INTRODUCTION
Peroxisomes are cytoplasmic organelles that can contain up to 50 different enzymes, including catalase that eliminates the hydrogen peroxide produced by peroxisomal oxidases. Although their functions vary among species and between different tissues, peroxisomes are involved in fatty acid α-oxidation and β-oxidation, ether-phospholipids biosynthesis, glyoxylate metabolism and purine catabolism, among other metabolic pathways (Islinger et al., Reference Islinger, Cardoso and Schrader2010; Smith & Aitchison, Reference Smith and Aitchison2013). Peroxisome research has been mainly focused on mammals (including human peroxisomal disorders) and fungi (Vamecq et al., Reference Vamecq, Cherkaoui-Malki, Andreoletti and Latruffe2014; Sibirny, Reference Sibirny2016), with several studies also dedicated to the peroxisomes of plants and some protozoans (Gabaldón, Reference Gabaldón2010; Hu et al., Reference Hu, Baker, Bartel, Linka, Mullen, Reumann and Zolmanh2012). Since their discovery in 1954, the biogenesis and evolutionary origin of peroxisomes have been much debated. Although these organelles most likely evolved non-symbiotically, molecular data suggest that peroxisomal β-oxidation enzymes had their origin in the alpha-proteobacteria ancestors of mitochondria (Bolte et al., Reference Bolte, Rensing and Maier2015). It was also shown that several environmental pollutants can cause peroxisome proliferation in fish and bivalves (Cajaraville et al., Reference Cajaraville, Cancio, Ibabe and Orbea2003). Nevertheless, despite some studies (Cancio & Cajaraville, Reference Cancio and Cajaraville2000) not much is known about the peroxisomes of molluscs and other invertebrates.
Due to the abundance of peroxisomes in the digestive gland this is the first choice organ to study peroxisomes in molluscs (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994). The digestive gland is a major organ of the digestive system in gastropods and other molluscs. Typically, this gland has a tubular structure, and the epithelium of its tubules is formed by digestive cells and basophilic cells with additional minor cell types reported in some species (Luchtel et al., Reference Luchtel, Martin, Deyrup-Olsen, Boer, Harrison and Kohn1997; Dimitriadis & Andrews, Reference Dimitriadis and Andrews2000). The digestive gland is connected to the stomach by ducts (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Oliveira, Alves, Coelho and Calado2011a) and seems to be the major source of extracellular digestive enzymes found in stomach and crop fluids (Oxford, Reference Oxford1977, Reference Oxford1979). Subsequently, the products of extracellular digestion are carried through the ducts into the digestive gland tubules where they are collected by endocytosis and their digestion is completed in the heterolysosomes of digestive cells (Lobo-da-Cunha, Reference Lobo-da-Cunha2000; Taïeb, Reference Taïeb2001). The basophilic cells present the ultrastructural features of protein-secreting cells, containing large amounts of rough endoplasmic reticulum cisternae and apical electron-dense secretory vesicles (Lobo-da-Cunha, Reference Lobo-da-Cunha1999; Dimitriadis & Andrews, Reference Dimitriadis and Andrews2000). These cells were sometimes called calcium cells when containing calcium concretions. Detoxification, storage of minerals and accumulation of lipid and glycogen reserves are other functions of the digestive gland of gastropods (Voltzow, Reference Voltzow, Harrison and Kohn1994; Luchtel et al., Reference Luchtel, Martin, Deyrup-Olsen, Boer, Harrison and Kohn1997). However, the digestive gland of gastropods can be morphologically and functionally diverse, for example digestive gland cells of sacoglossans retain functional chloroplast collected from algae (Pierce & Curtis, Reference Pierce and Curtis2012), and in some special cases this gland can even be entirely absent as in deep sea bathysciadiid limpets (Hartmann et al., Reference Hartmann, Hess and Haszprunar2011).
Despite some earlier studies in gastropods (Dannen & Beard, Reference Dannen and Beard1977; Beard & Holtzman, Reference Beard and Holtzman1985; Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994), peroxisomes were not yet reported in cephalaspideans (commonly known as bubble snails), a clade of marine heterobranch gastropods with or without external shell distributed worldwide, comprising about 634 species (Malaquias et al., Reference Malaquias, Bericibar and Reid2009; Wägele et al., Reference Wägele, Klussmann-Kolb, Verbeek and Schrödle2014; Oskars et al., Reference Oskars, Bouchet and Malaquias2015). Previous light and electron microscopy studies were focused on the salivary glands and digestive tract of cephalaspideans (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Batista and Oliveira2010a, Reference Lobo-da-Cunha, Malheiro, Alves, Oliveira, Coelho and Calado2010b, Reference Lobo-da-Cunha, Oliveira, Alves, Coelho and Calado2011a, Reference Lobo-da-Cunha, Oliveira, Ferreira, Coelho and Calado2011b, Reference Lobo-da-Cunha, Pereira-Sousa, Oliveira, Alves, Guimarães and Calado2014, Reference Lobo-da-Cunha, Santos, Oliveira, Alves, Coelho and Calado2016), but the digestive gland of these gastropods has not yet been studied in detail. To further proceed the investigation of the digestive system of cephalaspideans, the digestive gland was studied by light and electron microscopy in carnivorous and herbivorous species, representing four families of cephalaspideans. In this paper only the ultrastructural aspects of their peroxisomes are reported.
MATERIALS AND METHODS
Animal collection
Specimens of Bulla striata Bruguière 1792, Aglaja tricolorata Renier 1807 and Philinopsis depicta (Renier 1807) were collected on the southern coast of Portugal (Algarve). Specimens of Philine quadripartita Ascanius 1772 and Haminoea navicula (da Costa 1778) were collected in Ria de Aveiro, a coastal lagoon connected to the Atlantic Ocean on the central coastline of Portugal. Specimens were collected in May and June, and at least four of each species were analysed.
Morphology
Digestive gland samples were prepared for transmission electron microscopy as previously reported for other tissues of cephalaspideans (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Batista and Oliveira2010a). Small fragments were fixed with 2.5% glutaraldehyde and 4% formaldehyde (obtained from hydrolysis of para-formaldehyde), diluted with 0.4 M cacodylate buffer (final concentration 0.28 M), pH 7.4. After washing in buffer, samples were postfixed with 2% OsO4, dehydrated in ethanol and embedded in Epon. Semi-thin sections (2 µm) for light microscopy observations were stained with methylene blue and azure II. Ultrathin sections stained by uranyl acetate and lead citrate were observed in a JEOL 100CXII transmission electron microscope operated at 60 kV.
Histochemistry
The tetrazonium coupling reaction for protein detection was applied to 2 µm semi-thin sections of Epon embedded samples, as reported previously (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Batista and Oliveira2010a). Sections on glass slides were treated for 10 min with a freshly prepared 0.2% solution of fast blue salt B in veronal-acetate buffer (pH 9.2), then washed and treated for 15 min with a saturated solution of β-naphthol in veronal-acetate buffer (pH 9.2). After washing, Epon sections were air dried and mounted with DPX.
RESULTS
In the digestive gland of gastropods, basophilic cells can be identified by the presence of spherical secretory vesicles mostly accumulated in the cytoplasm above the nucleus. In semi-thin sections of Philinopsis depicta digestive gland, structures resembling peroxisomes with a diamond-shaped crystalline core were observed with the light microscope in the basal region of basophilic cells (Figure 1A). These organelles could reach 5 µm in diameter and the tetrazonium coupling reaction showed that their core was made of proteins (Figure 1A, inset). Using transmission electron microscopy, unusually large peroxisomes containing an electron-dense diamond-shaped core were seen in basophilic cells of P. depicta (Figure 1B), confirming the observations made in the semi-thin sections. These oval or round organelles were surrounded by cisternae of rough endoplasmic reticulum (Figure 1B). Peroxisomes were significantly smaller in the digestive cells of P. depicta digestive gland, with less than 1 µm in diameter, but also contained an electron-dense diamond-shaped core (Figure 1C).
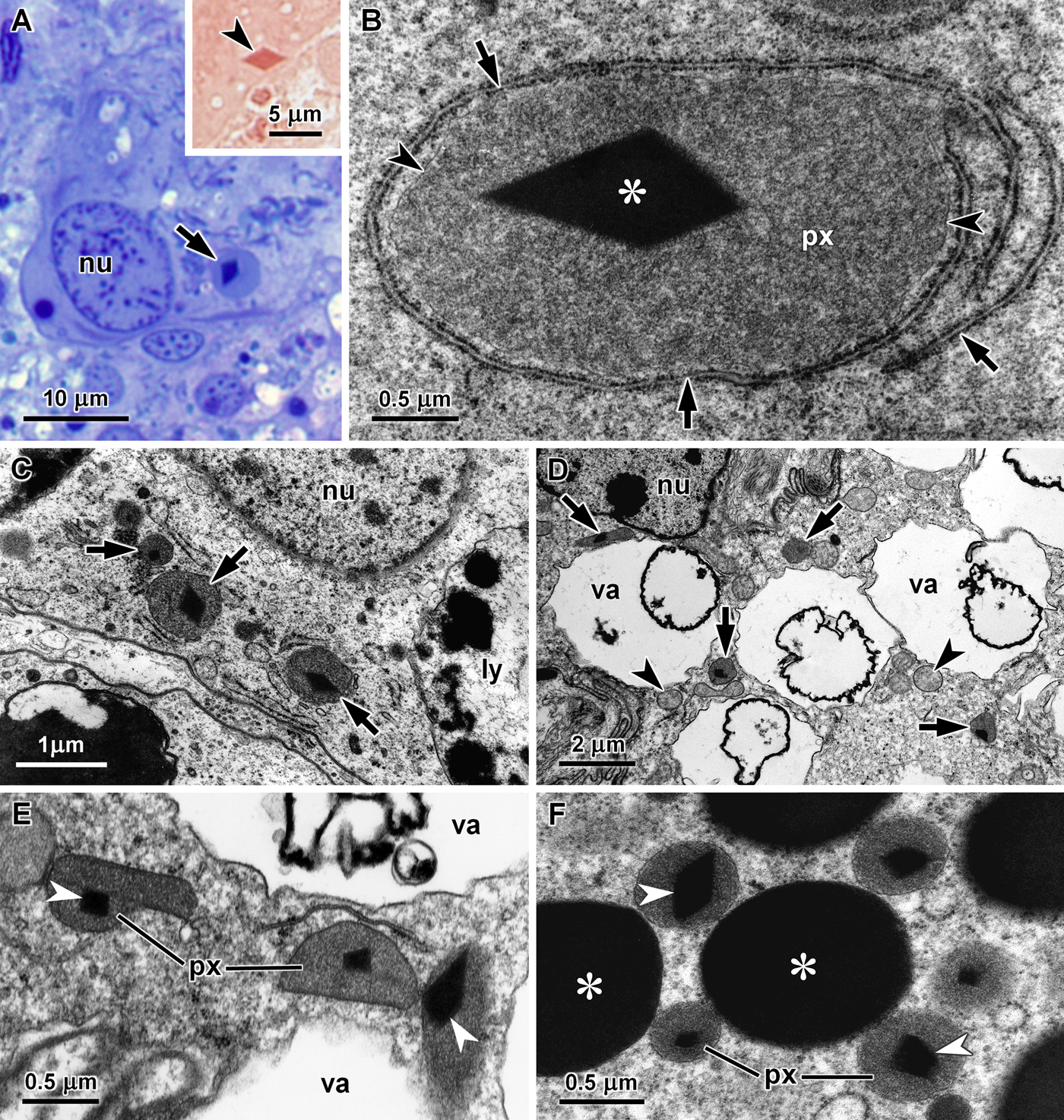
Fig. 1. Peroxisomes in the digestive gland of carnivorous cephalaspideans. (A) Basal region of a basophilic cell of Philinopsis depicta showing the nucleus (nu) and a structure looking like a very large peroxisome with a crystalline core (arrow) that could be stained by the tetrazonium coupling reaction for protein detection (arrowhead, inset); (B) Giant peroxisome (px) with a large core (asterisk) surrounded by rough endoplasmic reticulum cisternae (arrows) in a basophilic cell of P. depicta, arrowheads point to the peroxisomal membrane; (C) Peroxisomes with a diamond-shaped core (arrows) in the cytoplasm between the nucleus (nu) and the large heterolysosomes (ly) of a digestive cell of P. depicta; (D) Ultra-thin section of a basophilic cell of Aglaja tricolorata showing peroxisomes (arrows) among the vacuoles (va) that occupy most of the cytoplasm, the nucleus (n) and some mitochondria (arrowheads) are also visible; (E) Peroxisomes (px) with diverse shapes containing an electron-dense core (arrowheads) in the cytoplasm between the vacuoles (va) of a basophilic cell of A. tricolorata; (F) Several peroxisomes (px) with a diamond-shaped core (arrowheads) among secretory vesicles (asterisks) in a basophilic cell of Philine quadripartita.
Several peroxisomes were also present in the digestive gland cells of the other four species of cephalaspideans included in this study. However, due to their smaller dimensions they were not detectable in semi-thin sections stained with methylene blue and azure II, and were only observed by transmission electron microscopy. In Aglaja tricolorata, peroxisomes were observed in the cytoplasm around the vacuoles that could occupy a very large part of the basophilic cells (Figure 1D). These peroxisomes were more variable in shape, including round, oval and elongated forms usually with a length inferior to 1 µm in ultra-thin sections, and also contained an electron-dense core (Figure 1E). In basophilic cells of Philine quadripartita, round peroxisomes with a diameter about 0.5 µm were more abundant close to the electron-dense secretory vesicles (Figure 1F) and near mitochondria at the basal region of these cells. Two cores were found in the peroxisomes of Haminoea navicula (Figure 2A and B), which had an average diameter of 0.5 µm. In the digestive gland of this species, peroxisomes were also more frequently observed close to the secretory vesicles (Figure 2A) and at the basal region of basophilic cells (Figure 2B). These organelles were bigger in Bulla striata basophilic cells, frequently with a diameter between 1.0 and 1.5 µm (Figure 2C and D). In all five species, cores sections ranged from square to diamond-shaped, suggesting an octahedral crystal. They were all highly electron-dense without any visible internal crystalline structure. The morphological features of peroxisomes in the five species of cephalaspideans included in this study are summarized in Table 1.
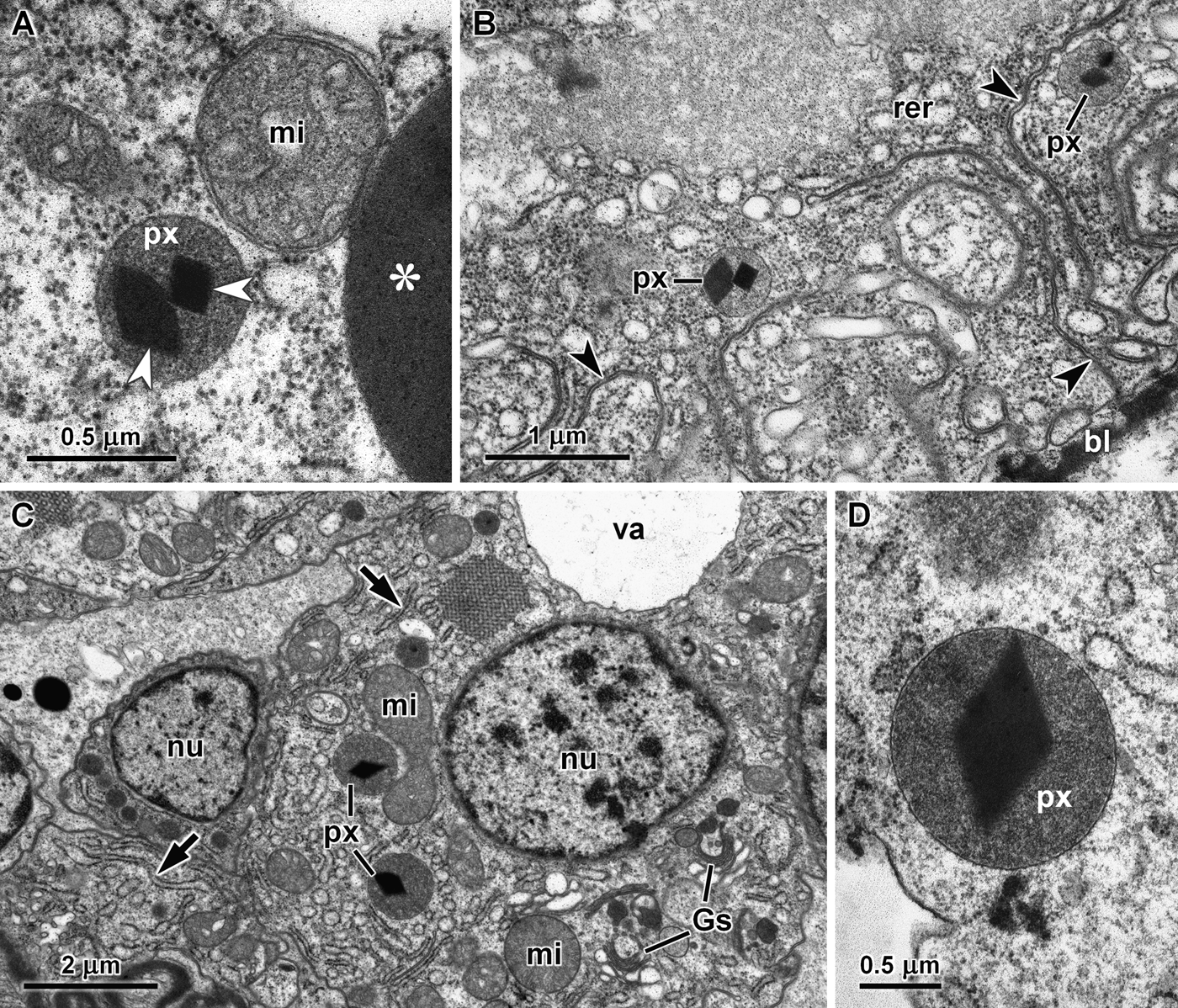
Fig. 2. Peroxisomes in the digestive gland of herbivorous cephalaspideans. (A) Two cores (arrowheads) are clearly visible within a peroxisome (px) close to mitochondria (mi) and a secretory vesicle (asterisk) in a basophilic cell of Haminoea navicula; (B) Basal region of a basophilic cells of H. navicula containing peroxisomes (px) and rough endoplasmic reticulum cisternae (rer), cell membrane invaginations (arrowheads) and the basal lamina (bl) are also visible; (C) Basal region of basophilic cells of Bulla striata showing nuclei (nu), peroxisomes (px), mitochondria (mi), Golgi stacks (Gs), rough endoplasmic reticulum cisternae (arrows) and a vacuole (va); (D) A peroxisome (px) of B. striata with a large diamond-shaped core.
Table 1. Morphological features of peroxisomes in basophilic cells of cephalaspideans.

DISCUSSION
Among molluscs, ultrastructural aspects of peroxisomes were reported in bivalves (Owen, Reference Owen1972; Cajaraville et al., Reference Cajaraville, Völkl and Fahimi1992), chitons (Lobo-da-Cunha, Reference Lobo-da-Cunha1997) and in some clades of gastropods (Dannen & Beard, Reference Dannen and Beard1977; Beard & Holtzman, Reference Beard and Holtzman1985; Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994; Lobo-da-Cunha, Reference Lobo-da-Cunha1999), but not in cephalaspideans.
Peroxisome abundance and dimensions in basophilic and digestive cells reveal the importance of these organelles in digestive gland metabolism. Catalase, D-amino acid oxidase, and the fatty acid β-oxidation enzymes acyl-CoA oxidase and multifunctional enzyme were reported in mussel peroxisomes (Cajaraville et al., Reference Cajaraville, Völkl and Fahimi1992; Cancio et al., Reference Cancio, Ibabe and Cajaravile1999, Reference Cancio, Völkl, Beier, Fahimi and Cajaraville2000). In addition to catalase and β-oxidation enzymes, urate oxidase, an enzyme involved in purine catabolism, was also detected in the scallop Placopecten magellanicus (Stewart et al., Reference Stewart, Carlin, Macdonald and Van Iderstine1994). In gastropods, in addition to catalase detected in marine and land species (Dannen & Beard, Reference Dannen and Beard1977; Malik et al., Reference Malik, Jones and Connock1987; Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994; Lobo-da-Cunha, Reference Lobo-da-Cunha1999), acyl-CoA oxidase and D-amino acid oxidase were reported in digestive gland peroxisomes of the land slug Arion ater (Malik et al., Reference Malik, Jones and Connock1987). These results suggest that peroxisomal metabolism in molluscs has similarities with the peroxisomal metabolism of vertebrates, which also contain these enzymes (Islinger et al., Reference Islinger, Cardoso and Schrader2010). In mammals and other vertebrates the liver is the richest organ in peroxisomes, typically occupying between 1 and 2% of the hepatocytes' volume (Rocha et al., Reference Rocha, Lobo-da-Cunha, Monteiro, Silva and Oliveira1999), and although the digestive gland of molluscs is an organ with different ontogeny and functions in both organs peroxisomes have an important metabolic role.
Although catalase is frequently used as a peroxisomal marker, the typical morphology of peroxisomes with crystalline cores can be sufficient to identify these organelles. Nevertheless, further experiments including catalase detection with antibodies or by cytochemical methods should be made to confirm the identification of these organelles as peroxisomes. Among gastropods, peroxisomal cores present a considerable morphological variability, including cores with diamond, hexagonal or rectangular sections (Dannen & Beard, Reference Dannen and Beard1977; Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994; Lobo-da-Cunha, Reference Lobo-da-Cunha1999). Although cores are typically present in peroxisomes, coreless peroxisomes were reported in basophilic cells of some gastropods (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994). On the other hand, peroxisomes with two cores were observed in bivalves and chitons (Owen, Reference Owen1972; Lobo-da-Cunha, Reference Lobo-da-Cunha1997), as in the basophilic cells of Haminoea navicula. In the digestive gland of the five cephalaspideans included in this study, all peroxisomal cores seem to have an octahedral shape suggesting that they are formed by the same proteins. More unusual hexagonal crystals have been found so far only in the digestive gland of Phorcus lineatus (=Monodonta lineata) and Gibbula umbilicalis, two species of the Trochidae family (Vetigastropoda) (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994).Thus, some relationships between the shape of peroxisomal cores and phylogenetic position may exist, but much more data are required to verify this hypothesis.
The core of mammalian liver peroxisomes contains urate oxidase (Völkl et al., Reference Völkl, Baumgart and Fahimi1988), but in gastropods the enzymes present in cores are still unknown. In the digestive gland of some marine gastropods the average diameter of peroxisomes is ~0.5 µm, nevertheless peroxisomes with diameters between 1.0 and 1.5 µm were frequent in basophilic cells of Trochidae (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Alves, Oliveira and Calado1994). But even these are smaller than the giant peroxisomes of the Philinopsis depicta digestive gland. Unusually large peroxisomes were also reported in the liver of the grey mullet (Mugil cephalus), which could reach 3 µm in diameter (Orbea et al., Reference Orbea, Beier, Völkl, Fahimi and Cajaraville1999). Even so, the peroxisomes with ~5 µm in diameter found in P. depicta basophilic cells surpass those, being among the biggest ever reported in animal cells. However, in the digestive cells of P. depicta peroxisomes have common sizes. In the anaspidean sea slug Aplysia depilans, peroxisomes are also larger in basophilic cells than in digestive cells, although these rarely exceed 0.7 µm in diameter (Lobo-da-Cunha, Reference Lobo-da-Cunha1999, Reference Lobo-da-Cunha2000). Although A. tricolorata and P. depicta both belong to the Aglajidae family, the peroxisomes in the A. tricolorata digestive gland are much smaller than the giant peroxisomes of P. depicta basophilic cells, showing the morphological variability of these organelles within this family of carnivorous cephalaspideans (Zamora-Silva & Malaquias, Reference Zamora-Silva and Malaquias2016). Peroxisomes were common in digestive gland cells of both carnivorous (A. tricolorata, P. depicta, P. quadripartita) and herbivorous cephalaspideans (B. striata, H. navicula). However, a quantitative study is required to verify if there is any significant difference in the abundance of these organelles between herbivorous and carnivorous cephalaspideans.
FINANCIAL SUPPORT
This work was supported by funds provided by ICBAS-UP.
CONFLICT OF INTEREST
The authors have no conflict of interest.





