Introduction
Influenza, also called the flu, is an extremely contagious respiratory disease, caused by the influenza virus (IV). The virus predominantly affects the upper respiratory tract (nose and throat), and in some cases may affect the lungs. The severity of clinical signs is variable, and they include fever, cough, sore throat, nasal congestion, difficulty breathing, muscle pain, headache, fatigue, nausea, vomiting, diarrhea, and lack of appetite (CDC, 2020a, 2020b).
IVs capable of infecting humans and causing epidemics and pandemics are viruses of the genera Influenzavirus A, B, and C, of the Orthomyxoviridae family. In addition to these, Influenzavirus D was identified in 2011. IVs infect different species, species A infects humans and animals in general, such as horses, birds, pigs, and dogs; species B infects humans, seals, and ferrets; species C infects humans, dogs and pigs; and species D was identified in goats, sheep, pigs, and cattle (Fig. 1). However, antibodies reactive to species D IV have already been identified in horses and humans (Jakeman et al., Reference Jakeman, Tisdale, Russell, Leone and Sweet1994; Youzbashi et al., Reference Youzbashi, Marschall, Chaloupka and Meier-Ewert1996; Osterhaus et al., Reference Osterhaus, Rimmelzwaan, Martina, Bestebroer and Fouchier2000; Matsuzaki et al., Reference Matsuzaki, Sugawara, Mizuta, Tsuchiya, Muraki, Hongo, Suzuki and Nakamura2002; Hause et al., Reference Hause, Ducatez, Collin, Ran, Liu, Sheng, Armien, Kaplan, Chakravarty, Hoppe, Webby, Simonson and Li2013; Ferguson et al., Reference Ferguson, Olivier, Genova, Epperson, Simth, Scheider, Barton, McCuan, Webby and Wan2016; Nedland et al., Reference Nedland, Wollman, Sreenivasan, Quast, Singrey, Fawcett, Christopher-Hennings, Nelson, Kaushik, Wang and Li2018). Although the pathogenesis of the latter virus has not been fully studied, some authors argue that humans, like pigs, can be infected with all IVs (Bailey et al., Reference Bailey, Choi, Fieldhouse, Borkenhagen, Zemke, Zhang and Gray2018; CDC, 2019). Pigs and chickens are considered two key reservoirs for IVs (Rajao et al., Reference Rajao, Vicent and Perez2018). However, the classification of natural reservoirs belongs mainly to water birds and wild sea birds (Yoon et al., Reference Yoon, Webby and Webster2014).
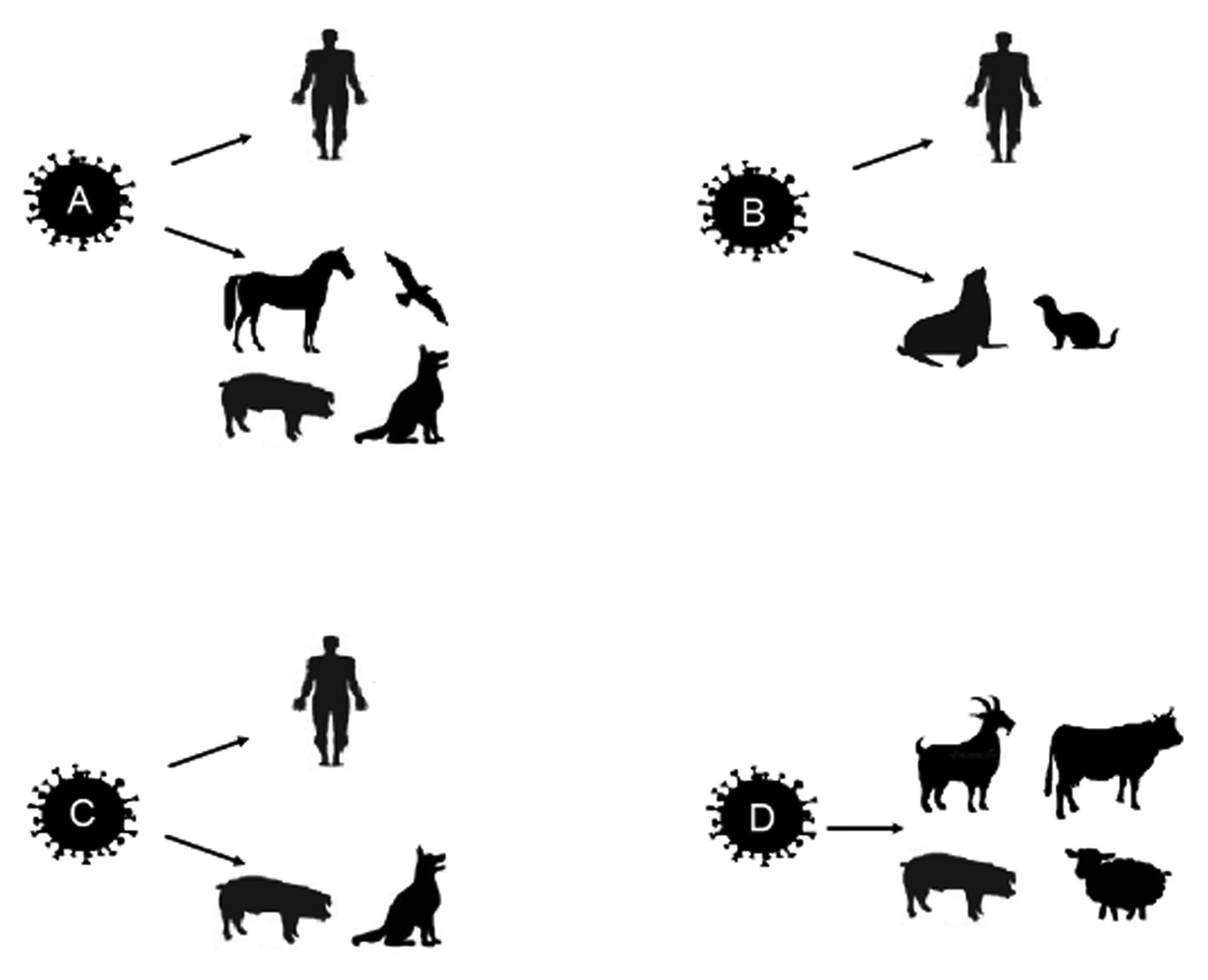
Fig. 1. Schematic representation of types A, B, C, and D of the influenza virus (IV) and the respective target species. IV A targets humans, horses, birds, pigs, and dogs; IV B affects humans, seals, and ferrets; IV C targets humans, pigs, and dogs; and IV D affects pigs, cows, goats, and sheep.
Genus A IV is considered the ancestral virus for all IVs (Doyle and Hopkins, Reference Doyle and Hopkins2011; Chambers, Reference Chambers2014). Being the most frequently found in circulation, this one is the main cause for the appearance of the disease, and has a greater predisposition to mutations and is the IV genus generally associated with epidemics and pandemics (Gasparini et al., Reference Gasparini, Amicizia, Lai, Bragazzi and Panatto2014a, Reference Gasparini, Amicizia, Lai, Bragazzi and Panatto2014b). IV A undergoes changes through the mutation, recombination, and rearrangement of its genetic material, constantly challenging the host's immune system (Webster et al., Reference Webster, Bean, Gorman, Chambers and Kawaoka1992; Mehle et al., Reference Mehle, Dugan, Taubenberg and Doudna2012). This virus has 13 proteins, among which are hemagglutinin (HA) and neuraminidase (NA), which represent 45% of the virus mass. Eighteen HA and 11 NA were identified, which are important in the classification of the strain (Aoyama et al., Reference Aoyama, Nobusawa and Kato1991, Hay et al., Reference Hay, Gregory, Douglas and Lin2001; Jagger and Digard, Reference Jagger and Digard2012; Cullinane and Newton, Reference Cullinane and Newton2013; Lewis et al., Reference Lewis, Anderson, Kitikoon, Skepner, Burke and Vicent2014). The disease caused by this virus affects a significant percentage of the world population, with epidemics and pandemics being described chronologically and geographically since ancient times, causing a total of ~10 million human deaths (Soema et al., Reference Soema, Kompier, Amorij and Kersten2015).
The first report of a disease that resembles influenza dates back to 412 BC, by Hippocrates, in his ‘Book of Epidemics’ with the name ‘fever of Perinthus’ or ‘cough of Perinthus.’ Some authors even claim that this is the first historical description of influenza (Kohn, Reference Kohn2007; Pappas et al., Reference Pappas, Kiriaze and Falagas2008). In 1173 and 1500 two outbreaks of influenza were described; however, the details of these episodes are not fully known (Kuszewski and Brydak, Reference Kuszewski and Brydak2000). The name ‘influenza’ appeared only in the 15th century, in Italy, due to an epidemic whose origin was attributed to the ‘influence of the stars,’ and the name spread throughout Europe, Asia, and Africa (Gintrac, Reference Gintrac1872).
Some authors and historians debate influenza on the American continent; that is to say, whether the disease already existed in this territory or whether it was introduced to the new world by infected pigs. There are some texts of Aztec origin that describe an outbreak of ‘pestilent catarrh’ between 1450 and 1456, in the current zone of Mexico. However, the hypotheses presented seem controversial and the manuscripts are difficult to translate for a complete and correct interpretation (de Souza, Reference de Souza2008). The first reliable documents date from 1510, describing a disease very similar to influenza and a virus that spread in Europe but originated in Africa. In 1557, the first large-scale epidemic occurred, but without contradiction the first pandemic dates from 1580. This pandemic originated in Asia and Russia, spreading to Europe through Asia Minor and North-West Africa, eventually affecting the American continent as well. The major tragedies occurred in Italy, where more than 8000 deaths were registered, and in Spain, where the disease even decimated entire cities (Potter, Reference Potter2001).
From the 15th century to the mid-19th century, 31 epidemics linked to influenza were recorded, including eight pandemics. Some of the outbreaks with the greatest impact occurred in 1729, from 1781 to 1782, from 1830 to 1833, from 1847 to 1848, and from 1898 to 1900. However, one of the most devastating outbreaks was the pandemic that occurred between 1918 and 1919, which was called the ‘Spanish’ flu, causing the death of more than 20 million people. Some authors described it as ‘the greatest medical holocaust in history’ (Waring, Reference Waring1971; Potter, Reference Potter2001). It was only in 1932/1933 that the virus was isolated for the first time, by collecting nasal secretions from infected patients (Smith et al., Reference Smith, Andrewes and Laidlaw1933). Subsequently, four pandemics related to swine IV were identified in humans in 1918, 1957, 1968, and 2009, namely H1N1, H2N2, H3N2, and H1N1, respectively (Scholtissek et al., Reference Scholtissek, Rohde, von Hoyningen and Rott1978; Crosby, Reference Crosby2003; Krueger and Gray, Reference Krueger and Gray2012; Mena et al., Reference Mena, Nelson, Quezada-Monroy, Dutta, Cortes-Fernández, Lara-Puente, Castro-Peralta, Cunha, Trovão, Lozano-Dubernard, Rambaut, van Bakel and García-Sastre2016) In 2018, >10 subtypes of swine IV circulating in the United States were reported (Walia et al., Reference Walia, Anderson and Vincent2018).
Equine influenza
Equine influenza (EI) is a highly infectious disease that affects the respiratory system of horses, with a high economic impact (Glass et al., Reference Glass, Wood, Mumford, Jessett and Grenfell2002; Arthur and Suann, Reference Arthur and Suann2011).
Etiology
The EI virus (EIV) belongs to the Influenzavirus A genus and the Orthomyxoviridae family and is considered the most significant respiratory pathogen in horses (Timoney, Reference Timoney1996). This was first identified in horses in 1956, in Prague. However, its presence was suggested as far back as 433 AD by the Greek veterinarian Absyrtus. In the year 1872, a huge outbreak was described that affected a considerable part of the horse population in North America, also affecting the entire economic situation and commercial services provided. EIV is considered a highly contagious agent, responsible for outbreaks of respiratory disease in horses in many countries, with high rates of transmission even among other species (Law, Reference Law1874; Sovinova et al., Reference Sovinova, Tomova, Pouska and Nemec1958; van Maanen and Cullinane, Reference van Maanen and Cullinane2002; Myers and Wilson, Reference Myers and Wilson2006).
The diameter of the virus ranges from 80 to 120 nm (Palese and Schulman, Reference Palese and Schulman1976; Ritchey et al., Reference Ritchey, Palese and Schulman1976; Timoney, Reference Timoney1996; Burnouf et al., Reference Burnouf, Griffiths, Padilla, Seddik, Stephano and Gutierrez2004; Krumbholz et al., Reference Krumbholz, Philipps, Oehring, Schwarzer, Eitner, Wutzler and Zell2010; Elton et al., Reference Elton, Bruce, Bryant, Wise, MacRae, Rash, Smith, Turnbull, Medcalf, Daly and Digard2013). The EIV genome is composed of eight negative-sense RNA segments, which are encapsulated by a nucleoprotein, giving them helical symmetry. Each of these RNA segments encodes up to 10 structural and non-structural proteins. The seventh segment is responsible for the shape of the EIV because it encodes the membrane protein. Certain more virulent strains encode the 11th protein. This protein has the ability to affect multiple systems, inducing apoptosis, promoting inflammation, and regulating viral polymerase activity.
The proteins encoded by the virus are HA, NA, matrix protein (M), nucleoprotein, three polymerase proteins [basic polymerase protein 1 (PB1), basic polymerase protein 2 (PB2) and acid polymerase protein (PA)], a nuclear export protein (NEP) and a non-structural protein (NS1) (Timoney, Reference Timoney1996; Elton and Bryant, Reference Elton and Bryant2011).
NS1 and PB1-F2 are proteins with an active role in viral replication but are not incorporated into the viral structure. PB1-F2 is a derivation of PB1 and is a smaller protein encoded by the open reading frame observed in some strains. NS1 is considered the most antagonistic protein in the immune response of target cells, interfering with type 1 interferons (INF) and thus reducing the production of IFN-β. NS1 is made up of 230 amino acids, however, it presents a different size when compared to that present in other species, especially with that observed in humans and swine. It also performs an RNA binding function and effector function (Suarez and Perdue, Reference Suarez and Perdue1998; Hale et al., Reference Hale, Randall, Ortin and Jackson2008; Wang et al., Reference Wang, Suarez, Patin-Jackwood, Mibayashi, García-Sastre, Saif and Lee2008; Boukharta et al., Reference Boukharta, Azlmat, Elharrak and Ennaji2015). NEP, which used to be considered as a non-structural protein called NS2, has now been shown to be linked to M protein, having been identified in the virion. Its action is essential for the release of viral ribonucleoproteins from the host cell, more specifically the nucleus (Paterson and Fodor, Reference Paterson and Fodor2012).
The HA glycoprotein is responsible for the response to host antibodies and the NA glycoprotein is responsible for the proliferation of the virus, by migration through the mucous membrane of the cells of the respiratory tract, which later, after proliferating, will release the viruses from the affected cells. These glycoproteins are responsible for the spikes that project outside the viral envelope (Timoney, Reference Timoney1996; Matrosovich et al., Reference Matrosovich, Matrosovich, Gray, Roberts and Klenk2004; Elton and Bryant, Reference Elton and Bryant2011).
The two subtypes of IV A which cause EI are H7N7 (subtype 1) and H3N8 (subtype 2), with H7N7 being the first to be identified in horses in Eastern Europe. This subtype H7N7 has not been isolated in horses since 1979 and is considered extinct (Sovinova et al., Reference Sovinova, Tomova, Pouska and Nemec1958; Webster, Reference Webster1993; Timoney, Reference Timoney1996). The nonexistence of the H7N7 subtype cases is a consequence of the strong bonds of the codon of this virus, made by a sequence of three nitrogenous bases of messenger RNA that encode an amino acid, without alterations based on the mutation or nucleotide composition. Some authors believe that it is far easier to induce protection against this virus and the widespread vaccination program contributed to the disappearance of this virus (Yarus et al., Reference Yarus, Widmann and Knight2009; Murcia et al., Reference Murcia, Wood and Holmes2011; Kumar et al., Reference Kumar, Bera, Greenbaum, Bhatia, Sood, Selvaraj, Anand, Tripathi and Virmani2016).
In 1963, H3N8 was isolated for the first time from horses. Initially an avian IV (AIV), it later diverged into two different lineages around 1980, which were called American and Eurasian (Daly et al., Reference Daly, Lai, Binns, Chambers, Barrandeguy and Mumford1996; Damiani et al., Reference Damiani, Scicluna, Ciabatti, Cardeti, Sala, Vulcano, Cordioli, Martella, Amaddeo and Autorino2008; Cullinane et al., Reference Cullinane, Elton and Mumford2010; Saenz et al., Reference Saenz, Quinlivan, Elton, Macrae, Blunden, Mumford, Daly, Digard, Cullinane, Grenfell, McCauley, Wood and Gog2010). Subsequently, the American lineage diverged into Florida, Kentucky, and Argentina sublineages. Among these three sublineages, there is a predominance of rotation, varying from year to year, which is called ‘re-cycling.’ This process is continuous and, after 3 years, it will repeat the predominance of the strain that started the cycle. It is believed that this mechanism allows for the survival of the virus and its perpetuation, changing the immunological target without evolution (Lai et al., Reference Lai, Chambers, Holland, Morley, Haines, Townsend and Barrandeguy2001, Reference Lai, Rogers, Glaser, Tudor and Chambers2004). In practice, the Florida line is predominant, because of the sequencing of HA, it was possible to identify two derivations of this line, which were classified as ‘clade 1’ and ‘clade 2.’ The major difference between each type is found in the sequence of HA, NA, and PA (Bryant et al., Reference Bryant, Rash, Russell, Ross, Cooke, Bowman, MacRae, Lewis, Paillot, Zanoni, Meier, Griffiths, Daly, Tiwari, Chambers, Newton and Elton2009, Reference Bryant, Rash, Woodward, Medcalf, Helwegen, Wohlfender, Cruz, Herrmann, Borchers, Tiwari, Chambers, Newton, Mumford and Elton2011; Murcia et al., Reference Murcia, Wood and Holmes2011). There are authors who defend the disappearance of pre-divergent strains and that these were overtaken by viruses that evolved in other strains and lineages. On the other hand, there are data that prove the similarity of strains identified in 2004 with strains that circulated before 1990, some with 99% compatibility (Martella et al., Reference Martella, Elia, Decaro, Di Trani, Lorusso, Campolo, Desario, Parisi, Cavaliere and Buonavoglia2007; Boukharta et al., Reference Boukharta, Azlmat, Elharrak and Ennaji2015).
The appearance of new strains is due to antigenic drift, which consists of the accumulation of mutant spots in the gene that encodes the surface of the HA and NA protein. They are small changes that occur in this protein, but the other proteins are also susceptible to this process. Usually, the result from these changes is a virus like the original, allowing the body to recognize and be able to have an immune response. When these changes are accumulated through time, they can result in a totally different virus. Antigenic shift is another type of process that can result in a new strain. This is a remarkable event in the viral genome, caused by a rearrangement of the genes, majoritarian at the level of NA or HA or both, that may result from a co-infection with another strain, and may even be secondary to a process of cross-infection. These alterations can be observable at various levels of amino acids which lead to the appearance of new strains, sometimes with similarities to old strains. These changes also affect the behavior of the virus at various levels (Lindstrom et al., Reference Lindstrom, Endo, Sugita, Pecoraro, Hiromoto, Kamada, Takahashi and Nerome1998; Lewis et al., Reference Lewis, Daly, Russell, Horton, Skepner, Bryant, Burke, Rash, Wood, Chambers, Fouchier, Mumford, Elton and Smith2011; Rash et al., Reference Rash, Morton, Woodward, Maes, McCauley, Bryant and Elton2017; CDC, 2021).
Epidemiology
Genetically, EIV and AIV are very similar, which may indicate the coexistence of IV in both horses and birds. The distinction between strains of EIV can be made by analysing a common element visible in all strains that belong to the American lineage. This is the presence of I194 V, which does not occur in strains derived from the Eurasian lineage (Cullinane and Newton, Reference Cullinane and Newton2013; Chambers, Reference Chambers2014; Landolt, Reference Landolt2014). Transmission of the virus between species, namely between horses and carnivores, can occur, as has already been identified in an outbreak of influenza in dogs. Laboratory analyses identified the presence of the H3N8 subtype in an EI outbreak in racing dogs (English Greyhounds) on a track in the United States and the United Kingdom. It was found that the transmission occurred due to proximity to the horses; however, there was no lateral transmission (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Crawford et al., Reference Crawford, Dubovi, Castleman, Stephenson, Gibbs, Chen, Smith, Hill, Ferro, Pompey, Bright, Medina, Johnson, Olsen, Cox, Klimov, Katz and Donis2005; Daly et al., Reference Daly, Blunden, Macrae, Miller, Bowman, Kolodziejek, Nowotny and Smith2008; Gibbs and Anderson, Reference Gibbs and Anderson2010; Kirkland et al., Reference Kirkland, Finlaison, Crispe and Hurt2010; Crispe et al., Reference Crispe, Finlaison, Hurt and Kirkland2011; Wang et al., Reference Wang, Wang, Hu, Sun, Pu, Liu and Sun2017).
EIV transmission is not solely confined to dogs, there is evidence of transmission in humans, camels, and zebras. It is assumed that the presence of this virus in other species, such as humans, can lead to its rearrangement with human IV, with the consequent appearance of new strains. When the virus infects humans, it cannot be attenuated and it is able to infect healthy horses with an infection capacity similar to that which exists between horses (Couch et al., Reference Couch, Douglas, Rossen and Kasel1969; Yondon et al., Reference Yondon, Zayat, Nelson, Heil, Anderson, Lin, Halpin, McKenzie, White, Wentworth and Gray2014; Larson et al., Reference Larson, Heil, Chambers, Capuanod, White and Gray2015; Na et al., Reference Na, Yeom, Yuk, Moon, Kang and Song2016). In China, EIV has also been isolated from two pigs. They did not have any symptoms, but they served as a vehicle for viral rearrangement and the emergence of a new strain (Solórzano et al., Reference Solórzano, Foni, Córdoba, Baratelli, Razzuoli, Bilato, del Burgo, Perlin, Martínez, Martínez-Orellana, Fraile, Chiapponi, Amadori, del Real and Montoya2015). In addition to the animals already mentioned, it has been possible to demonstrate that cats can also be infected through the experimental transmission of EIV H3N8 (Tu et al., Reference Tu, Zhou, Jiang, Li, Zhang, Guo, Zou, Chen and Jin2009; Su et al., Reference Su, Wang, Fu, He, Hong, Zhou, Lai, Gray and Li2014) (Fig. 2). There are no data on infection of horses with H1N1 AIV, but the same cannot be said for H3N8, whose avian strain A/equine/Jilin/1/1989, which appeared in China in 1989, was the cause of a high equine mortality rate and can be characterized as a more severe disease. EIV can affect dogs and, as it is a cross-transmission, causes serious illness and death (Webster and Yuanji, Reference Webster and Yuanji1991; Crawford et al., Reference Crawford, Dubovi, Castleman, Stephenson, Gibbs, Chen, Smith, Hill, Ferro, Pompey, Bright, Medina, Johnson, Olsen, Cox, Klimov, Katz and Donis2005).
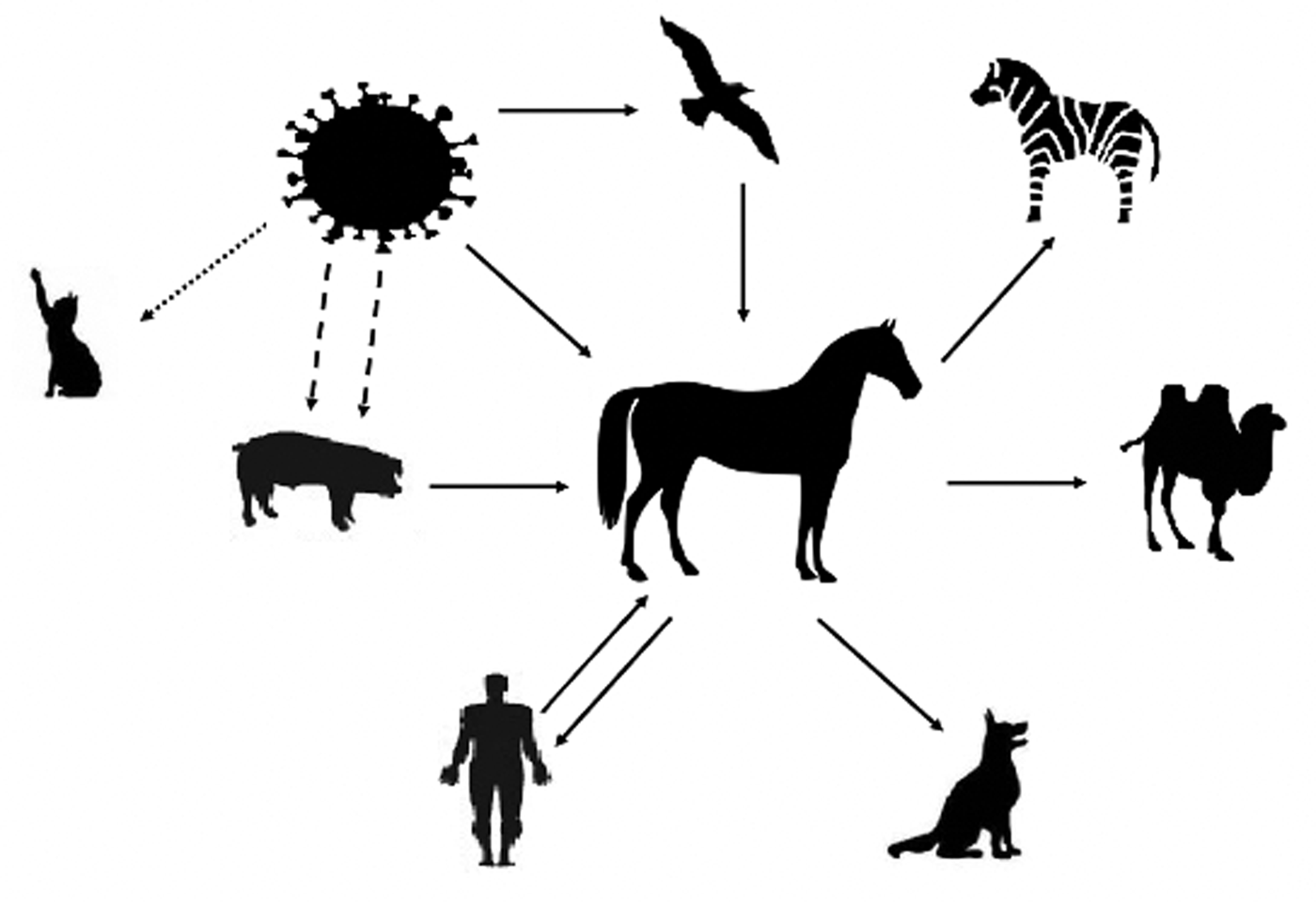
Fig. 2. The equine influenza virus (EIV) can directly infect the horse or come via other animals, such as pigs and birds. Pigs act as a vector for genetic rearrangement, allowing two strains to infect the animal, which will later result in a new strain. The presence of EIV has already been detected in zebras, camels, humans, and dogs. The virus can infect humans and infect other horses without losing its pathogenesis. Although cats can be infected, it has only been demonstrated at the laboratory level.
It is important to note that IV has restrictions on cross-infection due to HA. As it is a viral receptor-binding protein, it has the function of binding to the sialic acid of the host cell receptor. Sialic acid can appear as N-acetylneuramic acid or N-gluconeuramine. The bond depends on the sialic acid and the galactose portion, corresponding to the α(2→6) or α(2→3) linkage. It is possible to state that human IV, unlike animal IV (avian, canine, and equine), has a preference for the α(2→6)-gal linkage with N-acetylneuraminic acid (Ito and Kawaoka, Reference Ito and Kawaoka2000).
The differences between the EIV isolated from the horse and the dog occur so that these viruses correspond to the specificities of the target cell receptors of each species. However, the biology of the virus remains virtually unchanged when it moves to another host. There are changes in viruses whose effects are only visible when cross-infection occurs, as in the case of the strain that caused moderate pathogenesis in horses, and then originated the canine IV in Colorado. The observed change was the mutation in the acquired receptor for the virus to enter the target cell. Canine and equine H3N8 viruses show little difference from each other, with a mutation in the PA-X protein present in both (Collins et al., Reference Collins, Vachieri, Haire, Ogrodowicz, Martin, Walker, Xiong, Gamblin and Skehel2014; Feng et al., Reference Feng, Gonzalez, Deng, Yu, Tse, Huang, Huang, Wasik, Zhou, Wentworth, Holmes, Chen, Varki, Murcia and Parrish2015, Reference Feng, Sun, Iketani, Holmes and Parrish2016). The simultaneous circulation of the virus in dogs and horses allows bidirectional transmission between these species (Rivailler et al., Reference Rivailler, Perry, Jang, Davis, Chen, Dubovi and Donis2010).
Outbreaks of EI are characterized by a visible and rapid spread of the disease, with the peak occurring 1 week after the first case is identified and new cases will no longer be identified after 21–28 days. The spread of EI around the world is mostly due to the traffic of horses, with its reintroduction taking place in countries that no longer had the disease, as was the case in Japan (Mumford, Reference Mumford1990; Powell et al., Reference Powell, Watkins, Li and Shortridge1995; Morley et al., Reference Morley, Townsend, Bogdan and Haines2000; Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Yamanaka et al., Reference Yamanaka, Niwa, Tsujimura, Kondo and Matsumura2008a).
According to data from the World Organization for Animal Health (OIE), outbreaks of EIV (H3N8) strains were isolated and characterized in several countries such as Argentina, Germany, Chile, China, United States, France, Holland, Ireland, Nigeria, Sweden, United Kingdom, and Uruguay. In 2019, the same organization confirmed the occurrence of five new outbreaks in Italy, with the last outbreak recorded in that country in 1999 (OIE, 2019a, 2019d). According to the OIE, some countries have never reported cases of EI, such as Bangladesh, Belarus, Bolivia, Bulgaria, Ethiopia, Georgia, Greenland, French Guiana, Honduras, Iran, Iceland, Laos, Latvia, Lithuania, Malawi, Madagascar, Myanmar, New Zealand, Central African Republic, Sri Lanka, Swaziland, Sudan, Togo, Thailand, Chinese Taipei, and Zimbabwe (OIE, 2019b) (Fig. 3).
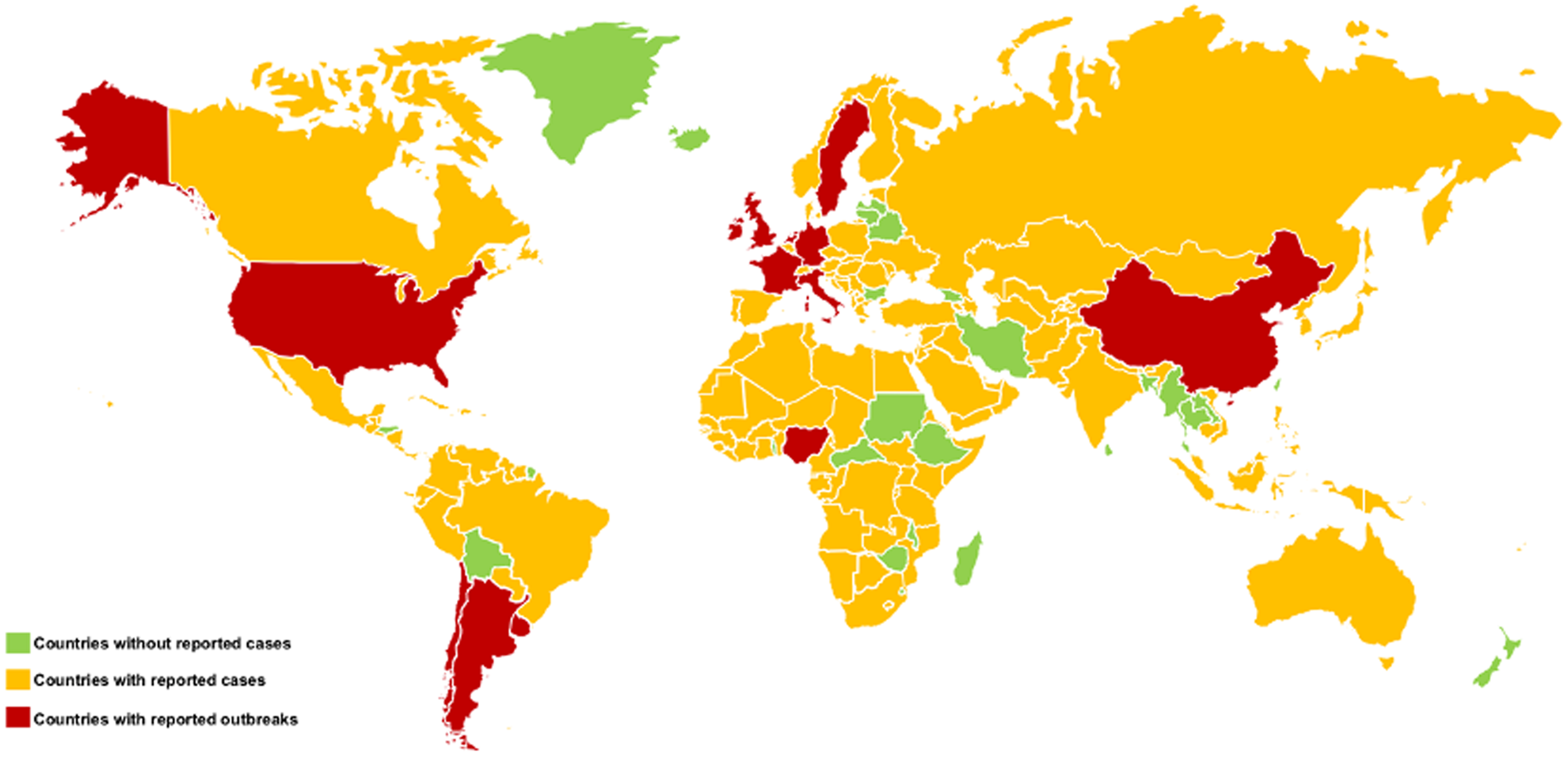
Fig. 3. Worldwide distribution of equine influenza (EI), where it is possible to identify countries without reported cases (green), countries with reported cases (yellow) and countries with reported outbreaks (red).
In the past, EI epidemics have been identified in several countries in Europe and North America at different times, such as the most prominent cases of the 1956 epidemics due to the H7N7 subtype, and the 1963, 1969, 1979, and 1989 epidemics due to the H3N8 subtype (van Maanen and Cullinane, Reference van Maanen and Cullinane2002; Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003). These outbreaks have been linked to the appearance of a new virus, as was the case in Miami in 1963, or to mutations in the strains, resulting in vaccine inefficiency. Another outbreak resulting from a vaccine failure was reported in Croatia, in 2004 (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Barbic et al., Reference Barbic, Madic, Turk and Daly2009). The H7N7 virus has also been linked to two outbreaks on the Asian continent, one in 1977 in Malaysia and the other in 1987 in India (Uppal and Yadav, Reference Uppal and Yadav1987; Uppal et al., Reference Uppal, Yadav and Sharma1987). The largest outbreaks of EI were recorded in China in 1989, where more than one million horses were affected by the A/equine/Jilin/1/1989 (H3N8JL89) strain, an avian-like virus, and in 1993/1994, due to the conventional strain of the H3N8 virus, very similar to the strain isolated in Europe in 1991 (van Maanen and Cullinane, Reference van Maanen and Cullinane2002). The Florida strain has been isolated in Europe since 2003, where a large-scale outbreak occurred in Newmark, with infection of vaccinated and unvaccinated horses (Newton et al., Reference Newton, Daly, Spencer and Mumford2006).
Most cases of EI identified in Europe, from 2006 to 2008, belong to the Florida clade 2 (FC2), because it was the predominant strain with a major outbreak in Sweden in 2007. On the other hand, the Florida clade 1 (FC1) has been identified more sporadically, and few outbreaks have been associated with this strain (Bryant et al., Reference Bryant, Rash, Russell, Ross, Cooke, Bowman, MacRae, Lewis, Paillot, Zanoni, Meier, Griffiths, Daly, Tiwari, Chambers, Newton and Elton2009, Reference Bryant, Rash, Woodward, Medcalf, Helwegen, Wohlfender, Cruz, Herrmann, Borchers, Tiwari, Chambers, Newton, Mumford and Elton2011; Gildea et al., Reference Gildea, Quinlivan, Arkins and Cullinane2012; Legrand et al., Reference Legrand, Pitel, Marcillaud-Pitel, Cuillinane, Couroucé, Fortier, Freymuth and Pronost2013; Woodward et al., Reference Woodward, Rash, Blinman, Bowman, Chambers, Daly, Damiani, Joseph, Lewis, McCauley, Medcalf, Mumford, Newton, Tiwari, Bryant and Elton2014). The FC1 is the main cause of outbreaks in the United States, whereas FC2 is more recently occurring in North America, having only been identified for the first time in a horse imported from Europe (Bryant et al., Reference Bryant, Rash, Woodward, Medcalf, Helwegen, Wohlfender, Cruz, Herrmann, Borchers, Tiwari, Chambers, Newton, Mumford and Elton2011; Pusterla, et al., Reference Pusterla, Mapes and Wademan2014).
Between 2007 and 2008, an outbreak of FC1 occurred in Japan and Australia, and in the same time span there was a major outbreak that affected China, Mongolia, and Kazakhstan. The outbreak occurring in Australia, a country that was free of influenza, resulted from the importation of a subclinically infected horse (EDS, 2008; Bryant et al., Reference Bryant, Rash, Russell, Ross, Cooke, Bowman, MacRae, Lewis, Paillot, Zanoni, Meier, Griffiths, Daly, Tiwari, Chambers, Newton and Elton2009; Watson et al., Reference Watson, Daniels, Kirkland, Carroll and Jeggo2011). Later, in 2012, an outbreak occurring in these countries was caused by strains derived from FC2. The number of infected animals in Australia was ~76,000 (Cullinane and Newton, Reference Cullinane and Newton2013; Paillot, Reference Paillot2014; Yondon, et al., Reference Yondon, Zayat, Nelson, Heil, Anderson, Lin, Halpin, McKenzie, White, Wentworth and Gray2014).
In 2011, the FC1 strain was isolated for the first time in Sweden, and since then only sporadic cases have appeared. Between 2012 and 2014 all cases of EI identified in Sweden were caused by FC2. The same occurred in Ireland, with the first case of FC1 being identified in 2010, and only FC2 cases have been identified since 2011. The 2011 outbreak in Sweden demonstrated the presence of both strains simultaneously, however with different origins (Gildea et al., Reference Gildea, Quinlivan, Arkins and Cullinane2012, Reference Gildea, Fitzpatrick and Cullinane2013; Back et al., Reference Back, Berndtsson, Gröndahl, Ståhl, Pringle and Zohari2016).
In France, something similar occurred, in which the predominant strain was FC2 between 2005 and 2010, with an outbreak of FC1 in 2009 attributed to a failure in vaccines (Legrand et al., Reference Legrand, Pitel, Marcillaud-Pitel, Cuillinane, Couroucé, Fortier, Freymuth and Pronost2013). In the United Kingdom, a case of FC1 occurred in 2006–2007, and from 2008 to 2009 the outbreaks were associated with both strains (Bryant et al., Reference Bryant, Rash, Russell, Ross, Cooke, Bowman, MacRae, Lewis, Paillot, Zanoni, Meier, Griffiths, Daly, Tiwari, Chambers, Newton and Elton2009, Reference Bryant, Rash, Woodward, Medcalf, Helwegen, Wohlfender, Cruz, Herrmann, Borchers, Tiwari, Chambers, Newton, Mumford and Elton2011). Between 2010 and 2012, only FC2 cases were detected in Germany and the United Kingdom (Woodward et al., Reference Woodward, Rash, Blinman, Bowman, Chambers, Daly, Damiani, Joseph, Lewis, McCauley, Medcalf, Mumford, Newton, Tiwari, Bryant and Elton2014).
FC2 is considered endemic in Europe and Asia, with periodic outbreaks, while FC1 is endemic in the United States, causing outbreaks which are sporadic and occur in different parts of the world (Back et al., Reference Back, Berndtsson, Gröndahl, Ståhl, Pringle and Zohari2016).
Currently, the virus circulates around the world, but South America is considered the epicenter of the spread of the H3N8 virus (Perglione et al., Reference Perglione, Golemba, Torres and Barrandeguy2016). These data led us to conclude that the prevalence of the disease in Europe has been changing from the Eurasian strain to the Florida strain, with an increase in cases of FC1 and FC2. Studies show that FC2 is diverging, and changes in strain antigens detected between 2013 and 2015 were characterized in a study conducted in the United Kingdom (Gildea et al., Reference Gildea, Quinlivan, Arkins and Cullinane2012; Rash et al., Reference Rash, Morton, Woodward, Maes, McCauley, Bryant and Elton2017). According to data from the National Institute for Agricultural and Veterinary Research (Instituto Nacional de Investigação Agrária e Veterinária – INIAV), in 2018 and 2019, two cases of EI were detected in Portugal caused by H3N8. According to the Animal Health Trust, in 2019, 21 cases of EI were identified in Ireland, 229 cases in the United Kingdom, and four cases in Sweden (Equineflunet, 2020). Conducting studies on the disease and the development of outbreaks in countries is extremely important for controlling the virus and identifying strains so that vaccines remain effective. Lack of monitoring can lead to the introduction of new lineages that have not been identified in such countries, triggering a mutation and leading to an outbreak of two lineages. The decrease or absence of control when new animals are brought into a country can lead to the occurrence of an outbreak, at a distance of 400 km, as was caused by the transport of horses across the Nordic countries (Back et al., Reference Back, Berndtsson, Gröndahl, Ståhl, Pringle and Zohari2016).
More recently, in December 2018 and January 2019, outbreaks were reported in several European countries, like France, Germany, and the UK (EFP, 2019; ICC, 2019; RESPE, 2019). The outbreak in France was identified to be like FC1, much more common in the American strains, and the virus identified in France was very similar to that of South America. The presence of this lineage in France has not been reported since 2009, and this situation changed by the introduction of unvaccinated and infected horses in French premises (Fougerolle et al., Reference Fougerolle, Legrand, Lecouturier, Sailleau, Paillot, Hans and Pronost2017; Paillot et al., Reference Paillot, Pitel, Pronost, Legrand, Fougerolle, Jourdan and Marcillaud-Pitel2019). The outbreak was confirmed until August in the UK and later in October, another report was presented involving an imported horse (British Veterinary Association, 2019).
Almost at the same time, later in 2018, several African countries reported mortality in donkeys and horses. Through field samples, EIV FC1 was identified in Niger and Senegal. The presence of EI was also suspected in Ghana, but not yet confirmed (Diallo et al., Reference Diallo, Souley, Ibrahim, Alassane, Issa, Gagara, Yaou, Issiakou, Diop, Diouf, Lo, Lo, Bakhoum, Sylla, Seck, Meseko, Shittu, Cullinane, Settypalli, Lamien, Dundon and Cattoli2020). The outbreaks in Niger, Senegal, and Nigeria, showed an EIV much more similar to the virus present in South America than that in Europe and the United States. This similarity supports an epidemiological link between South America and West Africa, and this is linked with the importation of horses from one region to the other (Sule et al., Reference Sule, Oluwayelu, Adedokun, Rufai, McCracken, Mansfield and Johnson2015; Diallo et al., Reference Diallo, Souley, Ibrahim, Alassane, Issa, Gagara, Yaou, Issiakou, Diop, Diouf, Lo, Lo, Bakhoum, Sylla, Seck, Meseko, Shittu, Cullinane, Settypalli, Lamien, Dundon and Cattoli2020).
The gender of the animal has no influence at all on predisposition to infection by EIV (Nyaga et al., Reference Nyaga, Wiggins and Priester1980; Gross et al., Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004). According to some studies, there seems to be some breed predisposition to infection by EIV and the development of the disease. Thoroughbred English horses appear to be more resistant to infection by EIV when compared to other breeds. Quarter Horses are the breed of horses most at risk of infection by EIV (Nyaga et al., Reference Nyaga, Wiggins and Priester1980; Gildea et al., Reference Gildea, Arkins and Cullinane2010). Regarding age, all age groups are susceptible to the development of the disease. However, the period of greatest susceptibility occurs between 2 and 6 months of age, due to the loss of antibodies acquired passively from 2 months of age. Some studies consider animals up to 5 years old more vulnerable to the disease (Nyaga et al., Reference Nyaga, Wiggins and Priester1980; Liu et al., Reference Liu, Pascoe, Chang and Zee1985; Landolt, Reference Landolt2014). Older horses are practically immune, due to natural exposure to the disease or vaccination (Morley et al., Reference Morley, Townsend, Bogdan and Haines2000). Vaccination history and exposure to the disease are also considered important factors. The higher the concentration of antibodies, the lower is the risk of developing the disease (Bogdan et al., Reference Bogdan, Morley, Townsend and Haines1993; Morley et al., Reference Morley, Townsend, Bogdan and Haines2000).
Transmission and pathogeny
The spread of EIV is considered to be one of the fastest among the other respiratory diseases that affect horses. The IV is relatively susceptible to environmental conditions; however, horses can be infected by the proximity of another sick animal through aerosols or by direct contact with contaminated equipment (fomites). Droplets from nasal discharges play a very important role in the spread of EIV and are one of the main causes for the spread of disease, not least because they lead to the creation of fomites (Timoney, Reference Timoney1996; Easterday et al., Reference Easterday, Hinshaw and Halvorson1997). Aerosols can propagate up to a distance of up to 35 m from the infected horse, and this may be greater, depending on the frequency of the cough. The lifetime of EIV in the environment as an aerosol is 24 to 36 h, but on surfaces, it can reach up to three days, whereas human IV survives only 15 h as an aerosol. Incubation time is one to three days, with a period of three to eight days (sometimes up to 10 days) of transmissibility to other animals (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Daly et al., Reference Daly, Newton and Mumford2004; Gross et al., Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004). Keeping animals in closed stables, with poor ventilation and a high concentration of animals, facilitates the spread of the virus (Morley et al., Reference Morley, Townsend, Bogdan and Haines2000). In unvaccinated horses, the rate of infection is ~100%. Partially immunized animals become subclinically infected and tend to spread the virus less when compared to non-immunized animals (Chambers, Reference Chambers2014; Landolt, Reference Landolt2014). Horses with clinical signs have a higher rate of infection spread when compared to asymptomatic animals (Cullinane and Newton, Reference Cullinane and Newton2013). After recovery, the animal does not have the virus and, therefore, the disease is considered self-limiting and sterile (Cullinane and Newton, Reference Cullinane and Newton2013).
EIV causes an infection in the upper and lower respiratory tract, and the development of lesions in the lungs of adult horses is common. In foals, the virus can cause severe pneumonia, which is sometimes fatal (Britton and Robinson, Reference Britton and Robinson2002; Gross et al., Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004; Peek et al., Reference Peek, Landolt, Karasin, Slack, Steinberg, Semrad and Olsen2004).
After inhalation, the virus adheres to epithelial cells through the HA glycoprotein spikes, which eventually fuse with the cell by adhering to the sialic acid receptors on the cell surface, thus allowing the viral particle to enter the cytoplasm in order to replicate. Horses have a mucus layer in the nasal cavity that can prevent HA virus binding, thereby inhibiting the virus from entering the cell (Scocco and Pedini, Reference Scocco and Pedini2008). The low pH inside the epithelial cell provides conditions for the fusion process between the virus and the cell membrane to take place. The acidic pH allows the alteration of HA, the opening of the ion channel, also called M2, and acidification of the virus nucleus, leading to the entry of viral RNA into the nucleus of the target cell. The presence of a high concentration of the Neu5Gc2-3Gal molecular complex essential for viral replication, which is present in the respiratory epithelium of the horse – and that NA also greatly favors – is essential for the rapid release of virions resulting from replication. Virions formed by viral replication are released by the infected cell, allowing the infection of new cells or propagation into the environment (Suzuki, Reference Suzuki2000; Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Takahashi et al., Reference Takahashi, Unuma, Kawagishi, Kurebayashi, Takano, Yoshino, Minami, Yamanaka, Otsubo, Ikeda and Suzuki2016).
This first phase of viral infection and replication occurs mainly in the nasopharyngeal mucosa. The virus can be detected in the entire respiratory tract 3–7 days after infection. After infection of the ciliated epithelial cells, the horse loses the ability to eliminate foreign substances that enter via this route. This viral process induces the death of the epithelial cells of the respiratory mucosa, inflammation, oedema and loss of the protective mucociliary barrier. Cell death occurs as a result of EIV-induced apoptosis of epithelial respiratory cells and systemic and local increase in type I INF (INF-α and INF-β) and interleukin-6 (Suzuki, Reference Suzuki2000; Lin et al., Reference Lin, Holland, Donofrio, McCoy, Tudor and Chambers2002; Wattrang et al., Reference Wattrang, Jessett, Yates, Fuxler and Hannant2003; Takahashi et al., Reference Takahashi, Unuma, Kawagishi, Kurebayashi, Takano, Yoshino, Minami, Yamanaka, Otsubo, Ikeda and Suzuki2016). The synthesis of INF is activated as a cellular defense mechanism by the presence of viral RNA (Jiao et al., Reference Jiao, Tian, Deng, Jiang, Liu, Liu, Bu, Kawaoka and Chen2008).
Infection with EIV allows for the emergence and proliferation of opportunistic bacteria, among which Streptococcus equi var. zooepidemicus stands out, due to the loss of regular response to their control. This secondary infection by bacteria leads to an increase in inflammation, which will also cause bronchopneumonia, which can lead to an increase in mortality (Wilson, Reference Wilson1993; Paillot, Reference Paillot2014). EIV has not been identified in any tissue other than the respiratory tract. However, when it comes to a strain resulting from the interspecies transmission, as was the case with A/equine/Jilin/1/1989 strain, atypical clinical signs may appear, with the virus having been identified in horses with enteritis (Webster and Yuanji, Reference Webster and Yuanji1991; Wilson, Reference Wilson1993; van Maanen and Cullinane, Reference van Maanen and Cullinane2002).
Diagnosis
Clinical diagnosis
The clinical diagnosis of this disease is based on the clinical signs presented by the infected animals, which can be confirmed by performing complementary diagnostic tests (Timoney, Reference Timoney1996). Diagnosis using complementary diagnostic imaging methods, such as ultrasound, is still not frequently used. The use of ultrasonography for the diagnosis of EI needs further studies, while radiography is difficult to use in adult horses (Gross et al., Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004). Among the most common clinical signs are nasal discharge, serous initially and subsequently mucopurulent, dry cough, fever, depression, and lack of appetite. The presence of these signs and their intensity may vary according to the age of the animal and the individual susceptibility to the disease (Timoney, Reference Timoney1996; Gross et al., Reference Gross, Hinchcliff, French, Goclan, Lahmers, Lauderdale, Ellis, Haines, Slemons and Morley1998, Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004; Morley et al., Reference Morley, Townsend, Bogdan and Haines2000; Cullinane and Newton, Reference Cullinane and Newton2013). The occurrence of abortion in pregnant mares is not frequent (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003).
The clinical signs present in previously unexposed horses infected with EIV include pyrexia with values between 38.5 and 41 °C, which appears after the incubation period of 24–72 h; depression in some animals; the refusal of food or anorexia; reluctance to move; dry and rough cough, which is one of the dominant clinical signs and appears soon after the temperature increases, lasting from 1 to 3 weeks, being easily stimulated through manual compression of the cranial portion of the trachea; pain on palpation of the submaxillary lymph nodes, particularly in younger animals; and mucous nasal discharge. Lung auscultation may present altered sounds, such as increased breathing intensity, crackles and wheezing. These changes to auscultation may be present in a horse with secondary bacterial pneumonia (Timoney, Reference Timoney1996; Gross et al., Reference Gross, Hinchcliff, French, Goclan, Lahmers, Lauderdale, Ellis, Haines, Slemons and Morley1998, Reference Gross, Morley, Hinchcliff, Reichle and Slemons2004; Morley et al., Reference Morley, Townsend, Bogdan and Haines2000; Cullinane and Newton, Reference Cullinane and Newton2013).
In foals, the disease presents a more severe form, with fever, severe breathing difficulties and acute interstitial pneumonia (Oxburgh and Klingeborn, Reference Oxburgh and Klingeborn1999; Britton and Robinson, Reference Britton and Robinson2002).
Horses that develop a secondary infection, usually Streptococcus zooepidemicus, present mucopurulent nasal discharges, persistent fever and more marked abnormal sounds on pulmonary auscultation. Rarer cases may present jaundice, signs of encephalitis, incoordination, and myoglobinuria (Wilson, Reference Wilson1993).
Laboratorial diagnosis
The diagnosis of EI in the field can be made using rapid antigen detection tests, which are also used in the detection of human influenza. This need for rapid identification of EI is due to the urgent need to introduce control measures for its spread. Tests such as Directigen Flu A are approved and have been validated for antigen detection in horses. Other tests have already been tested, but their sensitivity and specificity are considered to be low when compared to reverse transcriptase-polymerase chain reaction (PCR) (Chambers et al., Reference Chambers, Shortridge, Li, Powell and Watkins1994; Yamanaka et al., Reference Yamanaka, Tsujimura, Kondo and Matsumura2008b, Reference Yamanaka, Nemoto, Bannai, Tsujimura, Kondo, Matsumura, Gildea and Cullinane2015b). Insulated isothermal PCR was developed for the detection of HA3 from EIV, and can be performed in 1 h, using a portable device, with high sensitivity and specificity (Balasuriya et al., Reference Balasuriya, Lee, Tiwari, Skillman, Nam, Chambers, Tsai, Ma, Yang, Chang and Wang2014; Galvin et al., Reference Galvin, Gildea, Nelly, Quinlivan, Arkins, Walsh and Cullinane2014; Brister et al., Reference Brister, Barnum, Reedy, Chambers and Pusterla2019).
Rapid tests for the diagnosis of infection by EIV should not be considered substitutes for laboratory tests, because the performance of the latter leads to the identification of the virus, which allows the outbreak to be characterized, to carry out vaccine and epidemiological studies, and guarantees, with certainty, that an animal is infected with EIV (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003). The most commonly used laboratory test to detect the virus and diagnose the disease is the reverse-transcription PCR of samples collected from nasopharyngeal swab (Chambers et al., Reference Chambers, Shortridge, Li, Powell and Watkins1994; Oxburgh and Hagstrom, Reference Oxburgh and Hagstrom1999; Back et al., Reference Back, Berndtsson, Gröndahl, Ståhl, Pringle and Zohari2016; Gora et al., Reference Gora, Kwasnik, Zmudzinski and Rozek2017; OIE, 2019c). This technique was used, for example, in the detection of an outbreak in Mongolia in which the horses with EIV presented uncommon clinical signs. The primers used are specific to a specific region of the virus, allowing results to be obtained quickly and with great sensitivity, even when the excretion of the virus was weak. The primers are not exclusive, in other words, they can detect strains not yet studied (Alvarez et al., Reference Alvarez, Brunck, Boyd, Lai, Virtue, Chen, Bletchly, Heine and Barnard2008; Yondon et al., Reference Yondon, Heil, Burks, Zayat, Waltzek, Jamiyan, McKenzie, Krueger, Friary and Gray2013; Aeschbacher et al., Reference Aeschbacher, Santschi, Gerber, Stalder and Zanoni2015). Detection of the presence of double-stranded RNA and replication are signs of viral infection and triggers an exuberant antiviral defense mechanism (Daly and Reich, Reference Daly and Reich1993; Stark et al., Reference Stark, Kerr, Williams, Silverman and Schreiber1998; Jiao et al., Reference Jiao, Tian, Deng, Jiang, Liu, Liu, Bu, Kawaoka and Chen2008). The influenza virus is capable of counteracting the production of IFN-α/β by the host cell, by inhibiting the transcription factors involved in the activation of IFN and therefore attenuating host expression (Noah et al., Reference Noah, Twu and Krug2003; Garcia-Sastre, Reference Garcia-Sastre2006; Mibayashi et al., Reference Mibayashi, Martinez-Sobrido, Loo, Cardenas, Gale and Garcia-Sastre2007).
With the advancement of technology and based on the ‘One Health’ concept, diagnostic kits have been developed, such as the FluChip-8 G from InDevR Inc., which allow for the characterization and identification of influenza A or B subtypes in <10 h (Borkenhagen et al., Reference Borkenhagen, Salman, Mai-Juan and Gray2019). Tests are constantly being developed, such as lateral flow immunochromatography using colloidal silver as a revealer of the antigen–antibody interaction, which are highly sensitive and allow early detection of the virus, and pyrosequencing, which enables the differentiation of the strain in cases of the outbreak (Bernardino et al., Reference Bernardino, Mapes, Corbin and Pusterla2016; Yamanaka et al., Reference Yamanaka, Nemoto, Bannai, Tsujimura, Kondo, Matsumura, Fu, Fernandez, Gildea and Cullinane2017).
Measuring the concentration of antibodies against viral HA is important for confirming exposure to the virus, measuring the animal's vulnerability to infection and the effectiveness of a particular vaccine. The preferred test for this measurement is the simple radial haemolysis test when compared to the hemagglutination inhibition test. The enzyme-linked immunosorbent assay (ELISA) can also be used to detect anti-nucleoprotein antibodies, enabling the identification and differentiation of vaccinated animals and infected animals, because the antibodies resulting from the vaccination do not contain HA protein and are not detected in this test (Daly et al., Reference Daly, Newton and Mumford2004; Gildea et al., Reference Gildea, Arkins and Cullinane2010, Reference Gildea, Arkins and Cullinane2011; Kirkland and Delbridge, Reference Kirkland and Delbridge2011; Galvin et al., Reference Galvin, Gildea, Arkins, Wash and Cullinane2013; Chambers and Reedy, Reference Chambers and Reedy2014b; OIE, 2019c). The identification of antibodies against non-structural proteins of the virus in horses means it is possible to determine that the animal suffered a natural infection of the virus, as these antibodies are not identified when animals are immunized with an inactivated virus vaccine (Ozaki et al., Reference Ozaki, Sugiura, Sugita, Imagawa and Kida2001).
In case of pulmonary auscultation for suspected pneumonia, tracheal lavage should be performed. Animals with bronchitis or pneumonia have a high number of granulocytes, especially neutrophils in this secretion (Gross et al., Reference Gross, Hinchcliff, French, Goclan, Lahmers, Lauderdale, Ellis, Haines, Slemons and Morley1998). In terms of histology, it is possible to observe necrotic lesions in the bronchi and alveoli, infiltration of neutrophils, formation of hyaline membranes, squamous metaplasia and hyperplasia of the airway epithelium (Patterson-Kane et al., Reference Patterson-Kane, Carrick, Axon, Wilkie and Beegg2008).
The collection of material for laboratory analysis must be done carefully, as the quality of the sample can compromize the reliability of the results. As the density of the virus is higher in the nasopharynx than in the nasal cavities, swab collection should preferably be performed at the nasopharynx level. It is important that the transport of the samples is carried out under appropriate storage conditions (i.e. refrigerated), and if the transport to the laboratory takes longer than two days, they must be kept at −60 °C or lower (Chambers and Reedy, Reference Chambers and Reedy2014a; Gora et al., Reference Gora, Kwasnik, Zmudzinski and Rozek2017).
Differential diagnosis
As a differential diagnosis for EI, the following diseases should be considered: pasteurellosis, pleuropneumonia, and equine infectious adenitis, infection by equine rhinovirus and adenovirus, equine viral arteritis, equine rhinopenumonitis or equine herpesvirus, Hendra virus or equine morbilivirus (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Rush and Mair, Reference Rush and Mair2004; FAO, 2019).
When EIV infects animals which have been vaccinated or previously exposed to the virus, the disease is considered mild, because the horses are already immune and thus it can be clinically indistinguishable from an upper respiratory illness associated with other agents, such as equine herpesvirus-4, equine rhinitis virus, and arthritis virus.
Treatment
Treatment of a horse with EI is based on the management of the disease and rest for the animal. Although Amantadine has been tested for the treatment of EI, the existence of a specific antiviral available on the market to treat this disease has not yet been described. The use of NA inhibitors is indicated at an early stage, as it reduces the spread of the virus and limits its transmission to other animals (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Yamanaka et al., Reference Yamanaka, Cullinane, Gildea, Bannai, Nemoto, Tsujimura, Kondo and Matsumura2015a). Baloxavir marboxil is an enzyme inhibitor targeting the cap-dependent endonuclease activity of EIV and is an available option for the treatment of EI (Koszalka et al., Reference Koszalka, Tilmanis and Hurt2017). However, the usage of this antiviral agent has an ability to reduce, in long-term, the susceptibility of the virus to the treatment, because this agent induces mutations in EIV, at position 38 in polymerase acidic protein. Baloxavir marboxil can be used and can be useful in new outbreaks, but with more of its use the more mutation is induced (Omoto et al., Reference Omoto, Speranzini, Hashimoto, Noshi, Yamaguchi, Kawai, Kawaguchi, Uehara, Shishido, Naito and Cusack2018; Nemoto et al., Reference Nemoto, Tamura, Bannai, Tsujimura, Kokado, Ohta and Yamanaka2019a). The resting time indicated should be equivalent to the number of days the horse has presented a fever so that the respiratory epithelium can be recovered. In addition to the usual care taken with water, food and the horse's stall, it is necessary to pay particular attention so that the animal's recovery space has good ventilation. The bed must be made of materials that do not cause dust and the food must be of good quality without dust. After resting, work can be introduced gradually. Failure to comply with the rest period or the sudden introduction of high-stress work can lead to the development of chronic obstructive pulmonary disease and myocarditis (Chambers et al., Reference Chambers, Holland and Lai1995).
In horses with signs of secondary infection, antibiotherapy with broad-spectrum antibiotics is recommended, such as potentiated sulfonamides (e.g. Equibactin vet®), Ceftiofur (e.g. Cftiomax®), Penicillin G Procaine (e.g. Combiotic suspension for injection for cattle, sheep, pigs, and horses®), with or without Gentamicin. Penicillin can also be used in animals with secondary infection (Ensink et al., Reference Ensink, Klein, Barneveld, Vulto and van Miert1996, Reference Ensink, Smit and van Duijkeren2003). Although its efficacy is not fully known, animals with EI can be given mucolytics. The administration of corticosteroids and the use of antitussives are contraindicated due to their side effects and the possibility of masking complications, while the use of non-steroidal anti-inflammatory drugs should be considered (Kastner et al., Reference Kastner, Haines, Archer and Townsend1999). The use of antipyretics with non-steroidal anti-inflammatory action is not contraindicated, and the following drugs can be used: phenylbutazone, flunixin meglumine, or dipyrone (Wilson, Reference Wilson1993).
Prognosis
The prognosis of a horse with EI depends a lot on the vaccination status of the animal, the strain of the virus responsible for the infection, the age of the animal and the treatment implemented. In foals, a more severe form of the disease is reported, which is usually fatal when acute interstitial pneumonia appears (Britton and Robinson, Reference Britton and Robinson2002).
In general, the mortality rate is low, being considered <1%. However, due to the breakdown of immunity, after a viral infection, a bacterial infection can occur and usually this secondary infection is responsible for mortality. In 1989, the epidemic originating from an avian strain in China, showed a high rate of morbidity (80%) and mortality (20–35%), due to complications such as pneumonia and enteritis. The prognosis is also considered to be reserved when the viral strain responsible for the infection is the result of cross-infection (Webster and Yuanji, Reference Webster and Yuanji1991; Oxburgh and Klingeborn, Reference Oxburgh and Klingeborn1999; Britton and Robinson, Reference Britton and Robinson2002).
Most horses that are protected from the least favorable environmental conditions, which can cause immunosuppression, that have no complications, secondary infections or efforts before the recommended time, fully recover in 7–14 days. However, coughing may persist for a few weeks and horses with a more severe illness may take a month to recover (Morley et al., Reference Morley, Townsend, Bogdan and Haines2000; Cullinane and Newton, Reference Cullinane and Newton2013). Recovering horses that are transported, exercised or are exposed to adverse climatic conditions, may present with cough, severe bronchitis, pneumonia and may develop oedema of the limbs (Wilson, Reference Wilson1993).
Before the existence of vaccines, EI outbreaks in Mongolia had a mortality rate of 20%, having been reduced to 5% with the introduction of vaccination (Yin et al., Reference Yin, Lu, Guo, Qi, Ma, Zhu, Zhao, Pan and Xiang2013; Yondon et al., Reference Yondon, Heil, Burks, Zayat, Waltzek, Jamiyan, McKenzie, Krueger, Friary and Gray2013). Horses that are vaccinated or have previously been exposed to the virus are associated with low morbidity, mortality, and speed of spread of the disease. In an outbreak in Hong Kong in 1992, among vaccinated horses, 75% of the animals had positive serological tests, 37% had clinical signs and the mortality rate was only 0.2%. Horses from areas where the disease did not occur, such as New Zealand, had a morbidity rate of 52%. However, horses from the northern hemisphere had a morbidity rate of 20%, indicating previous exposure to EIV or vaccination (Powell et al., Reference Powell, Watkins, Li and Shortridge1995).
Prophylaxis
Vaccine surveillance and updating programs remain the best way to prevent and control EI (Gildea et al., Reference Gildea, Quinlivan, Arkins and Cullinane2012). Prevention of the disease must be done through the use of effective vaccines, this being more efficient in limiting the severity of clinical signs and their morbidity during outbreaks. Vaccines thus have an important role in controlling propagation, but they do not eliminate the chance of horses becoming infected, so it is important to carry out vaccine boosters (Powell et al., Reference Powell, Watkins, Li and Shortridge1995; Daly et al., Reference Daly, Newton and Mumford2004; Minke et al., Reference Minke, Audonnet and Fischer2004; Elton and Cullinane, Reference Elton and Cullinane2013). In the event of outbreaks, a strategic vaccination can be carried out in order to control the spread of the disease (Daly et al., Reference Daly, Newton, Wood and Park2013).
There are four types of vaccines: inactivated, sub-unit, attenuated, and viral vector. However, there are other vaccine types under development or under study, such as the case of the vaccine based on reverse genetics and others that have not shown benefits in relation to the existing ones, such as a DNA vaccine (Landolt et al., Reference Landolt, Hussey, Kreutzer, Quintana and Lunn2010; Daly et al., Reference Daly, Newton, Wood and Park2013; Rodriguez et al., Reference Rodriguez, Reedy, Nogales, Murcia, Chambers and Martinez-Sobrido2018). The first vaccine against EI appeared in the 1960s, and it was an inactivated virus vaccine (Daly et al., Reference Daly, Newton, Wood and Park2013). The choice of the most suitable vaccine must meet several requirements, such as inducing an immune response capable of being detected, demonstrating protection against natural or induced infection, containing significant and up-to-date viral strains, being safe and easy to administer (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003).
Inactivated vaccines provide protection to horses without releasing the virus, requiring boosters to be more effective. These vaccines are the most suitable for the vaccination of pregnant mares. Inactivated vaccines can be presented with an immunity-stimulating complex (ISCOM)–matrix adjuvant, which allows the duration of immunity to be increased (Bengtsson, Reference Bengtsson2013; Paillot et al., Reference Paillot, Prowse, Montesso, Huang, Barnes and Escala2013). Adjuvants promote cellular and humoral responses. Vaccines with ISCOMs demonstrated a reduction in clinical signs in horses, prevention of virus release, and the induction of specific IFN-γ production. The production of this IFN is possible through the activation of Th1 cells. The use of aluminium hydroxide gel adjuvant has been shown to be safe in inactivated vaccines, promoting good immunity. The administration of the vaccine combined with the inactivated equine herpes vaccine has shown an improvement in the immune response against EIV (Paillot et al., Reference Paillot, Kydd, Sindle, Hannant, Toulemonde, Audonnet, Minke and Daly2006, Reference Paillot, Grimmett, Elton and Daly2008; Horspool and King, Reference Horspool and King2013; Gildea et al., Reference Gildea, Higgins, Johnson, Walsh and Cullinane2016).
Subunit vaccines are composed of purified antigens, and can also be presented with ISCOM. In this case, they are particles derived from the combination of the viral protein with cholesterol, phospholipids, chylamine saponins, hydrophobic antigen, and membrane proteins. The response obtained with ISCOM in this type of vaccine is more prominent, observing the induction of strong antibody response with high levels of IFN-γ. If these are administered intranasally, as a vaccine booster, the animal presents high levels of immunoglobulin A (IgA) specific to the virus. This type of vaccine has a longer response duration when administered in a protocol combining vaccines for intramuscular administration (Sjolander et al., Reference Sjolander, Cox and Barr1998; Crouch et al., Reference Crouch, Daly, Henley, Hannant, Wilkins and Francis2005; Paillot et al., 2008; Elton and Bryant, Reference Elton and Bryant2011; Dilai et al., Reference Dilai, Piro, Harrak, Fourgerolle, Dehhaoui, Dikrallah, Legrand, Paillot and Fihri2018).
The attenuated vaccines aim to simulate a natural infection, and they are able to generate local and systemic immune responses. Due to the strain's ability to replicate only in the upper respiratory tract, the development of more severe clinical signs is avoided (Townsend et al., Reference Townsend, Penner, Watts, Cook, Bogdan, Haines, Griffin, Chambers, Holland, Whitaker-Dowling, Youngner and Sebring2001; Paillot, Reference Paillot2014).
Viral vector vaccines should be administered by intramuscular injection, with a six-monthly vaccination protocol. These are able to promote the production of a good amount of antibodies and are indicated for vaccination of pregnant mares, more specifically in the last stage of gestation, leading to the presence of considerable levels of antibodies in the colostrum. The canarypox vector allows antibodies to act only in HA, making it possible to distinguish between vaccinated animals and naturally infected animals in laboratory tests. This type of vaccine demonstrated rapid development of immunity, with a prolonged duration against the American strain, thus allowing the vaccination booster to take place after 1 year (Daly et al., Reference Daly, Newton and Mumford2004, Reference Daly, MacRae, Newton, Wattrang and Elton2011; Minke et al., Reference Minke, Toulemonde, Coupier, Guigal, Dinic, Sindle, Jessett, Black, Bublot, Pardo and Audonnet2007; Soboll et al., Reference Soboll, Hussey, Minke, Landolt, Hunter, Jagannatha and Lunn2010; Paillot and El-Hage, Reference Paillot and El-Hage2016).
In North America, a live modified virus vaccine is used, which has already demonstrated experimental effectiveness in preventing the disease in relation to heterologous viruses. In this type of vaccine, the virus maintains its ability to infect the host cell, stimulating long-lasting immunity. However, its use generates concern due to the possibility of reversion to normal virulence, with consequent impairment of the health of pregnant females and immunocompromized animals (Townsend et al., Reference Townsend, Penner, Watts, Cook, Bogdan, Haines, Griffin, Chambers, Holland, Whitaker-Dowling, Youngner and Sebring2001; Paillot et al., Reference Paillot, Lemaitre, Dancer, Thibault and Minke2014).
The greatest difficulty in combating EI lines is controlling the virus through vaccination and the existence of several animal reservoirs that enable it to reappear. Mutation of the virus also plays an important role, as it is a constant threat to the immune system and, therefore, to vaccines that try to predispose the organism to respond in the presence of the virus and limit its spread (Daly et al., Reference Daly, Newton, Wood and Park2013; Elton and Cullinane, Reference Elton and Cullinane2013). Previous studies have shown that vaccine efficacy can be compromized by changing a single amino acid (Legrand et al., Reference Legrand, Pitel, Marcillaud-Pitel, Cuillinane, Couroucé, Fortier, Freymuth and Pronost2013).
For good disease prevention, active surveillance is recommended, including a genetic and antigenic characterization of the virus detected, together with clinical and epidemiological information provided by veterinary associations, and also information on possible vaccine failures, which can culminate in an outbreak of the disease (Back et al., Reference Back, Berndtsson, Gröndahl, Ståhl, Pringle and Zohari2016; Daly and Murcia, Reference Daly and Murcia2018). The strains used in vaccines are recommended by the OIE. Every year, the sequencing and analysis of the HA of the strains that emerge allow us to evaluate the cross-protection provided by the vaccines in force. The data arising from this analysis are evaluated and reviewed by the Expert Surveillance Panel, which is made up of members representing the World Health Organisation and the OIE, enabling a decision to be made in the event that it is necessary to update the strain to be used in vaccines. Strains are updated only if the one currently being used is not able to provide adequate immunization (Cullinane et al., Reference Cullinane, Elton and Mumford2010). The OIE recommendations include the use of vaccines that contain viruses of the FC1 and FC2 lineage, as there have been no reports of influenza resulting from H7N7 and the Eurasian line. A/eq/South Africa/04/2003-like or A/eq/Ohio/2003-like should be administered for FC1; and for FC2, A/eq/Richmond/1/2007-like should be administered. Vaccines must be adapted to the existing strains in each country in order to improve the immune response to the virus. Some strains are not included in vaccines, although they are more recent, due to their vulnerability to mutations, which could compromise the desired response with vaccination (Gamoh and Nakamura, Reference Gamoh and Nakamura2017; OIE, 2019b). The vaccine must be administered strategically and in such a way that it provides the best possible immune response. The more recent the strain, the better the protection acquired (Radostits et al., Reference Radostits, Gray, Blood and Hinchcliff2003; Barbic et al., Reference Barbic, Madic, Turk and Daly2009; Daly and Murcia, Reference Daly and Murcia2018).
Vaccination of pregnant mares will protect the neonatal foal against the disease through passive antibody transfer. However, this transfer of antibodies may be a factor that compromises vaccine efficacy (van Maanen, et al., Reference van Maanen, Bruin, de Boer-Luijtze, Smolders and de Boer1992; Cullinane et al., Reference Cullinane, Weld, Osborne, Nelly, Mcbride and Walsh2001). Thus, vaccination plans start at 6 months of age to allow the level of maternal antibodies to decrease; however, a gap in immunity may occur. Passive immunity is short-lived and some foals with newly vaccinated mothers are seronegative at 4 weeks of age (Nelson et al., Reference Nelson, Schram and McGregor1998). It was also concluded that this gap can be reduced by starting the vaccination protocol at 3 months of age. (Perkins and Wagner, Reference Perkins and Wagner2015)
At this time, authorities in the UK, France, and Ireland recommend administering the first doses between 21 and 92 days apart, and the third dose with a gap of between 150 and 215 days after the second dose (Cullinane et al., Reference Cullinane, Gildea and Weldon2014). These intervals allow the immune response to be increased immediately before an increased risk of exposure to the virus. Longer time intervals increase the period of a possible gap in the immune system (Daly et al., Reference Daly, Newton, Wood and Park2013). With vaccination, young horses increase their antibody count, but only 75% of the population reaches values considered to be protective (Newton et al., Reference Newton, Lakhani, Wood and Baker2000a). The use of a semi-annual vaccination plan, instead of an annual one, reduces the risk of infections by the virus and, in turn, the appearance of outbreaks (Ryan et al., Reference Ryan, Gildea, Walsh and Cullinane2015). Accelerated vaccination, i.e. the reduction in the interval between vaccine doses, was successfully carried out in pre-outbreak situations in South Africa and Australia in 2007. In this situation, the administration of the second dose was given 14 days after the first vaccination and the third with an interval of 91 days after the second, showing identical antibody levels when using the regulated time and presenting a protective immune response more quickly. If the animal is infected after the first dose, the clinical signs are milder in relation to non-vaccinated animals, with the first administration having the ability to confer a certain degree of immunity (Arthur and Suann, Reference Arthur and Suann2011; El-Hage et al., Reference El-Hage, Savage, Minke, Ficorilli, Watson and Gilkerson2013).
Racehorses, due to their age, should receive more attention. When these animals arrive at the running stables, they usually have antibodies prior to vaccination, however, they are not considered sufficient for their protection (Newton et al., Reference Newton, Lakhani, Wood and Baker2000a). Studies carried out on English Thoroughbred horses showed that 95% of the animals at the same training center had already received more than one vaccine from different laboratories after the first immunization, having been associated with a high number of antibodies present in these animals compared to the rest. However, with the increase in vaccinations, average antibody levels have started to decline, and over-vaccination may lead to problems, such as the loss of pre-existing antibodies (Ryan et al., Reference Ryan, Gildea, Walsh and Cullinane2015).
Five vaccines against EI are marketed in Portugal, approved by the General Food and Veterinary Directorate (DGAV – Direção Geral de Alimentação e Veterinária): EquilisPrequenza, EquilisPrequenzaTe, Equip FT, ProteqFlu, and ProteqFlu-Te. The vaccines which are available and approved by the DGAV have been approved by the Committee for Medical Products for Veterinary Use (CVMP – Comité de Produtos Médicos de Uso Veterinário) (DGAV, 2019; EMA, 2020).
Outbreak control must be planned with an early and rapid vaccination program, and the creation of structures to quarantine infected horses is necessary. Therefore, it is necessary to set a deadline for the vaccination of all horses. The quarantine area must have restricted access and all people with responsibility must be trained in matters regarding biosafety, and the entrances and exits must be carefully controlled in order to avoid the spread of the disease (Arthur and Suann, Reference Arthur and Suann2011). In addition to vaccines, there are other methods of prevention; however, they are more directed at containing the spread of the virus or preventing its entry into a stable (Wilson, Reference Wilson1993; Daly et al., Reference Daly, Newton and Mumford2004).
Immunity
Immunity is achieved in two different ways, that is, by infection with the virus or through vaccination. The immunity acquired by exposure to the agent depends on the type of contact with the virus, the strain and the time of exposure. It is important to bear in mind that the resistance/immunity that the animal presents, derived from the disease or vaccination, is greater for homologous viruses in comparison to the heterologous virus (Yates and Mumford, Reference Yates and Mumford2000). Thus, animals with antibody concentrations within the standard of protection against the homologous virus are susceptible to disease caused by heterologous viruses (Newton et al., Reference Newton, Townsend, Wood, Sinclair, Hannant and Mumford2000b; van Maanen et al., Reference van Maanen, van Essen, Minke, Daly and Yates2003; Daly et al., Reference Daly, Newton and Mumford2004). After the last contact with breast milk, antibodies can remain for up to 1 year and in some cases can even reach 2 years, with a low incidence of the disease in foals. Partially immunized animals tend to become infected subclinically (Morley et al., Reference Morley, Townsend, Bogdan and Haines1999; Landolt, Reference Landolt2014).
Immunity by contact with the agent and by vaccination is characterized by the production of different Igs. In the case of natural infection, IgA, IgGa, and IgGb are produced. IgA is present in nasal secretions and the other Igs in serum. When immunized by vaccination, the serum contains only IgG(T) and the antibodies last for 3–4 months (Hannant et al., Reference Hannant, Mumford and Jessett1988, Reference Hannant, Jessett, O'Neil and Mumford1989; Wilson, Reference Wilson1993; Nelson et al., Reference Nelson, Schram and McGregor1998). Determining the antibody concentration enables the assessment of the animal's susceptibility to infection, and it is an indicator of the need for restructuring or application of a vaccination plan. The radial haemolysis test allows the analysis of immunity to a single strain and is predictive of disease resistance both at the field level and the experimental level (Newton et al., Reference Newton, Townsend, Wood, Sinclair, Hannant and Mumford2000b; van Maanen et al., Reference van Maanen, van Essen, Minke, Daly and Yates2003; Daly et al., Reference Daly, Newton and Mumford2004).
Most vaccines are composed of inactivated viruses or subunits of the virus combined with an adjuvant. This combination aims to maximize the immune response achieved through vaccination, thus an adjuvant is an important factor in the composition of the vaccine. It is important to note that the predominant factor in a vaccine is the inclusion of sufficient amounts of antigen from viral strains that have immunological value. The concentration of antigens included in the vaccine has a direct relationship to the magnitude and duration of the antibody response. Since the H7N7 virus is not detected, it should not be included in the vaccine (Daly et al., Reference Daly, Newton and Mumford2004; Minke et al., Reference Minke, Audonnet and Fischer2004). For preventive immunization, a constant update of the vaccine with the strains circulating in the equine population is required (Yates and Mumford, Reference Yates and Mumford2000; Daly et al., Reference Daly, Newton and Mumford2004; Elton and Cullinane, Reference Elton and Cullinane2013). A simple change, on the FC2 strains, in the antigenic site A position 144 of the HA gene can incapacitate a vaccine, i.e. lead to a vaccine failure. When this happens, there is no cross-protection between the existing strain and the vaccine that was administered, as reported by Nemoto et al. (Reference Nemoto, Yamayoshi, Bannai, Tsujimura, Kokado, Kawaoka and Yamanaka2019b) where the two vaccines had less cross-protection when the HA-144A underwent mutation and it was possible to see HA-A144 V or HA-A144 T (Yamanaka et al., Reference Yamanaka, Cullinane, Gildea, Bannai, Nemoto, Tsujimura, Kondo and Matsumura2015a; Nemoto et al., Reference Nemoto, Yamayoshi, Bannai, Tsujimura, Kokado, Kawaoka and Yamanaka2019b).
The use of live modified virus vaccines has immunity that lasts up to 6 months. In a study carried out to determine this interval, ponies showed milder clinical signs, compared to the unvaccinated control group, when exposed to a highly pathogenic virus 6 months after vaccination. Twelve months after the start of the study, a new data collection was carried out, in which the vaccinated animals showed a reduction in the temperature, concentration, and duration of the virus in relation to the non-vaccinated animals. According to the study, the use of this vaccine can lead to a marked reduction in the frequency, severity and duration of EI outbreaks in North America (Chambers et al., Reference Chambers, Holland, Tudor, Townsend, Cook, Bogdan, Lunn, Hussey, Whitaker-Dowling, Youngner, Sebring, Penner and Stiegler2001).
The recombinant vaccine with the canarypox vector was introduced in Europe, where it was widely embraced by Veterinarians and horse owners (Minke et al., Reference Minke, Audonnet and Fischer2004). This vaccine uses the viral vector to introduce the HA of H3N8 EIV genes into the host cell. The recombinant virus expresses the HA gene of the American FC1 and FC2 (Toulemonde et al., Reference Toulemonde, Daly, Sindle, Guigal, Audonnet and Minke2005; OIE, 2021). Infection of the cell with Canarypox is abortive, i.e. there is no production or expression of the vector; however, the EIV gene is expressed and is demonstrated through the main class 1 histocompatibility complex by the cell, giving rise to an immune response (Daly et al., Reference Daly, Newton and Mumford2004).
A multivalent vaccine with an inactivated virus is considered to fail when it does not produce a sufficient concentration of antibodies, indicating a lack of protection against infection by a heterologous IV (Newton et al., Reference Newton, Townsend, Wood, Sinclair, Hannant and Mumford2000b; van Maanen et al., Reference van Maanen, van Essen, Minke, Daly and Yates2003; Daly et al., Reference Daly, Newton and Mumford2004). Failure to immunize with the vaccine can occur due to factors such as the incorrect storage of the vaccine, incorrect administration, or the individual response of each animal, varying according to genetic factors, age, presence of infections, ongoing treatments, presence of maternal antibodies, nutritional status and stress (NOAH, 2020).
Economic impact
EI is associated with little economic loss, due to the low mortality of horses. However, when outbreaks occur in racehorse stables, affected animals must stop training, which can lead to suspension of race events for months in a given country, with associated economic losses (Powell et al., Reference Powell, Watkins, Li and Shortridge1995). Losses resulting from outbreaks affect industry and those who depend on racehorses and breeding, government, and individuals. The affected household will be in quarantine and the borders can close, to supress the virus (Smyth et al., Reference Smyth, Dagley and Tainsh2011).
The outbreak that occurred in Australia, in 2007, forced the implementation of a contingency plan that cost the government of that country about one billion Australian dollars. However, some authors argue that the cost associated with this outbreak may have been even greater (Cowled et al., Reference Cowled, Ward, Hamilton and Garner2009; Smyth et al., Reference Smyth, Dagley and Tainsh2011). Restricting the movement of animals has caused great disruption and economic loss for the entire Australian equine sector. In order to reduce the economic impact, New South Wales has instituted biosafety classes in the most affected areas. Biosafety protocols and vaccination programs were also created to control the disease (Arthur and Suann, Reference Arthur and Suann2011).
Acknowledgments
This work was supported by National Funds by FCT – Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020.






