boldsymbol {\cdot } \boldsymbol {v}_2/{{\hat {\boldsymbol {x}}}}\boldsymbol {\cdot } \boldsymbol {v}_2)$.
$\theta = \tan ^{-1}({{\hat {\boldsymbol {y}}}}\boldsymbol {\cdot } \boldsymbol {v}_2/{{\hat {\boldsymbol {x}}}}\boldsymbol {\cdot } \boldsymbol {v}_2)$.
Considering the general membrane boundary ![]() $S$, parameterized as in § 2, the inertia tensor above instead has eigenvalues
$S$, parameterized as in § 2, the inertia tensor above instead has eigenvalues
 \begin{gather} \lambda_1 =\frac{1}{4} \left\{{\rm \pi}+2 {\rm \pi}\varepsilon \sqrt{a_2^{2}+b_2^{2}}+\varepsilon ^{2} \left(3 \left(a_2^{2}+b_2^{2}\right)+2\frac{a_2^{(2)}a_2 +b_2^{(2)}b_2 }{\sqrt{a_2^{2}+b_2^{2}}}\right)\right\}+O(\varepsilon^{3}), \end{gather}
\begin{gather} \lambda_1 =\frac{1}{4} \left\{{\rm \pi}+2 {\rm \pi}\varepsilon \sqrt{a_2^{2}+b_2^{2}}+\varepsilon ^{2} \left(3 \left(a_2^{2}+b_2^{2}\right)+2\frac{a_2^{(2)}a_2 +b_2^{(2)}b_2 }{\sqrt{a_2^{2}+b_2^{2}}}\right)\right\}+O(\varepsilon^{3}), \end{gather} \begin{gather}\lambda_2 =\frac{1}{4} \left\{{\rm \pi}-2 {\rm \pi}\varepsilon \sqrt{a_2^{2}+b_2^{2}}+\varepsilon ^{2} \left(3 \left(a_2^{2}+b_2^{2}\right)-2\frac{a_2^{(2)}a_2 +b_2^{(2)}b_2 }{\sqrt{a_2^{2}+b_2^{2}}}\right)\right\}+O(\varepsilon^{3}), \end{gather}
\begin{gather}\lambda_2 =\frac{1}{4} \left\{{\rm \pi}-2 {\rm \pi}\varepsilon \sqrt{a_2^{2}+b_2^{2}}+\varepsilon ^{2} \left(3 \left(a_2^{2}+b_2^{2}\right)-2\frac{a_2^{(2)}a_2 +b_2^{(2)}b_2 }{\sqrt{a_2^{2}+b_2^{2}}}\right)\right\}+O(\varepsilon^{3}), \end{gather}
and then (E2) produces the deformation parameter in (3.6). The eigenvector associated with ![]() $\lambda _2$ has components
$\lambda _2$ has components
and then ![]() $\tan ^{-1}({{\hat {\boldsymbol {y}}}}\boldsymbol {\cdot } \boldsymbol {v}_2/{{\hat {\boldsymbol {x}}}}\boldsymbol {\cdot } \boldsymbol {v}_2)$ returns the inclination angle in (3.8).
$\tan ^{-1}({{\hat {\boldsymbol {y}}}}\boldsymbol {\cdot } \boldsymbol {v}_2/{{\hat {\boldsymbol {x}}}}\boldsymbol {\cdot } \boldsymbol {v}_2)$ returns the inclination angle in (3.8).
Appendix F. General solution to the inner expansion equations
The general solution to (4.11) is
for ![]() $m$ an integer and
$m$ an integer and ![]() $C_0$ an integration constant. At the next order (4.10) then produces
$C_0$ an integration constant. At the next order (4.10) then produces
 \begin{equation} \frac{{\rm d}}{{\rm d}\sigma}p_{inner}^{(1)} = \beta\left(\frac{1-C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}{1+C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}\right)\left(\alpha-\frac{\sigma ^{2}}{2}-2 \sigma p_{inner}^{(1)}\right), \end{equation}
\begin{equation} \frac{{\rm d}}{{\rm d}\sigma}p_{inner}^{(1)} = \beta\left(\frac{1-C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}{1+C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}\right)\left(\alpha-\frac{\sigma ^{2}}{2}-2 \sigma p_{inner}^{(1)}\right), \end{equation}and the solution
 \begin{align} p_{inner}^{(1)}(\sigma)&=\frac{C_1 \,{\rm e}^{\beta \sigma ^{2}}}{1+C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}-\frac{\sigma}{4}\nonumber\\ &\quad +\frac{{\rm \pi} ^{1/2} \,{\rm e}^{\beta \sigma ^{2}}}{8 \beta^{1/2}\left(1\!+\!C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}\right)}\left(C_0^{2} (1\!+\!4 \alpha\beta)\mbox{erfi}\left(\beta^{1/2}\sigma \right)\!+\!(1-4 \alpha \beta) \mbox{erf}\left(\beta^{1/2} \sigma \right)\right), \end{align}
\begin{align} p_{inner}^{(1)}(\sigma)&=\frac{C_1 \,{\rm e}^{\beta \sigma ^{2}}}{1+C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}}-\frac{\sigma}{4}\nonumber\\ &\quad +\frac{{\rm \pi} ^{1/2} \,{\rm e}^{\beta \sigma ^{2}}}{8 \beta^{1/2}\left(1\!+\!C_0^{2}\,{\rm e}^{2 \beta \sigma ^{2}}\right)}\left(C_0^{2} (1\!+\!4 \alpha\beta)\mbox{erfi}\left(\beta^{1/2}\sigma \right)\!+\!(1-4 \alpha \beta) \mbox{erf}\left(\beta^{1/2} \sigma \right)\right), \end{align}
where ![]() $C_1$ is an integration constant. The imaginary error function,
$C_1$ is an integration constant. The imaginary error function, ![]() $\mbox {erfi}(\beta ^{1/2}\sigma )=(2/\sqrt {{\rm \pi} })\int _0^{\beta ^{1/2}\sigma } \,{\rm e}^{t^{2}}\,{\rm d}t$, tends towards
$\mbox {erfi}(\beta ^{1/2}\sigma )=(2/\sqrt {{\rm \pi} })\int _0^{\beta ^{1/2}\sigma } \,{\rm e}^{t^{2}}\,{\rm d}t$, tends towards ![]() ${\rm e}^{\beta \sigma ^{2}}/\sqrt {{\rm \pi} \beta \sigma ^{2}}$ as
${\rm e}^{\beta \sigma ^{2}}/\sqrt {{\rm \pi} \beta \sigma ^{2}}$ as ![]() $|\sigma |\to \infty$.
$|\sigma |\to \infty$.
The approach in § 4.1 requires that ![]() $p_{inner}^{(0)}(0)+\varepsilon ^{1/2} p_{inner}^{(1)}(0)=\varepsilon ^{1/2}\varUpsilon +O(\varepsilon )$, with
$p_{inner}^{(0)}(0)+\varepsilon ^{1/2} p_{inner}^{(1)}(0)=\varepsilon ^{1/2}\varUpsilon +O(\varepsilon )$, with ![]() $\varUpsilon$ defined in (4.16), resulting in
$\varUpsilon$ defined in (4.16), resulting in
Here we see that ![]() $C_0$ cannot be
$C_0$ cannot be ![]() $O(1)$ as
$O(1)$ as ![]() $\varepsilon \to 0$, as in this case matching the initial data at
$\varepsilon \to 0$, as in this case matching the initial data at ![]() $\tau =3{\rm \pi} /2$ is not possible. But
$\tau =3{\rm \pi} /2$ is not possible. But ![]() $C_0$ cannot be zero or else either matching to the data above, or merging with the outer solution as
$C_0$ cannot be zero or else either matching to the data above, or merging with the outer solution as ![]() $\sigma \to \infty$, is not possible. The equation above is then to be seen as a signal that
$\sigma \to \infty$, is not possible. The equation above is then to be seen as a signal that ![]() $C_0$ is in fact
$C_0$ is in fact ![]() $O(\varepsilon ^{1/2})$ as
$O(\varepsilon ^{1/2})$ as ![]() $\varepsilon \to 0$.
$\varepsilon \to 0$.