I. INTRODUCTION
The pyrovanadates M 2V2O7, where M = Ca, Sr, Pb, Ba, Mg, Zn, Co, Ni, Cu, Mn, Cd, demonstrate promising magnetic (Bhatia et al., Reference Bhatia, Moharatra, Nirmala and Malik2010; Basiev et al., Reference Basiev, Voronko, Maslov, Sobol and Shukshin2011; Sanchez-Andujar et al., Reference Sanchez-Andujar, Yáñez-Vilar, Mira, Biskup, Rivas, Castro-García and Señarís-Rodríguez2011), optical (Cid-García et al., Reference Cid-García, Lozada-Morales, López-Calzada, Zayas Ma, Angel, Carmona-Rodriguez, Rodriguez-Melgarejo, Rubio-Rosas, Jiménez-Sandoval and Tomás2012; Dalal et al., Reference Dalal, Taxak, Lohra, Sangvan and Khatkar2015; Takahashi et al., Reference Takahashi, Hagiwara and Fujihara2016), and electrical (Cowin et al., Reference Cowin, Lan, Petit, Christophe, Zhang and Tao2011) properties. The physico-chemical properties and structures of divalent metal vanadates have been studied in depth. Double vanadates of nominal compositions MMIV2O7 and M 1.5MI 0.5V2O7, where M, MI = Ca, Sr, Pb, Ba, Mg, Zn, Co, Ni, Cu, Mn, Cd, were synthesized mainly in the period between 1985 and 1999. At the same time, not only the unit-cell parameters were determined for a number of compounds, but also the crystal structures were described (Table I). A systematic study of the crystal structure of divalent metal vanadates has cast doubt on the correctness of isolation of the pyroanion V2O74− in their chemical formula, since the data obtained showed a more complex anion structure (Zhuravlev et al., Reference Zhuravlev, Fotiev, Zhukov and Kristallov1982; Murashova et al., Reference Murashova, Velikodnyi and Trunov1989; Murashova et al., Reference Murashova, Velikodnyi and Trunov1991; Vogt and Muller-Buschbaum, Reference Vogt and Muller-Buschbaum1991a). In addition to vanadates with anionic groups V2O74−, compounds containing anionic groups V4O148− and [(V3O10)(VO4)]8− were also described. In Table I, a part of the formula of double vanadates is presented taking into account the data on the V–O distances in the vanadium–oxygen groups; the other part is presented in the form given in the original sources.
In this paper, the results of goal-oriented synthesis of double vanadates of calcium and zinc, lead, and cadmium (Table I) and their crystal structures are reported, and changes in the configuration and vanadium–oxygen polyhedra of related compounds are considered.
Table I. Crystallographic characteristics of double vanadates of divalent metals.
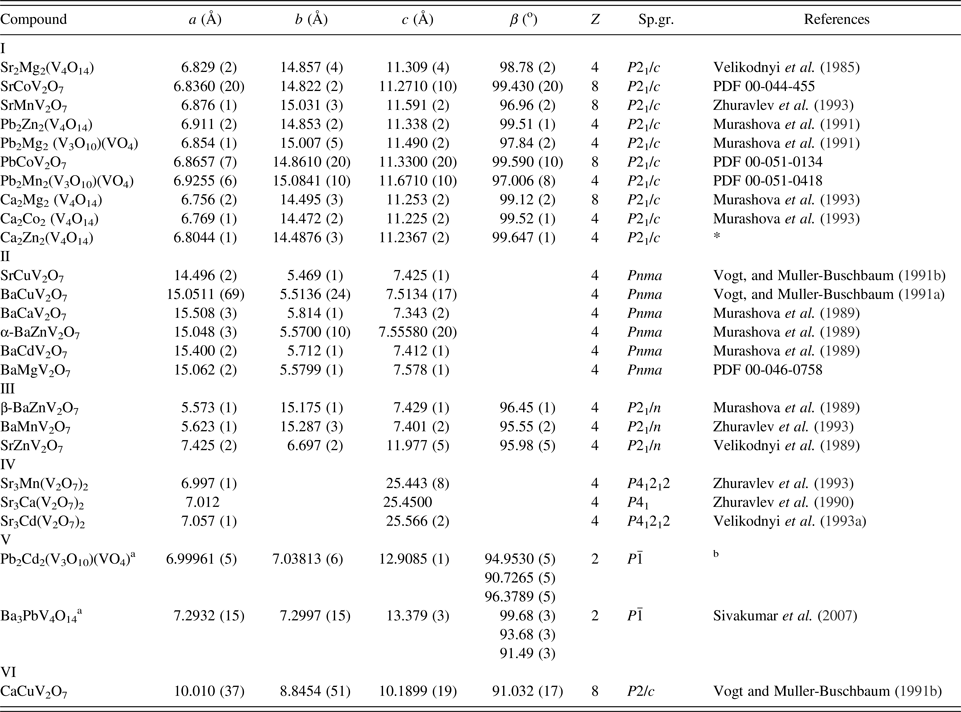
a The reduced cells are shown.
b Data obtained in this study.
II. EXPERIMENTAL
Samples of Ca2Zn2(V4O14) were synthesized using the citrate method. Pb2Cd2(V3O10)(VO4) was obtained via solid-phase synthesis. Calcium–zinc and calcium–manganese vanadates were prepared by dissolving an equimolar amount of manganese (zinc) oxides and calcium carbonate of reactive purity in nitric acid. An equivalent amount of V2O5 was separately dissolved in citric acid in the ratio 1:4 during heating. After complete dissolution of the V2O5 sample, the vanadium solution acquired a blue colour, indicating the formation of vanadyl citrate,
The formation of nitrate–citrate complexes followed the coacervation of vanadyl citrate solutions and nitrates of calcium, manganese, and zinc, preventing salting out and leading, under heating, to the formation of xerogels of variable composition. Evaporation was carried out at about 100 °C until a viscous mass was formed. After the formation of the xerogel, the temperature was raised to 250–300 °C, initiating a redox process in the reaction mass. The reaction proceeded without release of nitrogen oxides because of the excess of citric acid reducing agent. The resulting powder was brownish-yellow because of the presence of unburned carbon particles. The precursor was ground in an agate mortar and annealed at 500 and 650 °С, whereupon the product acquired a white colour. According to XRPD, it became single phase after annealing at ~650 °С.
Pb2Cd2(V3O10)(VO4) was synthesized using cadmium carbonate, lead oxide, and vanadium (V) oxide. The initial components were mixed and subjected to multistage annealing in the temperature range from 450 to 600 °С. The annealing time at the final stage of synthesis was 30 h.
The X-ray powder diffraction (XRPD) patterns were collected at room temperature on a STADI P (Stoe) diffractometer with transmission geometry, using CuK α 1 radiation and a linear mini-PSD detector in a 2θ range of 5–120° with a step of 0.02°. To obtain high-quality diffraction data, following the recommendations given by Stoe, very thin layers of powdered high absorbing materials were fixed on acetate foils by Elmers White Glue. The thickness of the layer was adjusted to get the transmission factor, I 0/I, equal to approximately 2–3, where I 0 is the flux of the primary beam, and I is the flux passing through the specimen. Polycrystalline silicon [a = 5.43075(5) Å] was used as an external standard. Possible impurity phases were checked by comparing their XRPD patterns with those in the PDF2 database (ICDD, 2016). The only impurity found was 1.5 mass% of Cd2V2O7 in Pb2Cd2(V3O10)(VO4). The XRPD pattern of Ca2Zn2(V4O14) was indexed using the TREOR program (Werner et al., Reference Werner, Eriksson and Westdahl1985) and compared with an analogous monoclinic unit cell of CaCoV2O7 (Murashova et al., Reference Murashova, Velikodnyi and Zhuravlev1993). The crystal structure refinement of the new compounds was carried out employing Rietveld analysis (Rietveld, Reference Rietveld1969) with the GSAS program suite using the XRPD data (Toby, Reference Toby2001; Larson and Von Dreele, Reference Larson and Von Dreele2004). The peak profiles were fitted with a pseudo-Voigt function, I(2θ) = x × L(2θ) + (1−x) × G(2θ) (where L and G are the Lorentzian and Gaussian parts, respectively). The angular dependence of the peak width was described by the relation (FWHM)2 = Utg 2θ + Vtg θ + W, where FWHM is the full line width at half maximum. The background level was described by a combination of 15-order Chebyshev polynomials. Since the background of XRPD pattern of Pb2Cd2(V3O10)(VO4) was difficult to fit, it was removed before the refinement. The absorption correction function for a flat plate sample in transmission geometry was applied. Since both models being refined have a large number of atomic positions, and correlations between a large number of variables are probable, the atomic displacement parameters of all oxygen atoms were constrained as single variable. The crystallographic data, atomic coordinates, atomic displacement parameters, and selected interatomic distances are given in Tables II–IV. Experimental, calculated, and difference XRPD patterns are shown in Figure 1.
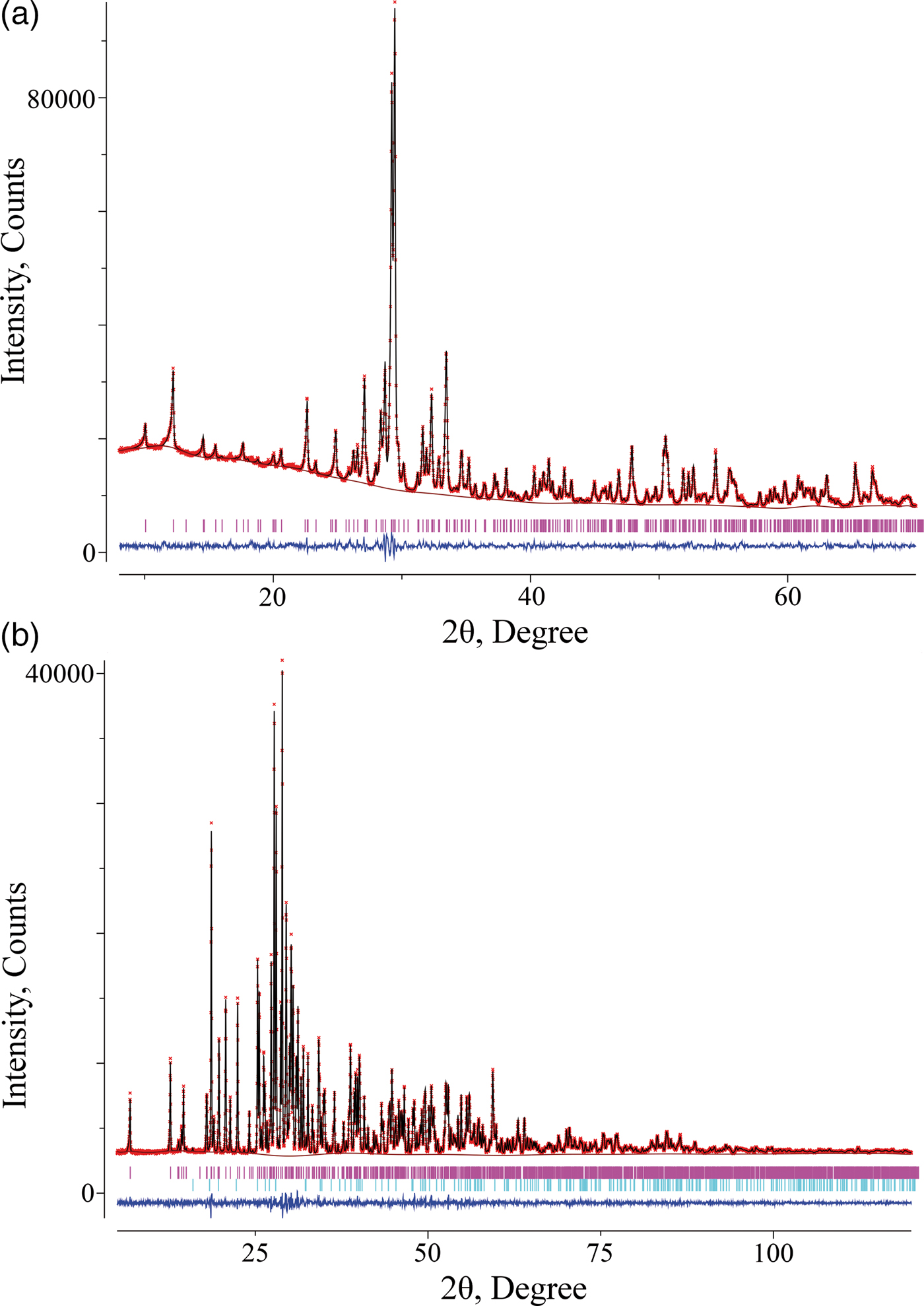
Figure 1. (Color online) Experimental (crosses), calculated (solid line), and difference (bottom line) XRPD patterns of (a) Ca2Zn2(V4O14) and (b) Pb2Cd2(V3O10)(VO4). The tick marks correspond to the Bragg reflections.
Table II. Crystal data and structure refinement for Ca2Zn2(V4O14) and Pb2Cd2(V3O10)(VO4).
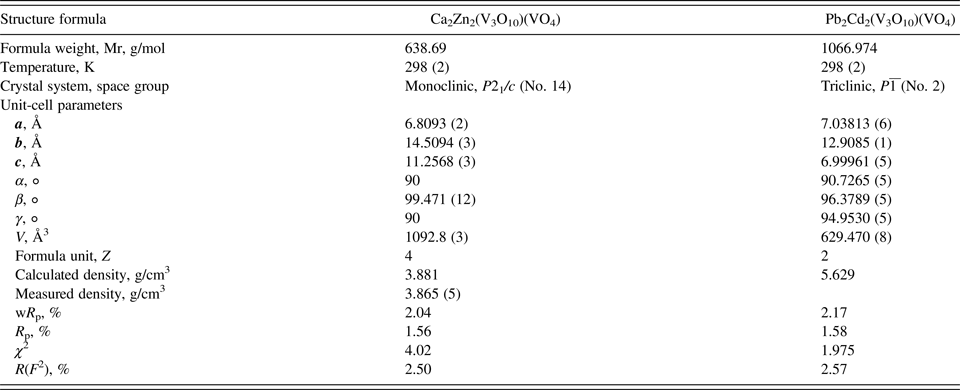
Table III. Atomic coordinates and isotropic displacement parameters (U iso × 100, Å2) for Ca2Zn2(V4O14) and Pb2Cd2(V3O10)(VO4).
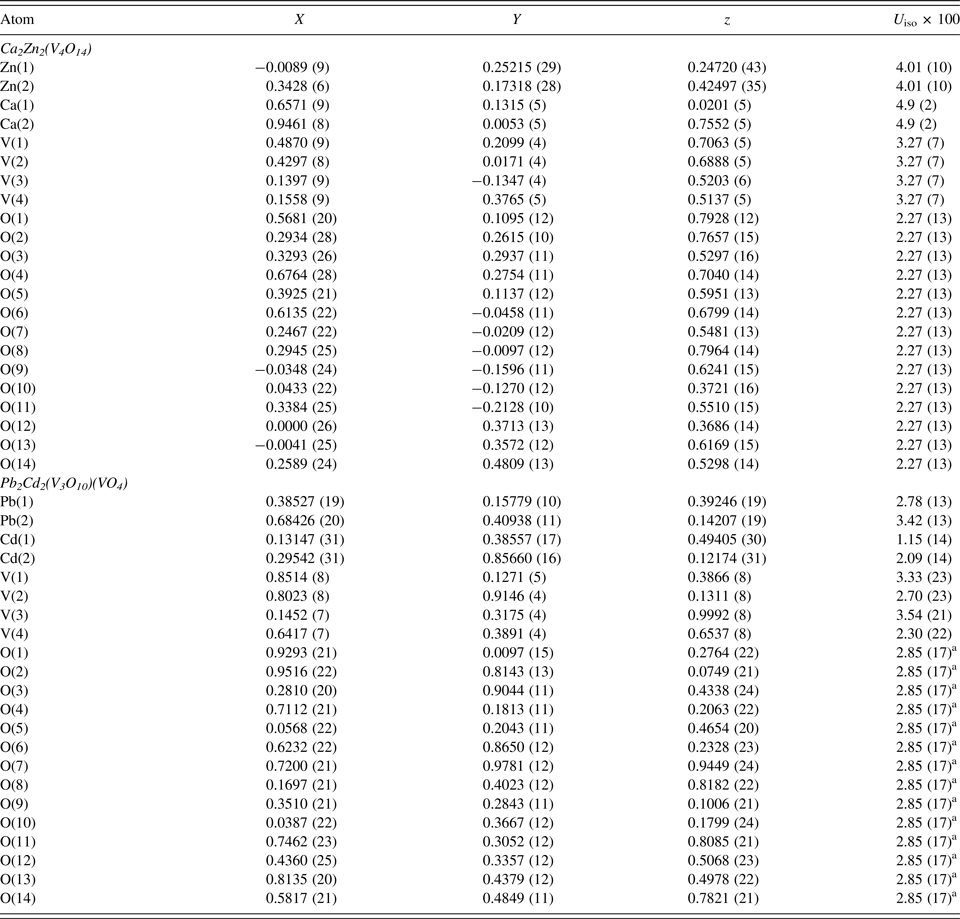
a Atomic displacement parameters of all oxygen atoms were constrained as single variable.
Table IV. Selected interatomic distances d (Å) and angles (°) for Ca2Zn2(V4O1) and Pb2Cd2(V3O10)(VO4).
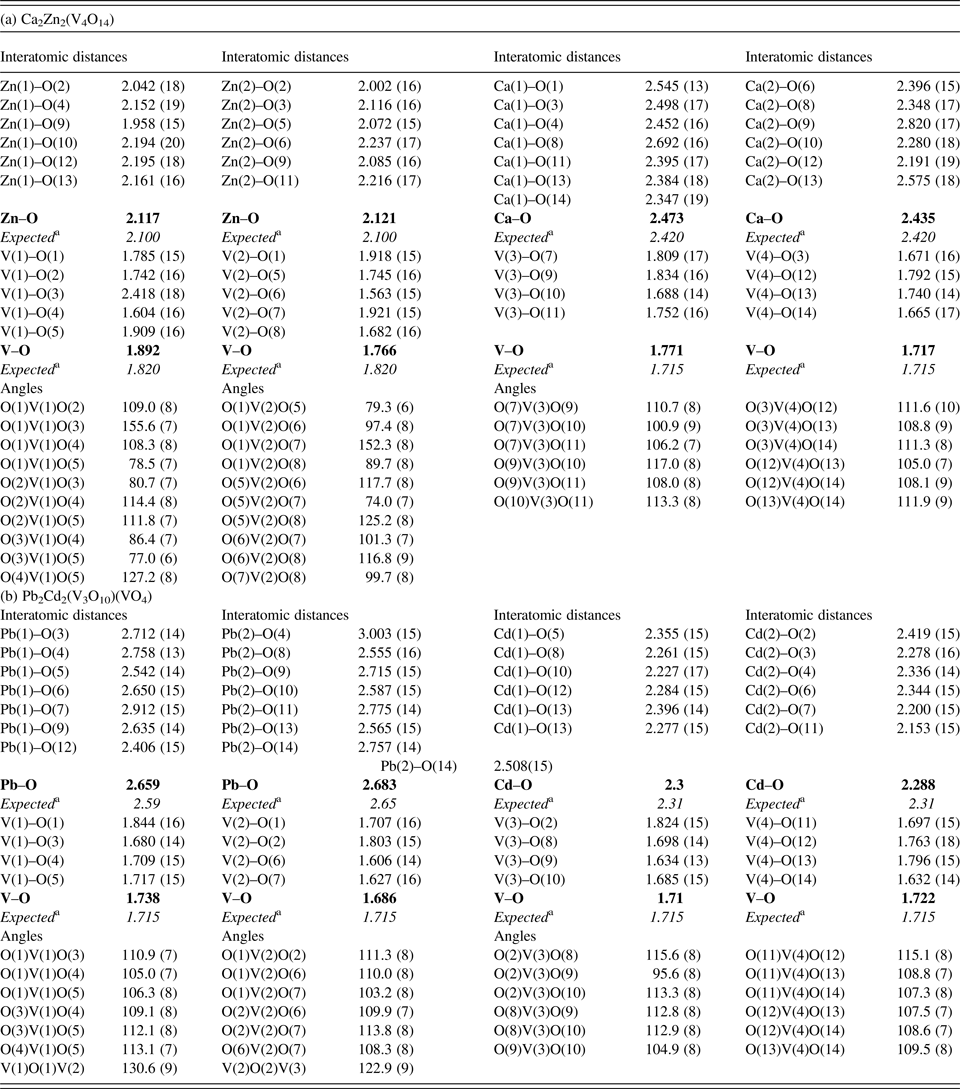
The average values are indicated by boldface type.
a The sum of the crystal radii according to [Shannon R.D. Acta Crystallogr. A 32 (1976) 751]: Pb+2 VII – 1.37 Å, Pb+2 VIII – 1.43 Å, Cd+2 VI − 1.09 Å, V+5 IV – 0.495 Å, O−2 III – 1.22 Å.
Density measurements were carried out on an AccuPyc II 1340 Gas Displacement Pycnometry System (Micromeritics Instrument Corporation, Norcross, Georgia, USA) with a sample chamber of 1 cm3. About 50–60% of the chamber volume was filled by the powder sample; 15 measurements were performed on the sample.
III. DISCUSSION
The XRPD pattern of Ca2Zn2(V4O14) was indexed in the monoclinic system with unit-cell parameters a = 6.8044(1) Å, b = 14.4876(3) Å, c = 11.2367(2) Å, β = 99.647(1)° [space group P21/c (14), Z = 4]. The most representative double vanadates belong to group I consisting of 10 compounds having an isotype structure that crystallizes in monoclinic system with space group P21/c. Their nominal anions V2O74− either condense into V4O148− tetramers when the oxygen coordination of the vanadium atoms increases to five [Figures 2(a) and 2(b)], or they form a pair of V3O105− trimer and orthovanadate ion VO43− [Figure 2(c)]. This division into the isotype series is rather arbitrary since the increased V–O distance varies from 2.24 Å in Pb2Zn2(V4O14) to 2.75 Å in the trimer–orthovanadate–ion chain in Pb2Mg2(V3O10)(VO4). This compound is represented as Ca2Zn2(V3O10)(VO4) (Babaryk et al., Reference Babaryk, Odynets, Khainakov, Garcia-Granda and Slobodyanik2015). Crystal structure of Ca2Zn2(V4O14) consists of layers containing V4O148− tetramers alternating along (10![]() $\bar 2$) direction with the layers of edge-sharing zinc–oxygen octahedra, Ca(1) and Ca(2) atoms fill empty sites in the latter with coordination numbers 7 and 6, respectively. V4O148− group consists of two highly distorted edge-sharing vanadium–oxygen polyhedra with CN = 5, which share corners with two terminal vanadium–oxygen tetrahedra [Figures 2(b)], wherein V(1)–O(3) distance, 2.418 Å, is much longer than others. This confirms the separation of the orthovanadate ion VO43− from V3O105− trimer, proposed by Babaryk et al. (Reference Babaryk, Odynets, Khainakov, Garcia-Granda and Slobodyanik2015). At the same time, the average metal–oxygen distances are in good agreement with the sums of the crystal radii according to Shannon (Reference Shannon1976).
$\bar 2$) direction with the layers of edge-sharing zinc–oxygen octahedra, Ca(1) and Ca(2) atoms fill empty sites in the latter with coordination numbers 7 and 6, respectively. V4O148− group consists of two highly distorted edge-sharing vanadium–oxygen polyhedra with CN = 5, which share corners with two terminal vanadium–oxygen tetrahedra [Figures 2(b)], wherein V(1)–O(3) distance, 2.418 Å, is much longer than others. This confirms the separation of the orthovanadate ion VO43− from V3O105− trimer, proposed by Babaryk et al. (Reference Babaryk, Odynets, Khainakov, Garcia-Granda and Slobodyanik2015). At the same time, the average metal–oxygen distances are in good agreement with the sums of the crystal radii according to Shannon (Reference Shannon1976).
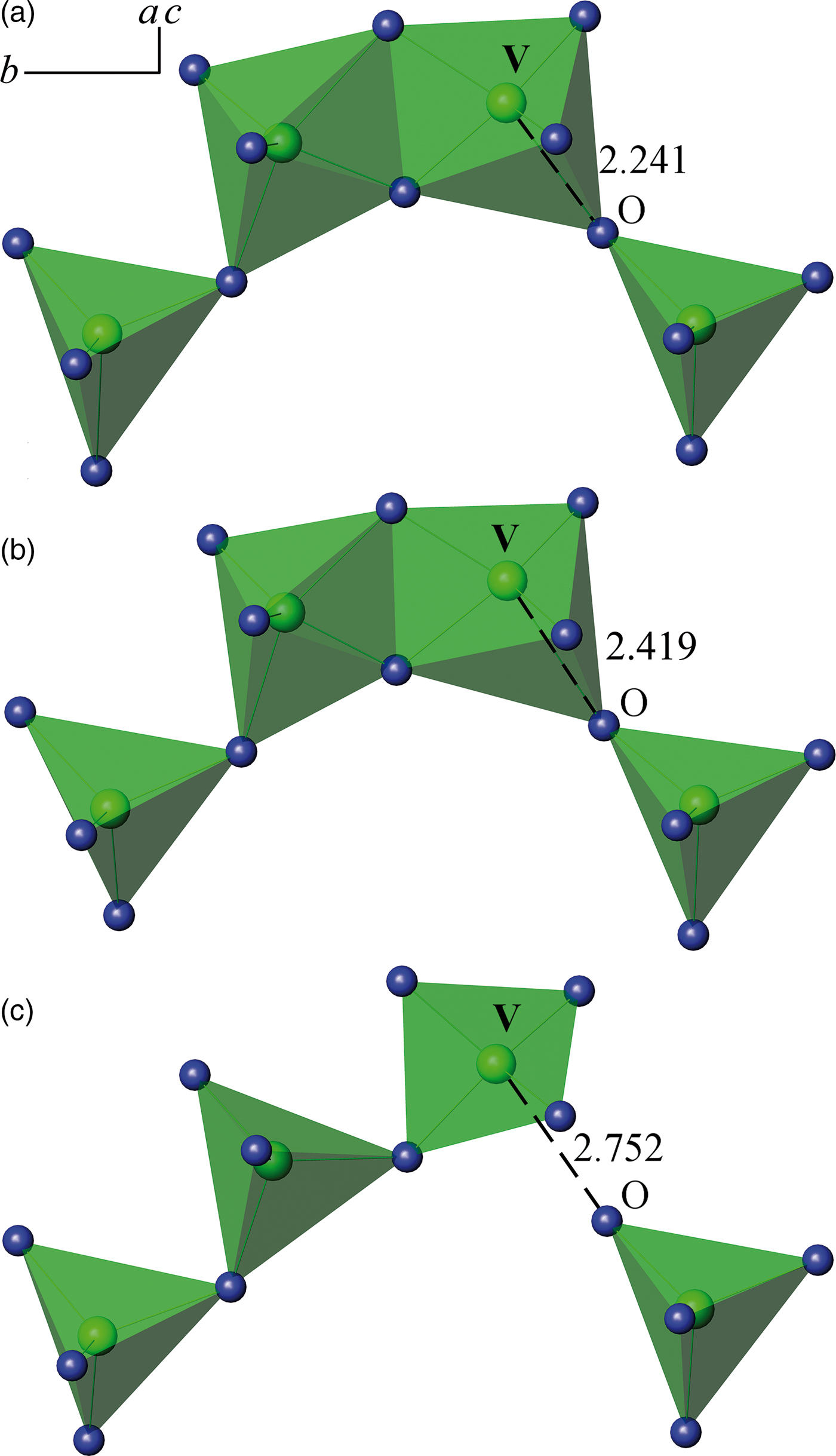
Figure 2. (Color online) The connection of anionic groups in vanadates Pb2Zn2(V4O14) (а), Ca2Zn2(V4O14) (b), Pb2Mg2(V3O10)(VO4) (d).
The XRPD pattern of the obtained Pb2Cd2(V3O10)(VO4) was found to be in good agreement with the data presented by Shpanchenko and Antipov in PDF2 (Entry 00-058-0617) for PbCdV2O7 and it was indexed with triclinic unit-cell parameters a = 7.03813(6) Å, b = 12.9085(1) Å, c = 6.99961(5) Å, α = 90.7265(5)°, β = 96.3789(5)°, γ = 94.9530(6)°, V = 629.470(8) Å3 (space group P ![]() $\bar 1$, No. 2, Z = 2), the reduced cell is a = 6.99961(5) Å, b = 7.03813(6) Å, c = 12.9085(1) Å, α = 94.9530(6)°, β = 90.7265(5)°, γ = 96.3789(5)°. Such unit-cell parameters may be indicative of a similarity of the structure to the B-type rare-earth disilicates RE2Si2O7 (Felsche, Reference Felsche1970). The crystal structure of PbCdV2O7 has been solved space group P
$\bar 1$, No. 2, Z = 2), the reduced cell is a = 6.99961(5) Å, b = 7.03813(6) Å, c = 12.9085(1) Å, α = 94.9530(6)°, β = 90.7265(5)°, γ = 96.3789(5)°. Such unit-cell parameters may be indicative of a similarity of the structure to the B-type rare-earth disilicates RE2Si2O7 (Felsche, Reference Felsche1970). The crystal structure of PbCdV2O7 has been solved space group P ![]() $\bar 1$ using the EXPO2013 program suite (Altomare et al., Reference Altomare, Cuocci, Giacovazzo, Moliterni, Rizzi, Corriero and Falcicchio2013) assuming two formula units per unit cell. The resulting model shown in Figure 3, similarly to the structure of Ca2Zn2(V4O14), consists of layers containing V4O148− groups alternating along the (1
$\bar 1$ using the EXPO2013 program suite (Altomare et al., Reference Altomare, Cuocci, Giacovazzo, Moliterni, Rizzi, Corriero and Falcicchio2013) assuming two formula units per unit cell. The resulting model shown in Figure 3, similarly to the structure of Ca2Zn2(V4O14), consists of layers containing V4O148− groups alternating along the (1![]() $\bar 1$ 1) direction with the layers of two edge-sharing Cd(1)–oxygen octahedra and separate Cd(2)O6 octahedra, Pb(1) and Pb(2) atoms fill empty sites in the latter with coordination numbers 7 and 8, respectively, forming a finite chain of four edge-sharing polyhedra. In Pb2Cd2(V3O10)(VO4), V4O148− group contains broken chains unusual for the vanadates consisting of single orthovanadate [V(4)O4]3− and linear trivanadate (V3O10)5−, formed of three sufficiently regular corner-sharing tetrahedra, alternating along (011) [Figure 3(e)]. The average metal–oxygen distances are in good agreement with the sums of the crystal radii according to Shannon (Shannon, Reference Shannon1976). Such a combination of triple tetrahedra (Si3O10)8− and single tetrahedra (SiO4)4− groups has been reported for Ho2Si2O7 (Felsche, Reference Felsche1972). Owing to the decrease in the unit-cell symmetry in Table I, the compound constitutes a separate group V. The compound Ba3PbV4O14 (Sivakumar et al., Reference Sivakumar, Chang and Halasyamani2007) is also referred to this group, although this contradicts the study of phase equilibria in the Ba2V2O7–Pb2V2O7 system. Primary solid solutions based on barium pyrovanadate were found to contain 0–30 mol% Pb2V2O7 (Zhuravlev and Velikodnyi, Reference Zhuravlev and Velikodnyi1997).
$\bar 1$ 1) direction with the layers of two edge-sharing Cd(1)–oxygen octahedra and separate Cd(2)O6 octahedra, Pb(1) and Pb(2) atoms fill empty sites in the latter with coordination numbers 7 and 8, respectively, forming a finite chain of four edge-sharing polyhedra. In Pb2Cd2(V3O10)(VO4), V4O148− group contains broken chains unusual for the vanadates consisting of single orthovanadate [V(4)O4]3− and linear trivanadate (V3O10)5−, formed of three sufficiently regular corner-sharing tetrahedra, alternating along (011) [Figure 3(e)]. The average metal–oxygen distances are in good agreement with the sums of the crystal radii according to Shannon (Shannon, Reference Shannon1976). Such a combination of triple tetrahedra (Si3O10)8− and single tetrahedra (SiO4)4− groups has been reported for Ho2Si2O7 (Felsche, Reference Felsche1972). Owing to the decrease in the unit-cell symmetry in Table I, the compound constitutes a separate group V. The compound Ba3PbV4O14 (Sivakumar et al., Reference Sivakumar, Chang and Halasyamani2007) is also referred to this group, although this contradicts the study of phase equilibria in the Ba2V2O7–Pb2V2O7 system. Primary solid solutions based on barium pyrovanadate were found to contain 0–30 mol% Pb2V2O7 (Zhuravlev and Velikodnyi, Reference Zhuravlev and Velikodnyi1997).
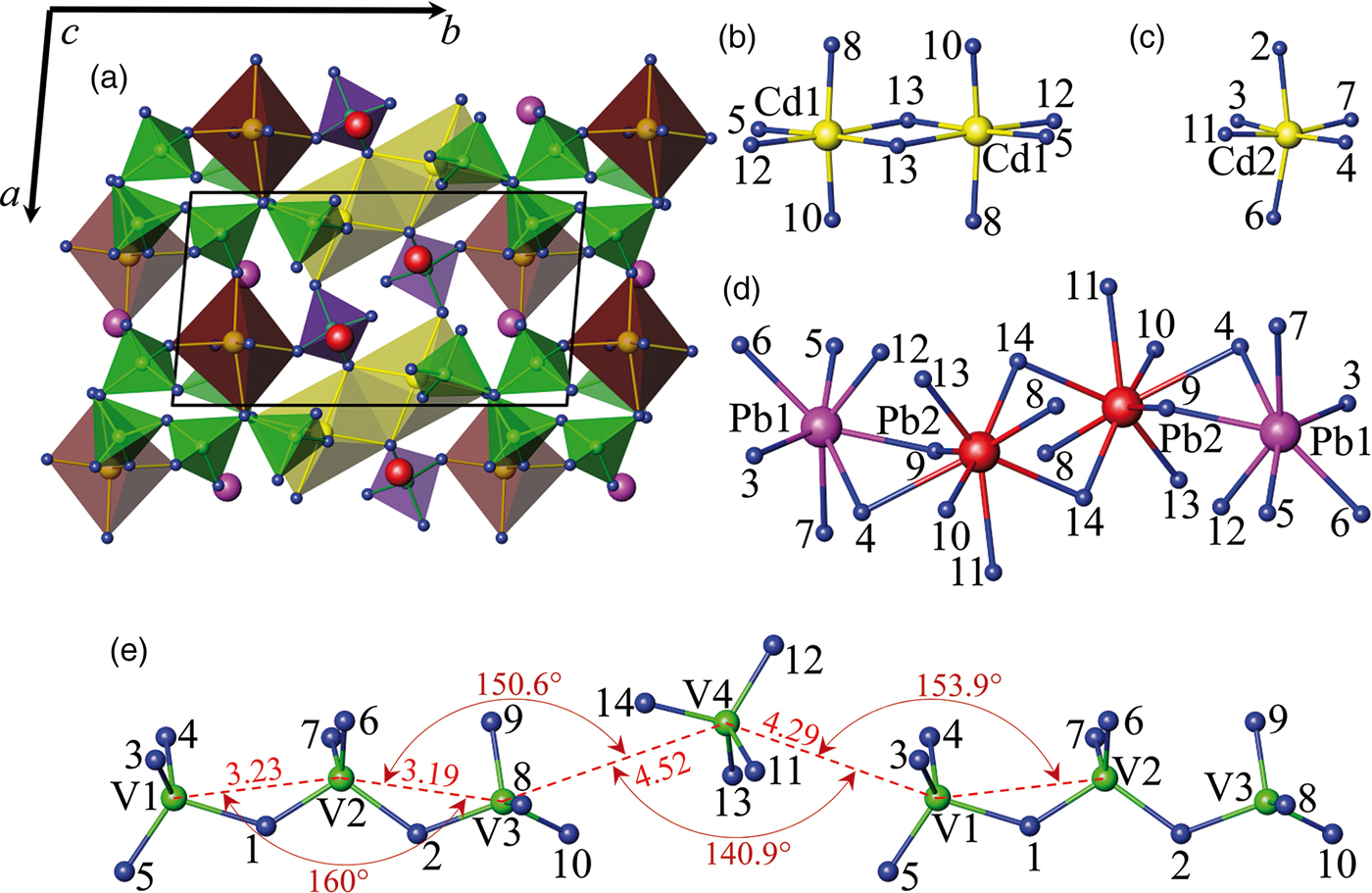
Figure 3. (Color online) Schematic drawing: (a) polyhedral view of the Pb2Cd2(V3O10)(VO4) structure, (b) and (c) stick and ball representation of the edge-sharing and isolated Cd(1)O6 and Cd(2)O6 octahedra, respectively, (d) isolated unit built of four edge-sharing Pb(1)O7 and Pb(2)O8 polyhedra, (e) broken chain consisting of alternating single orthovanadate (VO4)3− and linear trivanadate (V3O10)5− group. Green and purple tetrahedra indicate tri- and orthovanadates, yellow and brown colour indicate edge-sharing and single cadmium–oxygen octahedra. Blue, purple, red, yellow, and green spheres indicate oxygen, Pb(1), Pb(2), cadmium, and vanadium sites, respectively.
The authors also investigated the possibility of formation of Pb1.5Cd0.5(V3O10)(VO4) and Pb0.5Cd1.5(V3O10)(VO4) compounds. It was found that both compositions contain two phases: Pb2Cd2(V3O10)(VO4) and Pb2V2O7 or Pb2Cd2(V3O10)(VO4) and Cd2V2O7.
In group II of Table I, there are six compounds that crystallize in the orthorhombic system with space group Pnma. However, according to the V2O74− anion packing, four compounds isotypic with SrCuV2O7 (Vogt and Muller-Buschbaum, Reference Vogt and Muller-Buschbaum1991b) can be identified, with V2O74− dimers elongated along the a-axis [Figure 4(a)], while in the other two double pyrovanadates, BaCaV2O7 and α-BaZnV2O7 (high-temperature modification), V2O74− dimers are elongated along the c-axis [Figure 4(b)] (Murashova et al., Reference Murashova, Velikodnyi and Trunov1989).
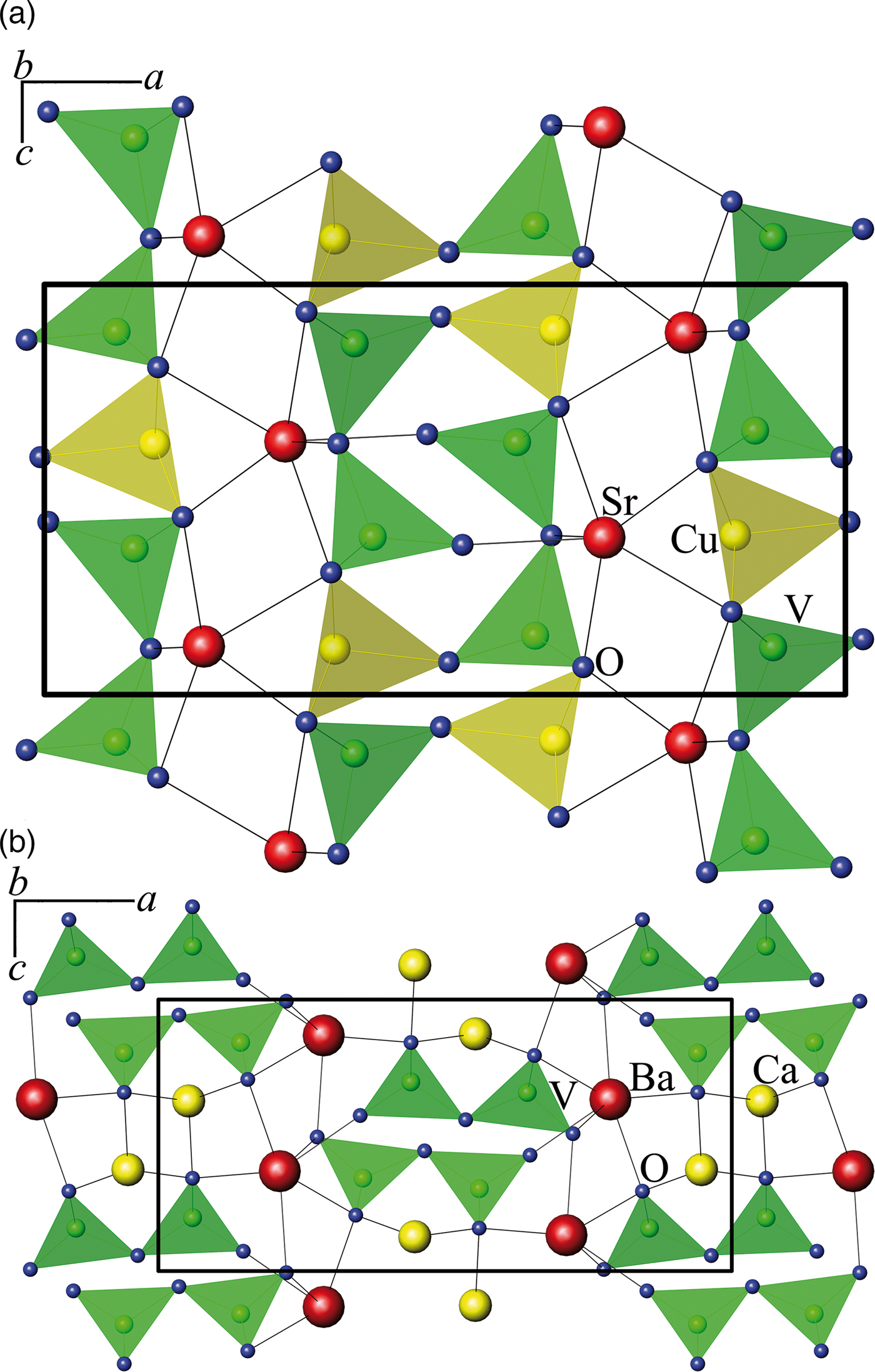
Figure 4. (Color online) The projection along the b-axis of the structure of (а) SrCuV2O7 (Velikodnyi and Murashova, Reference Velikodnyi and Murashova1992) and (b) BaCaV2O7 (Murashova et al., Reference Murashova, Velikodnyi and Trunov1989).
Three double vanadates in group III form compounds with monoclinic symmetry and space group P21/n. Figure 5 displays a typical structure of β-BaZnV2O7 (Murashova et al., Reference Murashova, Velikodnyi and Trunov1989). In group III, the vanadate SrZnV2O7 (Velikodnyi et al., Reference Velikodnyi, Murashova and Trunov1989) is also included, assuming the possibility of a different setting when indicating the parameters, namely: a ′ = c/2 = 5.9885 Å, b ′ = 2a = 14.85 Å, c ′ = b = 6.697 Å.
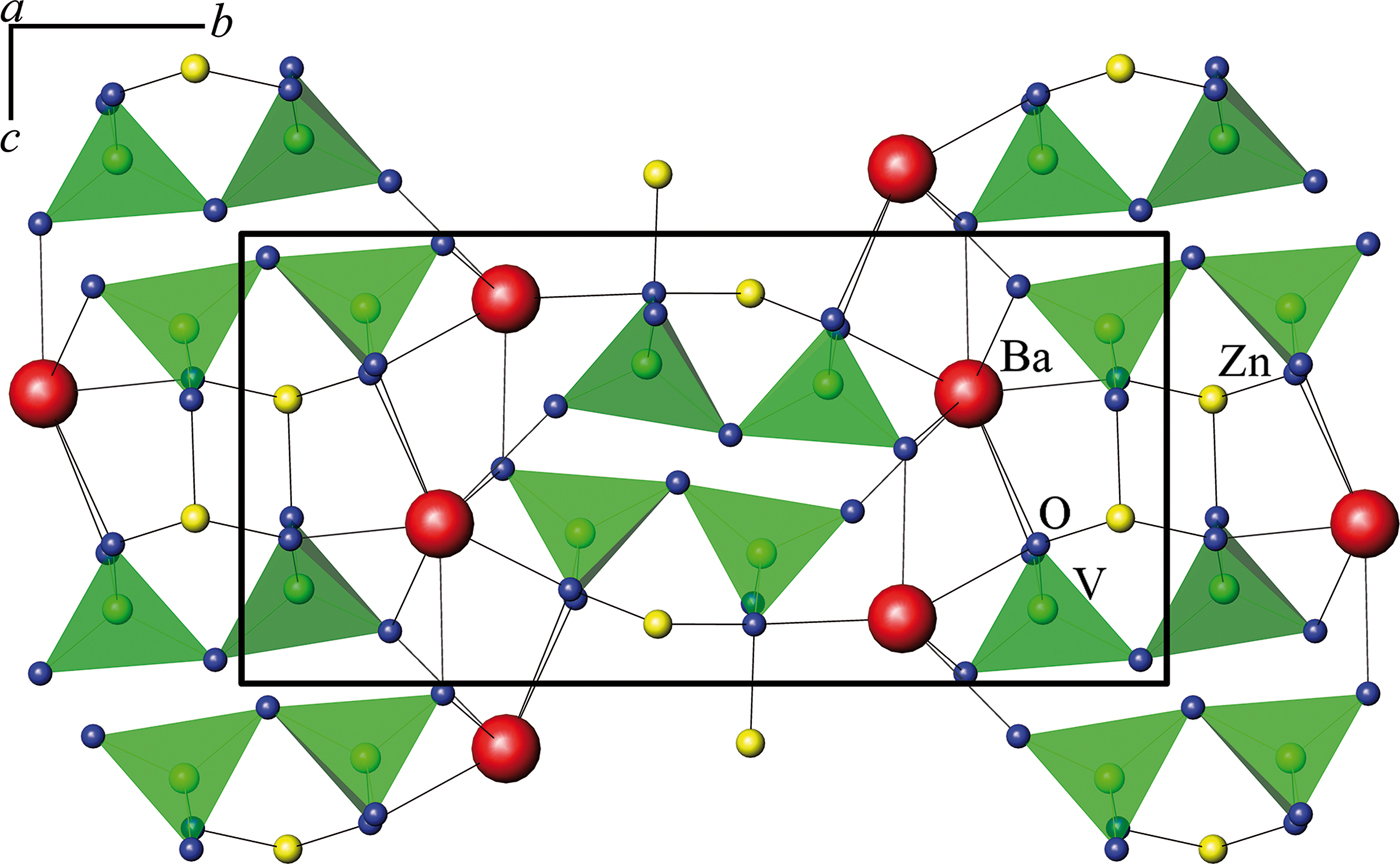
Figure 5. (Color online) Crystal structure of β-BaZnV2O7 (Murashova et al., Reference Murashova, Velikodnyi and Trunov1989).
The double vanadate CaCuV2O7 (Figure 6) stands apart, since its V2O74− chains are directed along the ac vector (Vogt and Muller-Buschbaum, Reference Vogt and Muller-Buschbaum1991a; Reference Vogt and Muller-Buschbaumb)
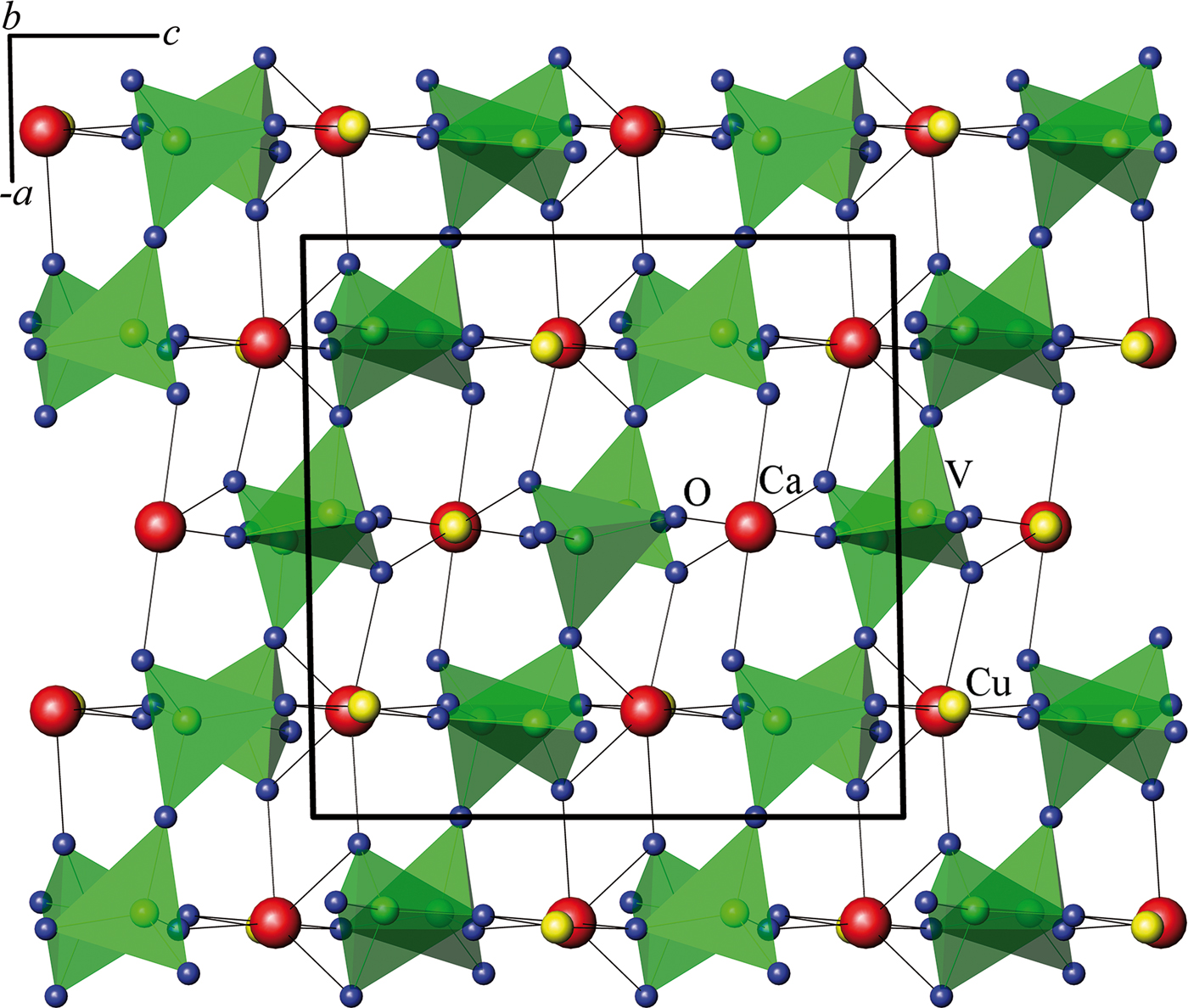
Figure 6. (Color online) Orientation of groups V2O74− in CaCuV2O7 (Vogt and Muller-Buschbaum, Reference Vogt and Muller-Buschbaum1991b).
The compound CaCdV2O7 (Krasnenko et al., Reference Krasnenko, Slobodin, Zabara, Fotiev and Kiseleva1990) is not shown in Table I because, in the authors” opinion, it comprises a part of the solid solution based on cadmium pyrovanadates. The limiting composition of the homogeneity region is restricted by the sample Ca1.22Cd0.78V2O7 (Murashova et al., Reference Murashova, Velikodnyi and Zhuravlev1994).
In the binary systems of vanadates of bivalent metals Sr2V2O7–MI 2V2O7 (MI = Mn, Ca, Cd) (Velikodnyi and Murashova, Reference Velikodnyi and Murashova1992; Zhuravlev et al., Reference Zhuravlev, Velikodnyi and Surat1993; Zhuravlev et al., Reference Zhuravlev, Surat and Velikodnyi1994), compounds with cation ratio Sr:MI = 3:1, crystallizing in tetragonal symmetry with space group P41212 in Sr3Mn(V2O7)2 and Sr3Cd (V2O7)2, and also Sr3Ca(V2O7)2 with space group P41 (Figure 7) are identified.
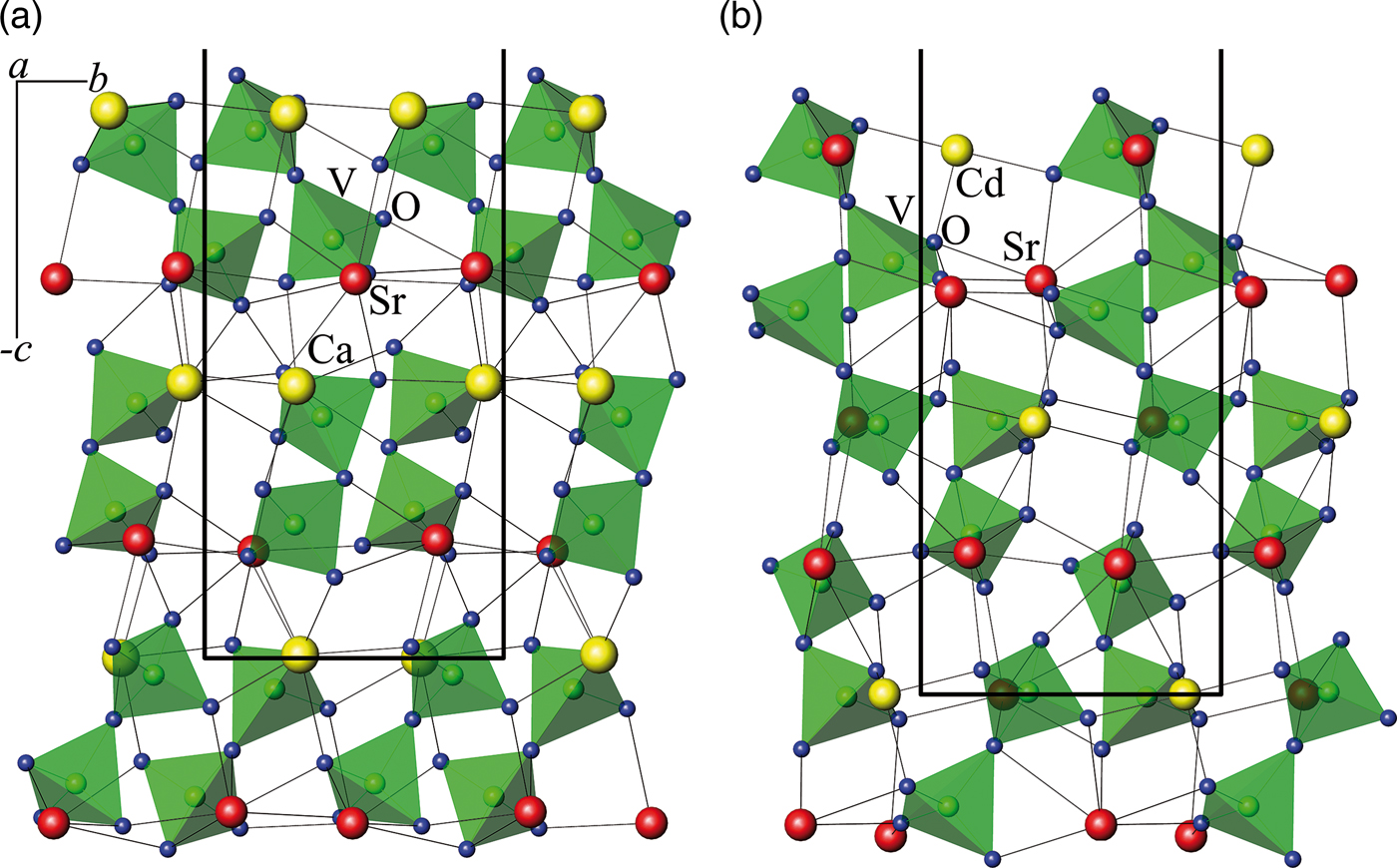
Figure 7. (Color online) The projection of fragments of the structure of (a) Sr3Ca(V2O7)2 and (b) Sr3Cd (V2O7)2 along the a-axis.
Proceeding from the arrangement (configuration) of oxygen polyhedrons in vanadium crystal lattices of double vanadates MMI 2V2O7, M 3MI (V2O7)2, MMI 2 (V4O14), M 3MI(V3O10) (VO4), they can be considered as derivatives of pyrovanadate M2V2O7 of the type of potassium dichromate (M 2V2O7, M = Ba, Sr, Co, Ni, Pb) or calcium pyrovanadate Ca2V2O7. The variety of crystal structures inside these two families is because of the arrangement of two types of bivalent cations and rotation of vanadium–oxygen polyhedrons. For pyrovanadates M 2V2O7 having a thortveitite structure, the formation of broad primary solid solutions in their binary systems is more typical. Both in the pyrovanadates M 2V2O7 and in the double vanadates of divalent metals, structural transformations associated with the evolution of “soft” metal–oxygen polyhedra can take place when the temperature or the composition are changed (Krasnenko et al., Reference Krasnenko, Rotermel and Samigullina2017; Rotermel and Krasnenko Reference Rotermel and Krasnenko2017).
IV. CONCLUSIONS
Two new double vanadates Ca2Zn2(V4O14) (I) and Pb2Cd2(V3O10)(VO4) (II) have been synthesized, and their crystal structures have been described. The double vanadates of divalent metals known to date can be classified in six different groups according to the proximity of their crystal structures to the structure of vanadium–oxygen anions. The crystal lattices of double vanadates of divalent metals can be regarded as being derived from two types of pyrovanadates M2V2O7 with the structure of potassium dichromate or calcium pyrovanadate.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715618000441.
ACKNOWLEDGEMENTS
The studies are carried out in accordance with the plans of the Institute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences (НИОКТР АААА-А16-116122810216-3, А16-116122810218-7).















