INTRODUCTION
During the last years of the 19th Century and the beginning of the 20th Century, several European nations conducted expeditions to Antarctica with the goals of promoting international prestige and reaching the South Pole. Scientific collections from these expeditions yielded a number of aeolid nudibranchs that were described as new species in several publications. Most of these papers were expedition reports in which specimens were studied years after collection. As a consequence, these descriptions were based on preserved animals and descriptive information on the external morphology and coloration is largely lacking; thus, it is very difficult to apply these descriptions to members of the Antarctic aeolid fauna collected in the present day. All expedition reports in which new aeolid Antarctic species were described are summarized in Table 1.
Table 1. Expedition reports in which new species of Antarctic aeolids were described, including reference, name of the expedition and new species described.

After a lapse of almost 30 years, a new emphasis in Antarctic research produced a series of papers dealing with Antarctic and sub-Antarctic aeolids, including Minichev (Reference Minichev1972), Burn (Reference Burn1973), Vicente (Reference Vicente1974) and Vicente & Arnaud (Reference Vicente and Arnaud1974), in which the new taxa Notaeolidia alutacea Minichev, Reference Minichev1972, Notaeolidia flava Minichev, Reference Minichev1972, Cuthona georgiana longipapillata Minichev, Reference Minichev1972, Cuthona paucicirra Minichev, Reference Minichev1972, Cuthona critina Minichev, Reference Minichev1972, Trinchesia macquariensis Burn, Reference Burn1973 and Cuthona claviformis Vicente in Vicente & Arnaud, Reference Vicente and Arnaud1974 were described. Many of these species were also described based on preserved animals and important descriptive information was lacking.
More recently, Wägele (Reference Wägele1990), Cattaneo-Vietti (Reference Cattaneo-Vietti1991) and Martynov (Reference Martynov2006) provided the first modern reviews of Antarctic aeolids. These included descriptions of living animals and morphological observations made with scanning electron microscopy. These papers focused on reviewing previously recognized species and synonymizing a handful of taxa, thus clarifying the taxonomy of the group. Two additional new species were introduced by Martynov (Reference Martynov2006), Guyvalvoria gruzovi Martynov, Reference Martynov2006 and Guyvalvoria savinkini Martynov, Reference Martynov2006.
In the present paper we present morphological data from an assortment of Antarctic aeolids that were recently collected by divers in the Ross Sea, photographed alive, and preserved for more detailed morphological and molecular analyses. Our main objectives here are to provide comprehensive descriptions of these taxa including colour, radular morphology and reproductive anatomy that will facilitate further research on the group. We assigned them to previously described species wherever possible, and describe a single new species.
MATERIALS AND METHODS
Specimens were collected by divers on SCUBA at several locations in McMurdo Sound, Antarctica. Specimens were collected by hand, brought back to McMurdo Station alive, and kept at –1°C until photographed. Live animals were photographed with a Nikon D-70S with 105 mm macro lens in a flow-through saltwater tank, measured, and preserved immediately in 99% EtOH.
Preserved specimens were dissected by dorsal incision. The internal features were examined and drawn using a Nikon SMZ-100 dissecting microscope with a camera lucida attachment. Special attention was paid to the morphology of the reproductive and digestive systems. The buccal mass was removed and dissolved in 10% sodium hydroxide until the radula and jaws were isolated from the surrounding tissue. The radula and jaws were then rinsed in water, dried, mounted and sputter-coated for examination with a scanning electron microscope (SEM) Hitachi S-3000N at the Natural History Museum of Los Angeles County.
The material examined was deposited at the Malacology Section of the Natural History Museum of Los Angeles County (abbreviated LACM).

Fig. 1. Photographs of living animals: (A) Eubranchus glacialis (Thiele, Reference Thiele and von Drygalski1912) (LACM 176411), dorsal view; (B) Eubranchus glacialis (Thiele, Reference Thiele and von Drygalski1912) (LACM 176411), frontal view; (C) Guyvalvoria paradoxa (Eliot, 1907) (LACM 176424) frontal view; (D) Guyvalvoria paradoxa (Eliot, 1907) (LACM 176424) dorsal view; (E) Cuthona elioti (Odhner, Reference Odhner1944) (LACM 176420), lateral view; (F) Cuthona crinita Minichev, Reference Minichev1972 (LACM 176422), dorsal view; (G) Cuthona modesta (Eliot, 1907) (LACM 176414), dorsal view; (H) Cuthona giarannae sp. nov., dorsal view. Photographs by C. Shields and L. Mullen (A, B, C, D, G & H); B. Miller (E); and Jonathan Sprague (F).

Fig. 2. Eubranchus glacialis (Thiele, Reference Thiele and von Drygalski1912) LACM 176411, scanning electron microscopy photographs of the radula and jaws. (A) Anterior radular teeth, arrows indicate alternating small denticles at the base of the central cusp; (B) posterior radular teeth; (C) jaw.
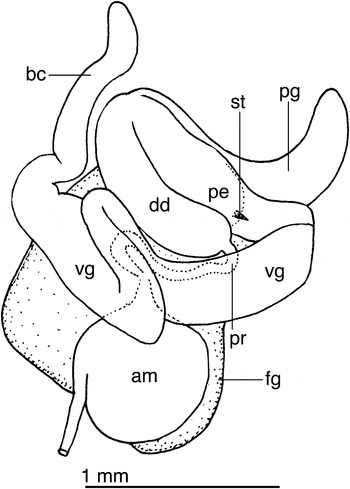
Fig. 3. Eubranchus glacialis (Thiele, Reference Thiele and von Drygalski1912) reproductive system of LACM 176411. am, ampulla; bc, bursa copulatrix; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate; st, stylet; vg, vagina.
Galvinella glacialis Thiele, Reference Thiele and von Drygalski1912: 223–224, text figure 9, pl. 19, figure 6.
MATERIAL EXAMINED
Arrival Heights, McMurdo Sound, Ross Sea, Antarctica (77°50′14.29″S 166°37′59.14″E), 16 November 2007, 1 specimen 7 mm preserved length (LACM 176410); 1 specimen 7 mm preserved length, dissected (LACM 176411). Dayton's Wall, McMurdo Sound, Ross Sea, Antarctica (77°51′02.31″S 166°39′56.07″E), 27 November 2007, 1 specimen, 4 mm preserved length (LACM 176412).
EXTERNAL MORPHOLOGY
Background colour golden-yellow (Figure 1A). Rhinophores and oral tentacles slightly paler than the rest of the body. Cerata translucent orange with opaque white apices. Digestive gland branches visible in the cerata as reddish-orange tubes.
Rhinophores and oral tentacles about the same length (Figure 1B). Cerata inflated, wider near the centre, narrowing towards both the base and the apex. Subapically, right where each ceras becomes white, the cerata widen slightly to narrow again towards the tip. Cerata arranged in 4 groups, the first one situated anterior to the pericardium and the rest posterior to the pericardium. Within each group, dorsal cerata tend to be larger than the more lateral ones. Reproductive opening on the right side of the body, in the middle of the first group of cerata. Anus dorsal, located behind the pericardium, slightly towards the right side of the body and anterior to the second group of cerata. Posterior end of the foot elongate with a rounded tip.
ANATOMY
Radular formula 56 × 1.1.1 in a 7 mm preserved length specimen. Rachidian teeth with a central cusp surrounded by 3–5 denticles on each side (Figure 2A, B). The cusp and denticles have a similar shape, but the central cusp is longer. In several teeth a small denticle is situated at the base of the central cusp, generally alternating between the left and right sides of the cusp (Figure 2A). The rachidian teeth are similar in shape and size throughout the radula. Lateral teeth plate like, with a pointy inner cusp and lacking denticles. All lateral teeth are similar in shape, but they decrease in size towards the posterior end of the radula. Jaws elongate (Figure 2C), no denticles on the masticatory border were observed.
Reproductive system with a short, wide ampulla (Figure 3). The hermaphroditic duct connects into the ampulla laterally, not distally. The ampulla connects to the female gland complex near the connection of the prostate. The prostate is a short, thin tube that expands into a large, muscular deferent duct. The distal end of the deferent duct contains the penis, which is armed with a short stylet. A long, curved prostate gland inserts on the mid-section of the deferent duct. The vagina is long and convoluted and connects directly into a very elongate bursa copulatrix.
REMARKS
Several species of Eubranchidae were described from Antarctic and sub-Antarctic waters. Most of these descriptions were based on preserved specimens and are therefore difficult to evaluate. Odhner (Reference Odhner1934) discussed these species and provided elements to distinguish them based on the radular teeth morphology. For example, the species Eubranchus fuegiensis Odhner, Reference Odhner1926 (from Argentina, Tierra del Fuego), Galvinella antarctica Eliot, 1907 (from Winter Quarters Bay, McMurdo Sound, Antarctica) and Galvinella glacialis Thiele, Reference Thiele and von Drygalski1912 (from the Gauss-Station, Davis Sea, Antarctica), all were described as having lateral teeth much broader than the rachidian, whereas Eubranchus adariensis Odhner, Reference Odhner1934 and Eubranchus falklandicus Eliot, 1907 had lateral teeth equal or narrower than the rachidian.
The specimens here examined show lateral teeth much broader than the rachidian and therefore fall within the first group. Galvinella antarctica is a peculiar species with the cerata very narrow mid-length and wider near the apex and the base. Eliot (Reference Eliot1907a) erected the genus name Galvinella for this species because of the unique shape of the cerata and the pointed anterior corners of the foot. Since its original description by Eliot (Reference Eliot1907a), this species has not been collected again and it is not clear whether the peculiar ceratal morphology was a true species characteristic or an artefact of preservation.
The second species of Galvinella, G. glacialis, was described by Thiele (Reference Thiele and von Drygalski1912) as having a brownish-grey colour with the apices of the cerata white. The anus was located dorsally on the centre right of the animal. The radular morphology, with a rachidian tooth having several large denticles and very elongate lateral teeth is consistent with the specimens here examined. Thiele (Reference Thiele and von Drygalski1912) assigned this species to Galvinella because of the conical shape of the cerata with rounded apices, but in the original description the cerata appeared to be club-shaped, very different from the drawings by Eliot (Reference Eliot1907a). All external and anatomical characteristics of G. glacialis are consistent with those of the specimens here examined, and therefore we assign them to this species. Miller (Reference Miller1971) stated that Galvinella was most likely a synonym of Eubranchus, and this opinion is now generally accepted. Examination of the type species of Galvinella (G. antarctica) and comparison to species of Eubranchus is necessary to confirm this synonymy.

Fig. 4. Cuthona modesta (Eliot, 1907) LACM 176416, scanning electron microscopy photographs of the radula and jaws: (A) radular teeth; (B) jaw.

Fig. 5. Cuthona modesta (Eliot, 1907) reproductive system of LACM 176416. bc, bursa copulatrix; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate; sr, possible seminal receptacle; vg, vagina.
Cuthonella modesta Eliot, Reference Eliot1907a: 25.
?Cuthona georgiana (Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886): Cattaneo-Vietti, Reference Cattaneo-Vietti1991: 224–228, figure 2.
MATERIAL EXAMINED
Dayton's Wall, McMurdo Sound, Ross Sea, Antarctica (77°51′02.31″S 166°39′56.07″E), 26 October 2007, 1 specimen, 8 mm preserved length (LACM 176413). Cape Evans Wall, McMurdo Sound, Ross Sea, Antarctica (77°38.413′S 166°31.120′E), 31 October 2007, 1 specimen 6 mm preserved length, dissected (LACM 176414). Intake Jetty, McMurdo Sound, Ross Sea, Antarctica (77°51′02.31″S 166°39′56.07″E), 15 November 2007, 1 specimen 8 mm preserved length, dissected (LACM 176415), 2 December 2007, 1 specimen 18 mm preserved length, dissected (LACM 176416).
EXTERNAL MORPHOLOGY
Background colour translucent grey, with the digestive and reproductive organs visible as an orange-grey mass (Figure 1G). Rhinophores and oral tentacles translucent grey with opaque white pigment on the distal half. Cerata translucent with opaque white apices. Digestive gland branches visible in the cerata as reddish-orange tubes.
Body relatively short and wide. Anterior end of the foot with two conspicuous, triangular foot corners. Rhinophores smooth, about 4 times as long as the oral tentacles. Cerata inflated, wider subapically, narrowing towards both the base and the apex. Cerata arranged in 8 rows, the first 4 situated anterior to the pericardium and the rest posterior to the pericardium. Within each group, dorsal cerata tend to be larger than lateral cerata. Reproductive opening on the right side of the body, behind the 3rd row of cerata. Anus latero-dorsal, located behind the pericardium, anterior to the 5th row of cerata. Posterior end of the foot elongate with a rounded tip.
ANATOMY
Radular formula 33 × 0.1.0 in a 7 mm preserved length specimen. Radular teeth narrow and elongate, with the region bearing denticles much narrower than the rest of the tooth (Figure 4A). Central cusp short and wide, shorter than the lateral denticles, with the distal end lower than that of the innermost denticles. Lateral denticles ranging from 5 to 6, comparatively short and wide (Figure 4A). Innermost lateral denticles juxtaposed, but outermost separated by small gaps. Jaws elongate (Figure 4B), with no observable denticles on the masticatory border.
Reproductive system (Figure 5) lacking a visibly differentiated ampulla. The hermaphroditic duct inserts directly into the female gland complex, which indicates that the ampulla is possibly embedded in the female gland tissue. The prostate emerges from the female gland mass and consists of a thin convoluted tube that opens directly into the short, conical penis. A short penial gland connects next to the connection between the deferent duct and the penis. The vagina is very long and wide and expands into a poorly defined, irregular bursa copulatrix. From the vagina a narrow, long duct emerges and appears to expand into a seminal receptacle and the female gland complex. These organs and connections are not well defined and with the material available it was not possible to identify them precisely.
REMARKS
Cuthonella modesta Eliot, 1907 (type locality: Hut Point, Winter Quarters Bay, Ross Sea) was described based on a single specimen 3.2 mm preserved length. Eliot (Reference Eliot1907a) described the living animal as uniform greenish-yellow, with stout, inflated cerata, a broad foot with a thick anterior margin with short lateral projections, and the genital opening situated right behind the rhinophores. All these characteristics were present in the material here examined. More importantly, the radular teeth of C. modesta were described as horseshoe shaped with a low, shorter than the lateral denticles cusp, which was also very similar to the radular teeth of our specimens. The only difference between the material here examined and the original description of C. modesta is the presence of white pigment on the tips of the cerata, rhinophores and oral tentacles in our specimens. Eliot (Reference Eliot1907a) never examined the live holotype and his description of colour was based on notes from collectors, who may have not described the specimens in detail.
The remarkable similarities in the external and anatomical features of C. modesta and the material here examined, as well as the fact that they were collected within 1 km of each other, provides support for the identification of our specimens.
The reproductive anatomy of the specimens of C. modesta examined here is unusual, however some of the organs could not be identified with certainty and this description should be regarded as tentative.
Specimens reported by Cattaneo-Vietti (Reference Cattaneo-Vietti1991) as Cuthona georgiana (Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886) and collected from Terra Nova Bay (Ross Sea) probably belong to the same species. The detailed external and anatomical descriptions by Cattaneo-Vietti (Reference Cattaneo-Vietti1991) coincide in great detail with our observations. A photograph of a live animal made available by Cattaneo-Vietti (personal communication) showed an animal very similar to the specimens here examined. The only substantial difference is the shape of the radular teeth that in Cattaneo-Vietti's (1991: figure 2C) drawing appear shorter than in our specimens and the central cusp is just slightly longer than the lateral denticles. A scanning electron micrograph of the radula provided by Cattaneo-Vietti (personal communication) confirmed these differences.
Odhner (Reference Odhner1944) transferred C. modesta to Cuthona, along with two other Antarctic species, C. antarctica and C. paradoxa. Odhner (Reference Odhner1944) argued that these three species have their cerata arranged in simple rows whereas Cuthonella (type species Cuthonella abyssicola Bergh, 1884) is characterized by having branches of the digestive gland with ceratal branches multiplying laterally. Our examinations of C. modesta and C. elioti (= C. antarctica) confirm that the anatomical characteristics of these two species are consistent with the diagnoses of Cuthona provided by Miller (Reference Miller1977) and Williams & Gosliner (Reference Williams and Gosliner1979).

Fig. 6. Cuthona elioti (Odhner, Reference Odhner1944) LACM 176419, scanning electron microscopy photographs of the radula and jaws: (A) radular teeth; (B) jaw; (C) masticatory borders of both jaws.
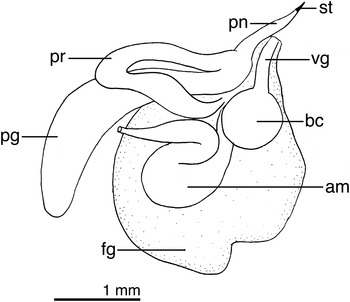
Fig. 7. Cuthona elioti (Odhner, Reference Odhner1944) reproductive system of LACM 176419. am, ampulla; bc, bursa copulatrix; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate; st, penial stylet; vg, vagina.

Fig. 8. Egg masses and embryos of Cuthona elioti. (A) Two field-collected masses attached to a bushy hydroid; (B) close-up view of embryos in a mass, taken along the long edge of the coiled ribbon (the top of the mass as pictured in A); (C) hydroids collected at Dayton's Wall, McMurdo Sound, containing a mass and adult of C. elioti. Photographs by B. Miller.
Cuthonella antarctica Eliot, Reference Eliot1907a: 23–24, text figures 25–26 (non-Aeolis antarctica Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886)
Cuthona elioti Odhner, Reference Odhner1944: 22 (replacement name for Cuthonella antarctica Eliot, 1907).
MATERIAL EXAMINED
Dayton's Wall, McMurdo Sound, Ross Sea, Antarctica (77°51′02.31″S 166°39′56.07″E), 26 October 2007, 1 specimen 3 mm preserved length (LACM 176417). Sewage outfall, McMurdo Sound, Ross Sea, Antarctica (77°51′31.95″S 166°40′47.51″E), 5 November 2007, 1 specimen 5 mm preserved length, dissected (LACM 176418). Cape Evans Wall, McMurdo Sound, Ross Sea, Antarctica (77°38.413′S 166°31.120′E), 22 November 2007, 1 specimen 6 mm preserved length, dissected (LACM 176419), 22 November 2007, 1 specimen 3 mm preserved length, dissected (LACM 176420).
EXTERNAL MORPHOLOGY
Colour uniformly translucent white with the digestive system visible as an orange-yellow mass (Figure 1E). Branches of the digestive system visible in the cerata, also orange-yellow, with the large cnidosacs opaque white. Dorsum, rhinophores and cerata covered with numerous tiny opaque white-bluish dots, more densely arranged near the apices of the cerata and on the head area in front of the rhinophores. Reddish-brown pigment present subapically on the cerata.
Body narrow, with relatively high profile. Anterior border of the foot rounded with no lateral projections. Rhinophores smooth, about 3–4 times as long as the oral tentacles. Cerata elongate and narrow, wider near the base. Cerata arranged in 7 rows, the two most posterior rows having the largest number of cerata. Reproductive opening situated right behind the second row of cerata. Anus opening between the fourth and fifth row, below the posterior end of the pericardium.
INTERNAL ANATOMY
Radular formula 14 × 0.1.0. Radular teeth narrow and elongate, with a pointy, sharp central cusp, and 3–4 narrow and pointy lateral denticles on each side of the cusp (Figure 6A). Lateral denticles not juxtaposed, but separated by small gaps. Innermost lateral denticles reaching almost the same height as the central cusp. Jaws elongate (Figure 6B), with a masticatory border bearing two rows of 13 well-defined denticles (Figure 6C), followed by an area with an undetermined number of irregular, worn-out teeth.
Reproductive system with a large ampulla folded twice (Figure 7), connecting with the female gland complex. Prostate short, tubular, emerging from the female glands and connecting with a short, conical penis and a long accessory penial gland. Penis with a short cuticular stylet. Vagina short, straight, connecting with a small, rounded bursa copulatrix.
BIOLOGY
The egg masses of Cuthona elioti are small (2.5–3 mm × 3–4 mm), white, thin, and sheet-like, and were attached along one long edge to bushy hydroids and loosely coiled into an upright barrel shape (Figure 8A). The sheets are 1–2 embryos thick and embryos are contained in individual capsules (Figure 8B). In most masses collected in October–December, embryos were at the veliger stage and had well developed vela and feet; embryos ranged in size from 250 to 340 µm in total length from velum to shell apex (Figure 8B). None contained veligers that appeared to be close to metamorphosis; combined with the large vela and small yolk volume of the embryos, suggesting that this species may have planktonic development. Masses were often clustered together on hydroids and adults were generally found nearby (Figure 8C). Egg masses were identified to species by COI sequence data, which were identical to adult Cuthona elioti (Shields et al., submitted).
REMARKS
Cuthonella antarctica Eliot, 1907 (type locality: Winter Quarters Bay, Ross Sea) was originally described as a uniformly yellowish-brown, fairly stout animal (Eliot, Reference Eliot1907a). The cerata of the preserved animals were described as irregular, the anterior end of the foot as rounded without traces of foot corners, and the rhinophores as much longer than the oral tentacles. One of the most distinctive characteristics of this species is the radular teeth, with sharp denticles, the outermost ones curved inwards. All these characteristics are very similar to those of the specimens here examined, which are therefore assigned to this species.
Odhner (Reference Odhner1944) considered that Eliot's species C. antarctica, C. modesta and C. paradoxa belong to the genus Cuthona. With this action, Odhner (Reference Odhner1944) made Cuthonella antarctica Eliot, 1907 a secondary homonym of Aeolis antarctica Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886 (ICZN, 1999: Article 57.3), which was transferred to Cuthona by Odhner (Reference Odhner1926). Thus, Odhner (Reference Odhner1944) proposed the new name Cuthona elioti for Cuthonella antarctica Eliot, 1907.
Our specimens assigned to C. elioti are characterized by having a penial stylet. In his review of the anatomical characteristics of Antarctic Tergipedidae, Cattaneo-Vietti (Reference Cattaneo-Vietti1991) indicated that only two Antarctic species possess a penial stylet, Cuthona macquariensis (Burn, Reference Burn1973) from Macquarie Island and Tergipes valentini (Eliot, 1907) from the Falkland Islands. These two species are easily distinguishable from C. elioti in several regards. Tergipes valentini is a species with just a few cerata, and an orange background colour with the viscera visible as a dark brown area (including the ceratal branches). Additionally, the apices of the rhinophores, oral tentacles and cerata are opaque white (Eliot, Reference Eliot1907b). This is very different from the uniformly translucent white colour of C. elioti with small bluish-white dots. The radular teeth of Tergipes valentini contain numerous lateral denticles (7–9), versus just a few (3–4) in C. elioti. Cuthona macquariensis is a pink species with radular teeth very different from those of C. elioti. The rachidian teeth of Cuthona macquariensis have a large central cusp with 7 small denticles on each side, whereas C. elioti has a narrow, sharp cusp with 3–4 narrow and pointy lateral denticles, curved inward.
Other Antarctic species of Tergipedidae with a reduced number of denticles on the radular teeth include Cuthona georgiana longipapillata Minichev, 1972, Cuthona paucicirra Minichev, Reference Minichev1972, Cuthona claviformis Vicente in Vicente & Arnaud, Reference Vicente and Arnaud1974 and Tergipes antarcticus Pelseneer, 1903. The radular teeth of all these other species are very different from those of Cuthona elioti. For example, Cuthona georgiana longipapillata has radular teeth much shorter than those of Cuthona elioti, and the teeth have very short lateral denticles with the central cusp reaching much more distally than the longest of the lateral denticles. In contrast in Cuthona elioti the apices of the innermost lateral teeth reach almost the same distance from the base as the central cusp. Cuthona paucicirra has an extremely elongate, almost triangular central cusp, and the radular teeth are wider than longer, very different from the elongate and narrow teeth of Cuthona elioti. Cuthona claviformis also has broad teeth with very short and broad denticles and central cusps. Finally Tergipes antarcticus, recently reviewed by Kiko et al. (Reference Kiko, Kramer, Spindler and Wägele2008) is a very different species with just a few short cerata adapted to live on sea ice.

Fig. 9. Cuthona crinita Minichev, Reference Minichev1972 LACM 176421, scanning electron microscopy photographs of the radula and jaws: (A) radular teeth; (B) jaw.

Fig. 10. Cuthona crinita Minichev, Reference Minichev1972, reproductive system of LACM 176421. am, ampulla; bc, bursa copulatrix; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate; vg, vagina.

Fig. 11. Egg mass of Cuthona crinita. (A) Gel ribbon with larvae that had hatched from their capsules. Larvae had well-developed propodia and were crawling; (B) hatched larva of Cuthona crinita, swimming.
?Cuthona schraderi var. bouvetensis Odhner, Reference Odhner1944: 27–29, pl. 1, figure 6, text figures 23, 26, 28, 30–32.
Cuthona crinita Minichev, Reference Minichev1972: 378–380, figure 10.
MATERIAL EXAMINED
Arrival Heights, McMurdo Sound, Ross Sea, Antarctica (77°50′14.29″S 166°37′59.14″E), 3 November 2006, 1 specimen 24 mm preserved length, dissected (LACM 176421), 1 specimen 19 mm preserved length, dissected (LACM 176422).
EXTERNAL MORPHOLOGY
Background colour opaque light grey, with the digestive and reproductive organs barely visible (Figure 1F). The buccal mass is partially visible as a pinkish tinge in the head. Rhinophores and oral tentacles light grey completely covered with densely arranged small opaque white spots. Cerata semi-translucent with the apical 1/3 covered with densely arranged small opaque white spots. Digestive gland branches visible in the cerata as irregular greyish-brown tubes.
Body broad, flattened dorso-ventrally. Rhinophores smooth, slightly longer and broader than the oral tentacles. Cerata narrow, elongate, wider near the base. Cerata arranged in 10 rows, 3 anterior to the genital opening, 3 situated between the genital opening and the posterior end of the pericardium, and the rest posterior to the pericardium. Within each row, dorsal cerata are much longer than the more lateral ones. Reproductive opening on the right side of the body, posterior to the third row of cerata. Anus latero-dorsal, located posterior to the fifth row of cerata. Posterior end of the foot short with a rounded tip.
ANATOMY
Radular formula 29 × 0.1.0 in a 25 mm preserved (contracted) length specimen. Radular teeth broad and elongate (Figure 9A). Central cusp sharp and wide, much longer than the lateral denticles. On each side of the cusp there are 6–8 sharp lateral denticles, the innermost ones merged with the basal sides of the cusp. Jaws elongate (Figure 9B), with no observable denticles on the masticatory border.
Reproductive system (Figure 10) with a small, convoluted ampulla that connects into the female gland complex. The prostate emerges from the female gland mass and consists of a broad convoluted tube that narrows into a long deferent duct and finally opens into the short, conical penis. A long penial gland connects next to the connection between the deferent duct and the penis. The vagina is short and expands into a spherical bursa copulatrix.
BIOLOGY
Only one mass of this common species was collected (in November) and the mass was at a very late stage of development (most of the embryos had hatched), suggesting this species spawns earlier in the year. Most of the mass had disintegrated but overall it appeared to be a long string, approximately 1.5 mm in diameter, strung over several filamentous hydroids with embryos contained singly in capsules in a tube inside the mass. Remaining embryos had large propodia, shells 340–450 µm in length, eyespots, and small vela (Figure 11). Most had already hatched from their capsules and were using their propodia to crawl in the gel. Once larvae were released in the laboratory, they were strong swimmers (Figure 11). The swimming ability of Cuthona crinita larvae suggests this species likely has a planktonic larval period though the small size of the vela indicates the duration of larval swimming may be short.
REMARKS
Cuthona crinita Minichev, Reference Minichev1972 was originally described by Minichev (Reference Minichev1972) based on preserved specimens collected from Mabus Point and Cape Khmara, Queen Mary Land, Antarctica. The preserved animals were large, up to 45 mm long, and described as colourless with a yellow tinge on the tips of the cerata. The identification of the specimens here studied as C. crinita is based on a comparison to the original description of this species by Minichev (Reference Minichev1972). The reproductive system of the material examined has a short ampulla, a long, wide prostate narrowing into an also very long and narrow deferent duct, a short, conical penis, and a very long penial gland. All these elements are also present in the drawings of the reproductive system of C. crinita by Minichev (Reference Minichev1972: fig. 10d) and not present in any other Antarctic species of Cuthona for which we have information about reproductive anatomy.
The radula of Minichev's (1972) specimens is also very similar to that of the animals here examined. The radular teeth of our specimens are broad and robust, with a sharp and wide central cusp, much longer than the lateral denticles. One each side of the cusp there are 6–8 sharp lateral denticles, the innermost ones merged with the basal sides of the cusp. This is very similar to the Minichev's (1972: figure 10g) drawings of the radula of C. crinita.
Another similar taxon that could constitute a synonym is Cuthona schradei var. bouvetensis Odhner, Reference Odhner1944 described based on a single specimen dredged in 200 m depth at Bouvet Island (Odhner, Reference Odhner1944). Odhner (Reference Odhner1944) indicated that the colour of the preserved animal was translucent and therefore different from the pale yellow original specimens of C. schraderi. The morphology of the reproductive system described by Odhner (Reference Odhner1944: fig. 32) for C. schradei var. bouvetensis is very similar to that of the material here examined. Both have a prostate that consists of a broad convoluted tube that narrows into a long deferent duct, which opens into the penis close to where the long penial gland connects. Also both have a short vagina that expands into a spherical bursa copulatrix. However, the penis of C. schradei var. bouvetensis is much longer that that of C. crinita and the specimens here examined. Another difference is that the radular teeth of C. schradei var. bouvetensis (Odhner, Reference Odhner1944: figure 30) are slightly narrower and have fewer lateral denticles than those of C. crinita and our specimens.
This is the second confirmed record of this species, and an expansion of the known range from Queen Mary Land into the Ross Sea. Some online photographs of nudibranchs found in the Antarctic Peninsula and identified as Notaeolidia gigas probably belong to this species (see Brueggeman, Reference Brueggeman1998).

Fig. 12. Cuthona giarannae sp. nov. LACM 3123, scanning electron microscopy photographs of the radula and jaws: (A) radular teeth; (B) jaw.

Fig. 13. Cuthona giarannae sp. nov. reproductive system of LACM 3124. am, ampulla; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate.
TYPE MATERIAL
Holotype: sewage outfall, McMurdo Sound, Ross Sea, Antarctica (77°51′31.95″S 166°40′47.51″E), 5 November 2007, 4 mm preserved length (LACM 3122).
Paratypes: Arrival Heights, McMurdo Sound, Ross Sea, Antarctica (77°50′14.29″S 166°37′59.14″E), 3 November 2006, 1 specimen 8 mm preserved length, dissected (LACM 3123). Sewage outfall, McMurdo Sound, Ross Sea, Antarctica (77°51′31.95″S 166°40′47.51″E), 5 November 2007, 1 specimen 8 mm preserved length, dissected (LACM 3124).
EXTERNAL MORPHOLOGY
Background colour translucent grey, with the digestive and reproductive organs visible as an opaque light grey mass (Figure 1H). Rhinophores and oral tentacles translucent grey completely covered with densely arranged small opaque white spots. Cerata translucent with opaque white apices. Digestive gland branches visible in the cerata as irregular greyish-brown tubes.
Body narrow and elongate. Foot broad with two small triangular corners on the anterior end. Rhinophores smooth, about twice as long as the oral tentacles and much wider. Cerata narrow, elongate, about the same width throughout. Cerata arranged in 7 rows, with 2–5 cerata each. The first two rows situated anterior to the pericardium and the rest posterior to the pericardium. Within each row, dorsal cerata tend to be larger than the more lateral ones. Reproductive opening on the right side of the body, below the second row of cerata. Anus latero-dorsal, located behind the pericardium, anterior to the third row of cerata.
ANATOMY
Radular formula 25 × 0.1.0 in a 5 mm preserved length specimen. Radular teeth narrow and elongate (Figure 12A). Central cusp generally shorter than the lateral denticles. Lateral denticles ranging from 6 to 8, relatively short and wide. In some teeth the two innermost lateral denticles are shorter than the central cusp. Jaws elongate (Figure 12B), with no observable denticles on the masticatory border.
Reproductive system (Figure 13) with a broad, short ampulla that connects into the female gland complex. The prostate emerges from the female gland mass and consists of a convoluted tube that narrows into a narrow deferent duct and finally opens into the elongate penis. A penial gland connects next to the connection between the deferent duct and the penis. There is no evidence of a vagina or bursa copulatrix.
ETYMOLOGY
This new species is named after Giar-Ann Kung, the SEM technician at the LACM, whose help was instrumental in the production of the scanning electron micrographs published in this and other papers.
REMARKS
Cuthona giarannae appears distinct from other Antarctic and sub-Antarctic species previously described and is therefore described herein as a new species.
The closest species in external appearance is Cuthona crinita, which also has a general whitish coloration with brownish-grey digestive branches in the cerata. These two species are easily distinguished because C. crinita is a much larger animal with relatively shorter rhinophores. Anatomically, C. crinita has broad and robust radular teeth with a sharp and wide central cusp, much longer than the lateral denticles. In contrast, the radular teeth of C. giarannae are narrow and elongate, with the central cusp generally shorter than the lateral denticles. The reproductive anatomy is also different, C. crinita having a much longer penial gland, more elongate prostate and a visible bursa copulatrix. However, the fact that a bursa copulatrix was not observed in C. giarannae could be due to the small size of the specimens examined. In addition to morphological differences, C. crinita and C. giarannae are genetically distinct (Shields et al., submitted).
The morphological characteristics of other Antarctic and sub-Antarctic species of Cuthona were summarized by Cattaneo-Vietti (Reference Cattaneo-Vietti1991). As mentioned throughout this paper, most species were described based on preserved specimens with limited anatomical information and therefore are very difficult to identify. Other species originally described from the Ross Sea (Cuthona modesta, C. elioti and C. paradoxa) are all re-described in this study, and all show significant differences from C. giarannae. Only C. modesta has similar radular morphology, with the central cusp shorter than the lateral denticles; however, the teeth of C. giarannae are much broader apically. In addition, these two species are very different externally. Cuthona modesta is a translucent species with opaque white pigment on the apices of the rhinophores and oral tentacles and the cerata have reddish-orange branches of the digestive glands. In contrast, C. giarannae has white pigment all over the rhinophores and oral tentacles and the digestive branches are greyish-brown.
Of all Antarctic and sub-Antarctic species of Cuthona, C. georgiana appears to have the widest geographical range. This species was originally described from South Georgia as Aeolis georgiana (Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886), based on 12 preserved specimens, the largest being 5 mm long. The original description contains limited information on the external morphology of this species. Subsequently, Odhner (Reference Odhner1926, Reference Odhner1944) re-described this species based on the type material and transferred it to the genus Cuthona. Odhner (Reference Odhner1926) illustrated the radula and Odhner (Reference Odhner1944) described the reproductive system of this species. These descriptions and illustrations confirm that C. georgiana is different from other species here described. The main differences between C. georgiana and C. giarannae are in the radular morphology (C. georgiana has a conspicuous central cusp longer than the lateral denticles) and the reproductive anatomy (C. georgiana has a distinct bursa copulatrix and a very large penial gland). Marcus (Reference Marcus1959) described one additional specimen of C. georgiana collected from Southern Chile. The preserved animal is anatomically different from the specimens studied by Odhner (Reference Odhner1926, Reference Odhner1944) and the specimens here examined. In the Marcus (Reference Marcus1959) specimen the radular teeth has lateral denticles arranged at the same level as the central cusp, although the central cusp is longer, whereas in Odhner's description only the innermost lateral denticles are at the same level as the central cusp, the rest being lower in the teeth. Also, the penial gland of the Marcus specimens appears to be comparatively smaller than that in Odhner's specimens. Finally, Cattaneo-Vietti (Reference Cattaneo-Vietti1991) cited C. georgiana for the first time from continental Antarctica and illustrated the radula and described the external morphology of live animals. Cattaneo-Vietti (Reference Cattaneo-Vietti1991) also considered Cratena exigua Thiele, 1912 to be a synonym. As mentioned above, we consider Cattaneo-Vietti's (1991) record to be C. modesta.

Fig. 14. Guyvalvoria paradoxa (Eliot, 1907) LACM 176423, scanning electron microscopy photographs of the radula and jaws: (A) radular teeth; (B) jaw; (C) detail of some radular teeth.

Fig. 15. Guyvalvoria paradoxa (Eliot, 1907) reproductive system of LACM 176423: (A) reproductive system; (B) detail of the bursa copulatrix, seminal receptacle and vagina connections. am, ampulla; bc, bursa copulatrix; dd, deferent duct; fg, female gland complex; pe, penis; pg, penial gland; pr, prostate; sr, seminal receptacle; vg, vagina.

Fig. 16. Masses and embryos of Guyvalvoria paradoxa. (A) Several masses attached to the stalk of a giant solitary hydroid Monocaulus parvula, photographed at the Dayton's Wall site in McMurdo Sound at approximately 30 m depth; (B) a single mass photographed in the laboratory; (C) embryos removed from the mass pictured in B and photographed under a compound microscope. Photographs by B. Miller.

Fig. 17. Guyvalvoria paradoxa on Monocaulus microrhiza, photographed in the field at Dayton's Wall, McMurdo Sound. Photograph by B. Miller.
?Aeolis schraderi Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886, p. 110–111, pl. 3, figure 7.
?Cuthona schraderi (Pfeffer in Martens & Pfeffer, Reference von Martens and Pfeffer1886): Odhner, Reference Odhner1926: 27–28, text figures 17–19.
Cuthonella paradoxa Eliot, Reference Eliot1907a: 24–25, text figure 27.
MATERIAL EXAMINED
Dayton's Wall, McMurdo Sound, Ross Sea, Antarctica (77°51′02.31″S 166°39′56.07″E), 28 November 2007, 1 specimen, 17 mm preserved length, dissected (LACM 176423), 29 November 2007, 1 specimen, 18 mm preserved length (LACM 176424).
EXTERNAL MORPHOLOGY
Colour uniformly translucent reddish-purple with the digestive system visible as a whitish mass in some parts of the body (Figure 1C, D), giving the body a general orange appearance. Some specimens substantially paler, possibly due to their diet. Branches of the digestive system visible in the cerata, as irregular, branched purple tubes. Cnidosacs opaque white. Dorsum, rhinophores and cerata covered with numerous opaque white dots, more densely arranged on the cerata. Rhinophores long, smooth, about 3–4 times as long as the oral tentacles. Cerata very elongate and narrow, wider near the base. Cerata arranged in 17 rows, 4 of them anterior to the genital aperture, 8 situated between the genital opening and the posterior end of the pericardium, and the rest posterior to the pericardium. Reproductive opening with the penial opening slightly anterior to the female opening. Anus dorsal, situated on the right side of the dorsum, posterior to the pericardium, behind the 10th row of cerata.
INTERNAL ANATOMY
Radular formula 11 × 0.1.0 in a 15 mm preserved length specimen. Radular teeth narrow and elongate, with a large, prominent, conical central cusp. On the sides of the cusp there are 13–15 small denticles, covering about 1/5 of the length of each tooth (Figure 14A, C). Jaws elongate (Figure 14B), with no masticatory border denticles visible.
Reproductive system with a large ampulla folded once (Figure 15), connecting with the female gland complex. Prostate long, tubular, emerging from the female gland complex and connecting with a short, wide penis and a large, folded accessory penial gland. Penis with no cuticular stylet. Vagina long, straight, connecting with a small, rounded bursa copulatrix and an elongate seminal receptacle (Figure 15).
BIOLOGY
The egg masses of Guyvalvoria paradoxa are thick, curved gelatinous bands between 3–6 mm in diameter and between 1 and >5 cm in length, ranging in colour from white to beige, pink, or pale purple (Figure 16A, B). Embryos in most masses observed were at the late veliger stage and were whitish, 350–390 µm in length, and each embryo was contained in an ovoid capsule that was slightly larger than the embryo (Figure 16C) (anterior edge of velum to shell apex). Occasionally, a mass has capsules that contained two, or rarely three embryos instead of one. Masses were locally abundant in October–December and were laid on hydroids, most frequently the stalks of the giant solitary species Moncaulus microrhiza or Monocaulus parvula (Figure 17). Egg masses were identified to species by COI sequence data, which were identical to adult Guyvalvoria (Shields et al., submitted).
The egg masses were mostly at the late veliger stage when collected, but some were at earlier stages of development (blastulae or trochophores). The large vela and small yolk volume of the embryos suggest this species may have planktonic development. Several different types of similarly sized larvae belonging to opisthobranch species have been reported from plankton samples in the Southern Ocean, though they have not been identified to species (Stanwell-Smith et al., Reference Stanwell-Smith, Hood and Peck1997).
REMARKS
Eliot (Reference Eliot1907a) described Cuthonella paradoxa Eliot, 1907 (type locality: Winter Quarters Bay, McMurdo Sound, Antarctica) as a yellow species with traces of reddish brown, some minute reddish dots on the larger cerata, and the intestines and hepatic diverticula seen through the integuments are reddish brown. A radular tooth is illustrated (Eliot, Reference Eliot1907a: text figure 27) as having a large central cusp with a series of denticles situated on each side of the cusp. Both the radular morphology and the description of the live animals are very similar to those of the specimens here examined, also collected from McMurdo Sound. Thus, we regard our specimens as belonging to Cuthonella paradoxa.
Martynov (Reference Martynov2006) reviewed the status of polar species of Tergipedidae with the anus situated caudally in comparison to most species of Cuthona. He resurrected the name Guyvalvoria for three Antarctic species and introduced the new name Murmania for an Arctic species having a dorsal triangular ridge-like wedging between the rhinophores. Martynov (Reference Martynov2006) regarded Cuthonella paradoxa as a synonym of Guyvalvoria francaisi Vayssière, Reference Vayssière1906 (type locality: ‘Île Wandel’ = Renaud Island, Antarctic Peninsula) and verified the morphology of the type material of both names. Martynov (Reference Martynov2006) also introduced two new species names of Guyvalvoria, G. gruzovi Martynov, Reference Martynov2006 (type locality: Amery Ice Shelf) and G. savinki Martynov, Reference Martynov2006 (type locality: off Kerguelen Island). None of these species has ever been illustrated or described alive, thus it is difficult to compare them with the live material here examined. Martynov's (2006) descriptions are comprehensive and include SEM of the radulae as well as illustrations of the reproductive anatomy, making anatomical comparisons possible.
Both the radular morphology and reproductive anatomy of Guyvalvoria francaisi illustrated by Martynov (Reference Martynov2006) are very different from those of the specimens of Cuthonella paradoxa here examined. For example, the radular teeth of G. francaisi have a very narrow, sharp cusp with large denticles on each side (Martynov, Reference Martynov2006: figure 3A, B), whereas in C. paradoxa the central cusp is much wider and the lateral denticles much smaller than those of G. francaisi. The reproductive system of G. francaisi has a wide, muscular prostate (Vayssière, Reference Vayssière1906: pl. 2, figure 23; Martynov, Reference Martynov2006: figure 5D) and appears to lack a seminal receptacle and bursa copulatrix (Martynov, Reference Martynov2006: figure 5D), whereas in C. paradoxa the prostate is a narrow tube much more similar to that G. savinkini, and it has a large, distinctive bursa copulatrix and seminal receptacle, which are absent in G. francaisi. Thus, we consider C. paradoxa to be distinct from G. francaisi. However, and in the absence of a comprehensive phylogenetic analysis we will follow Martynov's (2006) classification and regard C. paradoxa as a member of Guyvalvoria.
The specimens of Guyvalvoria paradoxa here examined display many similarities to specimens described by Pfeffer in Martens & Pfeffer (Reference von Martens and Pfeffer1886) as Aeolis schraderi (type locality: South Georgia Island) and later re-described by Odhner (Reference Odhner1926, Reference Odhner1944) as Cuthona schraderi. These similarities include external features and the radular morphology. Odhner (Reference Odhner1926, Reference Odhner1944) re-examined Pfeffer's specimens borrowed from the Zoological Museum in Hamburg, Germany, and considered specimen No. 6973 as the ‘type’ of the species (this decision makes this specimen the lectotype of Aeolis schraderi—ICZN, 1999, Article 74.5). Odhner (Reference Odhner1926, Reference Odhner1944) described in detail the external morphology of this specimen. Odhner (Reference Odhner1926) also described the radula of specimen No. 7534, which according to Odhner (Reference Odhner1944) clearly belongs to C. schraderi. The lectotype of Aeolis schraderi (specimen No. 6973), specimen No. 7534, and the specimens here examined both have the genital aperture with the male opening slightly separated from the female opening, a similar number of rows of cerata, the anus situated close to the 9th or 10th row of cerata, and radular teeth with a large central cusp with multiple denticles on each side (the lectotype teeth have not been described or illustrated). The colour of Aeolis schraderi was described by Pfeffer in Martens & Pfeffer (Reference von Martens and Pfeffer1886) as orange in the live animals and brown in the preserved state, which also resembles the colour of the living animals here examined. However, the descriptions and re-descriptions of Cuthona schraderi do not contain enough information to determine positively whether or not they are conspecific with Guyvalvoria paradoxa.
Odhner (Reference Odhner1944) also mentioned three other specimens collected at the German station in South Georgia (No. 7977, No. 8088 and No. 7534), which are either mixed lots or their identification is unclear. The radula of specimen No. 8088 illustrated by Odhner (Reference Odhner1944: text figure 29) is clearly different from that of specimen No. 7534 illustrated by Odhner (Reference Odhner1926: text figure 18). Odhner (Reference Odhner1944) explained the differences as intraspecific variation. The reproductive system and position of the anus of specimen No. 8088 (Odhner, Reference Odhner1944: text figures 22 & 32) are very different from the specimens here examined and more consistent with those of a species of Cuthona. For instance, specimen No. 8088 lacks a seminal receptacle and has a very elongated and straight penial gland connected to a long penis, similar to those described here for Cuthona crinita. Additionally, Odhner (Reference Odhner1944) described Cuthona schraderi var. bouvetensis Odhner, Reference Odhner1944, which has radular morphology similar to that of specimen No. 8088, but very different from the original specimens assigned to Aeolis schraderi. The identity of all these specimens is unclear.
ACKNOWLEDGEMENTS
We thank ANUDE team members P. Kitaeff, B. Miller, L. Mullen, J. Sprague, and E. Schreiber for assistance with collecting and photographing specimens, R. Robbins and S. Rupp for collecting specimens and dive support, and the science support staff of McMurdo Station for facilitating this research. Riccardo Cattaneo-Vietti kindly provided us with a photograph of the live animal and a scanning electron micrograph of the radula of C. modesta. Lindsey Groves (LACM) assisted with the curation of the specimens. This project was supported by National Science Foundation grants ANT-0551969 to A.L.M., ANT-0440577 to H.A.W., and DEB-0329054 to T.M. Gosliner and A.V.




















