Introduction
Clinical and experimental data have revealed deleterious adaptations in the vasculature as a hallmark of the fetal programming process.Reference Faienza, Brunetti and Delvecchio 1 – Reference Khorram, Chuang and Pearce 11 These responses are attributed to insults (e.g. maternal diet manipulation, hypoxia, and uteroplacental insufficiency) that occur during the critical window of fetal development.Reference Holemans, Gerber and Meurrens 5 – Reference Kane, Herrera and Camm 12 Indeed, intrauterine growth restriction (IUGR) is associated with abnormalities in the modulatory ability of the vascular endothelium, resulting in reduced endothelium-dependent relaxation.Reference Torrens, Hanson and Gluckman 7 – Reference Itani, Skeffington and Beck 9 Several mechanisms are involved in vascular programming; increased reactive oxygen species (ROS) levels and reduced antioxidant capacity have been observed in IUGR animals.Reference Franco, Akamine and Dimarco 8 – Reference Tarry-Adkins, Martin-Gronert and Chen 10 , Reference Oliveira, Akamine and Carvalho 13 In addition, decreased endothelial nitric oxide synthase (eNOS) messenger RNA expression, methylation of the NOS3 promoter, and lower nitric oxide synthase (NOS) activity can contribute to NO reduction in different vascular beds.Reference Herrera, Cifuentes-Zuniga and Figueroa 4 , Reference Franco Mdo, Arruda and Dantas 14 Together, these adaptations may lead to an increase in cardiovascular events in adult life.
Asahara et al. have isolated and characterized putative endothelial progenitor cells (EPCs) from the peripheral blood (PB) to investigate their possible roles.Reference Asahara, Murohara and Sullivan 15 EPCs are a heterogeneous population of cells in different states of maturation derived from the bone marrow (BM).Reference Shi, Rafii and Wu 16 , Reference Zhang, Malik and Rehman 17 These cells exhibit the capacity to proliferate and migrate to injured sites where they play a role in several physiological repair processes such as reendothelization and vascular integrity maintenance.Reference Van Zonneveld and Rabelink 18 – Reference Jansen, Yang and Hoelscher 20 Indeed, EPCs can differentiate into mature endothelial cells or activate resident endothelial cells by releasing paracrine factors.Reference Hill, Zalos and Halcox 21 Cardiovascular risk factors can reduce both EPC number and functions.Reference Hill, Zalos and Halcox 21 , Reference Vasa, Fichtlscherer and Aicher 22 Furthermore, the properties of EPCs can be impaired under pathological conditions such as hypertension, vascular dysfunction, and heart failure, thus contributing to the prognosis of cardiovascular diseases.Reference Imanish, Kobayashi and Hano 23 – Reference Oikonomou, Siasos and Zaromitidou 26
It is known that IUGR could induce harmful changes in EPCs.Reference Ligi, Simoncini and Tellier 27 Ligi et al. have extracted EPCs from the umbilical cord of newborns with IUGR and found a significant reduction in functional capacity, evidenced by the lower number of colony-forming units (CFUs) and the longer time in establishing colonies in vitro.Reference Ligi, Simoncini and Tellier 27 In addition, the angiogenic capacity of EPCs was reduced, and senescence was enhanced.Reference Ligi, Simoncini and Tellier 27 Therefore, IUGR may be an additional risk factor for EPC dysfunction. Given that EPC dysfunction is also present in cases of altered flow-mediated vasodilation,Reference Miura, Numaguchi and Ishii 28 – Reference Palombo, Kozakova and Morizzo 30 we hypothesized that EPC functional impairment could be a possible link between IUGR and reduced endothelium-dependent vasodilation in rats. Therefore, this study investigated the possible effects of IUGR on the vascular reactivity, EPC number, and EPC function of adult male rat offspring in vitro.
Materials and methods
All procedures were approved by the Ethical Committee for Animal Research (Protocol Number: 836.880) at the Federal University of São Paulo and were in accordance with the guidelines for ethical conduct in the care and use of animals proposed by the Brazilian Society of Laboratory Animal Science (SBCAL/COBEA).
Adult male and female Wistar rats purchased from the Institute of Biomedical Sciences of the University of São Paulo were kept at a controlled room temperature in a light–dark cycle (12:12 h) with free access to standard rat chow and tap water. As previously described, female Wistar rats were mated with male Wistar rats (age range: 14–16 weeks) overnight. The presence of spermatozoa in the vaginal smear was considered as the day of conception (day 0). Each pregnant rat was individually housed in standard cages and randomly divided into one of two groups: the control group (CT, n=10), which were fed a standard laboratory animal diet (Nuvilab CR1; based on the recommendation of the National Research Council and National Institutes of Health, USA) ad libitum, and the restricted group (RT, n=11), which were fed 50% of the typical daily food intake (determined by the amount of food consumed by the CT group during the gestation period). The pups were weighed immediately after they were born, and the mothers from the RT group were fed an ad libitum diet. At birth, litter size was standardized to eight pups (four males and four females) for both groups to prevent alterations in neonatal growth due to decreased milk availability during suckling. The offspring were nursed by their mothers until weaning at day 21. The present study was conducted only on male offspring (age range: 19–20 weeks). To avoid litter effects, two animals from each litter were chosen for each experiment. Female offspring were euthanized (age range: 14–16 weeks) for use in another study unrelated to the present research.
Resting arterial blood pressure evaluation
Resting systolic blood pressure was noninvasively determined using a computerized tail-cuff system (PowerLab 4/S; ADInstruments Pty Ltd., Castle Hill, Australia). Rats were acclimatized to the apparatus in daily sessions over 5 days (1 week before measurements).
Assessment of vascular reactivity in vitro
Under anaesthesia (50 mg/kg sodium thiopental, administered intraperitoneally), the thoracic aorta was quickly harvested and cleaned to remove connective tissues in cold Krebs–Henseleit solution (118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2·2H2O, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 11 mM glucose, and 0.01 mM EDTA; pH 7.4). Two segments of the thoracic aorta from each animal (4 mm in length) were mounted in an isolated chamber containing Krebs–Henseleit solution, gassed with 95% O2 and 5% CO2, and maintained at 37°C. A basal tension of 1.5 g was applied to each segment of the thoracic aorta. Isometric tension was recorded using an isometric force transducer (TRI 210; Letica, Barcelona, Spain) connected to an acquisition system (PowerLab 8/30; ADInstruments Pty Ltd.). The preparations were equilibrated for 1 h (the Krebs–Henseleit solution was changed every 20 min), followed by tension adjustments. After equilibration, the cumulative concentration-response curves to the agonists acetylcholine (ACh: 10−9–10−5 M) and sodium nitroprusside (SNP: 10−9–10−5 M) were obtained in pre-contracted (noradrenaline: 10−7 M) endothelium-intact aorta rings. Arterial integrity was assessed by stimulation of the vessels with potassium chloride (KCl: 120 mM). Endothelial integrity was assessed by evaluating the relaxant effect of ACh (10−6 M) in vessels pre-contracted with noradrenaline (10−7 M). The agonist concentration-response curves were fitted using a nonlinear interactive fitting software (GraphPad Prism 5.0; GraphPad Software Inc., USA). The maximal response (Emax) and potency (−LogEC50) are expressed as mean±s.e.m. and a confidence interval, respectively.
Thoracic aorta preparation for NO and Western blotting assays
Under anaesthesia (50 mg/kg sodium thiopental, administered intraperitoneally), the thoracic aorta was quickly harvested and cleaned to remove connective tissues in cold Krebs–Henseleit solution. Then, it was immediately frozen and stored at −80°C. The tissues were prepared using lysis buffer (100 mM Tris-HCl at pH 7.4, 100 mM sodium pyrophosphate, 10 mM sodium orthovanadate, 100 mM NaF, 10 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 0.01 mg/ml aprotinin, and 1% Triton X-100) and centrifuged (15,000 g , 30 min, 4°C), and the supernatant was collected. The protein content of the lysates was determined using Pierce BCA (bicinchoninic acid) Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA).
NO measurement
NO is extremely unstable; thus, the nitrite and nitrate in thoracic aorta homogenates were re-converted to NO via reaction with vanadium. NO was measured by the chemiluminescence method using a NO analyser (Sievers Instruments Inc., Boulder, CO, USA), which is based on the gas-phase chemiluminescence reaction between NO and ozone. The results were analysed by software, and the concentration of each sample was normalized to their respective protein concentrations.Reference Hampl, Walters and Archer 31
Western blotting analysis
The supernatant from thoracic aorta homogenates (70 µg) was treated with Laemmli buffer containing 200 mmol/l dithiothreitol and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis with 7.5% polyacrylamide gels. Following electrophoresis, proteins were electro-transferred overnight onto nitrocellulose membranes (BioRad, Hercules, CA, USA). After blocking nonspecific sites with T-TBS buffer containing 3% bovine serum albumin at room temperature for 2 h, the membranes were incubated overnight at 4°C with the primary antibody anti-p-eNOSSer1177 (1∶1000; Cell Signaling, USA), anti-p-eNOSThr495 (1:1000; Millipore, USA), or anti-t-eNOS (1∶1000; Cell Signaling). The primary antibodies were detected using peroxidase-conjugated secondary anti-rabbit IgG (1:1500; Jacskon ImmunoResearch Inc., West Grove, PA, USA) and anti-mouse IgG (1:10000; Jackson ImmunoResearch Inc.) antibodies. The immune complexes were detected using an enhanced luminol chemiluminescence system (ECL Plus; GE Healthcare, Little Chalfont, UK) and subsequently photographed (Gel Logic 2200 Pro; Nova Analítica, Brazil). The bands were analysed using ImageJ software (National Institutes of Health, Bethesda, MD,USA). t-NOS was expressed as the ratio between its mean optical density and the optical density of Ponceau staining.Reference Romero-Calvo, Ocón and Martínez-Moya 32 , Reference Aldridge, Podrebarac and Greenough 33 The expression of p-eNOSSer1177 and p-eNOSThr495 was normalized to that of t-eNOS. The results are expressed as the optical density ratio of p-eNOSSer1177 and p-eNOSThr495 to t-eNOS.
Isolation of PB and BM mononuclear cells
Under anaesthesia (50 mg/kg sodium thiopental, administered intraperitoneally), 5–6 ml of PB was collected from the abdominal aorta in ethylenediaminetetraacetic acid (EDTA) tubes, and both tibias and femurs were obtained and placed in sterile phosphate-buffered saline (PBS) solution containing penicillin–streptomycin (100 µl/l; Sigma-Aldrich Co., USA). Under sterile conditions, tibia and femur cavities were flushed with Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, USA) to obtain the BM. Both the PB and BM were fractionated by density gradient centrifugation (2500 rpm, 25 min, 20–22°C) (Ficoll-Paque; GE Healthcare, Munich, Germany). Then, monoculear cells (MNCs) obtained from the samples were quantified, and their in vitro function and senescence were evaluated.Reference Fernandes, Nakamuta and Magalhães 34
EPC immunophenotyping and quantification by flow cytometry
The MNCs derived from the PB and BM were considered as EPCs (CD34+/VEGFR2+ cells). Briefly, 1×106 MNCs were individually incubated at room temperature in the dark for 30 min with the antibodies anti-CD34-PE-Cy7 (Bioss, Woburn, MA, USA) and VEGFR2-FITC (Bioss) or the isotype controls anti-IgG-PE-Cy7 (BioLegend, San Diego, CA, USA) and IgG-FITC (BioLegend). The negative control was also incubated at room temperature in the dark for 30 min. After incubation, the cells were washed with PBS and fixed in 1% paraformaldehyde solution. Fixed cells were stored at 4°C in the dark for 15–20 h and evaluated using a flow cytometer (FACSCanto; BD Biosciences, USA) by collecting 1,000,000 events. The data were analysed using BD FACSDiva software (BD Biosciences). Gates were established on the forward-scatter and side-scatter (FSC/SSC) plots corresponding to selected MNCs, followed by CD34-PE-Cy7 and VEGFR2-FITC (Supplementary material Figure). The number of EPCs (CD34+/VEGFR2+) was calculated as the percentage of MNC events.Reference Fernandes, Nakamuta and Magalhães 34
Assay of EPC functional capacity in vitro
The CFUs of MNCs from the PB and BM were evaluated in vitro. Briefly, 5×106 MNCs were cultured (37°C, 5% CO2, 95% humidity) on six-well plates pre-coated with fibronectin (BioCoat; BD Biosciences), seeded with EndoCult medium (STEMCELL Technologies, Vancouver, Canada). After 48 h, 1×106 non-adherent cells were transferred in triplicate to 24-well plates pre-coated with fibronectin and cultured for 72–96 h (37°C, 5% CO2, 95% humidity) in EndoCult medium. Subsequently, the CFUs were manually counted by two blinded observers using an inverted microscope (Nikon Instruments Inc., USA).Reference Fernandes, Nakamuta and Magalhães 34
Assay of EPC senescence in vitro
Initially, 5×106 MNCs from the BM were cultured (37°C, 5% CO2, 95% humidity) on six-well plates pre-coated with fibronectin (BioCoat), seeded with EndoCult medium (STEMCELL Technologies). After 48 h, 1×106 non-adherent cells were washed twice with PBS and fixed in 2% paraformaldehyde at room temperature for 3 min. Then, the cells were washed with PBS and incubated in duplicate (37°C, 5% CO2, 95% humidity) for 24 h in β-galactosidase solution with EGM-2 medium (Lonza Group Ltd., Basel, Switzerland). Cells positive for SA-β-Gal exhibited a blue phenotype and were considered as senescent cells, and they were manually counted by two-blinded observers using an inverted microscope (Nikon Instruments Inc., USA).Reference Fernandes, Nakamuta and Magalhães 34
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., USA). Results are expressed as mean±s.e.m. The data were analysed by an independent Student’s t‐test, and the significance level was set at P<0.05.
Results
At birth, the body weight of pups in the RT group was significantly reduced compared with that of pups in the CT group (CT: 6.6±0.11 g v. RT: 4.5±0.14 g; n=16; P=0.001). However, between 19 and 20 weeks of age, both groups exhibited similar body weights (CT: 398.40±11.24 g v. RT: 396.1 g±18.82 g; n=16; P=0.787). Blood pressure levels were significantly higher in RT offspring than in CT offspring (CT: 110±3 mmHg v. RT: 123±2 mmHg; n=7; P=0.004).
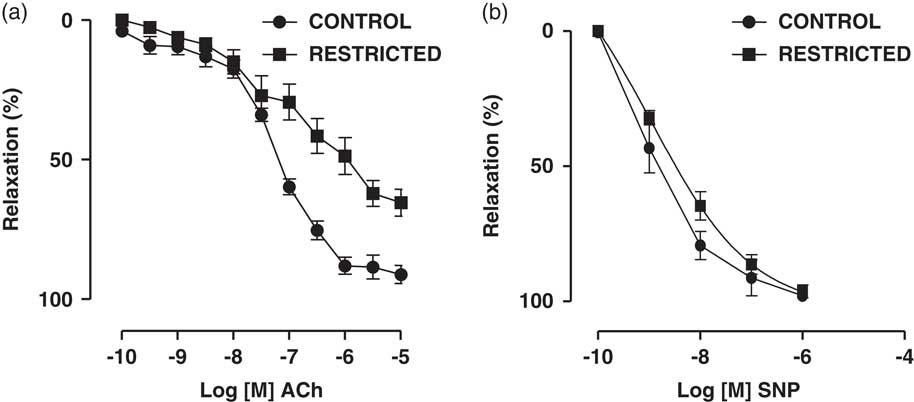
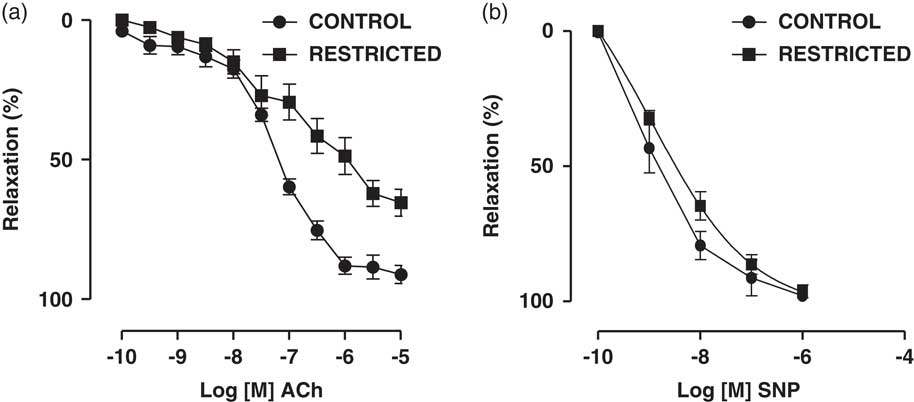
Fig. 1 Dose-dependent relaxations to (a) acetylcholine (ACh) and (b) sodium nitroprusside (SNP) in thoracic aorta rings from control and restricted offspring. Data are expressed as the percentage of noradrenaline pre-contraction.
IUGR-induced reduction in endothelium-dependent relaxation in thoracic aorta rings
The endothelium-dependent vasodilator ACh induced a concentration-dependent relaxation in noradrenaline-pre-contracted thoracic aorta rings from RT and CT offspring. On the other hand, we found that the maximal vasodilation response to ACh was significantly reduced in the RT group when compared with that in the CT group (CT: 90.2±1.7% v. RT: 65.0±4.4%; n=8; P=0.001) (Fig. 2a). The NO donor SNP also promoted a concentration-dependent vasodilation in noradrenaline-pre-contracted thoracic aorta rings from RT and CT offspring; however, there were no significant differences in the maximal vasodilation response (CT: 99.6±0.5% v. RT: 99.0±0.7%; n=8; P=0.238) or sensitivity (EC50) [CT: 8.1 (9.6–6.5) v. RT: 8.9 (9.9–7.9); n=8; P=0.819] to SNP between the groups (Fig. 1).
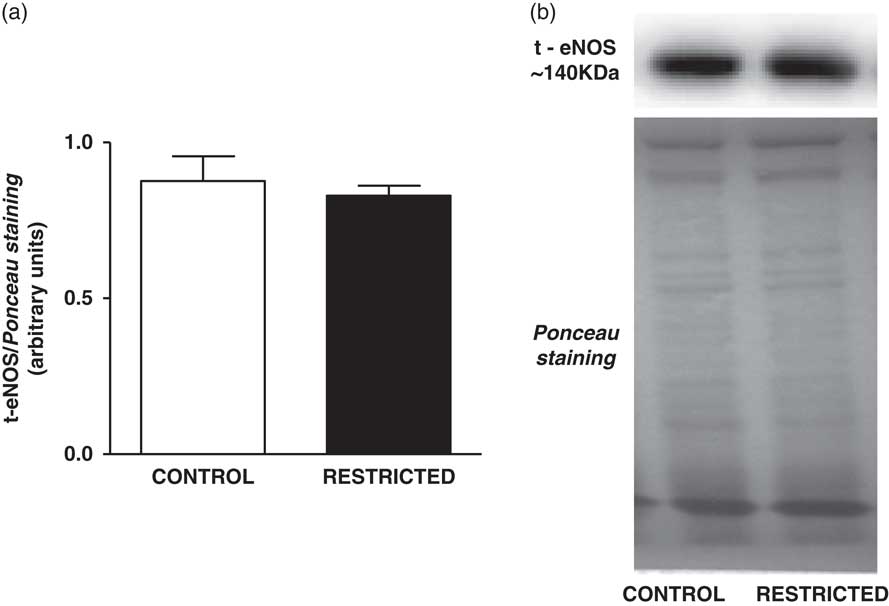
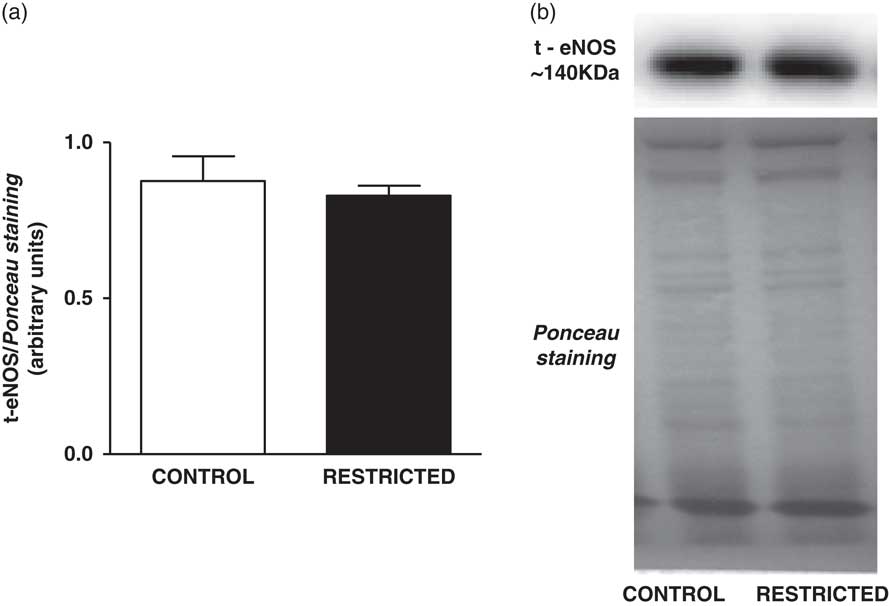
Fig. 2 Histograms show the densitometric analysis of the Western blotting for t-eNOS. Data are expressed as mean±s.e.m. of t-eNOS/Ponceau staining (six animals/group). Student’s unpaired t-test: *P<0.05, restricted v. control. Representative immunoblots of t-eNOS/Ponceau staining of the thoracic aorta from control and restricted offspring.
Effects of IUGR on NO content and eNOS expression in the thoracic aorta
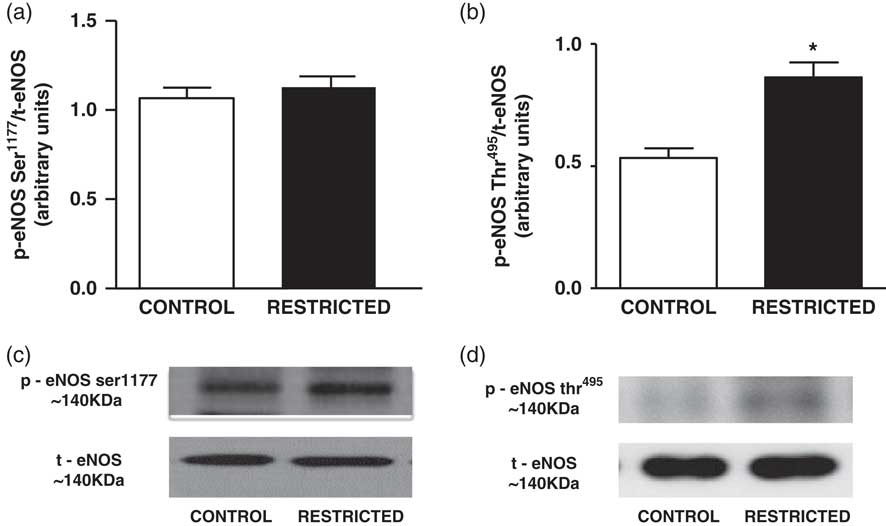
The NO content found in the thoracic aorta homogenates from RT offspring was lower than that in those from CT offspring (CT: 426±63 µM/mg of protein v. RT: 202±21 µM/mg of protein, n=8; P<0.05). IUGR did not affect the protein expression of total eNOS (Fig. 2) or eNOSSer1177 (Fig. 3a); however, there was a significant increase in eNOSThr495 phosphorylation (Fig. 3b) in the thoracic aorta from RT offspring.

Fig. 3 Histograms show the densitometric analysis of the western blotting for (a) eNOSSer1177 and (b) eNOSThr495. Data are expressed as mean±s.e.m. of the ratio of eNOSSer1177/total eNOS and eNOSThr495/total eNOS (six animals/group). Student’s unpaired t-test: *P<0.05, restricted v. control. Representative immunoblots of (c) eNOSSer1177/total eNOS and (d) eNOSThr495/total eNOS of the thoracic aorta from control and restricted offspring.
Effects of IUGR on EPC number, functional capacity and senescence in vitro
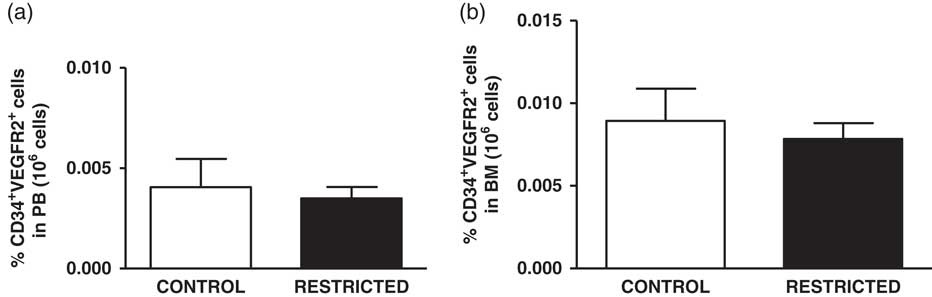
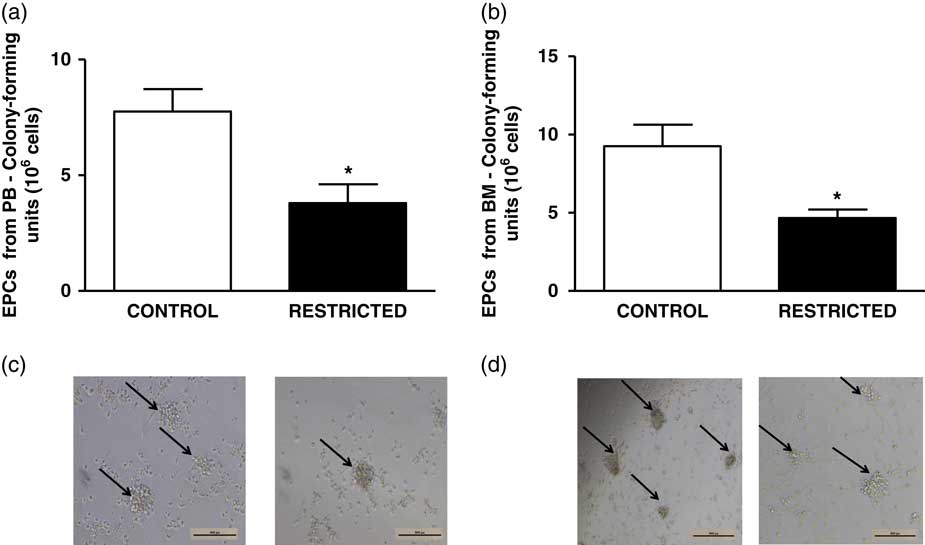
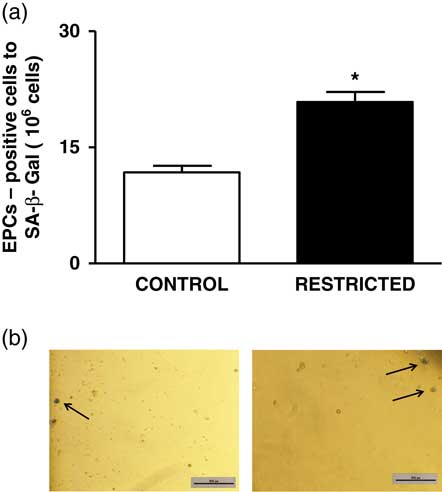
The percentage of EPCs in the PB and BM was similar between both groups (Fig. 4a and 4b). However, in comparison with the CT group, the RT group demonstrated a reduced number of CFU-EPCs in the PB and BM in vitro (Fig. 5a and 5b). The number of senescent cells from the BM was significantly higher in the RT group than in the CT group (Fig. 6a).
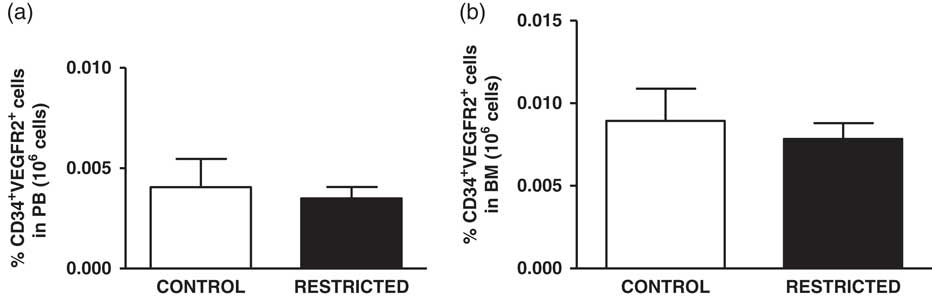
Fig. 4 Histograms show the amount of (a) peripheral blood-derived endothelial progenitor cells and (b) bone marrow-derived endothelial progenitor cells from control and restricted offspring. Data are expressed as the percentage of endothelial progenitor cells (CD34+/VEGFR2+ cells) in 106 cells (7–10 animals/group). Student’s unpaired t-test: *P<0.05, restricted v. control.
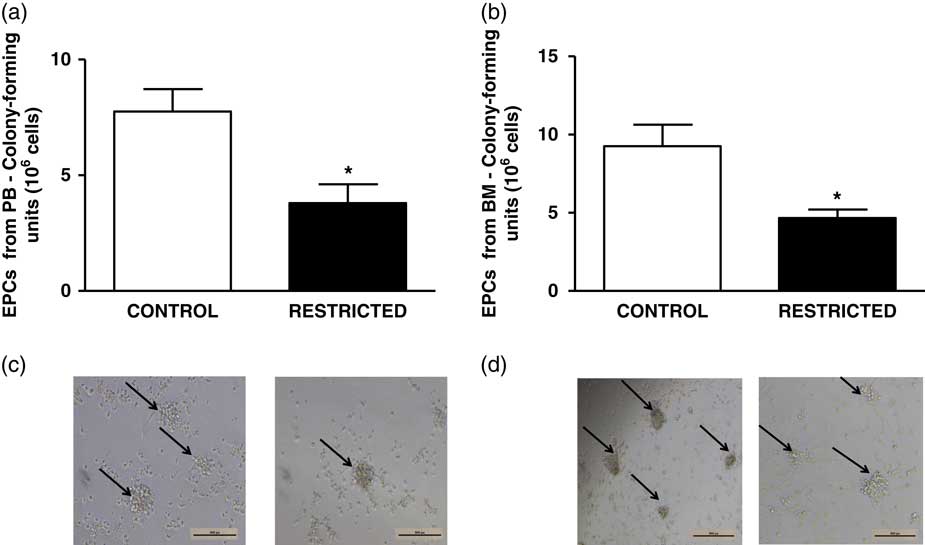
Fig. 5 Histograms show the number of colony-forming units of (a) peripheral blood-derived endothelial progenitor cells and (b) bone marrow-derived endothelial progenitor cells from control and restricted offspring. Data are expressed as mean±s.e.m. (five animals/group). Student’s unpaired t-test: *P<0.05, restricted v. control. (c) Black arrows show the colony-forming units of peripheral blood-derived endothelial progenitor cells and (d) black arrows show the colony-forming units of bone marrow-derived endothelial progenitor cells from control and restricted offspring.
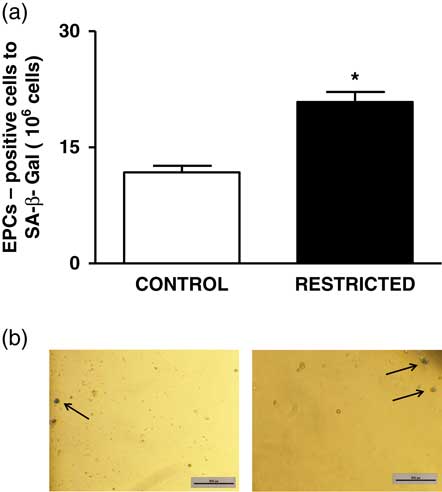
Fig. 6 (a) Histograms show the number of senescent endothelial progenitor cells derived from the bone marrow from control and restricted offspring. Data are expressed as mean±s.e.m. (five animals/group). Student’s unpaired t-test: *P<0.05, restricted v. control. (b) Black arrows show the senescent cells derived from the bone marrow from control and restricted offspring.
Discussion
IUGR causes deleterious changes in vascular function, contributing to increased cardiovascular risk.Reference Faienza, Brunetti and Delvecchio 1 – Reference Herrera, Cifuentes-Zuniga and Figueroa 4 The mechanisms involved have been investigated; however, little is known about the effect of IUGR on EPC properties. The present study revealed novel IUGR-induced deleterious adaptations in EPCs derived from the PB and BM. In particular, we demonstrated in vitro that EPC function was reduced, and the number of senescent cells from the BM was increased. In addition, IUGR did not affect the EPC number of adult male offspring.
In the present study, we observed that IUGR reduced ACh-induced vasodilation in thoracic aorta rings; however, there were no changes in SNP-induced relaxation, which was consistent with the findings of previous studies.Reference Torrens, Hanson and Gluckman 7 , Reference Franco, Akamine and Dimarco 8 , Reference Franco Mdo, Arruda and Dantas 14 The reduced endothelium-dependent vasodilation via ACh in IUGR rats could be partly caused by lower bioavailability and/or NO production; thus, the concentration of NO was assessed. The results revealed that RT rats had lower NO levels in the thoracic aorta, which may be attributed to changes in the vascular bed, specifically increased ROS levels and reduced eNOS activity.Reference Oliveira, Akamine and Carvalho 13 , Reference Franco Mdo, Arruda and Dantas 14 eNOS activity can be modulated by post-translational mechanisms including protein phosphorylation.Reference Qian and Fulton 35 It is known that Ser1177 phosphorylation activates eNOS, whereas Thr495 phosphorylation inhibits its activity.Reference Church and Fulton 36 – Reference Fleming, Fisslthaler and Dimmeler 39 Therefore, these phosphorylation sites were evaluated in the aorta. Our data showed that IUGR did not affect the phosphorylation of eNOS at Ser1177; however, the phosphorylation at Thr495 was increased, suggesting that this phosphorylation site can play a role in reducing NO levels in the RT group. Although the possible mechanisms by which IUGR induces an increase in phosphorylation at Thr495 have not been elucidated, previous data suggest that Rho-kinase and protein kinase Cα (PCKα) could be involved. Indeed, Rho inhibition has been found to result in decrease in eNOSThr495 expression, while nitrite concentration was increased.Reference Watts and Motley 40 In addition, IUGR seems to decrease PCKα expression.Reference Sugden and Langdown 41 For this reason, it is plausible suggest that IUGR-induced NO levels could in part resulting from changes in PCKα-induced eNOSThr495phosphorylation.Reference Li, Zhang and Zhang 42
EPCs are essential for maintaining vascular healthy and endothelium repair, whereas the capacity of mature endothelial cell is limited.Reference Asahara, Murohara and Sullivan 15 , Reference Werner, Koriol and Schiegl 43 Furthermore, clinical findings have emphasized the association between abnormal flow-mediated vasodilatation and impairment in EPCs number, bioavailability and/or function under pathological disorders.Reference Miura, Numaguchi and Ishii 28 – Reference Palombo, Kozakova and Morizzo 30 Therefore, circulating EPCs has emerged as a robust marker predictor of cardiovascular diseases.Reference Werner, Koriol and Schiegl 43 , Reference Schmidt-Lucke, Rossig and Fichtlscherer 44 Extending these observations, experimental data have demonstrated that reduction in ACh-induced vasodilatation is followed by lower circulating EPCs amount in conditions as ageing, and hyperhomocysteinemia.Reference Sun, Dong and Wang 45 , Reference Dong, Sun and Liu 46 In addition, a significantly improvement in endothelial function was observed in hypertensive rats after EPCs transplantation from normotensive rats.Reference Yu, Shao and Yan 47 Our data evidenced that IUGR did not change the percentage of EPCs derived from PB and BM. Similar observations was reported by Ligi et al. They observed that IUGR has no effect on number of EPCs extracted from human umbilical cord.Reference Ligi, Simoncini and Tellier 27
On the other hand, IUGR seems to play a role in functional activity of EPCs. Indeed, we showed that RT offspring had a lower CFUs-EPCs number in PB and BM, reflecting a deteriorated function in vitro. These results agreed with previous findings demonstrating that umbilical cord EPCs of babies, restricted in utero, had a significant reduction in CFUs number.Reference Ligi, Simoncini and Tellier 27 Data about the effects of IUGR on EPCs proprieties are scarce. However, animal models which showed endothelial dysfunction also had reduced CFU–EPCs number, or the EPCs capacity to forming colonies was completely abolished.Reference Imanish, Moriwak and Hano 24 , Reference Fernandes, Nakamuta and Magalhães 34 , Reference Yu, Shao and Yan 47 , Reference Yoshida, Fukuda and Maeshima 48 These previous findings support the hypothesis that EPCs dysfunction could be at least in part involved in vascular dysfunction. The mechanisms underlying negative effects in EPCs function induced by IUGR, remains to be elucidated. Nevertheless, it is known that several aspects may affect the functional capacity of EPCs.Reference Imanishi, Tsujioka and Akasaka 49 Among them, accelerated senescence process of these cells seems to be involved.Reference Vassallo, Simoncini and Ligi 50 , Reference Liu, Li and Peng 51 It is important to note the EPCs viability, and hence its function is partially controlled by an adequate senescence process which, under physiological limits, play a key role as a factor for the cells survival.Reference Ben-Porath and Weinberg 52 In the present study we demonstrated that RT group showed increased number of senescent EPCs derived from BM, as evidenced by the positive staining by SA-β-Gal. It should be considered that senescent cells remain viable, but considerable changes on its morphology and function are observed.Reference Wright and Shay 53 – Reference Sitte, Merker and Von Zglinicki 55 The high senescent cells number found in RT rats may have contributed partially to reduce EPCs function in vitro. Cells can become senescent promptly, independently of rate of cells division.Reference Robles and Adami 56 Factors as high ROS levels and lack of nutrients can induce senescence, which is designated as stress-induced premature senescence.Reference Kaneko, Tahara, Taguchi and Kondo 57 , Reference Chen and Ames 58 Accelerated senescence in EPCs has been attributed in part to increased oxidative stress, due to NADPH activation, that induces upregulation in p38 mitogen activated protein kinase phosphorylation.Reference Kuki, Imanishi and Kobayashi 59 , Reference Seeger, Haendeler and Walter 60 In summary, our data provides evidence that IUGR rats show deleterious changes in EPCs, as reduction in EPCs function and accelerated senescence in vitro. These findings may collaborate in part to clarify the possible mechanisms involved in endothelial dysfunction induced by fetal programming.
Acknowledgements
The authors thank Dr Maria Aparecida Dalboni from Federal University of São Paulo for her excellent support in flow cytometer analysis.
Financial Support
This research was supported by Grant from the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil) (Project Number: 2013/03139-0). Vanessa Oliveira is supported by a fellowship from the FAPESP (2013/00311-6).
Conflicts of Interest
None.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174417000484