Introduction
Various methods used for the control of insect pest have a variety of concepts behind their mode of action. Since the negative effects of insecticide application are known and acknowledged far and wide, environment-friendly insect pest control strategies are gaining importance. Insects are known to have a variety of biological responses to various wavelengths of the light spectrum (Tariq et al., Reference Tariq, Noor, Saeed and Zhang2015). Many insect control strategies utilize knowledge of light-dependent behavior of pest insects. Nocturnal insect are attracted towards light (including UV and visible light), which provides the basis for electric insect killers. UV-emitting insect traps effectively attract insects and prevent their entry to greenhouses. This environment-friendly insect pest-control method is being used as a tool in integrated pest management (IPM) programs (Johansen et al., Reference Johansen, Vänninen, Pinto, Nissinen and Shipp2011; Ben-Yakir et al., Reference Ben-Yakir, Antignus, Offir and Shahak2013). A plethora of literature reports the negative impact of Ultraviolet (UV) radiation on the biology of pest insects (Tariq et al., Reference Tariq, Noor, Saeed and Zhang2015). After penetrating cells, UV light induces the production of reactive oxygen species that cause damage to nucleic acids, membrane of lipids, and proteins (Jurkiewicz & Buettner, Reference Jurkiewicz and Buettner1994; Vile & Tyrrell, Reference Vile and Tyrrell1995). A recent study revealed that blue light (400–500 nm) induced more harmful effects than UV light to insect pests of various orders including Diptera (Hori et al., Reference Hori, Shibuya, Sato and Saito2014) suggesting that blue light can also be used in IPM programs. However, for insect pest control through blue light, its effects on insect physiology must be understood, though such information is largely lacking.
Animals protect themselves from harmful effect of UV radiation through the deposition of melanin, which is a photo protective compound (Ortonne, Reference Ortonne2002). In animals, melanin perform key roles in immune system, camouflage, wound healing and cuticle hardening (Roulin, Reference Roulin2014). Phenoloxidase (PO) enzyme, a part of the pro-PO cascade, is produced by hemocytes, and is responsible for the production and regulation of melanin in insects (Sugumaran, Reference Sugumaran2002). A recent study revealed that UV light impaired adult immune function of damselfly as a carryover effect of larval exposure, the exposed larvae invested more in cuticular melanin, which caused immunosuppression in adult flies (Debecker et al., Reference Debecker, Sommaruga, Maes and Stoks2015). However, it is still unknown whether insects protect themselves from blue light exposure through the deposition of melanin or there is any effect of blue light irradiation on immune function of insects.
Different studies showed that larval stressors can bridge metamorphosis and can severely affect adult fitness-related traits (Pechenik et al., Reference Pechenik, Wendt and Jarrett1998; Pechenik, Reference Pechenik2006; Campero et al., Reference Campero, De Block, Ollevier and Stoks2008a). As previous studies documented that blue light is more harmful to insects than UV light (Hori et al., Reference Hori, Shibuya, Sato and Saito2014), we propose that blue light exposure may have effect on cuticular melanin synthesis and subsequently cause immunosuppression in Bactrocera dorsalis. Larval stressors can also change adult traits with or without affecting metamorphic traits (Marjan De Block, Reference Marjan De Block2005; Allen et al., Reference Allen, Briggs, McCoy and Vonesh2010). However, the effect of blue light exposure at larval stage, on metamorphic traits and adult fitness is poorly understood.
In this study, we evaluated the effect of blue light exposure on cuticular melanin at larval stage and adult immune response of B. dorsalis. In addition, we investigated the effects of blue light exposure at larval stage on age and mass at metamorphosis and the mediatory role of cuticular melanin in carryover effects of larval stressors across metamorphosis. The immune response in the adults was evaluated by using melanotic encapsulation technique, which is commonly used to measure immuno-competence in invertebrates (Rantala & Roff, Reference Rantala and Roff2005) and by the quantification of hemocytes and PO activity. It is known that natural pathogens trigger immune response more robustly than artificial challenge (Adamo, Reference Adamo2004), and can be used to evaluate the efficiency of insect immune system (Neyen et al., Reference Neyen, Bretscher, Binggeli and Lemaitre2014). Thus, adult immune status was directly measured through survival after exposure to a natural pathogen, Beauvaria bassiana (Bals.). Beauveria bassiana (Bals.) has been extensively explored against fruit fly (Ekesi et al., Reference Ekesi, Maniania and Lux2002; Dimbi et al., Reference Dimbi, Maniania, Lux, Ekesi and Mueke2003; Daniel & Wyss, Reference Daniel and Wyss2009; Cossentine et al., Reference Cossentine, Thistlewood, Goettel and Jaronski2010) as a potent entomopathogen. We assessed the immune performance of adult flies through conidial exposure to B. bassiana.
Materials and methods
Insect cultures
The second-instar larvae of B. dorsalis were obtained from the colony reared in our laboratory according to the methods described by Zheng et al. (Reference Zheng, Peng, He and Zhang2012). Larvae were transferred to liquid-based artificial diet (Chang et al., Reference Chang, Vargas, Caceres, Jang and Cho2006) at 25–26°C with 12:12 h light: dark photoperiod and 60–65% RH. The larvae used in this experiment were synchronous and were randomly attributed between treatments (blue light and control), which minimized the effect of size difference and initial instar collection on test results.
Experimental design
A blue light emitting diode (LED) arrays (light emission surface: 150 × 150 mm; 360 LEDs were equally arranged on a panel; LED type: φ3-mm plastic mould; Langtuo Biological Technology Co. Ltd., Hangzhou, China) that emits radiation in the range of 400–500 nm (center wavelength: 460 nm) was used as the source to irradiate larvae. The distance between the light source and the larvae was 50 cm; the emitted energy of 460 nm was at an intensity of 0.12 W m−2 measured by a radiometer (AR823; Digital Lux Meter Co. Ltd., China). Each larvae was confined in a small Petri dish (5 cm diameter) containing 0.5 gram of liquid diet (Hanife, Reference Hanife2008). During measurements, the distance between the light source and the radiometer sensor was approximately the same as it was between the light source and insects in the incubator.
The insects were irradiated inside Petri dishes covered with glass lids. The same glass lid was used to determine the intensity of the light prior to insect radiation. Insects were irradiated inside incubator. The light source was connected with digital timer switches (HTS-AT10, Han-Seung, Daegu, Korea) to allow the treatment of insects for the exact periods of time. The Petri dishes containing larvae were placed directly under the light source during irradiation.
At the start of exposure period, 240 larvae were equally divided into four groups (A, B, C and D). After exposure the group A larvae were used for life history parameters, melanin quantification, encapsulation response, PO activity and hemocyte number counts, and the group B larvae were used at their adult stage for conidial exposure to B. bassiana, while the group C and D larvae were kept without exposure, and were used as controls along with the group A and B larvae. The final sample sizes for each response variable are shown in the figures.
The second-instar larvae were randomly assigned to blue light treatments and were individually exposed to radiations for 7 days with the duration of 7 hours per day. The temperature and relative humidity (RH) of the irradiated area was set at 25–26°C and 60–65%, respectively. The control larvae were kept in incubator without exposure. After irradiation, larvae were allowed to continue their life cycles. The late third-instar larvae were allowed to pupate in Petri dishes containing humid sand.
Life history
Prior to use in experiments and at the end of last exposure period, the larvae were weighted to about 12.5 mg using an electronic balance (Sartorius BSA124S, Germany). The individual larval growth rates were calculated by the following formula (Stoks et al., Reference Stoks, Swillen and De Block2012): (the final wet mass – the initial wet mass)/number of days.
The developmental time of larvae was defined as the number of days to reach the final-instar (Debecker et al., Reference Debecker, Sommaruga, Maes and Stoks2015). After eclosion and before immune challenge, the adults were weighted. Four larvae died during the larval stage, two adults did not emerge successfully and two adults showed deformations, and were not further analyzed.
Melanin quantification
Melanin present in the final-instar’s exuviae was measured. The exuviae of successfully emerged flies were collected, the melanin extraction protocol described by Zhou et al. (Reference Zhou, Shang, Ping and Zhao2012) was used. The extraction of melanin was carried out by adding 1 M NaOH/10% DMSO in each individual exuvia, the volume for each exuvia was adjusted (extraction volume in μl = 200 × exuvial mass in mg) as described by Debecker et al. (Reference Debecker, Sommaruga, Maes and Stoks2015). The samples were incubated at 80°C for 2 h so that melanin can dissolve in solvent followed by centrifugation at 12,000 g for 15 min. Each sample (20 µl) was loaded onto a 384-well plate with three replications and absorbance was read at 380 nm using microplate spectrophotometer (XMark™, BIO-RAD). Synthetic melanin (Sigma-Aldrich, St. Louis, MO, USA) Cat. No. M8631) was dissolved in 1 M NaOH/10% DMSO to prepare concentration gradients (0–500 µg ml−1) for making a standard curve (range of 1–100 µg ml−1). Exuvial melanin was measured by extrapolating with standard curve from synthetic melanin. The cuticular melanin content was calculated as the amount of melanin in the exuviae (in μg) per mg larval wet mass.
Encapsulation assay
Encapsulation rate followed by melanization is an effective innate immune response against parasites. This innate immune response of insects can be triggered by inserting a nylon filament (inert antigen) (Rantala et al., Reference Rantala, KoskimÌki, Taskinen, Tynkkynen and Suhonen2000; Therry et al., Reference Therry, Nilsson-Örtman, Bonte and Stoks2014) in the thorax of the insect. The ability of immune system to encapsulate artificial object (nylon filament) is correlated with the ability to encapsulate parasites (Smilanich et al., Reference Smilanich, Dyer and Gentry2009). After the adult exoskeleton was hardened a nylon filament (mean length of insert ± SE: 1.00 ± 0.02 mm, diameter: 0.20 mm; rubbed with fine sandpaper) was inserted into the thorax of flies to quantify the encapsulation rate (Nagel et al., Reference Nagel, Mlynarek and Forbes2011). Adults were kept for 24 h in the same conditions, and then encapsulated filaments were removed and stored at −20°C until the quantification of the deposited melanin. The adult flies were also frozen at −80°C for the analysis of PO activity and the hemocyte number. To quantify the melanin deposited on filaments, we used the same protocol used for the extraction of melanin of the exuviae. The melanin deposited on the filaments was desorbed in fixed volume of 50 µl of NaOH/10% DMSO, and the fixed sample was loaded onto a 384-well plate with two replications (2 × 20 µl). Absorbance was read at 380 nm using microplate spectrophotometer (XMark™, BIO-RAD, USA). The degree of melanization could be affected by the length difference of the inserted filaments, so encapsulation rate was expressed as the amount of melanin per unit length (μg mm−1) of the inserted filament. The filaments were photographed under microscope (Olympus B × 51, Tokyo, Japan), and the length was measured using Image J. software (US National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/).
Quantification of hemocytes
To quantify the hemocytes, hemolymph was extracted from adult flies by perfusing in 120 µl ice-cold cacodylate buffer (0.01 M Na-Coc and 0.005 M CaCl2) as described by Campero et al. (Reference Campero, Block, Ollevier and Stoks2008b). A total of 20 µl of each hemolymph sample was placed into a well of multi-well microscopic slide, stained with 5 µl ethidium bromide (2.5 mM), and incubated at 4°C for 2 h. Afterwards, three images of each well was captured using fluorescence microscope (Olympus B × 51, Tokyo, Japan) at magnification of 400×. Hemocytes were counted by Image Pro-Plus software (Image Pro-Plus 6.0; Media Cybernetics, Silver Spring, MD, USA). The average of the three counts was taken to represent the value of each test sample. The hemocyte number was expressed as the total count per mg of adult wet mass.
PO assay
The PO activity was measured by spectrophotometric assay (Shi & Sun, Reference Shi and Sun2010). The remaining volume of hemolymph sample (100 µl) was centrifuged at 15,000 g for 10 min at 4°C, After centrifugation, 30 µl of the supernatant was mixed with 110 µl milli-Q water, 30 µl phosphate buffered saline (PBS buffer:250 ml distilled water, 2 g NaCl, 50 mg KCl, 360 mg Na2HPO4, 360 mg K2HPO4, and pH 7.2) and 30 µl L-Dopa (Acros Organic, Morris Plains, NJ, USA, 4 mg ml−1 ultrapure water) as a substrate, and was kept at 30°C for 90 min, the absorbance was measured at 490 nm every 20 s in duplicate using SpectraMax reader (Molecular Devices Corp., California, USA). The enzyme activity was determined as the slope of the linear part of the reaction curve and expressed in enzyme units (U), one unit represents 1 mM dopachrome formed per min per mg adult wet mass.
Immune efficiency assessment through conidial exposure
The immune competency of adult flies (that had been previously exposed to blue light at their larval stage) was further evaluated through conidial exposure to B. bassiana. The adults were treated with LC50 [3.12 × 10−6 conidia ml−1 (De La Rosa et al., Reference De La Rosa, Lopez and Liedo2002)] of B. bassiana. One-day old adult flies from group B and D were treated with B. bassiana. The entomopathogenic fungus B. bassiana strain Bb24 originally isolated from a species of fruit fly was purchased from China General Microbiological Culture Collection Center Beijing, and was used for the experiment. The fungal strains were cultured on Sabouraud dextrose agar medium, in sterilized test tubes and Petri dishes and incubated for 15 days at 27 ± 1°C, 80 ± 5% RH and 14 h photophase. The conidia were harvested after 15 days and stored at 4°C. Before performing the bioassays, the viability of the conidia was confirmed as described by Lane et al. (Reference Lane, Humphreys, Thompson and Trinci1988). The 55 successfully emerged flies from group B and D were sprayed with 0.5 ml of a suspension of the fungal strain containing 3.12 × 10−6 conidia ml−1 for 30 s, which is the Mean Lethal Concentration (LC50) for fruit flies as reported by De La Rosa et al. (Reference De La Rosa, Lopez and Liedo2002). The treated flies were placed on absorbent paper towels to absorb excess fungal suspension. Adult flies of each group were kept in plastic flasks and maintained under 25–26°C with 12:12 h light: dark photoperiod and 60–65% RH. Sterile water and food were provided to flies daily as described by Zheng et al. (Reference Zheng, Peng, He and Zhang2012). Flies survival was scored on daily basis for 10 days post-inoculation. The Kaplan–Meier survival test was used to calculate the survival rates.
Statistical analysis
The effects of blue light exposure on different response variables were tested by running ANOVA using R software (R CoreTeam, 2013). Data for development time and encapsulation rate were log-transformed and square root-transformed, respectively, and the growth rate and hemocyte number were log(x + 1)-transformed. The effects of blue light on mass at adult emergence could be well explained by the difference in cuticular mass between treated and un-treated flies. ANOVA was run on the sum of the masses (mass at emergence and cuticular mass). Survival statistics were performed using log rank analysis and the Gehan–Breslow–Wilcoxon test using GraphPad Prism v. 5.0 (GraphPad Software, USA).
Results
Blue light exposure did not affect significantly larval growth rate (ANOVA, F 1,114 = 1.56, P = 0.12, fig. 1A). However, blue light-irradiated animals showed longer development time (F 1,114 = 9.4, P = 0.0001, fig. 1B) and lower mass at emergence (F 1,107 = 6.18, P = 0.0001, fig. 1C) as compared with animals reared without blue light exposure. The results revealed that blue light significantly inhibited larval cuticular melanin. The blue light-exposed larvae incorporated lower melanin in their cuticle (F 1,110 = 15.4, P = 0.0001, fig. 2A), and at adult stage they showed reduced encapsulation of nylon filaments (F 1,107 = 30.8, P = 0.0001, fig. 2B). The adults that emerged from blue light-exposed larvae showed lower PO activity (F 1,110 = 21.03, P = 0.0001, fig. 2C) however, no significant change in hemocyte number was detected (F 1,110 = 1.23, P = 0.21, fig. 2D) as compared with the control animals.
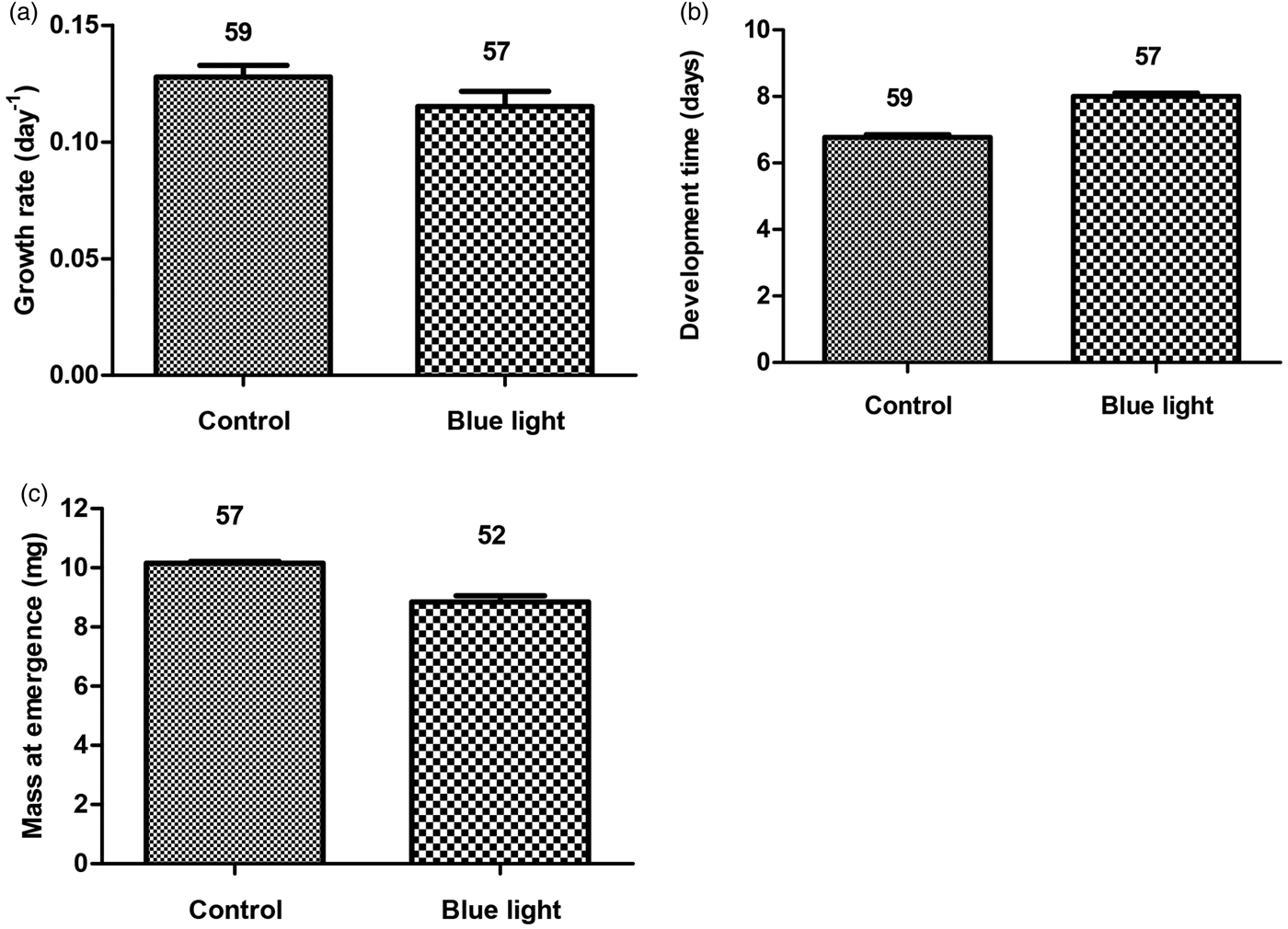
Fig. 1. Mean larval growth rate (A), larval development time (B), and adult mass at emergence (C) as a function of larval blue light exposure in the B. dorsalis. Given numbers are observed means ± SE.
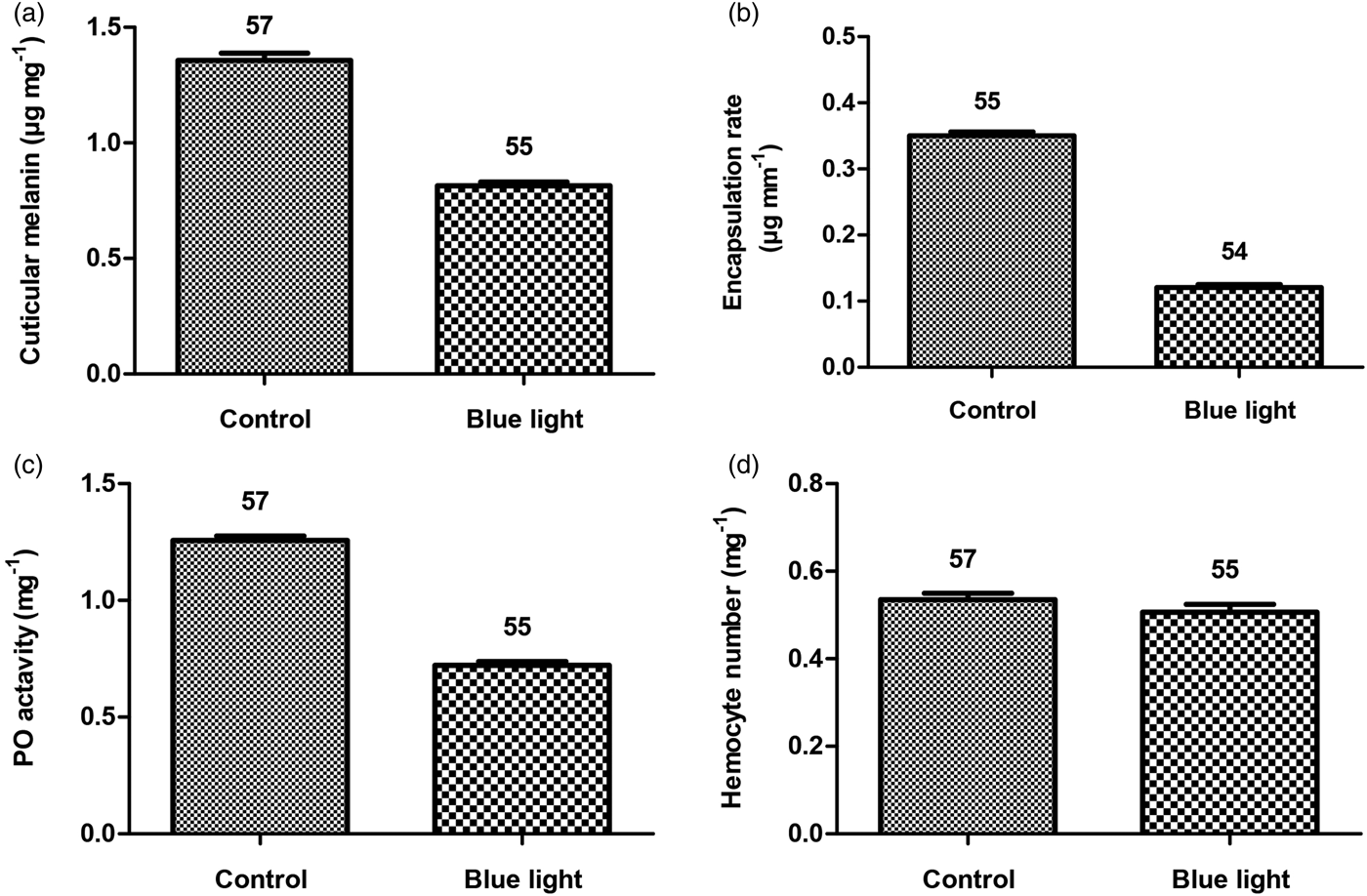
Fig. 2. Larval cuticular melanin content (A), adult encapsulation rate (B), adult PO activity (C), and adult hemocyte number (D) as a function of larval blue light exposure in the B. dorsalis. Given numbers are observed means ± SE.
The immune competency of blue light-treated and non-treated flies was further measured at larval stage through conidial exposure of B. bassiana. Survival assays showed a significant (P = 0.0001) increase in susceptibility of blue light-exposed insects to B. bassiana over control individuals (fig. 3).
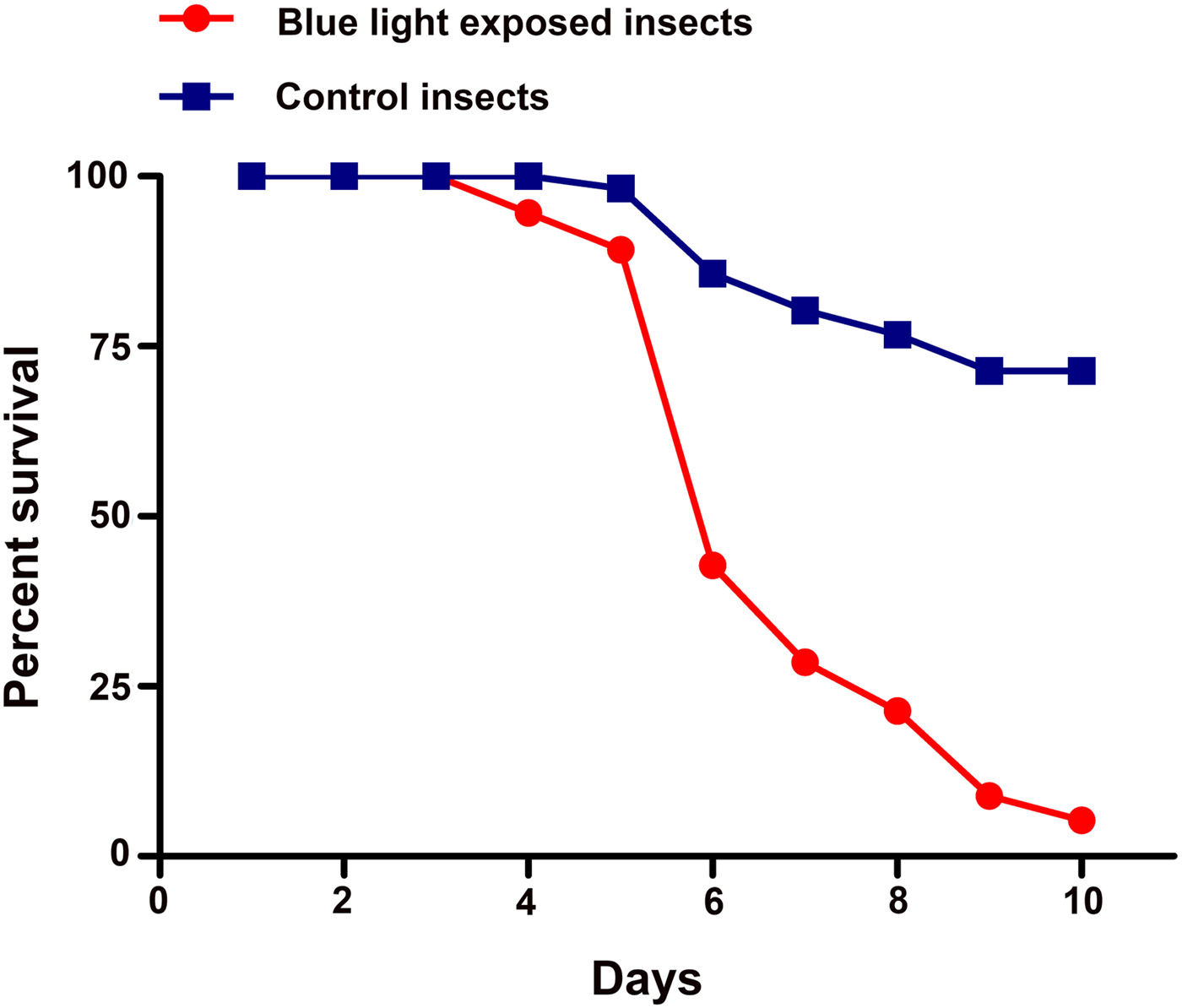
Fig. 3. Insects exposed to blue light at their larval stage are more susceptible to B. bassiana infections. Adults of B. dorsalis were challenged with B. bassiana (strain Bb24). Graph represents percent survival as calculated by the Kaplan–Meier method. The log rank analysis and Gehan–Breslow–Wilcoxon Test indicated that the survival of blue light exposed insects was significantly different than control flies (P < 0.0001).
Discussion
Hori et al. (Reference Hori, Shibuya, Sato and Saito2014) showed for the first time that blue light induced more harmful effects than UV light to insects, and blue light irradiation killed insect pests of various orders including Diptera. However, the effects of blue light on the physiology of insects are still largely unknown. In the present study we found that blue light irradiation inhibited cuticular melanin in the larvae of B. dorsalis, and exposed-larvae showed longer development time, with a lower mass at emergence. The carryover effects of larval blue light exposure were observed in the adult stage in terms of reduced melanotic encapsulation response, and lower PO activity, while no significant change was detected in hemocytes numbers. The blue light exposure resulted in lower encapsulation response and higher susceptibility of adult flies to B. bassiana clearly suggesting that blue light impaired the adult immune function of B. dorsalis.
Melanin performs several key functions in insects including immune responses (Ortonne, Reference Ortonne2002; Roulin, Reference Roulin2014; Debecker et al., Reference Debecker, Sommaruga, Maes and Stoks2015). The biosynthesis of melanin was inhibited in melanoma cells that were irradiated with blue light (Ohara et al., Reference Ohara, Kobayashi, Fujiwara, Kitajima, Mitsuoka and Watanabe2004). Moreover, a recent study revealed that blue light exposure decreased the viability of epithelial cells and ultimately lower melanin content (Chiarelli-Neto et al., Reference Chiarelli-Neto, Ferreira, Martins, Pavani, Severino, Faião-Flores, Maria-Engler, Aliprandini, Martinez, Di Mascio, Medeiros and Baptista2014). Our results showed that blue light-exposed larvae incorporated lower melanin in their exoskeletons suggesting the effect of blue light on cell vitality (Chiarelli-Neto et al., Reference Chiarelli-Neto, Ferreira, Martins, Pavani, Severino, Faião-Flores, Maria-Engler, Aliprandini, Martinez, Di Mascio, Medeiros and Baptista2014), inhibition of DNA synthesis, and cell division (Ohara et al., Reference Ohara, Kobayashi, Fujiwara, Kitajima, Mitsuoka and Watanabe2004). Debecker et al. (Reference Debecker, Sommaruga, Maes and Stoks2015) reported that UV exposed-larvae of damsel fly invested more in melanin synthesis which caused impair immune function of adult flies. We found that exposure of B. dorsalis larvae to blue light also impaired adult immune function but the mode of impairment was different from that with UV. Unlike UV, blue light inhibited larval cuticular melanin and impaired immune function at adult stage. Several studies reported the associations between cuticular melanization and immune function (Robb et al., Reference Robb, Forbes and Jamieson2003; Hagen et al., Reference Hagen, Sørlibråten, Ims and Yoccoz2006; Prokkola et al., Reference Prokkola, Roff, Kärkkäinen, Krams and Rantala2013) but they are largely unknown in the context of blue light exposure.
PO enzyme regulates the formation of melanin in insects (Sugumaran, Reference Sugumaran2002). Reduced PO activity was detected in adult flies that had been exposed to blue light at their larval stage; the low PO activity may be cause of reduced cuticular melanin. In insects, melanization is an innate immune response against pathogens in terms of encapsulation (Balasubramanian et al., Reference Balasubramanian, Hao, Toubarro, Nascimento and Simões2009), and PO activity is a key immune function parameter (Siva-Jothy et al., Reference Siva-Jothy, Moret and Rolff2005; González-Santoyo & Córdoba-Aguilar, Reference González-Santoyo and Córdoba-Aguilar2012). Lower cuticular melanin and PO activity recorded in this study might explain the reduced encapsulation response and higher mortality against B. bassiana in blue light-exposed insects. Furthermore, blue light exposure strongly inhibited melatonin biosynthesis (Bonmati-Carrion et al., Reference Bonmati-Carrion, Arguelles-Prieto, Martinez-Madrid, Reiter, Hardeland, Rol and Madrid2014), which regulates a wide range of physiological functions including immune system (Carrillo-Vico et al., Reference Carrillo-Vico, Lardone, Álvarez-Sánchez, Rodríguez-Rodríguez and Guerrero2013) this inhibitory effect could well explain the impaired immune function in blue light-exposed animals.
Generally, negative correlation between cuticular coloration and immuno-competence has been observed (Robb et al., Reference Robb, Forbes and Jamieson2003; Hagen et al., Reference Hagen, Sørlibråten, Ims and Yoccoz2006). Our mechanistic observations regarding the carryover effects of blue light-exposed larvae on adult immune function are therefore considered to be general, widespread and may express important cost of blue light exposure in an insect. While in vitro experiments showed that blue light inhibited melanin contents (Ohara et al., Reference Ohara, Kawashima, Watanabe and Kitajima2002; Ohara et al., Reference Ohara, Kobayashi, Fujiwara, Kitajima, Mitsuoka and Watanabe2004; Chiarelli-Neto et al., Reference Chiarelli-Neto, Ferreira, Martins, Pavani, Severino, Faião-Flores, Maria-Engler, Aliprandini, Martinez, Di Mascio, Medeiros and Baptista2014), our study reveals for the first time, this effect in vivo in an insect.
Blue light exposure affected age and mass at metamorphosis, the delayed metamorphosis with smaller mass negatively affected the fitness of adult damselflies (Thompson et al., Reference Thompson, Hassall, Lowe and Watts2011; Stoks & Cordoba-Aguilar, Reference Stoks and Cordoba-Aguilar2012). Blue light irradiation cause stress and can be lethal for insect pests of various orders including Diptera (Hori et al., Reference Hori, Shibuya, Sato and Saito2014). To reduce the detrimental effects, the organism may prolong its developmental time (Zhang et al., Reference Zhang, Meng, Wang, Zhu and Lei2011). We found that blue light-exposed larvae had longer development time and a lower mass at metamorphosis. The prolonged developmental time could be a strategy to reduce the harmful effects of radiations. The prolonged development time was observed in B. dorsalis after the inhibition of PO activity occurred (Bai et al., Reference Bai, Chen, Shen, Wei, Wei and Wang2014) indicating the lowered PO activity may prolong development time in blue light-exposed larvae. Blue light had no significant effect on larval growth rate which seems to be in conflict with delayed metamorphosis at a smaller mass. Based on the growth rates of the larvae measured during the 7-day of blue light exposure, the growth rate does not seem to be affected during the exposure period but some days later after the exposure.
In life history study, the theory was that larval stressors could be carried over to the adult stage through age and mass at metamorphosis (Rowe & Ludwig, Reference Rowe and Ludwig1991; Abrams et al., Reference Abrams, Leimar, Nylin and Wiklund1996; Relyea, Reference Relyea2007), although it was not upheld in current study, but there was little evidence in our study that described the mediatory role of the developmental time and mass at metamorphosis to the carryover effects of irradiated-larvae for the adult stage.
In conclusion, our study provides the first evidence that blue light exposure inhibits cuticular melanin, PO activity and impaired immune function in an insect. Furthermore, research revealed that blue light-exposed larvae had the carryover effects in adult stage, in terms of developmental time and mass at metamorphosis. It was documented that stressors such as temperature (Prokkola et al., Reference Prokkola, Roff, Kärkkäinen, Krams and Rantala2013), food shortage (Lee et al., Reference Lee, Simpson and Wilson2008), crowding (Hagen et al., Reference Hagen, Sørlibråten, Ims and Yoccoz2006) and drought (Talloen et al., Reference Talloen, Van Dyck and Lens2004) can affect cuticular melanin. Our results highlight that, for future consideration, melanin serving as a mediator of larval carryover effects for adult stage may provide better insight that how larval stressors shape adult fitness (Pechenik, Reference Pechenik2006; Stoks & Cordoba-Aguilar, Reference Stoks and Cordoba-Aguilar2012; O'Connor et al., Reference O'Connor, Norris, Crossin and Cooke2014), which is still largely enigmatic to date.
Acknowledgments
This research was supported by the earmarked fund for China Agriculture Research System (No.CARS-27) and The Fundamental Research Funds for the Central Universities (NO.2014PY005).





