1. Introduction
Eurypterids (so-called ‘sea scorpions’) are an extinct group of aquatic predatory chelicerate arthropods that flourished in the Palaeozoic oceans and rivers. Eurypterids possessed elongate, dorsoventrally flattened bodies, consisting of two tagmata (Dunlop & Lamsdell, Reference Dunlop and Lamsdell2017). The anterior prosoma (cephalothorax) is composed of a fused dorsal shield bearing paired compound eyes and median ocelli as well as six pairs of appendages, the chelicerae (appendage I) and five locomotory appendages (appendages II–VI) inserting ventrally. The posterior opisthosoma (abdomen) consists of seven preabdominal segments, five postabdominal segments and a terminal telson (Størmer, Reference Størmer and Moore1955). Eurypterids are divided into two suborders: Stylonurina Diener, Reference Diener1924 and Eurypterina Burmeister, Reference Burmeister1843, conventionally distinguished by the form and function of appendage VI. In Stylonurina this appendage retains a walking function, whereas in Eurypterina it is modified into a swimming paddle (Lamsdell et al. Reference Lamsdell, Braddy and Tetlie2010b; Lamsdell, Reference Lamsdell2011).
Phylogenetic analysis supports the monophyly of both Eurypterina (Tetlie & Cuggy, Reference Tetlie and Cuggy2007; Lamsdell et al. Reference Lamsdell, Hoşgör and Selden2013, Reference Lamsdell, Briggs, Liu, Witzke and McKay2015; Lamsdell & Selden, Reference Lamsdell and Selden2013) and Stylonurina (Lamsdell et al. Reference Lamsdell, Braddy, Loeffler and Dineley2010a, b; Lamsdell, Reference Lamsdell2013; Lamsdell & Selden, Reference Lamsdell and Selden2017). Stylonurina is diagnosed by the possession of transverse sutures on the ventral plates and the lack of a modified podomere 7a on appendage VI. Stylonurina first appear in the early Late Ordovician (460 Ma) (Størmer, Reference Størmer1951) and persist into the late Permian (Ponomarenko, Reference Ponomarenko1985).
Here we describe semi-articulated juvenile stylonurine eurypterid specimens and isolated fragments that may represent adults, from the Upper Famennian (Upper Devonian) Strud locality, Belgium. This early continental ecosystem has until now yielded an abundant and diversified flora including early cupulate seed plants (Prestianni et al. Reference Prestianni, Steel, Thorez, Gerrienne, Steemans and Javaux2007), vertebrates including early tetrapod remains (Clément et al. Reference Clément, Ahlberg, Blieck, Blom, Clack, Poty, Thorez and Janvier2004; Olive et al. Reference Olive, Ahlberg, Pernègre, Poty, Steurbaut and Clément2016a), placoderms (Olive, Reference Olive2015; Olive et al. Reference Olive, Clément, Daeschler and Dupret2015a, Reference Olive, Clément, Daeschler and Dupret2016b), actinopterygian, acanthodian and sarcopterygian fishes (Clément & Boisvert, Reference Clément and Boisvert2006), as well as an aquatic arthropod fauna. The arthropods, mostly recovered from fine shales deposited in a calm and confined floodplain environment including ephemeral pools, have recently been reported in several publications. They include a putative insect (Garrouste et al. Reference Garrouste, Clément, Nel, Engel, Grandcolas, D’Haese, Lagebro, Denayer, Gueriau, Lafaite, Olive, Prestianni and Nel2012, Reference Garrouste, Clément, Nel, Engel, Grandcolas, D’Haese, Lagebro, Denayer, Gueriau, Lafaite, Olive, Prestianni and Nel2013; Hörnschemeyer et al. Reference Hörnschemeyer, Haug, Béthoux, Beutel, Charbonnier, Hegna, Koch, Rust, Wedmann, Bradler and Willmann2013), various crustaceans including eumalacostracans (Gueriau et al. Reference Gueriau, Charbonnier and Clément2014a, b) and notostracans, spinicaudatan and anostracan branchiopods (Lagebro et al. Reference Lagebro, Gueriau, Hegna, Rabet and Budd2015; Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016, Reference Gueriau, Rabet and Du Tien Hat2018), as well as the eurypterid material presented herein, which has been mentioned on several occasions but not yet subjected to detailed study.
Although numerous eurypterids are known from the Carboniferous of Belgium (Tetlie & Van Roy, Reference Tetlie and Van Roy2006), only two taxa have been formally described from Upper Devonian rocks, both at the Pont-de-Bonne Modave quarry (Liège Province). Cyrtoctenus dewalquei Fraipont, Reference Fraipont1889 was a member of the large, sweep-feeding mycteropoids (Størmer & Waterston, Reference Størmer and Waterston1968; Lamsdell et al. Reference Lamsdell, Braddy and Tetlie2009), while the single specimen of Adelopthalmus (?) lohesti (Delwalque in Fraipont, Reference Fraipont1889) has been suggested to be more properly interpreted as a stylonurine (Størmer & Waterston, Reference Størmer and Waterston1968; Tetlie & Van Roy, Reference Tetlie and Van Roy2006), although its exact affinities are uncertain. These fossils were recovered from deltaic deposits including channel-filling sequences, most likely more distal than the Strud facies discussed herein. Although most of the vertebrate fauna remains to be described, several taxa have been found both in Pont-de-Bonne Modave and Strud, in particular the lungfishes Soederberghia cf. S. groenlandica Lehman, Reference Lehman1959 and Jarvikia Lehman, Reference Lehman1959 (Clément & Boisvert, Reference Clément and Boisvert2006) and some placoderms (Olive, Reference Olive2015), indicating links between both localities. However, it is not surprising to find such similarities between the Modave and Strud assemblages since the whole Condroz area consisted of a single deltaic unit during the Late Famennian, suggesting that vertebrates and arthropods may have settled in all or most of the river branches (as also demonstrated by the discovery of a new tetrapod-bearing locality in Becco; Olive et al. Reference Olive, Clément, Denayer, Derycke, Dupret, Gerienne, Gueriau, Marion, Mottequin and Prestianni2015b, Reference Olive, Ahlberg, Pernègre, Poty, Steurbaut and Clément2016a). Nevertheless, Cyrtoctenus is a genus of generally very large eurypterids and there seem to be no similarities between this taxon and the new fossils recovered from the Strud locality.
2. Geological setting
The Strud locality (Namur Province, Belgium; 50° 26′ 43.32″ N, 5°03′ 24.86″ E) exposes a 1.4 m thick fining-upward channel-filling succession with no evidence for marine influence. According to regional stratigraphic correlations, these deposits belong to the Upper Famennian Bois des Mouches Formation (see Denayer et al. Reference Denayer, Prestianni, Gueriau, Olive and Clément2016 for a detailed stratigraphical and palaeoenvironmetal survey of the locality). A miospore assemblage (VCo Oppel Rugospora radiata interval biozone) confirms a late Famennian age (Denayer et al. Reference Denayer, Prestianni, Gueriau, Olive and Clément2016). Unlike the other arthropods from the locality that have been recovered from the stratigraphically highest fine shales interpreted as floodplain and temporary pond deposits (Fig. 1), eurypterid specimens described herein are not restricted to these horizons. The fine shales yielded small and largely complete specimens, while fragments of much larger specimens have been recovered in association with well-preserved plant remains and isolated complete vertebrate bones from coarser dark sandy layers (Fig. 1) interpreted as a higher-energy habitat of the floodplain such as rivers.
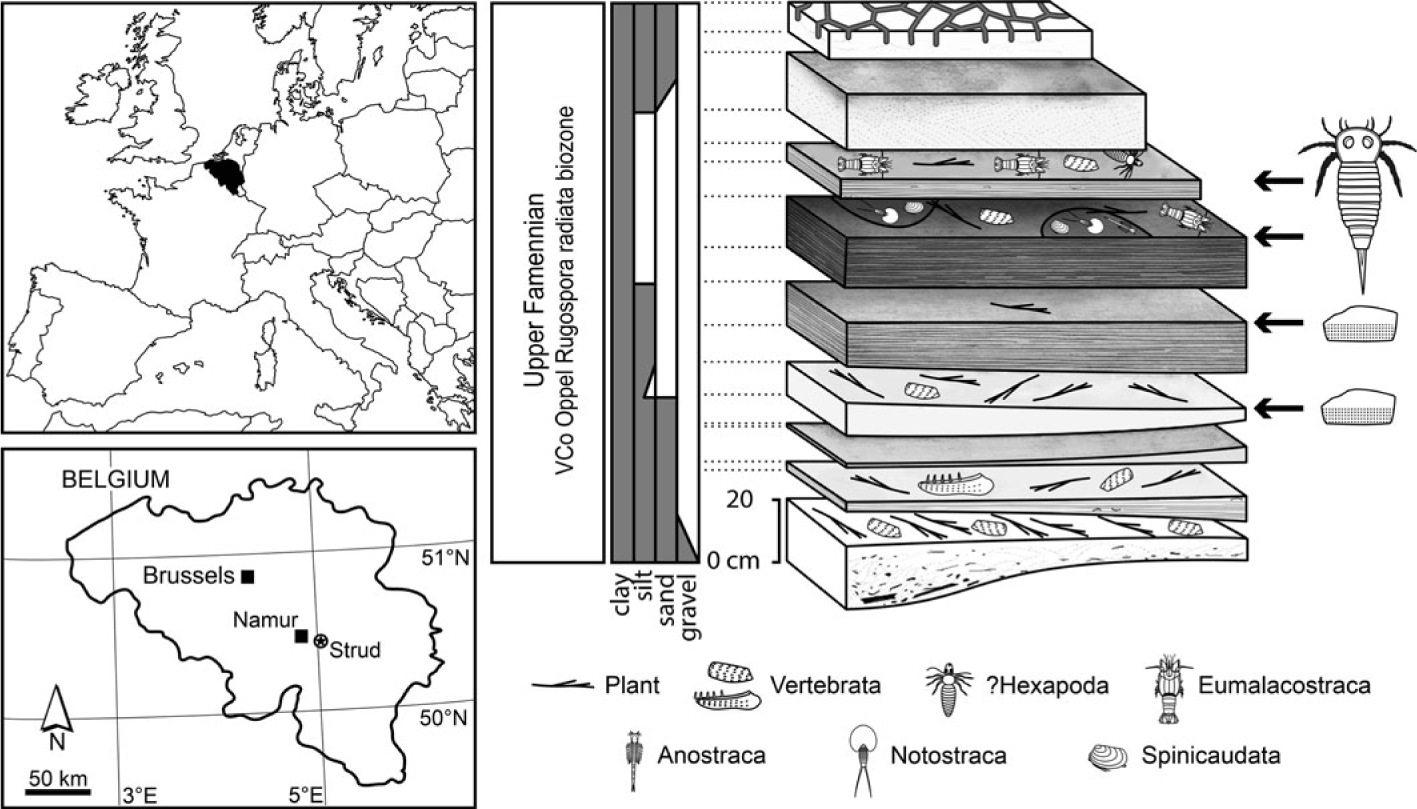
Fig. 1. Location and stratigraphy of the Strud channel-filling deposits (Late Devonian, Belgium) showing their main fossiliferous content, including the eurypterid material (small, largely articulated specimens in fine shales, and isolated cuticular fragments in coarser deposits). Modified from Gueriau et al. (Reference Gueriau, Charbonnier and Clément2014a) and Denayer et al. (Reference Denayer, Prestianni, Gueriau, Olive and Clément2016).
3. Materials and methods
3.a. Fossil material
The present study is based on seven specimens (IRSNB a 13223–13229) that were collected during successive field campaigns. The material is housed at the Royal Institute of Natural Sciences, Brussels (Belgium). The specimens were photographed with a Canon EOS 5D Mark III camera coupled with a Canon MP-E 65 mm macro lens, using a polarizing filter. In order to increase the contrast between the matrix and the fossils, we used polarized light and photographed the specimens in ethanol. The photographs reproduced in Figure 2b are part–counterpart composites. Drawings were produced using Adobe Photoshop and Adobe Illustrator. Measurements were made using the software ImageJ.
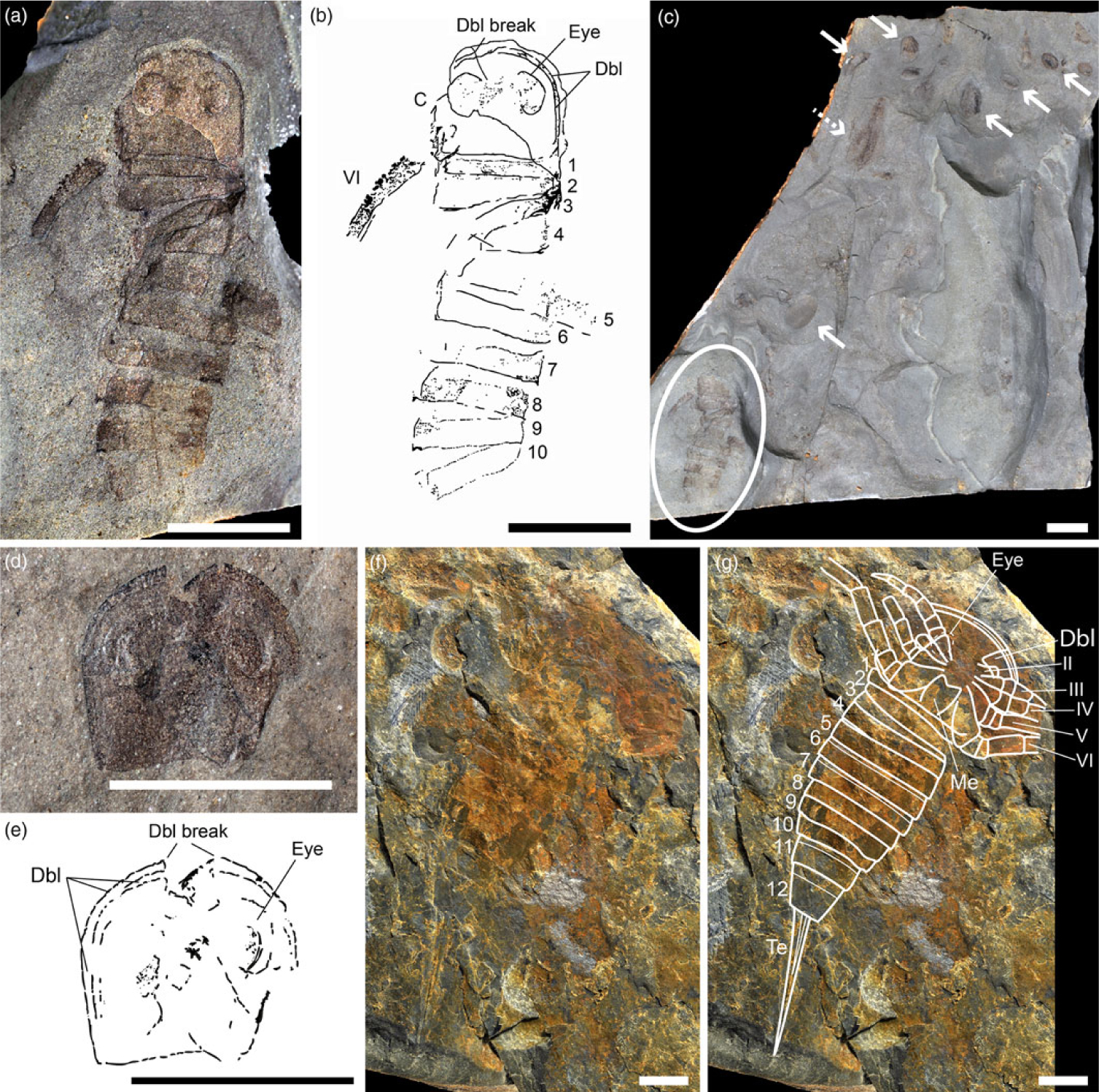
Fig. 2. Juvenile eurypterids from the Late Devonian of Strud, Belgium. (a, b) IRNSB a 13224b, composite photograph (part + counterpart) of an almost complete specimen in dorsal view and interpretative drawing, respectively; (c) IRNSB a 13224b (circle) and associated branchiopod crustaceans in temporary pond deposits (notostracan, dotted arrow; spinicaudatans, solid arrows); (d, e) IRNSB a 13225b, isolated carapace in dorsal view and interpretative drawing, respectively; (f, g) IRSNB a 13223a, cast of a complete specimen in ventral view and interpretative line drawing. Scale bars 5 mm. Abbreviations: C, carapace; Dbl, doublure; Eye, compound eye; Me, metastoma; Te, telson. Arabic numerals indicate opisthosomal segments 1–12, roman numerals indicate prosomal appendages I–IV.
3.b. Preservation
Specimens from the fine floodplain and temporary pool deposits preserve delicate structures such as appendages, and are largely articulated (Fig. 2), which is evidence for absence of (or very limited) transport in the calm and confined environment of the floodplain (Gueriau et al. Reference Gueriau, Charbonnier and Clément2014a, Reference Gueriau, Rabet and Du Tien Hat2018). Their opisthosomal segments are ‘telescoped’ and the only preserved prosomal appendage is disarticulated. The gut is not preserved. IRSNB a 13224 and IRSNB a 13225 (Fig. 2a–e) are likely to represent exuviae judging by their incompleteness and non-preservation of the mid-gut (Tetlie et al. Reference Tetlie, Brandt and Briggs2008), unlike many Strud crustaceans from the same layers that regularly preserve it (Lagebro et al. Reference Lagebro, Gueriau, Hegna, Rabet and Budd2015; Gueriau et al. Reference Gueriau, Rabet and Du Tien Hat2018; see also discussion in Tetlie et al. Reference Tetlie, Brandt and Briggs2008 about ecdysis in eurypterids). On the other hand, IRSNB a 13223 (Fig. 2f, g) could represent the cast of a carcass based on its relative completeness and position of the appendages. Larger specimens from the other horizon are represented by isolated and fragmented cuticular remains.
3.c. Morphological terminology
Eurypterid terminology largely follows Tollerton (Reference Tollerton1989) for morphology of the carapace, metastoma, lateral eyes, prosomal appendages, genital appendage, opisthosomal differentiation, telson, and patterns of ornamentation. The terminology of the ventral plate morphologies follows Tetlie et al. (Reference Tetlie, Brandt and Briggs2008). Terminology for prosomal structures and cuticular sculpture, and the labelling of the appendages, follows Selden (Reference Selden1981) with additions from Lamsdell et al. (Reference Lamsdell, Briggs, Liu, Witzke and McKay2015). Minor modifications to the terminology used in these papers follow Lamsdell (Reference Lamsdell2011).
4. Systematic palaeontology
ARTHROPODA Siebold, Reference Siebold, Siebold and Stannius1848
CHELICERATA Heymons, Reference Heymons1901
EURYPTERIDA Burmeister, Reference Burmeister1843
STYLONURINA Diener, Reference Diener1924
KOKOMOPTEROIDEA Kjellesvig-Waering, Reference Kjellesvig-Waering1966
HARDIEOPTERIDAE Tollerton, Reference Tollerton1989
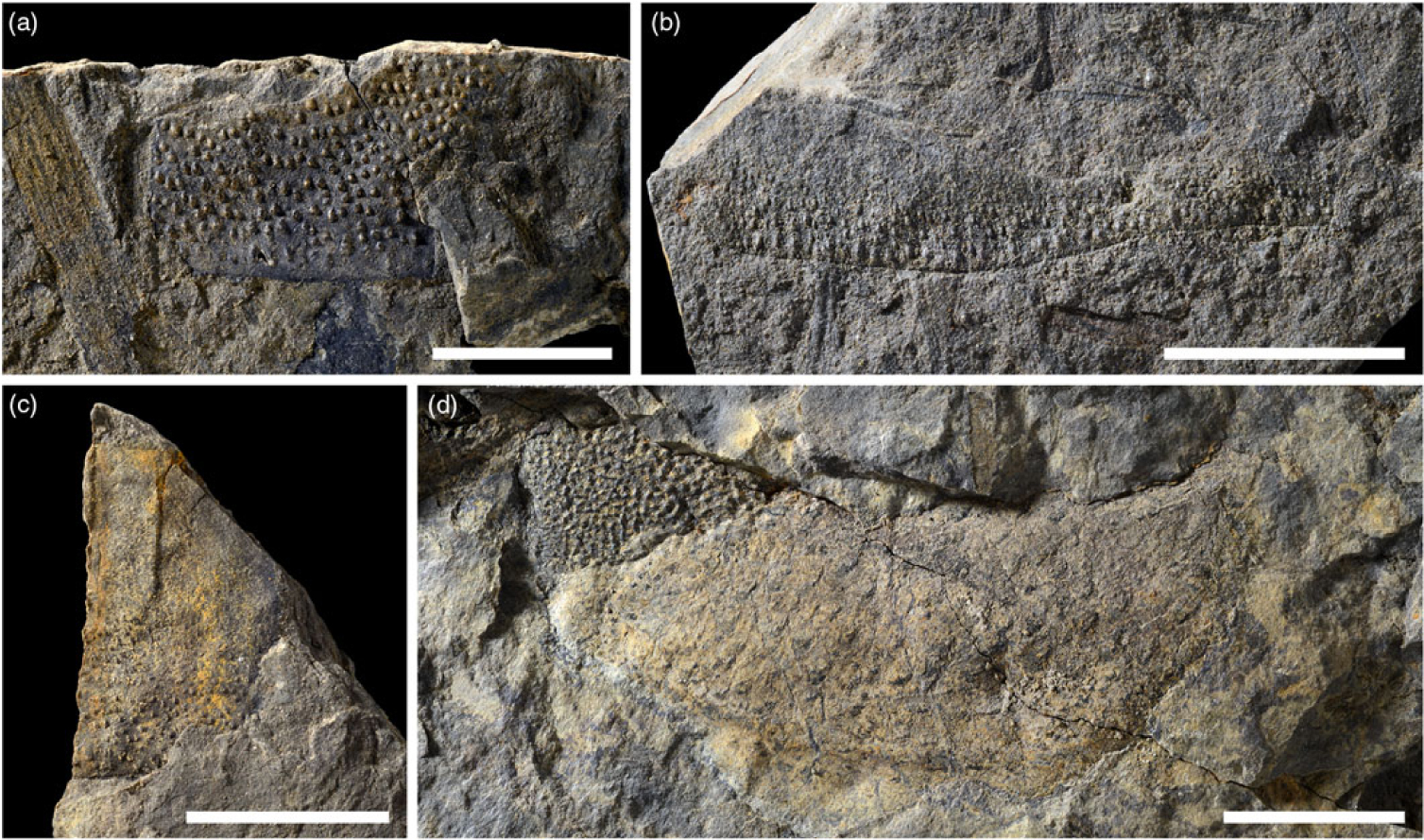
Fig. 3. Isolated fragments of adult eurypterids from the Late Devonian of Strud, Belgium. (a) IRNSB a 13227a; (b) IRNSB a 13226a; (c) IRNSB a 13228a; (d) IRNSB a 13229a. Scale bars 20 mm.
Description. One fully articulated specimen (IRSNB a 13223a: Fig. 2f, g), one semi-articulated (IRSNB a 13224b: Fig. 2a–c), one isolated dorsal prosomal shield (IRNSB a 13225b: Fig. 2d, e) and four isolated tergite fragments (IRNSB a 13226a–13229a: Fig. 3) have been recovered from the locality. Based on the size of the articulated specimens (total length including telson, 49.2 mm), the partially articulated specimen and the isolated shield are estimated to have been c. 20 mm long in life (see Table 1 for measurements). Two large crescentic eyes are located in a centrimesial position, with large palpebral lobes giving them an overall circular outline (‘ovocrescentic’ sensu Tollerton, Reference Tollerton1989). Ocelli are not well preserved, although a slight swelling in between the lateral eyes likely indicates their position. The dorsal prosomal shield is subquadrate, with blunt posterior angles that approximate a right angle. The doublure bears parallel striae, with paired sutures located either side of an epistomal plate (Fig. 2b, g) indicating the doublure is of Hallipterus type (Tetlie et al. Reference Tetlie, Brandt and Briggs2008).
Table 1. Measurements for Strud juvenile eurypterid specimens. All measurements are in mm. Abbreviations: max., maximum; seg. largest opisthosomal segment; est., estimated

* The length / width measurements for the eyes represent the average between the left and right eyes.
† Eye position is described by four distances (1 / 2 / 3 / 4) measured from the centre of the eye: (1) distance (following length axis) to anterior carapace margin; (2) distance (perpendicular to length axis) to the closest lateral carapace margin; (3) distance (following length axis) to posterior carapace margin; (4) distance (perpendicular to length axis) to the other eye centre.
The chelicerae are not preserved. One specimen (Fig. 2f, g) displays the proximal podomeres of appendages II–VI, although the coxae are poorly preserved and do not display the gnathobases. The distal portions of the appendages are preserved in outline only, indicating they are walking limbs with no apparent armature. Appendage III is preserved in its entirety, revealing it consists of seven podomeres. Appendage VI is not expanded into a swimming paddle. This is corroborated by Appendage VI in IRNSB a 13224, which is incomplete but also interpreted as a walking limb with small denticles proximally on its anterior margin (Fig. 2a, b). The metastoma is potentially preserved as an imprint on the articulated specimen (Fig. 2f, g), being broad with a deep anterior notch but not preserving the posterior margin, possibly pararectangular in shape (see Tollerton, Reference Tollerton1989, fig. 5).
The opisthosoma (Fig. 2a–c, f, g) is slender, with the maximum width at the second or third segment approximately equal to the width of the carapace. There is no clear difference in dimension between the pre- and postabdominal tergites, although the tergites of the postabdomen bear short epimeral spurs. The three posterior-most segments (tergites 10–12) are differentiated from the preceding homonomous tergites, becoming successively narrower and longer. Neither spines nor setae are seen on any of the segments, and the genital appendage is not preserved.
The telson is estimated to be between 1/4 and 1/3 of the total body length and is xiphous, narrowing marginally from its base before expanding and subsequently narrowing to its termination, with a medial ridge (Fig. 2f, g).
The isolated tergite fragments are significantly larger than the partially articulated fossils (up to 80 mm long). Three of the four fragments are covered in tubercles, as is typical for hardieopterids (Fig. 3a–c). The fourth fragment displays two layers of cuticle; the underlying layer has a pitted surface likely representing the underside of tubercles, and the superimposed layer is covered in scales (Fig. 3d).
5. Discussion
5.a. Phylogenetic affinities
The eurypterid material from Strud is incomplete and fragmentary; however, the presence of an epistoma in one of the smaller specimens and the pustulose ornament of the large fragments indicate an assignment to the stylonurine family Hardieopteridae. The new specimens exhibit some similarities to the broadly contemporaneous Stylonurus (?) arnoldi Ehlers, Reference Ehlers1935 from the Upper Devonian of Pennsylvania, USA (generic assignment discussed below). These include the pustulose ornamentation, enlarged lateral eyes, rounded anterior margin of the prosoma, and a narrow opisthosoma that does not expand beyond the width of the prosoma. The lateral eyes in both Stylonurus (?) arnoldi and the Strud material are identical in morphology to the lateral eyes of hardieopterids, although they are far larger in comparison to carapace size than in any previously known species. Stylonurus (?) arnoldi is, however, differentiated from the material described herein by the possession of a row of large scales that runs across the posterior margin of each tergite.
Both the Strud eurypterids and Stylonurus (?) arnoldi share a number of characteristics with rhenopterids, a clade of Late Ordovician – Early Devonian stylonurines that include the oldest known representatives of Stylonurina (Størmer, Reference Størmer1951). The rhenopterid species Brachyopterella ritchiei Waterston, Reference Waterston1979 and Kiaeropterus ruedemanni, Størmer, Reference Størmer1934 also exhibit long, narrow and relatively undifferentiated opisthosomas, as in the Strud specimens. Rhenopterids also tend to possess rather bulbous eyes (e.g. Kiaeropterus cyclophthalmus (Laurie, Reference Laurie1892), Brachyopterella pentagonalis (Størmer, Reference Størmer1934), Kiaeropterus ruedemanni), although they are more anteriorly positioned and not as large as in the Strud material, being c. 1/4 rather than 1/2 the carapace length. Brachyopterella pentagonalis and Kiaeropterus ruedemanni also possess an epistoma, although in these taxa the sutures defining the plate diverge markedly anteriorly and the transverse suture of the doublure curves posteriorly as it crosses onto the epistoma. Rhenopterids are further differentiated from Stylonurus (?) arnoldi and the Strud specimens by the possession of a median ocellar area (Tetlie et al. Reference Tetlie, Anderson and Poschmann2007), which is lacking in the material described herein, and by their lack of pustulose ornamentation.
The articulated Strud specimens most likely represent juveniles, as indicated by their small size and relatively large eyes. Juvenile eurypterids are relatively uncommon in the fossil record, but eurypterids are believed to have nine postembryonic ontogenetic stages, which is also the case in Limulus (Andrews et al. Reference Andrews, Brower, Gould and Reyment1974), and numerous studies have documented postembryonic changes in eurypterid morphology, including relative allometric proportions (Andrews et al. Reference Andrews, Brower, Gould and Reyment1974; Brower & Veinus, Reference Brower and Veinus1974, Reference Brower and Veinus1978; Cuggy, Reference Cuggy1994; Lamsdell & Selden, Reference Lamsdell and Selden2013). Consistently, juveniles possess relatively large lateral eyes, elongate prosomal appendages with poorly developed armature, and a more rounded anterior margin of the dorsal prosomal shield (Andrews et al. Reference Andrews, Brower, Gould and Reyment1974; Lamsdell & Selden, Reference Lamsdell and Selden2013). As such, a number of characteristics of the smaller Strud specimens are likely influenced by their early ontogenetic stage, such as the extreme rounding of the anterior portions of the carapace and the lack of appendage armature. Stylonurus (?) arnoldi also likely represents a juvenile (Lamsdell, pers. obs.) and occurs in the same strata as Hallipterus excelsior (Hall, Reference Hall1884). Hallipterus is also a hardieopterid (Tetlie, Reference Tetlie2008), and it is possible that Stylonurus (?) arnoldi is a juvenile form (and junior synonym) of Hallipterus excelsior, as supported by their shared carapace ornament of pustules with a row of enlarged pustules across the posterior carapace margin.
Among hardieopterids, only Tarsopterella has a subquadrate carapace, although carapace shape is known to change throughout eurypterid ontogeny (Andrews et al. Reference Andrews, Brower, Gould and Reyment1974; Brower & Veinus, Reference Brower and Veinus1974, Reference Brower and Veinus1978; Cuggy, Reference Cuggy1994), and if Stylonurus (?) arnoldi is a juvenile Hallipterus then the carapace shape changes from horseshoe-shaped to triangular over the course of its postembryonic development. An epistoma, revealed in the Strud specimens (Fig. 2) where the doublure has separated anteromedially, is also known in Hallipterus (Tetlie, Reference Tetlie2008). The doublure of Hardieopterus lacks an epistoma, having a single median suture between the two lateral plates (Waterston, Reference Waterston1979), while the condition of the doublure of Tarsopterella is unknown. The Strud specimens therefore show closer affinity to Tarsopterella and Hallipterus than they do to Hardieopterus. The Strud hardieopterid is, however, distinct from the known species of Tarsopterella and Hallipterus in lacking enlarged scale rows across the posterior margin of the prosomal dorsal shield and tergites.
5.b. Palaeoecology
The occurrence of eurypterids in an Upper Devonian non-marine setting at Strud corroborates previous observations that eurypterids underwent a marine to freshwater transition during the Devonian (Lamsdell & Braddy, Reference Lamsdell and Braddy2010; Lamsdell & Selden, Reference Lamsdell and Selden2017), with all Famennian eurypterids either known from fluvial settings (Hall, Reference Hall1884; Ehlers, Reference Ehlers1935) or from near-shore lagoons and deltas alongside plant material indicating the eurypterid material was swept in from continental settings (Lamsdell et al. Reference Lamsdell, Braddy and Tetlie2009; Plax et al. Reference Plax, Lamsdell, Vrazo and Barbikov2018). This transition occurs independently in several eurypterid groups (Lamsdell & Braddy Reference Lamsdell and Braddy2010; Lamsdell & Selden, Reference Lamsdell and Selden2017), as well as in the other aquatic chelicerate groups Xiphosura (Lamsdell, Reference Lamsdell2016) and Chasmataspidida (Lamsdell & Briggs, Reference Lamsdell and Briggs2017), suggesting that some external (potentially environmental) factor drove a concerted shift to non-marine habitats (Lamsdell et al. Reference Lamsdell, Congreve, Hopkins, Krug and Patzkowsky2017).
Previously, it has been recognized that juvenile eurypterids are found more often in near-shore and lagoonal environments than are adults (Braddy, Reference Braddy2001). This mirrors the life habits of modern horseshoe crabs, where larval and juvenile horseshoe crabs remain in coastal waters while adults disperse further offshore (Botton et al. Reference Botton, Tankersley and Loveland2010). Among Carboniferous eurypterids, it has been suggested that juveniles may have developed separately from adult populations in isolated ‘nursery’ pools that effectively formed a sheltered crèche for the small, vulnerable juveniles (Jeram & Selden, Reference Jeram and Selden1994). The same phenomenon is also suggested in Strud, where the juvenile material is recovered from a pond-like environment, while the large fragments likely from adults were collected stratigraphically lower in the succession together with plant and vertebrate remains in a coarser deposit from a higher-energy environment such as a river. Placoderms also used habitats (though different) of the Strud alluvial plain as a nursery (Olive et al. Reference Olive, Clément, Daeschler and Dupret2016b).
Upon reaching maturity, eurypterids could have migrated from the ponds to rivers during periods of flooding, or maybe even left the pools and traversed land to reach adjacent rivers, although evidence for terrestrial eurypterid excursions is exceedingly rare and indicates that individuals had extreme difficulty moving without the buoyancy afforded by water (Whyte, Reference Whyte2005). This would explain why the small specimens are found in association with the branchiopod crustaceans whereas the adult fragments are found in strata deposited elsewhere. The advantage of living in small shallow ponds is clear – here the young eurypterids were safe from predators (fish) but had access to prey (the smaller branchiopods and/or their eggs). The discovery of such a shift in life habit among the Strud eurypterids is important as it corroborates previous observations of ontogenetic migrations among Carboniferous hibbertopterids and indicates that this behaviour may have been common among freshwater eurypterid groups.
Author ORCIDs
James C Lamsdell 0000-0002-1045-9574
Acknowledgements
We thank the Gesves local council staff and field workers of the Strud expeditions. We acknowledge O. Béthoux and P. Loubry (French National Museum of Natural History (MNHN)) for help with photographs, and A. Folie and A. Drèze (Royal Belgian Institute of Natural Sciences (IRSNB)) for our requests of catalogue numbers. J. Denayer (Liège University) is warmly acknowledged for discussion about the depositional environment of the Pont-de-Bonne Modave quarry, and S. Olive (IRSNB) for discussion about the Famennian vertebrate assemblage. This work is a contribution to the French National Agency for Research TERRES project (ANR-2010-BLAN-607-03 grant), which supported P.G. and the Strud expeditions. LL and GEB were supported by the Swedish Research Council (VR: 621-2011-4703).
Conflict of interest
None.






