Published online by Cambridge University Press: 16 April 2004
Here we describe extracellular matrix alterations in footpad lesions and draining lymph nodes caused by Leishmania (L.) amazonensis in mouse strains with distinct susceptibilities to this parasite: BALB/c (susceptible), C57BL/6 (intermediate), and DBA/2 (resistant). Changes in ECM were observed mainly in BALB/c mice that, in general, presented tissue damage associated with high parasite burden. Under polarized light, Sirius Red revealed type I collagen that was predominant in the primary lesion in all strains studied at the early phase of infection, but gradually decreased and was replaced by abundant type III collagen fibres in chronic phase lesions. The presence of type III collagen seemed to provide support to inflammatory cells, mainly vacuolated and parasitized macrophages. Laminin expression was not altered during infection by L. (L.) amazonensis in any of the mouse strains studied. Furthermore, the decreased fibronectin expression, in all strains, in areas where amastigotes have been found, indicated that this decline was also not related to the genetic background.
Leishmania is the causative agent of a broad spectrum of human diseases ranging from single self-healing cutaneous lesions to anergic diffuse cutaneous lesions to fatal visceral leishmaniasis. Clinical manifestations of leishmaniasis depend on a fine interaction between the parasite and the host genetic backgrounds (Handman, 2001). Several parasites produce proteases that degrade matrix proteins, facilitating the breaching of the dermal barrier to spread the infection. When Leishmania promastigotes are inoculated into the host skin, a loosening of the dermis connective tissue matrix occurs enabling then the establishment of infection (Lira, Rosales-Encina & Arguelos, 1997).
The extracellular matrix (ECM) consists of fibrous proteins (collagen and elastin), proteoglycans, glycosaminoglycans, and structural proteins. Based on their chemical nature, the fibrous components may be divided into two distinct systems: elastic and collagen (Montes, 1996). ECM plays an essential role in cell anchorage, migration, division, differentiation, and also in cellular death. Furthermore, ECM takes part in tissue fluid dynamics and provides mechanical support for both rigid and elastic tissues (Rodgers & Irving-Rodgers, 2002). Studies have shown that certain enzymes such as metalloproteinases can modify the functions of extracellular matrices not only by degrading matrix proteins, but also by affecting growth factors, cytokines and cell adhesion molecules (Yamada & Kemler, 2002). Laminin, a large mosaic protein of the extracellular matrix, is important in the development and maintenance of cellular organization (Becker, Hunter & Engel, 1990). Recent work suggests that migration of Leishmania through the ECM precedes macrophage attachment, and that cell entry is mediated by the interaction of laminin with parasite laminin receptors (Bandyopadhyay et al. 2001). Fibronectin is also a key component in several biological processes (Wyller, 1987). It has several functional domains that enable interactions with cells, heparin, fibrin, collagen, immunoglobulins, and also with parasites (Wyller et al. 1985). It increases the phagocytic capacity of macrophages and neutrophils, since it enhances chemotaxis, phagocyte adherence, and phagocytosis (Proctor, 1987; Vannier-Santos et al. 1992). Studies in both Leishmania spp. and Trypanosoma cruzi have provided strong evidence that these protozoan parasites use host fibronectin to bridge their association with the host monocytes and macrophages (Wyller, 1987).
Based upon histopathological and immunological studies, a broad spectrum of host responses to L. (L.) amazonensis has been observed in man (Barral et al. 1991). Similar spectral aspects could be reproduced when mouse inbred strains were infected with the H21 MHOM/BR/76/MA-76 strain of L. (L.) amazonensis, as was previously published by our group (Calabrese & Gonçalves da Costa, 1992; Cupolilo et al. 2003). Our primary interest in the present study has been to investigate whether extracellular matrix changes due to L. (L.) amazonensis infection are related to the genetic background of the host. Thus, in this study, we have examined extracellular matrix changes in three mouse strains (BALB/c, C57BL/6, and DBA/2) that display distinct susceptibilities to the cutaneous infection by L. (L.) amazonensis amastigotes. Whereas BALB/c mice are highly susceptible and DBA/2 resistant, C57BL/6 show intermediate susceptibility to L. (L.) amazonensis infection.
Female BALB/c, C57BL/6, and DBA/2 mice with ages ranging from 4 to 6 weeks were obtained from the animal facilities of Instituto Oswaldo Cruz. We used 6 animals per experimental group.
L. (L.) amazonensis, H21 MHOM/BR/76/MA-76 strain was isolated from a patient with diffuse cutaneous leishmaniasis (DCL) and maintained by serial passages in mice in our laboratory: amastigotes were removed from footpad lesions, purified by filtering and inoculated subcutaneously (106 parasites/0·05 ml) in normal mice footpads. Evaluation of the percentage viability of the parasites was made using erythrosine B stain, as described elsewhere (Hodgkinson, Herman & Semprevivo, 1980).
Normal mice were subcutaneously infected in the left footpad by injecting 104 L. (L.) amazonensis amastigotes. Three animals of each group were killed in accordance with guidelines for experimental procedures of Fundação Oswaldo Cruz (Process no. P0062-00), at 20, 60 and 90 days post-infection. Normal uninfected mice were used as controls. The results presented here are representative of 3 independent experiments that gave essentially the same results.
Skin fragments from the inoculation site and draining lymph node were collected during necropsy and fixed in 10% neutral-buffered formalin, routinely processed for paraffin embedding and stained with one of the following reagents: Haematoxylin-Eosin (H&E), Sirius Red (Direct Red 80, Aldrich, Milwaukee, WI 53233, USA), Gordon and Sweet's method for reticulin fibres, and Weigert's resorcin-fucsin for elastic tissue. Cell types were scored based on their characteristic morphology.
For confocal laser-scanning microscopy, fragments collected in parallel were embedded in tissue-freezing medium (OCT Compound-embedding medium for frozen specimens, Milles Inc., USA) and immediately frozen. Five μm thick cryosections were obtained and fixed in cold acetone for 15 min. For double labelling procedures, sections were blocked in PBS containing 0·2% gelatin, 0·1% NaN3, and 0·1% saponin (PGN-saponin), incubated with human polyclonal anti-Leishmania serum diluted 1[ratio ]600, washed 3 times with PBS and then incubated with secondary antibodies conjugated with Cy3 (Sigma). After this, the material was washed again. The slides were subsequently incubated with polyclonal antibodies (PharMingen, San Diego, CA, USA) to the ECM proteins fibronectin (FN) and laminin (LN) diluted 1[ratio ]200. Secondary goat anti-rabbit FITC-conjugated antibodies were used. All incubations were performed for 40 min with antibodies diluted in PGN-saponin. The nuclei were stained with 4,6-di-amino-2-phenylindole fluorescent DNA-binding probe (DAPI, Molecular Probes, Eugene, OR). After the washes in PBS, the slides were mounted in glycerol containing 0·1% p-phenylenediamine (Sigma). The slides were examined on a BioRad 1024 (UV) confocal scanning system coupled to a Zeiss Axiovert 100 microscope, using a 40× 1·2 N.A. PlanApochromatic water immersion objective.
At the early phase of infection there was a predominance of type I collagen (thick red fibres) in the primary lesion of all mouse strains studied, which was evidenced by Sirius Red staining under polarized light (data not shown). Also, in uninfected control animals, type I collagen was highly abundant (Fig. 1A). The relative abundance of type I collagen gradually decreased and type III collagen (thin and greenish fibres) was a major component at 90 days post-infection (Fig. 1B). In BALB/c mice, however, these changes were more evident and lesions contained an intense inflammatory infiltrate composed mainly of heavy parasitized macrophages, culminating in substantial loss of the normal dermal architecture (Fig. 1C). The presence of type III collagen deposited around the parasitized macrophages (Fig. 1D) was confirmed by Gordon and Sweet's method for reticulin impregnation. No alterations in the lymph nodes of all mice strains were observed, except where parasites have been detected (not shown).

Fig. 1. Histological analysis of the footpad (primary lesion) of BALB/c mouse infected with 104 Leishmania (L). amazonensis amastigotes, H21 MHOM/BR/76/MA-76. (A) Control group showing predominance of type I collagen (thick red fibre) – Sirius Red – under polarized light (arrows). (B) Infected mouse, substantial amounts of type III collagen are visible (thin greenish fibre) – Sirius Red – under polarized light (arrows). (C) Severe tissue damage is observed close to large numbers of vacuolated macrophages filled with amastigotes (arrows); (Sirius Red). (D) Type III collagen deposits around parasitized macrophages (arrows) providing support for these cells (Gordon and Sweet's method for reticulin).
Confocal analysis of skin and lymph node material revealed that, regardless of the infection stage or mouse strain, the expression of laminin did not show substantial alterations, even in those animals that presented severe lesions (Fig. 2B, D, F and H). On the other hand, the expression of fibronectin was variable in susceptible and resistant mice. It was discrete in DBA/2 and moderate in BALB/c and C57BL/6 mice, particularly in areas where amastigotes were found in large numbers (Fig. 3B, D, F and H).
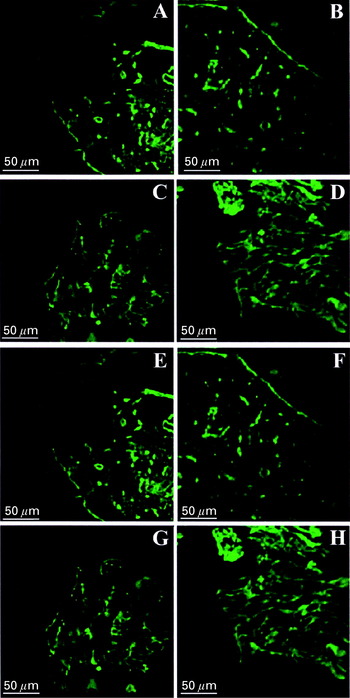
Fig. 2. Confocal analysis of lymph node and primary footpad lesion of BALB/c (A, B, C, D) and DBA/2 (E, F, G, H) mice infected with 104 Leishmania (L.) amazonensis amastigotes (H21 MHOM/BR/76/MA-76). (A) Laminin distributed mainly around blood vessels and in the basal membrane (green-FITC) of lymph node. (B) Unaltered laminin distribution in the draining lymph node of infected mice (green-FITC). (C) Skin of control group footpad shows normal LN distribution (green-FITC). (D) Normal laminin distribution in primary footpad lesion (green-FITC). (E) Laminin distribution mainly around blood vessels and in the basal membrane (green-FITC) of the draining lymph node from uninfected mouse. (E) Unaltered laminin distribution in the lymph node of infected mice (green-FITC). (G) Laminin distribution in skin of control group footpads (green-FITC). (H) Unaltered laminin distribution in primary footpad lesion (green-FITC).

Fig. 3. Confocal analysis of BALB/c (A, B, C, D) and C57BL/6 (E, F, G, H) mice of lymph node and primary lesion infected with 104 Leishmania (L.) amazonensis amastigotes (H21 MHOM/BR/76/MA-76). (A) Well-preserved fibronectin (green-FITC) in samples of lymph nodes of control mice. (B) Unaltered fibronectin in the lymph node of infected mice (green-FITC). (C) Normal fibronectin distribution in footpad skin of the control group (green-FITC). (D) Unaltered fibronectin distribution in the skin enclosing the primary lesion (green-FITC). (E) Control group lymph nodes with well-preserved fibronectin distribution (green-FITC). (F) Decreased fibronectin expression (green-FITC) in the draining lymph node of infected mice with visible parasites (red-Cy3). (G) Normal fibronectin distribution in the footpad skin of control mice (green-FITC). (H) Large number of parasites (red-Cy3) and moderate decrease of fibronectin (green-FITC) in footpad.
No changes could be found in control animals throughout the experiments (Figs 1A, 2A, C, G, E and 3A, C, G, E).
Three different mouse strains with distinct resistance profiles to L. (L.) amazonensis infection were used in this study. BALB/c and C57BL/6 mice were highly susceptible, whereas DBA/2 was the most resistant mouse, being able to control the infection, avoiding the spread of parasites to internal organs. Some cases of self-cure were also reported in DBA/2 mice at 3 months post-infection (Abreu-Silva, 2003).
Our results showed that in the strains examined here, changes in ECM organization pattern were more evident in areas of high parasite load. This occurred mainly in BALB/c mice that developed severe tissue destruction presenting areas rich in type III collagen associated with parasitized macrophages. The expression of type I collagen seemed to decrease concomitant with the time-course of infection in these animals, whereas type III collagen fibres became more abundant. Type III collagen fibres were found chiefly around the parasitized histiocytes. It was reported that L. (L.) mexicana promastigotes can bind to type I collagen, indicating that this interaction could be important in the pathogenesis of the infection at the onset of specific parasite tropism for host skin (Lira et al. 1997). However, our data suggest that in chronic murine experimental infection, type III collagen provides support to inflammatory cells, predominantly vacuolated and parasitized histiocytes. The occurrence of collagen type III was also demonstrated in hepatic granuloma of mice infected with Leishmania donovani (Leite & Croft, 1996; Ghosh et al. 1996). By contrast, in hepatic granuloma induced by Schistosoma mansoni the reverse occurs and type III collagen is replaced by type I fibres that give support to preserve the structural integrity of the granuloma (Al Adnani, 1985).
Neither the expression nor the overall distribution of laminin in the footpad lesions, as well as lymph nodes, was affected in experimental murine L. (L.) amazonensis infection in the three different mouse strains. A slight decrease in fibronectin expression was observed in areas with high amastigote loads in lesions of DBA/2 mice, a strain that usually presents lower parasite burdens when compared to BALB/c or C57BL/6 mice. These results could suggest that a reduced fibronectin expression might be more dependent on the local parasite burden, in spite of the host strain. It is tempting to speculate that amastigotes could be directly involved in the degradation of extracellular matrix components as recent studies have suggested (McGwire, Chang & Engman, 2003). Severe tissue damage observed in Entamoeba histolytica infection also appears to be associated with its ability to degrade extracellular matrix (particularly glycoproteins and collagen components) through released cysteine proteases (Rhoads & Fetterer, 1996).
The presence of laminin bound to the parasite surface seems to enhance macrophage infectivity, while both laminin and anti-LBP (Laminin Binding Protein – GP63) antibodies have decreased the infectivity in the BALB/c mouse model, apparently by blocking laminin-binding protein sites on the host cell surface. These findings represent evidence in favour of the notion that Leishmania migration through the ECM network, prior to macrophage attachment and entry, is mediated in part by the interaction of cell–parasite (Ghosh et al. 1999; Bandyopadhyay et al. 2001, 2002).
We would like to thank Arlindo Caldeira da Rocha (in memorium) for histological preparations. This work was supported by grants from CNPq and Instituto Oswaldo Cruz/Ministry of Health, Brazil.

Fig. 1. Histological analysis of the footpad (primary lesion) of BALB/c mouse infected with 104 Leishmania (L). amazonensis amastigotes, H21 MHOM/BR/76/MA-76. (A) Control group showing predominance of type I collagen (thick red fibre) – Sirius Red – under polarized light (arrows). (B) Infected mouse, substantial amounts of type III collagen are visible (thin greenish fibre) – Sirius Red – under polarized light (arrows). (C) Severe tissue damage is observed close to large numbers of vacuolated macrophages filled with amastigotes (arrows); (Sirius Red). (D) Type III collagen deposits around parasitized macrophages (arrows) providing support for these cells (Gordon and Sweet's method for reticulin).

Fig. 2. Confocal analysis of lymph node and primary footpad lesion of BALB/c (A, B, C, D) and DBA/2 (E, F, G, H) mice infected with 104 Leishmania (L.) amazonensis amastigotes (H21 MHOM/BR/76/MA-76). (A) Laminin distributed mainly around blood vessels and in the basal membrane (green-FITC) of lymph node. (B) Unaltered laminin distribution in the draining lymph node of infected mice (green-FITC). (C) Skin of control group footpad shows normal LN distribution (green-FITC). (D) Normal laminin distribution in primary footpad lesion (green-FITC). (E) Laminin distribution mainly around blood vessels and in the basal membrane (green-FITC) of the draining lymph node from uninfected mouse. (E) Unaltered laminin distribution in the lymph node of infected mice (green-FITC). (G) Laminin distribution in skin of control group footpads (green-FITC). (H) Unaltered laminin distribution in primary footpad lesion (green-FITC).

Fig. 3. Confocal analysis of BALB/c (A, B, C, D) and C57BL/6 (E, F, G, H) mice of lymph node and primary lesion infected with 104 Leishmania (L.) amazonensis amastigotes (H21 MHOM/BR/76/MA-76). (A) Well-preserved fibronectin (green-FITC) in samples of lymph nodes of control mice. (B) Unaltered fibronectin in the lymph node of infected mice (green-FITC). (C) Normal fibronectin distribution in footpad skin of the control group (green-FITC). (D) Unaltered fibronectin distribution in the skin enclosing the primary lesion (green-FITC). (E) Control group lymph nodes with well-preserved fibronectin distribution (green-FITC). (F) Decreased fibronectin expression (green-FITC) in the draining lymph node of infected mice with visible parasites (red-Cy3). (G) Normal fibronectin distribution in the footpad skin of control mice (green-FITC). (H) Large number of parasites (red-Cy3) and moderate decrease of fibronectin (green-FITC) in footpad.