Introduction
Sarcocystis is an intracellular protozoan parasite that was first reported in 1843 (Miescher, Reference Miescher1843). To date, more than 200 species have been recognized in this genus that infects a wide array of domestic and wild animals, resulting in considerable health and production losses in farmed animals worldwide (Tenter, Reference Tenter1995; Dubey, Reference Dubey2015). Sarcocystis spp. complete their life cycles in two hosts, i.e. a definitive (predator) and an intermediate (prey) host and they have higher host-specificiy for their intermediate hosts than definitive hosts (Fayer, Reference Fayer2004). Sarcocystis hominis, Sarcocystis heydorni and Sarcocystis suihominis are known to infect humans where cattle (S. hominis and S. heydorni) and pigs (Sarcocystis suihominis) serve as their intermediate hosts (Poulsen and Stensvold, Reference Poulsen and Stensvold2014; Dubey, Reference Dubey2015). Sarcocystis nesbitti has also been reported to infect humans (as intermediate host) following the ingestion of food/water contaminated with reptile excreta as snakes appear to be the potential definitive hosts (AbuBakar et al. Reference AbuBakar, Teoh, Sam, Chang, Johari, Hooi, Lakhbeer-Singh, Italiano, Omar and Wong2013; Lau et al. Reference Lau, Chang, Tan, Fong, Mahmud and Wong2014). The development of asexual stages of Sarcocystis spp. occur in intermediate hosts, including mammals (e.g., herbivorous animals such as camels, sheep and cattle; primates including humans), birds and poikilothermic animals where sarcocysts appear mainly in striated muscles of the diaphragm, heart, oesophagus and skeletal muscles (Hidron et al. Reference Hidron, Vogenthaler, Santos-Preciado, Rodriguez-Morales, Franco-Paredes and Rassi2010; Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a) and less frequently in the central nervous system and smooth muscle of the intestine (Fayer, Reference Fayer2004; Miller et al. Reference Miller, Barr, Nordhausen, James, Magargal, Murray, Conrad, Toy-Choutka, Jessup and Grigg2009; Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a). Definitive hosts (e.g. canids, felids, marsupials and primates) acquire the infection by ingesting intermediate host tissue infected with mature sarcocysts, then following a phase of sexual reproduction in the definitive host, oocysts/sporocysts are excreted into the environment via feces which can then be ingested by appropriate intermediate hosts.
The dromedary or one-humped camel (Camelus dromedarius) is widely distributed in the hot, arid areas of the Middle East, Africa, South Asia and Central Australia and Canary Islands of Spain while the Bactrian or two-humped camel (Camelus bactrianus) is found in China, Canary Islands of Spain, India, Kazakhstan, Mongolia and Russia (Kalmykistan) and (Faye, Reference Faye2014; Kadim et al. Reference Kadim, Mahgoub and Mbaga2014). The total camel population is estimated over 28 million worldwide (FAOSTAT, 2016). Camels are adapted to adverse climatic and harsh environmental conditions such as high temperatures, intense solar radiation, water scarcity and poorly digestible vegetation which can adversely affect the performance of other meat-producing animals (Kadim et al. Reference Kadim, Mahgoub and Purchas2008; Faye, Reference Faye2014), thereby making camels suitable for commercial meat and milk production in a wide range of agroclimatic zones. The annual worldwide camel meat and milk productions have been recorded as 532 198 and 2 696 337 tonnes, respectively (FAOSTAT, 2016). Camel meat is preferred in some countries due to its lower fat and cholesterol contents compared with beef, lamb and ostrich meat and other perceived health benefits (Kadim et al. Reference Kadim, Mahgoub and Purchas2008, Reference Kadim, Mahgoub and Mbaga2014). Thus, camel meat potentially holds a high value for local as well as international meat markets due to the camel’s production ability in harsh environments and the increasing demand for camel meat. However, the presence of sarcocysts in camel meat could potentially downgrade its quality for human consumption as camels serve as an intermediate host for at least two Sarcocystis spp. and the infection is common in the dromedary camel throughout its distribution globally (Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011; Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013; Omer et al. Reference Omer, Alzuraiq and Mohammed2017). Infected camels generally exhibit subclinical infections, although Sarcocystis spp. can induce significant pathology or even mortality in these animals (Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008; Omer et al. Reference Omer, Alzuraiq and Mohammed2017). However, little is known about various aspects of sarcocystosis in camels, including its molecular biology, pathology, molecular epidemiology, life cycle, economic impact and public health significance.
This paper provides an update on the taxonomy, epidemiology, pathogenesis and diagnosis of Sarcocystis spp. that infect camels. Furthermore, it highlights areas for future research that could enhance our knowledge on sarcocystosis in camels. The word camel(s) refers to the dromedary or one-humped camel(s) throughout this paper, unless otherwise mentioned as the Bactrian camel.
Taxonomy of Sarcocystis spp. in camels
There has been considerable confusion regarding the nomenclature of Sarcocystis spp. in camels, and at least six different names have been used for Sarcocystis spp. in camels, including Sarcocystis cameli, Sarcocystis ippeni, Sarcocystis camelicanis, Sarcocystis camelocanis, Sarcocystis miescheri and Sarcocystis meischeri.
Mason (Reference Mason1910) first time described sarcocysts in camels from Egypt as white lines (12 × 1 mm) in striated muscles with either thick or thin walls. Although the wall thickness of these sarcocysts was different, Mason (Reference Mason1910) thought that these cysts belonged to the one species of Sarcocystis, S. cameli. Subsequently, based on their morphological features, thick- and thin-walled sarcocysts in camels were renamed as S. cameli (Dubey et al. Reference Dubey, Speer and Fayer1989) and S. ippeni (Odening, Reference Odening1997), respectively. Other studies also confirmed the presence of both types of sarcocysts in camels from Saudi Arabia (Fatani et al. Reference Fatani, Elsebaie and Hilali1996) and Sudan (Ishag et al. Reference Ishag, Majid and Magzoub2006). Ishag et al. (Reference Ishag, Majid and Magzoub2006) observed two different sizes of Sarcocystis sporocysts excreted in feces of dogs experimentally fed with camel meat. The smaller (13.2–13.6 × 6.5–9.5 µm) sporocysts with a longer patent period (55–57 days) were considered as S. cameli whereas the larger (16.0 × 9.9–11.5 µm) sporocysts with a shorter patent period (37–45 days) were named as S. camelocanis (Ishag et al. Reference Ishag, Majid and Magzoub2006). Subsequently, the name S. camelicanis was introduced for sporocysts excreted in dog feces, fed with camel meat without providing any further detail (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009). Sarcocystis miescheri was reported as a new Sarcocystis sp. in camels, based on oocyst features excreted by dogs fed with sarcocyst-infected camel meat (Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011), and the same name was used and misspelled as S. meischeri in a subsequent study (Abd-Elmalek et al. Reference Abd-Elmalek, Abed and Mandour2015).
Overall these studies indicate that the taxonomy of Sarcocystis spp. of camels remains debatable, primarily due to the unavailability of type specimens and inadequate structural descriptions. Dubey et al. (Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b) reviewed the literature on Sarcocystis spp. infecting camels, and based on their morphological features, proposed that S. cameli and S. ippeni were the only two valid species that affect camels worldwide. However, in our review, we have used the original names of Sarcocystis spp. as used by various authors (Tables 1 and 2) for clarity.
Table 1. Morphology of Sarcocystis spp. in dromedary camels.
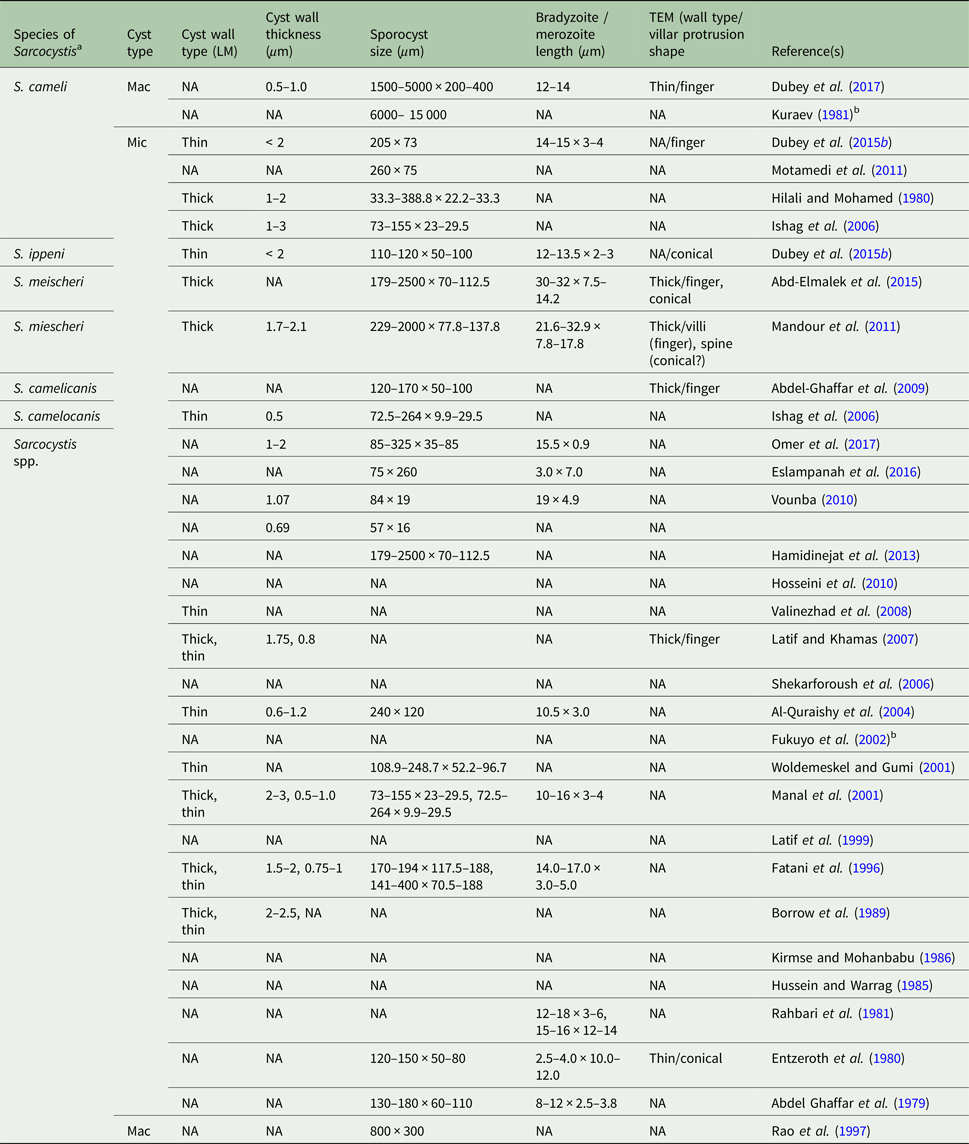
LM, light microscopy; Mac, macroscopic; Mic, microscopic; NA, not available/applicable; TEM, transmission electron microscopy.
a The name used by the authors.
b The study did not mention the type (dromedary/Bactrian) of camels used or used both types of camels and did not correlate the result with any of the two types.
Table 2. Studies aimed at assessing the prevalence of Sarcocystis spp. in dromedary camels.

a The name used by the authors.
b Decimal points were rounded off; Br, brain.
c The study did not mention the type (dromedary/Bactrian) of camels used or used both types of camels and did not correlate the result with any of the two types; D, diaphragm; E, oesophagus; H, heart; His, histology; Mc, muscle compress/squash/squeeze; NA, not available; Pd, pepsin digestion; Sm, skeletal muscle; T, tongue; Td, trypsin digestion
Structure of sarcocysts found in camels
Both macroscopic and microscopic sarcocysts have been reported in camels, though the latter is more common (Table 1).
Macroscopic sarcocysts appear as small (1.5–5.0 × 0.2–0.4 mm), white structures embedded in various muscle tissues of camels (Dubey et al. Reference Dubey, A'Aji, Mowery, Verma and Calero-Bernal2017). However, microscopic sarcocysts vary in size depending on the type of tissue in which they are present (Table 1). Furthermore, the shape of microscopic sarcocysts could also vary (round/spherical, elongated, ellipsoid, spindle-shaped, spiral etc.). For instance, cysts from cardiac muscle (154 × 62 µm) and skeletal muscle of the shoulder (108.9 × 52.2 µm) were smaller than those (248.7 × 96.7 µm) observed in oesophageal striated or smooth muscle (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001).
Generally, each sarcocyst is surrounded by a primary cyst wall, containing numerous villar protrusions into the cyst ground substance. The ground substance divides the cyst cavity into multiple compartments within which metrocytes and merozoites/bradyzoites are packed. Generally, metrocytes are located at the periphery while bradyzoites occupy the interior of each cyst (Abdel Ghaffar et al. Reference Abdel Ghaffar, Entzeroth, Chobotar and Scholtyseck1979; Al-Quraishy et al. Reference Al-Quraishy, Bashtar, Al-Rasheid and Abdel-Ghaffar2004; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011). The size and shape of bradyzoites vary depending on the location within camel tissues. For instance, elongated/banana-shaped (12–18 × 3–6 µm) and spherical (15–16 × 12–14 µm) bradyzoites have been observed in muscle of heart, diaphragm and oesophagus, respectively (Rahbari et al. Reference Rahbari, Bazargani and Rak1981; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011). The nucleus in banana-shaped bradyzoites is located either centrally or towards the blunt end (Fatani et al. Reference Fatani, Elsebaie and Hilali1996; Ishag et al. Reference Ishag, Majid and Magzoub2006; Latif and Khamas, Reference Latif and Khamas2007; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011).
The morphology of the primary cyst wall of microcysts is used as the most important criterion to classify Sarcocystis spp. (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009; Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a, Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbasb). Two distinct types of primary cyst walls have been reported in camels (one with finger-like and other with cone-like villar protrusions). Various authors have used different names to describe these two types of the cyst (see Table 1). For clarity, we have designated them as Variety A and Variety B cyst walls.
Variety A (S. cameli) cyst walls
The microscopic cysts of S. cameli have smooth walls, with variable length (e.g., 130–180) and width (60–110 µm). Abdel-Ghaffar et al. (Reference Abdel Ghaffar, Entzeroth, Chobotar and Scholtyseck1979) first described the ultrastructure of microscopic sarcocysts collected from camels in Egypt and found that the electron-dense cyst wall had finger-like villar protrusions (1.2–1.6 × 0.5 µm) which harboured knob-like elevations at their surfaces. Each villar protrusion was characterized by the presence of internal fibrillar elements (microtubules). Elongated bradyzoites measured 8–12 × 2.5–3.8 µm in size (Abdel Ghaffar et al. Reference Abdel Ghaffar, Entzeroth, Chobotar and Scholtyseck1979). Subsequent studies found that each villar protrusion contained about 16–18 knob-like structures (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009). Similar sarcocyst wall structure was reported in other studies from Iran (Motamedi et al. Reference Motamedi, Dalimi, Nouri and Aghaeipour2011), Jordan (Latif and Khamas, Reference Latif and Khamas2007) and Saudi Arabia (Al-Quraishy et al. Reference Al-Quraishy, Bashtar, Al-Rasheid and Abdel-Ghaffar2004), although they used different names for the parasite (see Table 1).
Dubey et al. (Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b) described similar sarcocyst wall structure in camels and named it as S. cameli. Sarcocysts of this species had thin walls, with no visible projections. The cyst wall had finger-like villar protrusions (3.0 × 0.5 µm), with rows of knob-like projections that appeared to be interconnected, and bradyzoites (14–15 × 3–4 µm) in the centre. Dubey et al. (Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a) named this cyst type as ‘type 9j’ and proposed dogs as a potential definitive host for S. cameli (Dubey et al. Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b).
The first ultrastructure of macroscopic sarcocysts from camels in Iraq was recently described by Dubey et al. (Reference Dubey, A'Aji, Mowery, Verma and Calero-Bernal2017). They observed a thin cyst wall with ‘type 9j’ or finger-like villar protrusions (2.5 µm) and named the parasite as S. cameli.
Variety B (S. ippeni) cyst walls
Entzeroth et al. (Reference Entzeroth, Ghaffar, Chobotar and Scholtyseck1980) described the second type of microscopic sarcocysts from camels in Egypt harbouring a cyst wall with cone-like villar protrusions (0.5–1.4 µm) and bradyzoites (2.5–4.0 × 10.0–12.0 µm) with cristae. Dubey et al. (Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b) observed similar cyst walls in camels from Egypt and named it as S. ippeni. Sarcocysts of S. ippeni are characterized by thick (2.3–3.0 µm) cyst walls containing conical villar protrusions, harbouring electron dense knobs. Each villar protrusion harbours smooth, criss-crossed microtubules without any granules/dense areas and bradyzoites measure 12.0–13.5 × 2.0–3.0 µm in size (Dubey et al. Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b).
Therefore, it appears S. cameli can form both macroscopic and microscopic sarcocysts in camels. Cysts possess a cyst wall characterized by finger-like villar protrusions with knob-like projections on each villar protrusion. Sarcocystis ippeni forms microscopic sarcocysts characterized by conical villar protrusions harbouring electron dense knobs.
Life cycle of Sarcocystis spp.
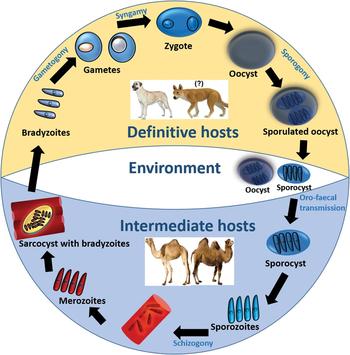
To establish the definitive host(s) of Sarcocystis spp. that infect camels, various authors have conducted experimental infection studies by feeding dogs and cats with Sarcocystis-infected camel meat (see Table 3). To date, only sporocysts have been found in feces of dogs, suggesting that dogs are the potential definitive host for Sarcocystis spp. that infect camels. Although the complete life cycle of Sarcocystis spp. of camels remains to be explored, a general life cycle of Sarcocystis spp. is illustrated in Fig. 1.
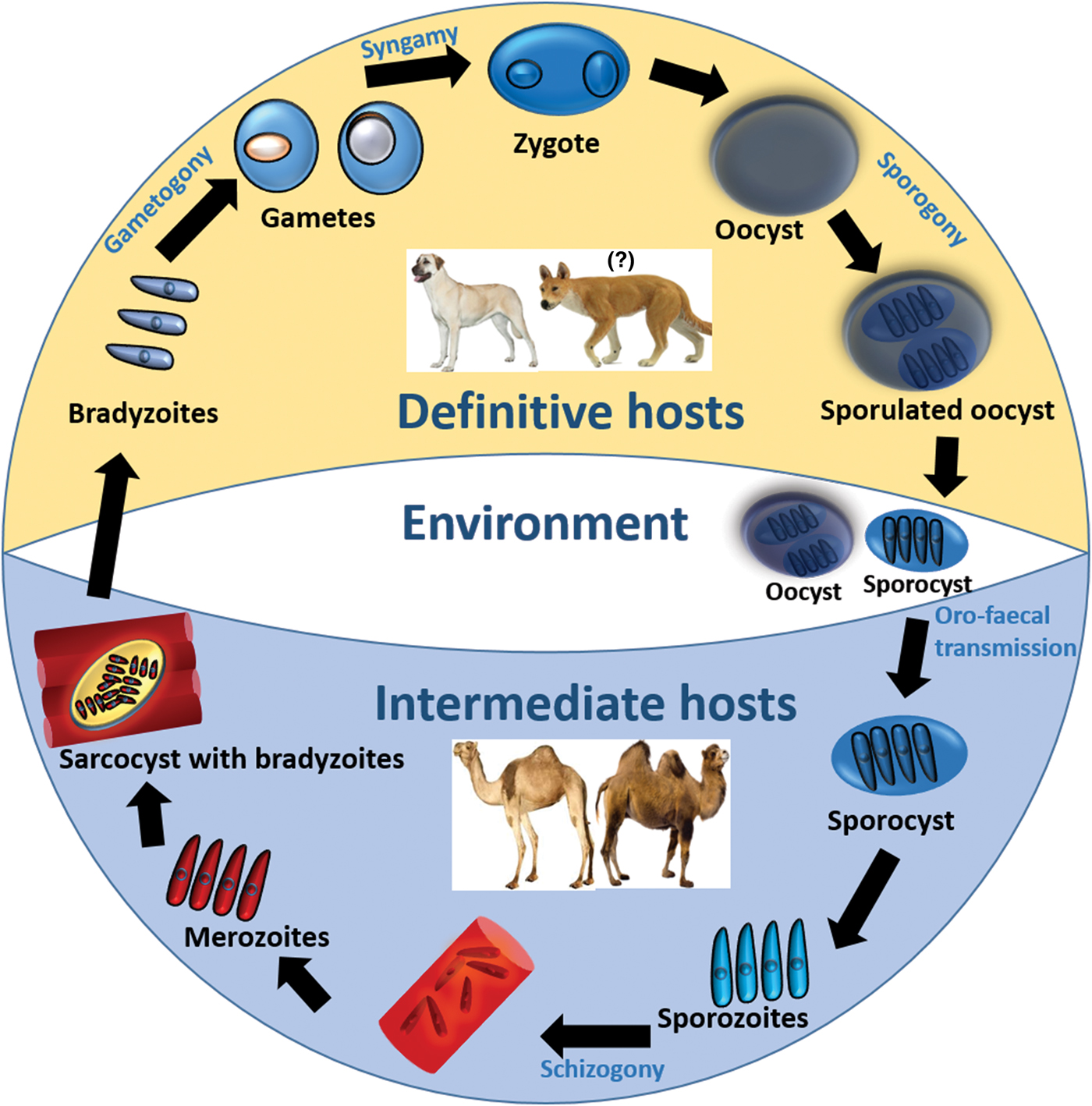
Fig. 1. Life cycle of Sarcocystis spp. in camels.
Table 3. Experimental studies on Sarcocystis spp. infecting dromedary camels.

NA, not available/applicable.
a The name used by the authors.
b Cats were also used as an experimental animal in some studies although they were found refractory to experimental infection.
A definitive host such as a dog becomes infected following the ingestion of sarcocysts contained in infected camel tissue. The prepatent period could vary from 7 to 15 days (see Table 3). Following cyst rupture in the posterior third of the small intestine, bradyzoites are released into the lumen, penetrate the lamina propria and undergo various sexual developmental stages (Hilali et al. Reference Hilali, Imam and Hassan1982). Micro- and macrogametes are differentiated by 2–3 days post-infection (dpi) and are spherical to ovoid, although microgametes are fewer in number and may contain up to 30 nuclei. A zygote is formed following the fusion of a macro- and microgamete and may be observed 5 dpi having a dense cell wall and relatively a large nucleus (Hilali et al. Reference Hilali, Imam and Hassan1982; Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009). Each zygote develops into an oocyst which contains two completely sporulated sporocysts and appears in the lamina propria by 8 dpi (Hilali et al. Reference Hilali, Imam and Hassan1982). Each sporocyst contains four sporozoites and a residual body (Hilali et al. Reference Hilali, Nassar and Elghaysh1992; Ishag, Reference Ishag2003; Ishag et al. Reference Ishag, Majid and Magzoub2006; Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009). The sizes of sporocysts observed in various experimental infection studies in dogs are presented in Table 3. Individual sporocysts or sporulated oocysts are released in the feces of the definitive host, which may then contaminate camel food and water and can readily infect them.
Camels may acquire a Sarcocystis infection by ingesting food/water or their own feces contaminated with sporulated oocysts or sporocysts shed by a definitive host such as a dog. Each sporocyst releases four sporozoites in the digestive tract of camels which penetrate the gut wall and enter endothelial cells of blood vessels (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009; Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a). Following a few generations of asexual reproduction (i.e. schizogony), a huge number of merozoites are produced which migrate in the blood and finally develop into sarcocysts in myocytes. Each sarcocyst contains millions of infective bradyzoites which are protected from host cell defense mechanisms by a parasitophorous vacuole, made from the host cell plasma membrane (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009).
Given that both S. cameli and S. ippeni can form microcysts in camels and the nomenclature of these species was unclear at the time when these studies were conducted, it is not possible to relate the type of sarcocyst or Sarcocystis sp. with the dog as their definitive host. Nevertheless, the current evidence suggests that the dog is the potential definitive host for at least one of the Sarcocystis spp. in camels.
Pathogenesis of sarcocystosis in camels
Despite the high prevalence (discussed in the following section) of Sarcocystis in camels, there is a paucity of information on the pathogenesis of sarcocystosis in these animals. Several factors, including the immune status of the host, the number of oocysts/sporocysts ingested, and Sarcocystis spp. involved, determine the number and distribution of sarcocysts in an intermediate host (Wernery et al. Reference Wernery, Kinne and Schuster2014; Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a). However, these factors have been mainly unexplored for Sarcocystis spp. in camels. Thick- (1–3 µm) and thin- (0.5 µm) walled sarcocysts have been observed in muscles of infected camels (Ishag et al. Reference Ishag, Majid and Magzoub2006), indicating that multiple Sarcocystis spp. or more than one parasitic forms could exist in the same camel host.
Microscopic sarcocysts, the most common form reported in camels, appear in different tissues of the host such as diaphragm, oesophagus and skeletal muscles (e.g. limb muscle, masseter muscle), heart, tongue, etc. (Table 2). Although the disease is usually asymptomatic or subclinical in camels (Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013; Omer et al. Reference Omer, Alzuraiq and Mohammed2017), significant pathology has been ascribed to microscopic sarcocystosis. For instance, following the ingestion of Sarcocystis sporocysts, camels can develop pyrexia, anorexia and restlessness and may exhibit anaemia, low packed cell volume and decreased albumin, globulin, haemoglobin and serum protein concentrations (Fatani et al. Reference Fatani, Elsebaie and Hilali1996). Haemorrhages may be observed in the omentum, mesenteric lymph nodes, urinary bladder, myocardium, brain, lungs and skeletal muscles (Fatani et al. Reference Fatani, Elsebaie and Hilali1996; Manal et al. Reference Manal, El Amin and Osman2001). Initial inflammatory changes induce necrosis and degeneration in musculoskeletal tissues which may ultimately lead to healing with fibrosis and scarring (Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008). Mostly, the affected tissues may exhibit no degenerative or inflammatory changes in host tissues. However, in some of the infected tissues, degenerated sarcocysts could be associated with necrosis and an inflammatory response between muscle fibres which is characterized by infiltration of polymorphonuclear cells, lymphocytes, macrophages, eosinophils and fibroblasts (Manal et al. Reference Manal, El Amin and Osman2001; Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008).
Overall these studies indicate that sarcocystosis could be associated with significant pathology, with mild or no clinical symptoms in infected camels. However, this is worth mentioning that the sample size in almost all previous studies was very small (Table 3) that warrants further studies with large sample size with proper experimental controls to better understand the pathogenesis of Sarcocystis spp. in camels. Future studies are required to comprehensively understand the pathogenesis of sarcocystosis and its impact on the cardiac/musculoskeletal functioning and productivity of camels, economic importance and animal welfare. Furthermore, pathogenesis associated with macroscopic sarcocysts need to be examined in camels.
Epidemiology of sarcocystosis in camels
The prevalence of sarcocystosis in camels has been reported from different regions of the world, including the Middle East, Africa and Asia (Table 2). Natural infections with Sarcocystis spp. in camels are usually asymptomatic (Hosseini et al. Reference Hosseini, Ataeie, Rahimi and Jafarian2010; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011; Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013; Abd-Elmalek et al. Reference Abd-Elmalek, Abed and Mandour2015; Omer et al. Reference Omer, Alzuraiq and Mohammed2017), despite the presence of significant histopathology in affected tissues (Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008). The prevalence of sarcocystosis in camels has been estimated in Afghanistan (61%), Iran (52–84%), Egypt (23–64%), Jordan (22%), Kazakhstan (32%), Saudi Arabia (28–88%), Somalia (82%) and Sudan (81%) (Table 2). Furthermore, infection rates may vary considerably among different organs examined (Supplementary Table S1). In general, the probability of detecting a sarcocyst increases when more than one organ is examined (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001). However, the prevalence of sarcocystosis may vary in different studies depending on the differences in diagnostic methods used, sampling location, animal age, type of organs/tissue(s) analysed and the number of samples studied. Thus, these factors should carefully be evaluated when comparing the prevalence data from different studies.
Herd management practices, including sanitary conditions and the presence of dogs, play a crucial role in the epidemiology of Sarcocystis in camels as the infection depends on the frequency of contact between camels and dogs (or their excreta). For instance, sarcocystosis has been reported more frequently in areas where camels are reared in the presence of pastoral dogs (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001; Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008). Similar findings have been reported for the presence of higher Sarcocystis infection rates in llamas (another camelid) kept with pastoral dogs and under poor sanitary conditions (Romero et al. Reference Romero, Carletti, Franco, Moré, Schnittger and Florin-Christensen2017; Saeed et al. Reference Saeed, Rashid, Vaughan and Jabbar2018). Likewise, road-side slaughter and disposal of camel carcasses in the wild are common practices in developing countries and facilitates easy access of dogs and wild carnivores to infected tissues. This increases the likelihood of shedding sporocysts in feces of these animals which could contaminate camel feed and water (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001).
Longevity in camels has been found to be associated with Sarcocystis infections as older animals have had a long time to be exposed to oocysts/sporocysts in infected pastures. For example, a higher prevalence of sarcocystosis was observed in adult (e.g. 8–12 years) camels than young (e.g. < 2 years) ones from different parts of the world (Fatani et al. Reference Fatani, Elsebaie and Hilali1996; Latif et al. Reference Latif, Al-Delemi, Mohammed, Al-Bayati and Al-Amiry1999; Al-Quraishy et al. Reference Al-Quraishy, Bashtar, Al-Rasheid and Abdel-Ghaffar2004; Shekarforoush et al. Reference Shekarforoush, Shakerian and Hasanpoor2006; Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013; Omer et al. Reference Omer, Alzuraiq and Mohammed2017). No significant variation was observed in infection rates between male and female camels in many studies (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001; Shekarforoush et al. Reference Shekarforoush, Shakerian and Hasanpoor2006; Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008; Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013), however, a higher prevalence was recently reported in female camels (Omer et al. Reference Omer, Alzuraiq and Mohammed2017). This may have been due to a difference in age rather than sex, as all males used in this study were 2–4 years younger than females (Omer et al. Reference Omer, Alzuraiq and Mohammed2017). Studies in other camelids, such as alpacas and llamas, have shown that females are more susceptible to Sarcocystis infections, possibly due to their lower immunity during gestation and around parturition (Romero et al. Reference Romero, Carletti, Franco, Moré, Schnittger and Florin-Christensen2017; Saeed et al. Reference Saeed, Rashid, Vaughan and Jabbar2018). However, further investigations are required to test this hypothesis in camels.
Diagnosis of sarcocystosis in camels
Diagnosis of acute sarcocystosis in camels is challenging due to the lack of a commercial standard diagnostic test, asymptomatic or generalized nature of the disease and the presence of hidden (microscopic) sarcocysts. Virtually all camels, regardless of clinical involvement, have been reported with some microscopic sarcocysts in muscles during meat inspection (Table 2). However, a variety of conventional methods, including muscle squash, muscle squeeze, pepsin digestion, trypsin digestion and histopathological examination have been used for the diagnosis of microscopic sarcocysts in camels. The muscle squash method involves a firm crushing of small pieces of meat between two glass slides and the muscle squeeze method uses a metal instrument to crush and squeeze muscle tissue to obtain the fluid for microscopic examination (Latif et al. Reference Latif, Al-Delemi, Mohammed, Al-Bayati and Al-Amiry1999; Latif and Khamas, Reference Latif and Khamas2007; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011). Enzymatic digestion of muscle using pepsin or trypsin enzymes or histological examination of tissues are other commonly used methods to study microscopic sarcocysts in camels (Valinezhad et al. Reference Valinezhad, Oryan and Ahmadi2008; Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009; Hamidinejat et al. Reference Hamidinejat, Hekmatimoghaddam, Jafari, Sazmand, Haddad Molayan, Derakhshan and Mirabdollahi2013; Omer et al. Reference Omer, Alzuraiq and Mohammed2017). It is important to consider that some of these methods could produce superior results to others. For instance, pepsin or trypsin digestion methods were found to be more sensitive than muscle squash/squeeze or histological diagnostic methods (Borrow et al. Reference Borrow, Mohammed and Di Sacco1989; Latif et al. Reference Latif, Al-Delemi, Mohammed, Al-Bayati and Al-Amiry1999; Omer et al. Reference Omer, Alzuraiq and Mohammed2017). This could be due to the use of larger pieces of muscle during enzymatic digestion steps allowing the release of a larger number of bradyzoites for microscopic examination.
The molecular diagnosis could be another useful method for the detection of Sarcocystis spp.; however, there is a paucity of information on the molecular diagnosis of Sarcocystis spp. in camels. The first molecular identification of S. cameli was undertaken for microscopic sarcocysts in camels from Iran where the 18S rRNA gene fragment was amplified from bradyzoite DNA using conventional polymerase chain reaction (PCR) followed by sequencing (GenBank: GU074011.1) and restriction fragment length polymorphism (RFLP) analyses (Motamedi et al. Reference Motamedi, Dalimi, Nouri and Aghaeipour2011; Eslampanah et al. Reference Eslampanah, Motamedi, Dalimi, Noori, Habibi, Aghaeepour and Niroumand2016). The RFLP method was more cost-effective than DNA sequencing or electron microscopic procedures. Recently, the 18S rRNA fragment was amplified from microscopic sarcocysts in camels, although no phylogenetic analyses were provided (Omer et al. Reference Omer, Alzuraiq and Mohammed2017). This indicates that extensive studies are required to bridge the knowledge gap on molecular profiling of Sarcocystis spp. which could provide a foundation to develop a molecular test for the diagnosis of these parasites in camels.
Molecular detection of sarcocysts is invaluable in identifying Sarcocystis spp.; however, this holds little value as an early diagnostic method in camels since the DNA is extracted from a developed cyst at necropsy. Studies are required to develop a molecular test for the detection of Sarcocystis spp. from blood, and this could be an invaluable diagnostic tool for early diagnosis of sarcocystosis in camels.
Control of sarcocystosis in camels
Anticoccidial drugs have been trialled to treat intestinal stages of Sarcocystis spp. during acute infections not only in camels and other intermediate hosts but also in their definitive hosts. For example, camels treated with amprolium were protected from the severe form of experimental sarcocystosis (Ishag et al. Reference Ishag, Majid and Magzoub2006). The prophylactic use of salinomycin protected sheep from experimental infections with Sarcocystis sp. (Leek and Fayer, Reference Leek and Fayer1983). Similarly, halofuginone reduced the severity or prevented disease in sheep and goats infected with Sarcocystis spp. (Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a). Given that it is not possible to diagnose acute sarcocystosis under field conditions and these drugs only affect intestinal stages of the parasite, chemotherapy holds little or no value in treating muscular sarcocystosis in camels. Furthermore, some antiparasitic drugs (such as salinomycin) may be highly toxic to camels, causing weakness, limb incoordination and even mortality (Anderson, Reference Anderson2008).
Immunisation against Sarcocystis spp. could be another solution to protect camels and other intermediate hosts from sarcocystosis; however, no commercial vaccines are available thus far. Immunisation studies in cattle, sheep, goats and pigs indicate that animals inoculated with small numbers of sporocysts exhibited protective immunity against a subsequent challenge with Sarcocystis spp. (Fayer and Dubey, Reference Fayer and Dubey1984; Ford, Reference Ford1985; Abdel-Baki et al. Reference Abdel-Baki, Allam, Sakran and El-Malah2009). Thus, the development of a vaccine against Sarcocystis spp. in camels and other domestic animals is practicable. However, a thorough understanding of the immune mechanisms underlying host–pathogen interactions is crucial for the development of a successful vaccine against Sarcocystis spp. and this aspect has been completely overlooked in camels. Given that no vaccine is available against sarcocystosis and chemotherapy might not be effective, preventative measures provide the only practical solution to protect camels against Sarcocystis spp.
In camels, the main method of Sarcocystis transmission appears to be an environment contaminated with dog/carnivore feces facilitating the fecal-oral route. Therefore, the disruption of this cycle is essential to break the life cycle and control sarcocystosis in camels. The following steps could be undertaken to potentially disrupt the Sarcocystis life cycle in camels: (a) camel feed/water and bedding storage areas should be kept free from dogs and wild carnivores and their excreta; (b) dogs should not be fed with uncooked/untreated camel meat as it may contain sarcocysts; freezing or boiling could significantly reduce or eliminate sarcocysts in camelid meat (Godoy et al. Reference Godoy, Vilca, Gonzáles, Leyva and Sam2007; Saeed et al. Reference Saeed, Rashid, Vaughan and Jabbar2018); and (c) any camel tissues, including carcasses and placental/fetal material, should be buried or incinerated to prevent ingestion by definitive hosts such as dogs and wild carnivores (d) abattoirs and slaughtering plots should be kept free from stray dogs.
Conclusions and future directions
Sarcocystosis is a parasitic disease of camels. Camels serve as an intermediate host for at least two morphologically distinct Sarcocystis spp., S. cameli and S. ippeni. Both Sarcocystis spp. can form microscopic sarcocysts which appear in diaphragmatic, oesophageal, cardiac and skeletal muscles; however, S. cameli can also produce macroscopic sarcocysts. Sarcocystosis usually remains asymptomatic, although significant pathologies have been observed in affected camels. Animal age, the presence of pastoral dogs and poor sanitary conditions are the main predisposing factors for sarcocystosis in camels. Several conventional methods, including muscle squash/squeeze, pepsin/trypsin digestion and histopathological examination are used for the diagnosis of Sarcocystis spp. in camels, however, no methods are available for an early diagnosis of Sarcocystis spp. Furthermore, prevention is the only pragmatic approach to control sarcocystosis in camels as there is no effective treatment once an animal is infected.
This article provides an in-depth analysis of the existing knowledge on sarcocystosis in camels; however, several aspects of sarcocystosis in camels require further investigations. For instance, sarcocystosis has not been well-studied in the Bactrian (two-humped) camel, although one study reported Sarcocystis in Bactrian camels from Kazakhstan (Kuraev, Reference Kuraev1981). Similarly, little is known about sarcocystosis in camels from other regions of Asia (e.g., China, India and Pakistan) and Africa (e.g., Somalia) which represents the highest population of dromedary camels in the world. Furthermore, the prevalence data on macroscopic sarcocysts and the susceptibility of different breeds of camels are scarce. Further studies are required to improve our understanding of the impact of sarcocystosis on camel health and reproductive performance, meat production and productivity, predisposition to other diseases and associated economic losses.
The taxonomy of Sarcocystis spp. infecting camels is primarily based on the morphological characterization of the cyst wall of sarcocysts and no molecular methods have been used in this regard. The cyst walls of S. cameli and S. ippeni are characterized by finger-like and conical villar protrusions, respectively. Morphological features of sarcocysts may be affected by factors such as the age of cyst, the location of cyst within the host, and the tissue processing/fixation methods; therefore, the description of a new Sarcocystis sp. using morphological characters (e.g. the structure of cyst wall) only, could be insufficient and may create confusion during species identification processes. For instance, various shapes of villar protrusions (conical, finger-like and stubby) were observed within the same sarcocyst (Dubey et al. Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b) (see Fig. 5 in this paper). Furthermore, the ‘thick or thin’ cyst wall character also varied in different studies (Table 1), although various authors described a similar shape of villar protrusions (Al-Quraishy et al. Reference Al-Quraishy, Bashtar, Al-Rasheid and Abdel-Ghaffar2004; Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009; Mandour et al. Reference Mandour, Rabie, Mohammed and Hussein2011; Abd-Elmalek et al. Reference Abd-Elmalek, Abed and Mandour2015; Dubey et al. Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b, Reference Dubey, A'Aji, Mowery, Verma and Calero-Bernal2017). This likely explains why there are at least six different names for Sarcocystis spp. that infect camels. For instance, Dubey et al. (Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b), characterized and named only thin-walled Sarcocystis spp. and did not include any thick-walled sarcocysts from camels which may point towards the existence of more than two Sarcocystis spp. and leave taxonomic classification as subject to change based on the availability of more information. Another limitation of these studies is the use of small number and old (stored for decades) cyst samples to describe new species of Sarcocystis in camels (Dubey et al. Reference Dubey, Hilali, Van Wilpe, Calero-Bernal, Verma and Abbas2015b, Reference Dubey, A'Aji, Mowery, Verma and Calero-Bernal2017). Thus, it is essential to utilize molecular characterization besides morphological criteria, with large numbers of fresh samples, including both thick- and thin-walled sarcocysts for more authentic and robust taxonomy of Sarcocystis spp. that infect camels.
Based on experimental infection studies, dogs are known as definitive hosts for Sarcocystis spp. that infect camels (Abdel-Ghaffar et al. Reference Abdel-Ghaffar, Mehlhorn, Bashtar, Al-Rasheid, Sakran and El-Fayoumi2009). However, sarcocystosis has also been reported from camels in Ethiopia in the absence of dogs (Woldemeskel and Gumi, Reference Woldemeskel and Gumi2001), suggesting that other carnivores (e.g. wild canids) could also serve as the potential definitive host for these parasites and warrants for further studies. Little is known about the developmental stages (e.g. merozoites, bradyzoites, microgametes, microgametes, etc.) of Sarcocystis spp. in both camels and dogs. Experimental infection studies are required to improve our understanding of the developmental stages of Sarcocystis spp. in camels as well as their definitive host(s) for designing effective control strategies.
The lack of standard diagnostic tests is another major hindrance to fully understand the disease biology of Sarcocystis spp. in camels. Since microscopic sarcocysts (the most common form reported) are hidden within muscles of the camel, sensitive diagnostic tools are required to detect Sarcocystis preferably from body fluids (e.g. blood) for an early diagnosis of Sarcocystis in these animals. Serological methods have been developed for the determination of anti-Sarcocystis antibodies in sera of other camelid hosts such as alpacas and llamas (Viscarra et al. Reference Viscarra, Rushton, González and López2003; More et al. Reference More, Pardini, Basso, Marin, Bacigalupe, Auad, Venturini and Venturini2008; Romero et al. Reference Romero, Carletti, Franco, Moré, Schnittger and Florin-Christensen2017). A high inter-species cross-reactivity was observed among different Sarcocystis spp. of ruminants in these studies, highlighting the possibility of developing a similar serological test for the diagnosis of sarcocystosis in camels. Likewise, a range of molecular methods has been developed for the evaluation of genetic diversity as well as the diagnosis of Sarcocystis spp. in other animals (Stojecki et al. Reference Stojecki, Karamon, Sroka and Cencek2012). For instance, a semi-nested PCR has been developed to detect Sarcocystis DNA, allowing the detection of as low as 100 bradyzoites per mL of llama blood (Martin et al. Reference Martin, Franco, Romero, Carletti, Schnittger and Florin-Christensen2016). Experimental infection studies in cattle have shown that Sarcocystis merozoites can be detected in buffy coat preparations from infected animals as early as the third week of infection (Dubey et al. Reference Dubey, Calero-Bernal, Rosenthal, Speer and Fayer2015a). Although this method holds little value for the routine diagnosis of Sarcocystis spp. in animals due to the short time merozoites circulate in the blood soon after initial infection, this highlights the possibility of detecting Sarcocystis DNA from camel blood with regular PCR. However, none of these methods has been trialled for early diagnosis of sarcocystosis in camels.
As mentioned earlier, the only known species with zoonotic potential include S. hominis, S. heydorni and S. suihominis (Poulsen and Stensvold, Reference Poulsen and Stensvold2014; Dubey, Reference Dubey2015). Despite a high prevalence of Sarcocystis spp. in camels, their zoonotic potential and public health significance have not been evaluated. It has been suggested that the consumption of uncooked meat infected with Sarcocystis could cause gastrointestinal and respiratory problems in humans (Leguía, Reference Leguía1991; Poulsen and Stensvold, Reference Poulsen and Stensvold2014). Similarly, camelid meat infected with Sarcocystis spp. can produce a pronounced pathology in dogs and toxicity in rabbits (Leguía, Reference Leguía1991; Godoy et al. Reference Godoy, Vilca, Gonzáles, Leyva and Sam2007; Vilca et al. Reference Vilca, Durán, Ramos and Lucas2013). However, the impact of Sarcocystis-infected camel meat on human or animal (i.e., dog) health remains an untouched area of study. Physical and chemical methods have been utilized to treat infected camelid meat to neutralize the toxicity and the viability of sarcocysts. For instance, boiling (100 °C for 10 min), baking (105 °C for 65 min), and frying methods were used to decontaminate llama meat infected with Sarcocystis and fed to young dogs (Godoy et al. Reference Godoy, Vilca, Gonzáles, Leyva and Sam2007; Vilca et al. Reference Vilca, Durán, Ramos and Lucas2013). The puppies that ate treated meat did not excrete any sporocysts in their feces unlike those who received untreated meat. These results indicate that boiling, baking and freezing could be applied to neutralize the viability of sarcocysts. Similar studies are required in camels to assess the effectiveness of these methods to decontaminate camel meat infected with Sarcocystis spp.
Our knowledge on sarcocystosis in camels is limited and further studies are required to understand the epidemiology and disease biology of Sarcocystis spp. and their impact on camel health, immunity, reproduction and productivity. The development of validated serological and molecular diagnostic tools for the early detection of Sarcocystis spp. from body fluids (e.g. blood) in camels would be a breakthrough in parasitology research. Molecular characterization of Sarcocystis spp. infecting camels could assist in developing an early diagnostic test, describing a more authentic taxonomy and determining phylogenetic relationships of these parasites with Sarcocystis spp. from other camelid hosts as well as other coccidian protozoa.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018000239.
Acknowledgements
We are grateful to Professor Ian Beveridge for the translation of articles in French language. We are thankful to Miss Sahar T. Shairani for her technical assistance.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.