Introduction
The spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), was first detected in Europe and in the contiguous United States in 2008 (Goodhue et al., Reference Goodhue, Bolda, Farnsworth, Williams and Zalom2011; Hauser, Reference Hauser2011; Walsh et al., Reference Walsh, Bolda, Goodhue, Dreves, Lee, Bruck, Vaughn, O'Neal and Zalom2011; Calabria et al., Reference Calabria, Máca, Bächli, Serra and Pascual2012). In the following years, D. suzukii continued to rapidly spread throughout large parts of North and South America and Europe and has been identified in North Africa, Middle East and Oceania (Asplen et al., Reference Asplen, Anfora, Biondi, Choi, Chu, Daane, Gibert, Gutierrez, Hoelmer, Hutchison, Isaacs, Jiang, Kárpáti, Kimura, Pascual, Philips, Plantamp, Ponti, Vétek, Vogt, Walton, Yu, Zappalà and Desneux2015; Nikolouli et al., Reference Nikolouli, Colinet, Renault, Enriquez, Mouton, Gibert, Sassu, Cáceres, Stauffer, Pereira and Bourtzis2017; EPPO, 2021). Unlike most other drosophilids, females of D. suzukii have a serrated and highly sclerotized ovipositor that enables them to deposit eggs in healthy, ripening fruits (Mitsui et al., Reference Mitsui, Beppu and Kimura2010; Hauser, Reference Hauser2011). The resulting larvae feed on the fruit tissue, and secondary damage by pests and pathogens could initiate from the oviposition sites, making the fruits unmarketable, causing major economic losses in soft and stone fruits (Walsh et al., Reference Walsh, Bolda, Goodhue, Dreves, Lee, Bruck, Vaughn, O'Neal and Zalom2011; Rombaut et al., Reference Rombaut, Guilhot, Xuéreb, Benoit, Chapuis, Gibert and Fellous2017). This species has a high fecundity and a wide host range (Lee et al., Reference Lee, Bruck, Curry, Edwards, Haviland, Van Steenwyk and Yorgey2011; Kenis et al., Reference Kenis, Tonina, Eschen, van der Sluis, Sancassani, Mori, Haye and Helsen2016). Depending on various factors, including crop, cultivar and location, damage caused by D. suzukii varies from negligible to 80% of harvest losses (Bolda et al., Reference Bolda, Goodhue and Zalom2010). More recently, Mazzi et al. (Reference Mazzi, Bravin, Meraner, Finger and Kuske2017) estimated revenue losses of up to €64,000 ha−1 for sweet cherry production in Switzerland. Various integrated pest management programs are under development to control this pest (Haye et al., Reference Haye, Girod, Cuthbertson, Wang, Daane, Hoelmer, Baroffio, Zhang and Desneux2016; Mazzi et al., Reference Mazzi, Bravin, Meraner, Finger and Kuske2017; Nikolouli et al., Reference Nikolouli, Colinet, Renault, Enriquez, Mouton, Gibert, Sassu, Cáceres, Stauffer, Pereira and Bourtzis2017). Understanding the complex environmental effects on the biological responses of D. suzukii and on thermal (cold) tolerance in general is crucial for estimating population dynamics over the seasons and anticipating population growth at the beginning of the growing season. Such information can be used to develop predictive models that are an essential part of sustainable management programs (Asplen et al., Reference Asplen, Anfora, Biondi, Choi, Chu, Daane, Gibert, Gutierrez, Hoelmer, Hutchison, Isaacs, Jiang, Kárpáti, Kimura, Pascual, Philips, Plantamp, Ponti, Vétek, Vogt, Walton, Yu, Zappalà and Desneux2015; Hamby et al., Reference Hamby, Bellamy, Chiu, Lee, Walton, Wiman, York and Biondi2016).
To characterize cold tolerance of insects, several metrics are often used, including the supercooling point, the critical thermal minimum (CTmin), the chill coma recovery time (CCRT), the lower lethal temperature and the lower lethal time (Sinclair et al., Reference Sinclair, Coello Alvarado and Ferguson2015). Since most Drosophilidae, including D. suzukii (Jakobs et al., Reference Jakobs, Gariepy and Sinclair2015; Stephens et al., Reference Stephens, Asplen, Hutchison and Venette2015; Enriquez and Colinet, Reference Enriquez and Colinet2017), are chill susceptible and quickly die as a result of non-freezing cold exposures, their supercooling point (i.e. the temperature at which the insect's body fluids freeze) has little ecological value (Bale, Reference Bale1993; Andersen et al., Reference Andersen, Manenti, Sørensen, MacMillan, Loeschcke and Overgaard2015; Sinclair et al., Reference Sinclair, Coello Alvarado and Ferguson2015). Both CTmin (i.e. the low temperature provoking the loss of neuromuscular coordination) and CCRT (i.e. the time it takes to recover from chill coma) are ecologically relevant measures for insect thermal performance and have therefore been used in many Drosophila studies (e.g. Gibert and Huey, Reference Gibert and Huey2001; Hazell and Bale, Reference Hazell and Bale2011; MacMillan and Sinclair, Reference MacMillan and Sinclair2011). Furthermore, Andersen et al. (Reference Andersen, Manenti, Sørensen, MacMillan, Loeschcke and Overgaard2015) found CTmin, lower lethal temperature and lower lethal time to be the best predictors of the estimated cold tolerance and the geographical distribution of Drosophila species. The lower lethal temperature (i.e. the low temperature at which a certain percentage of the test population dies) and the lower lethal time (i.e. the time required to kill a predefined percentage of individuals at a certain temperature) characterize mortality caused by the accumulation of direct and indirect chill injuries, respectively, and can be used to describe the acute and chronic cold tolerance of insects (Denlinger and Lee, Reference Denlinger and Lee2010; Andersen et al., Reference Andersen, Manenti, Sørensen, MacMillan, Loeschcke and Overgaard2015; Sinclair et al., Reference Sinclair, Coello Alvarado and Ferguson2015).
Cold hardiness is species-specific, and numerous factors are known to affect this trait, such as the sex or the exposure duration and intensity. Survival at low temperature depends highly on the temperature to which the insect was exposed prior, during and after the cold temperature event (Colinet and Hoffmann, Reference Colinet and Hoffmann2012; Grumiaux et al., Reference Grumiaux, Andersen, Colinet and Overgaard2019; Enriquez et al., Reference Enriquez, Ruel, Charrier and Colinet2020). Additionally, the duration of exposure, the cooling and rewarming rate, and the frequency of these low-temperature events also affect insect survival (Danks, Reference Danks1996; Chown and Terblanche, Reference Chown and Terblanche2006; Colinet et al., Reference Colinet, Sinclair, Vernon and Renault2015). Other ecological variables are known to influence the thermal tolerance of insects, including the relative humidity, wind, photoperiod or solar radiation (Danks, Reference Danks1996; Chown and Terblanche, Reference Chown and Terblanche2006; Andersen et al., Reference Andersen, Findsen and Overgaard2013). Food deprivation can also alter insect cold tolerance. Indeed, several studies have shown that starvation can affect the resistance to long-term cold stress in D. melanogaster (Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015; Pathak et al., Reference Pathak, Munjal and Parkash2018), but the underlying mechanisms remained unresolved.
In recent years, a plethora of studies have focused on the overwintering biology of D. suzukii. Winter phenology studies indicate that D. suzukii flies most likely overwinter as dormant females in natural or man-made refuges (e.g. Zerulla et al., Reference Zerulla, Schmidt, Streitberger, Zebitz and Zelger2015; Pelton et al., Reference Pelton, Gratton, Isaacs, Van Timmeren, Blanton and Guédot2016; Rossi-Stacconi et al., Reference Rossi-Stacconi, Kaur, Mazzoni, Ometto, Grassi, Gottardello, Rota-Stabelli and Anfora2016; Thistlewood et al., Reference Thistlewood, Gill, Beers, Shearer, Walsh, Rozema, Acheampong, Castagnoli, Yee, Smytheman and Whitener2018). Several studies have suggested the occurrence of reproductive dormancy in D. suzukii, most likely a quiescence rather than a true diapause (e.g. Toxopeus et al., Reference Toxopeus, Jakobs, Ferguson, Gariepy and Sinclair2016; Wallingford et al., Reference Wallingford, Lee and Loeb2016; Wallingford and Loeb, Reference Wallingford and Loeb2016; Zhai et al., Reference Zhai, Lin, Zhang, Zhang, Zheng and Yu2016; Everman et al., Reference Everman, Freda, Brown, Schieferecke, Ragland and Morgan2018). In addition, studies have evaluated cold tolerance of different life stages and populations (e.g. Kimura, Reference Kimura2004; Jakobs et al., Reference Jakobs, Ahmadi, Houben, Gariepy and Sinclair2016; Plantamp et al., Reference Plantamp, Salort, Gibert, Dumet, Mialdea, Mondy and Voituron2016; Ryan et al., Reference Ryan, Emiljanowicz, Wilkinson, Kornya and Newman2016; Enriquez and Colinet, Reference Enriquez and Colinet2017), as well as the impact of different acclimation responses and other environmental factors on the thermal (cold) susceptibility of D. suzukii (e.g. Jakobs et al., Reference Jakobs, Gariepy and Sinclair2015; Shearer et al., Reference Shearer, West, Walton, Brown, Svetec and Chiu2016; Toxopeus et al., Reference Toxopeus, Jakobs, Ferguson, Gariepy and Sinclair2016; Enriquez et al., Reference Enriquez, Renault, Charrier and Colinet2018; Stockton et al., Reference Stockton, Wallingford and Loeb2018; Grumiaux et al., Reference Grumiaux, Andersen, Colinet and Overgaard2019; Enriquez and Colinet, Reference Enriquez and Colinet2019a, Reference Enriquez and Colinet2019b).
Despite the suspected role of food deprivation in shaping the aspects of insect's cold tolerance, including in drosophilids, via cross-tolerance and hormetic responses (e.g. Salin et al., Reference Salin, Renault, Vannier and Vernon2000; Nyamukondiwa and Terblanche, Reference Nyamukondiwa and Terblanche2009; Andersen et al., Reference Andersen, Findsen and Overgaard2013; Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Scharf et al., Reference Scharf, Wexler, MacMillan, Presman, Simson and Rosenstein2016), so far, no study has addressed whether starvation can alter the cold tolerance of D. suzukii. Therefore, in the present study, we assessed the effects of various periods of starvation (0, 12, 24 and 36 h) on subsequent cold tolerance of adults (reared at 25°C, LD 12:12 h), using several classical cold tolerance metrics (CCRT, CTmin, acute and chronic cold survival). The body composition and energetic reserves (mass, water content, total lipid, glycerol, triglycerides, glucose and soluble proteins) were measured in control and starved individuals to attest that starvation treatments had detectable effects, assuming that starvation would strongly affect lipids and cold tolerance (Hoffmann et al., Reference Hoffmann, Hallas, Anderson and Telonis-Scott2005).
Materials and methods
Mass rearing and starvation treatments
A 1-year-old laboratory stock culture of D. suzukii flies was used. It was established from a field collection of flies on blueberries and raspberries in Thorigné Fouillard, France (48°3′41.8″N, 1°14′19.3″W), in September 2016. The colony was reared in 100 ml glass bottles containing an artificial diet (per litre water: 15 g agar, 50 g sucrose, 30 g inactive dry brewer's yeast, 20 g cornmeal, 50 g carrot powder, 1.2 g methyl 4-hydroxybenzoate (Nipagin) dissolved in 12 ml ethanol, 2.22 g tartaric acid, 0.89 g ammonium sulphate, 0.22 g magnesium sulphate and 0.67 g potassium phosphate) and kept in an incubator (MIR-154-PE, Panasonic, Healthcare Co., Ltd., Gunma, Japan) set at 25°C, LD 12:12 h and 70% RH. Newly emerged adults were collected daily and maintained on the same artificial diet. Three- to four-day-old adults were randomly taken from the raring stock, were transferred in groups of approximately 30 individuals to 40 ml plastic Drosophila vials (25 × 95 mm, VWR International, France) and divided into four experimental treatments in which they were either given access to medium ad libitum ( = control group, or 0 h starvation) or deprived of food for increasing periods (12, 24 or 36 h). Vials of the control group contained 5 ml of the artificial medium described above, whereas those of the other treatments were filled with 5 ml agar-water medium (1 litre distilled water, 15 g agar and 1.2 g methyl 4-hydroxybenzoate dissolved in 12 ml ethanol) to induce starvation without desiccation. Mortality due to starvation was checked before the experiments and never exceeded 2%.
Critical thermal minimum
The CTmin of flies submitted to increasing starvation periods was studied using a long (52 × 4.7 cm), vertically positioned glass knockdown column containing several cleats to help flies hold on to the column while still awake. The column was connected to a thermostatic cooling bath (Lauda ECO RE 630S, Lauda Dr R. Wobser GmbH & Co. KG, Lauda-Königshofen, Germany) that pumped monopropylene glycol through the double-walled column. For each treatment group and each sex separately, approximately 60 flies, randomly selected within each starvation treatments, were placed in the upper end of the column, after which temperature was gradually decreased from 20 to −5°C at a rate of 0.5°C min−1 (n = approximately 60 flies), a rate considered as a standard when assessing CTmin (Sinclair et al., Reference Sinclair, Coello Alvarado and Ferguson2015). The temperature inside the column was monitored continuously using a thermocouple type K connected to a Comark Tempscan C8600 scanning thermometer (Comark Instruments, Norwich, Norfolk, UK). The CTmin values were individually recorded at the moment flies lost muscular functions due to cold-induced paralysis, also known as chill coma, and hence fell out of the column. This experiment was conducted twice with flies of two successive generations (i.e. n total per starvation treatment and per sex = approximately 120 flies).
Chill coma recovery time
For each starvation treatment and each sex separately, chill coma was induced by placing 50 flies, randomly selected within each treatment, in a 35 ml glass test tube that was placed inside an ice-water slurry already at 0°C for 8 h (n = 50). This temperature and exposure time were chosen according to previous work on the thermal tolerance of D. suzukii (Enriquez and Colinet, Reference Enriquez and Colinet2017) and are classically used in Drosophila (Sinclair et al., Reference Sinclair, Coello Alvarado and Ferguson2015). Upon removal, adults were supinely positioned on a table in a temperature-regulated room at 24 ± 1°C using a fine paintbrush, and the time to regain the ability to stand (i.e. chill coma recovery time, CCRT) was monitored individually. After 1 h, the experiment was stopped. Flies that were not on their legs after 1 h of recovery were considered not recovered. Measurements of CCRT were done in two repeated experiments using adult D. suzukii of two successive generations (i.e. n total per starvation treatment and sex = 100 flies).
Acute cold tolerance assay
For each starvation treatment and sex separately, 100 flies randomly selected within each treatment were separated into ten replicates of ten individuals (n = 100 per treatment and sex). Flies were exposed to acute cold stress in 35 ml empty glass test tubes (n = 10 per tube) directly placed in a cryostat (Lauda ECO RE 630S, Lauda Dr R. Wobser GmbH & Co. KG) set at −5°C for 1 h. This temperature and exposure time were chosen according to previous work on the thermal tolerance of D. suzukii (Enriquez and Colinet, Reference Enriquez and Colinet2017). Afterwards, flies were transferred back to food vials and placed in an incubator (MIR-154-PE, Panasonic, Healthcare Co., Ltd.) set at rearing conditions. Survival was visually assessed 48 h after cold exposure. Acute cold tolerance assays were performed twice in two successive generations (i.e. n total per starvation treatment and sex = 200 flies).
Chronic cold tolerance assay
To assess the chronic cold tolerance, for each starvation treatment and each sex separately, 100 flies randomly selected within each treatment were separated into ten replicates of ten individuals (n = 100 per treatment and sex). Flies were placed in empty glass test tubes (10 per tube) and directly exposed to chilling in an ice-water slurry at 0°C for 8 h. This temperature and exposure time were chosen according to previous work on the thermal tolerance of D. suzukii (Enriquez and Colinet, Reference Enriquez and Colinet2017). Following chronic cold treatment, all individuals were transferred to an incubator (MIR-154-PE, Panasonic, Healthcare Co., Ltd.) maintained at rearing conditions in food vials. Like in the acute cold tolerance assay, the number of survivors was counted 48 h later. Chronic cold tolerance assays were performed twice in two successive generations (i.e. n total per starvation treatment and sex = 200 flies).
Gravimetric measurements
For each starvation treatment, fresh mass of 30 randomly chosen males and females (n = 30) was quantified with 0.001 mg accuracy using an XP2U microbalance (Mettler Toledo International Inc., Greifensee, Switzerland). Flies were briefly anaesthetized with CO2 during the procedure. Next, adults were placed individually in 1.5 ml microcentrifuge tubes and stored at −80°C for approximately 2 h. The flies were then dried in a universal oven (UNE 200, Memmert GmbH & Co. KG, Schwabach, Germany) at 80°C for 48 h. Afterwards, all flies were reweighed to determine their dry mass. The body water content of each individual was then calculated as the difference between the fresh and dry mass and quantified as a percentage of fresh mass.
Lean dry mass was measured by the addition of 1.5 ml Folch mixture ( = 2:1 chloroform:methanol) to the tubes containing the dried flies, after which the tubes were placed horizontally on a shaker (Polymax 1040, Heidolph Instruments GmbH & Co. KG, Schwabach, Germany). Three days later, the liquid phase containing total lipids was removed and flies were redried in the oven at 80°C for 24 h. Samples were reweighed to quantify the lean dry mass. The total lipid content of the individual corresponds to the dry mass subtracted by its lean dry mass and divided by its fresh mass, respectively.
Triglyceride and glycerol quantification
For each starvation treatment and sex, eight biological replicates, each consisting of a pool of five adults (n = 8) were used to detect triglyceride (TAG) and glycerol concentrations by means of a colourimetric assay with triglyceride reagent (Sigma-Aldrich, France; T2449) as described by Tennessen et al. (Reference Tennessen, Barry, Cox and Thummel2014). This method is commonly used to quantify TAG. However, it should be kept in mind that this colourimetric assay not only releases glycerol from triglycerides but also from mono- and diglycerides. Flies were snap-frozen and homogenized in liquid nitrogen using a pellet pestle to obtain a fine powder. This powder was then diluted in 200 μl of PBST buffer solution (phosphate-buffered saline (PBS) + 0.05% Tween). After heat inactivation of the enzymes (10 min at 70°C), two sets of aliquots were taken from each sample. Free glycerol reagent (Sigma-Aldrich; F6428) was added to all aliquots, while triglyceride reagent, containing a lipoprotein lipase that cleaves glycerides into glycerol and fatty acids, was only added to one set of aliquots. The optical density of these samples was measured at 540 nm using a 96-well plate reader (VersaMax Microplate Reader, Molecular Devices, Sunnyvale, CA, USA). Conversion of the absorbance in each sample to its total glycerol concentration was done based on the triolein-equivalent standard curve (0–1 mg ml−1 range) (Sigma-Aldrich; G7793). The total amount of glycerides was then calculated by subtracting the glycerol concentration in the aliquots containing triglyceride reagent from that in the aliquots without triglyceride reagent (i.e. the initial concentration of free glycerol). Finally, TAG and glycerol levels were converted and expressed as quantities (μg) per adult fly.
Glucose and protein quantification
For each starvation treatment and sex, free glucose levels were determined using the Glucose Oxidase (GO) assay kit (Sigma-Aldrich; GAGO-20), following the protocol described by Tennessen et al. (Reference Tennessen, Barry, Cox and Thummel2014). For this colourimetric assay, eight biological replicates, each consisting of five pooled adults, were homogenized into a fine powder using liquid nitrogen and a pellet pestle (n = 8). After adding 100 μl of PBS, samples were incubated at 70°C for 10 min, and diluted 1:8 in PBS. Then, 30 μl of clear supernatant was transferred to a 96-well microplate. To each well, 100 μl of GO reagent consisting of glucose oxidase and peroxidase (Sigma-Aldrich; G3660), and o-dianisidine (Sigma-Aldrich; D2679) was added. These samples were incubated at 37°C for 30 min, after which time the enzymatic reaction was stopped by the addition of 100 μl of 12N sulphuric acid. The optical density was measured at 540 nm using a 96-well plate reader (VersaMax Microplate Reader, Molecular Devices), with the intensity of the colour being proportional to the original glucose concentration in the sample. Quantification was done using the calibration curve from the glucose standard solution (0−0.16 mg ml−1 range) (Sigma-Aldrich; G3285) and converted to μg per adult fly.
To measure the soluble protein content, 10 μl of the homogenized samples from the glucose assay was subjected to low-spin centrifugation (500 × g, 5 min, 4°C) to allow gentle sedimentation of cell debris. From each sample, 5 μl of clear supernatant was taken, diluted three times in PBS and transferred to a 96-well microplate, together with 250 μl of Bradford micro-assay reagent (Sigma-Aldrich; B6916) for measurement of the optical density at 595 nm (VersaMax Microplate Reader, Molecular Devices). The quantity of soluble proteins was determined based on a standard curve using a bovine serum albumin standard (Sigma-Aldrich; P0834 and P0914) (0–1.25 mg ml−1 range) and converted to μg per adult fly.
Statistical analysis
All analyses were performed in R version 3.4.4 (R Core Team, 2018). To determine if there was a significant difference between the two replicated experiments of cold tolerance, a generalized linear model (GLM) was fitted to the data, with ‘repetition’ as a factor. When data between both replicated experiments did not differ significantly (P > 0.05), they were pooled, and a GLM was used to describe the effects of ‘sex’, ‘starvation’ (i.e. fed or starved for various durations, coded as a categorical variable) and ‘sex by starvation’ interaction. When a significant difference was found between the two repeated experiments, a generalized linear mixed-effects model (GLMM) was fitted to the data (‘glmer’ function in ‘lme4’ package) via restricted maximum likelihood (REML), with ‘repetition’ as a random effect. For acute and chronic cold survival data, regression models with binomial error distribution and logit link function were used to analyse the data. For survival data, we specified the number of failures (i.e. dead) as well as the numbers of successes (i.e. alive) in a two-vector binomial response variable. As individuals from the same starvation treatment were divided into ten vials during the exposures, we build a first GLMM model with ‘vial’ as a random factor to account for any uncontrolled variability among the vials. We also build a classical GLM model without this random factor, and next, we compared both models using the ‘model.sel’ function from the ‘MuMin’ package. Based on smaller AIC, the GLM models were chosen. For the continuous and positive data (i.e. CTmin and CCRT), a significant difference was found between the two repeated experiments, hence a GLMM was used, with γ error distribution and identity link. For all models, the statistical significance of each variable was determined by an analysis of deviance via the ‘Anova’ function implemented in the ‘car’ package (Fox and Weisberg, Reference Fox and Weisberg2011). Differences among groups of ‘starvation’ or ‘sex by starvation’ variables were computed using estimated marginal means (EMMs) in ‘emmeans’ package (Lenth, Reference Lenth2018) and were considered significantly different when P ≤ 0.05.
Data obtained from gravimetric measurements and TAG, glycerol, glucose and soluble protein were checked for normality and homogeneity of variances. When these assumptions were not fulfilled, data were log-transformed or non-parametric tests were used. Total lipid, glucose and TAG were analysed with (parametric) ANOVA with ‘starvation’, ‘sex’ and ‘sex by starvation’ interaction as factors. Post-hoc tests were then conducted on significant terms via EMMs. Due to heteroscedasticity of variances, the data of lean dry mass and body water were analysed with Welch's ANOVA, using the ‘oneway.test’ function implemented in the ‘stats’ package (R Core Team, 2018). Pairwise comparisons of starvation groups were done with the Games–Howell post hoc test using ‘posthocTGH’ function in ‘userfriendlyscience’ package (Peters, Reference Peters2017). The Kruskal–Wallis test was applied to analyse the data of fresh mass, dry mass, glycerol and soluble protein content, and Dunn's post-hoc tests were used to determine differences among all the ‘sex by starvation’ combinations (in ‘Dunn.test’ package). The Kruskal–Wallis test cannot be applied to a factorial structure, hence, when these tests were used, we did not report separate effects of ‘starvation’, ‘sex’ and their ‘interaction’. Outliers were identified based on the interquartile range criterion and removed if present.
Results
Critical thermal minimum
Fasting significantly affected the CTmin of adults (GLMM, χ2 = 44.60 df = 3, P < 0.001), whereas sex did not (GLMM, χ2 = 1.69, df = 1, P = 0.19). No significant interaction was detected between sex and starvation (GLMM, χ2 = 3.40, df = 3, P = 0.334), therefore EMMs were not performed on the interaction between starvation and sex. Males and females starving for periods of 12, 24 or 36 h had a higher CTmin (on average 5.2, 5.4 and 5.4°C, respectively) than control flies fed ad libitum (4.8°C) (EMMs, P < 0.001 for all treatments) (fig. 1). Even if fasting globally increased CTmin compared to control only, comparisons of CTmin within the starved flies (i.e. 12, 24, 36 h) were not different (EMMs, P > 0.05) (fig. 1).

Figure 1. Boxplots of the critical thermal minimum (CTmin) of Drosophila suzukii following a starvation period of 0, 12, 24 and 36 h in females (n = 134, 124, 135 and 117, respectively; dark grey) and in males (n = 137, 120, 130 and 129, respectively; light grey). The data combined two replicated experiments. Observations within the 25–75 percentile range are represented by the boxes. The horizontal lines inside the boxes display the medians and the crosses represent the means. Boxes with different letters indicate differences among the starvation periods (i.e. significant effect of ‘starvation period’ followed by EMMs post-hoc tests, P ≤ 0.05).
Chill coma recovery time
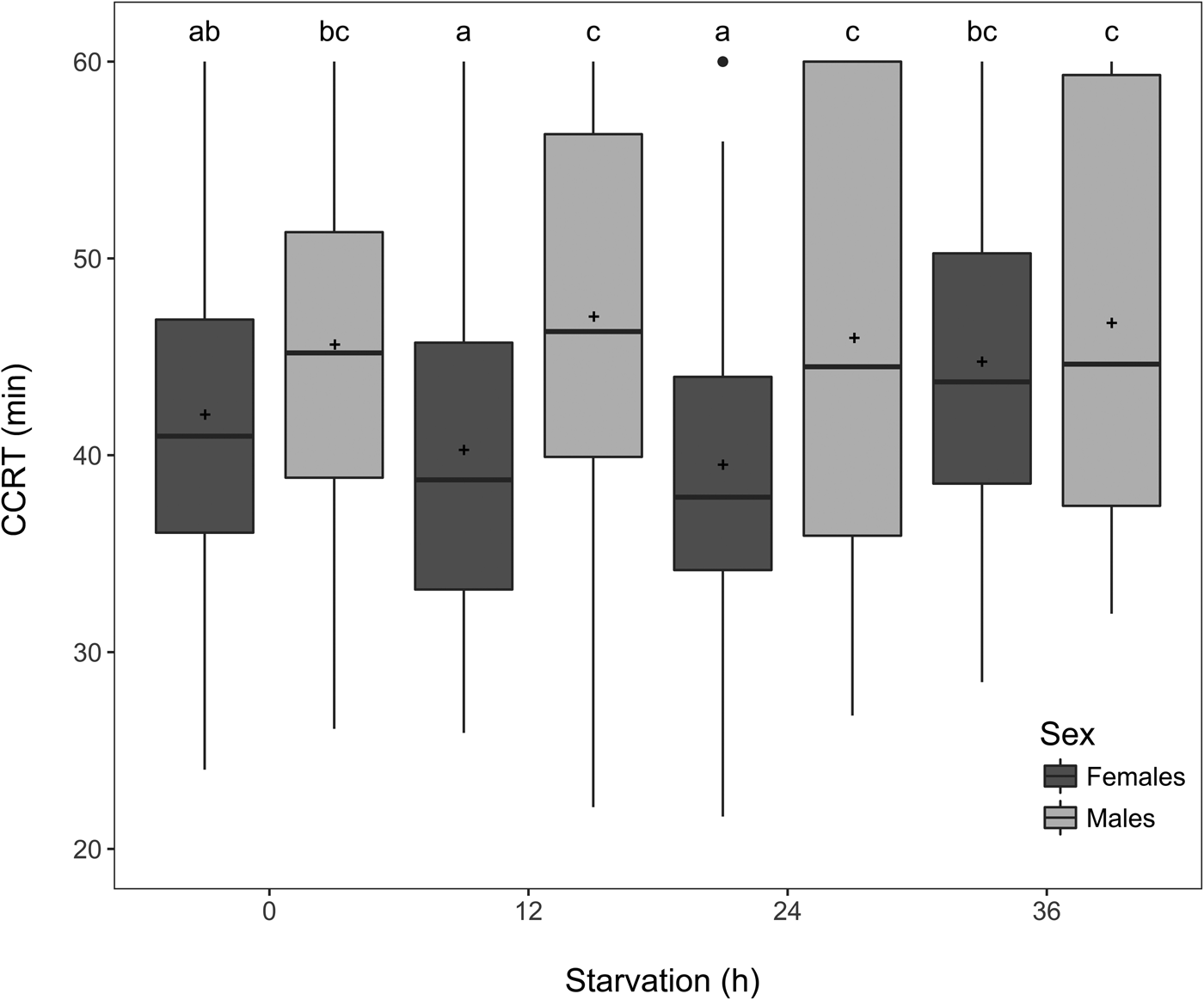
Starvation had a global significant effect on CCRT (GLMM, χ2 = 20.69, df = 3, P = 0. 0001, fig. 2), However, no clear pattern was observed among the different starvation treatments for males. Yet, females starved for 36 h needed more time to recover from chill coma than those fasting for 12 h (EMMs, P = 0.011) or 24 h (EMMs, P < 0.001) (fig. 2). Males had globally a higher CCRT than females (GLMM, χ2 = 8.17, df = 1, P = 0.004), especially when flies were starved for 12 or 24 h (EMMs, all P values < 0.001) (fig. 2). A small, but significant interaction between the duration of starvation and sex (GLMM, χ2 = 8.85, df = 3, P = 0.031) indicated that the effect of starvation on CCRT differed between sexes.

Figure 2. Boxplots of the chill coma recovery time (CCRT) of Drosophila suzukii following a starvation period of 0, 12, 24 and 36 h in females (n = 100; dark grey) and in males (n = 100; light grey). The data combined two replicated experiments. Observations within the 25–75 percentile range are represented by the boxes. The horizontal lines inside the boxes display the medians and the crosses represent the means. Boxes with different letters indicate differences among all the combined treatments (i.e. significant ‘sex by starvation’ interaction followed by EMMs post-hoc tests, P ≤ 0.05).
Acute cold tolerance assay
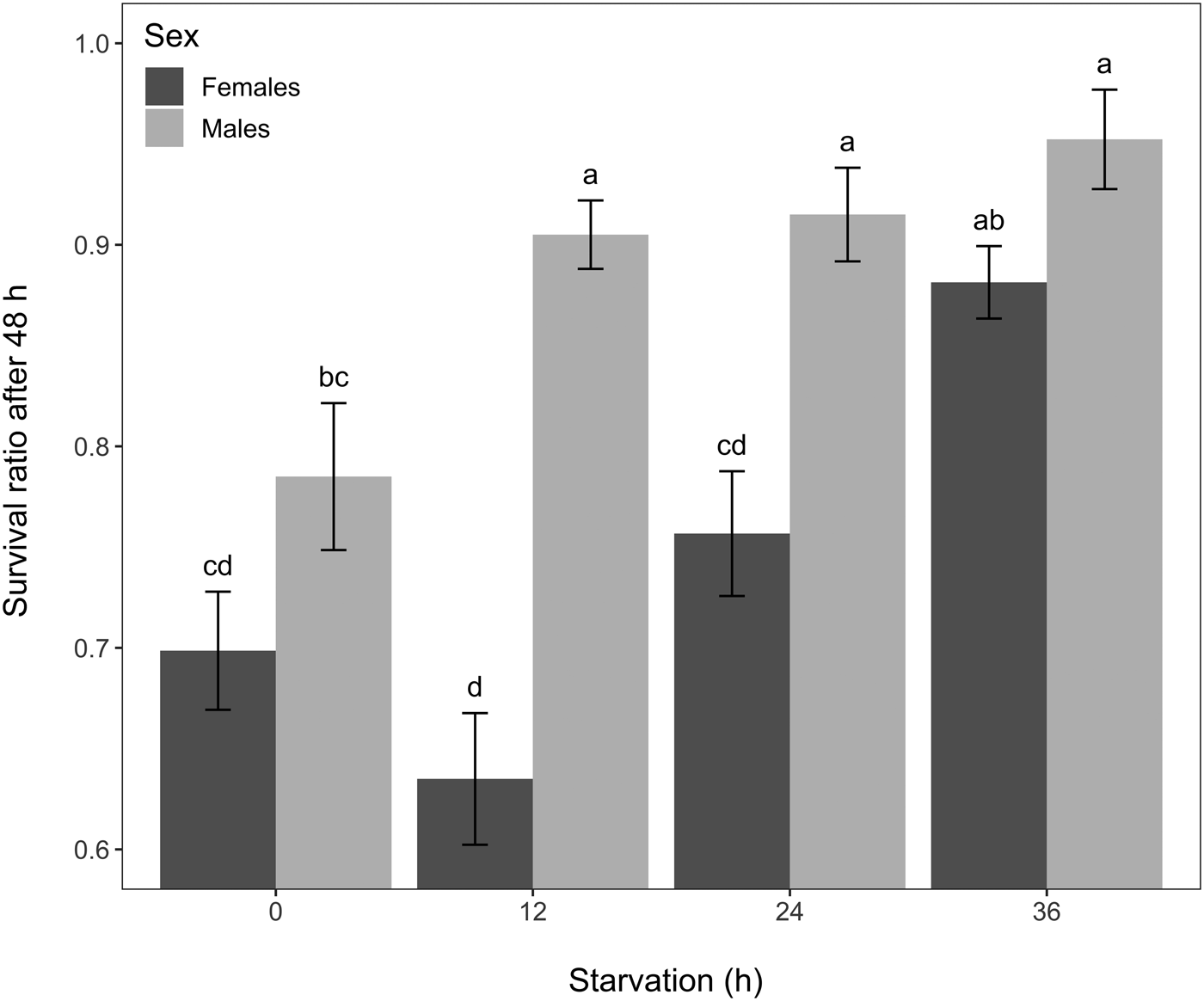
Starvation significantly increased acute cold survival (GLM, χ2 = 36.73, df = 3, P < 0.001). There was only a marginal overall difference between sexes (GLM, χ2 = 3.79, df = 1, P = 0.05), and survival was affected by the interaction between starvation and sex (GLM, χ2 = 12.85, df = 3, P = 0.005). A fasting period of 36 h resulted in female flies being more resistant to acute cold exposure than females that were starved for 12 or 24 h or that were fed ad libitum (EMMs, P < 0.05 for all treatments) (fig. 3). Results also showed that males starved for 12, 24 and 36 h were more cold tolerant than controls and >90% were still alive after the cold treatment (EMMs, all P values < 0.05) (fig. 3). Overall, males appeared to slightly better cope with acute cold exposure than females; the survival rates being different between sexes for flies starved for 12 h (EMMs, P < 0.001) and 24 h (EMMs, P = 0.001) (fig. 3).

Figure 3. Mean survival ratio of Drosophila suzukii 48 h after an acute cold exposure (1 h at −5°C) according to sex and starvation group (n = 200 individuals for each bar). Error bars represent standard errors of the means. Bars with different letters indicate differences among all the treatment combinations (i.e. significant ‘sex by starvation’ interaction followed by EMMs post-hoc tests, P ≤ 0.05).
Chronic cold tolerance assay
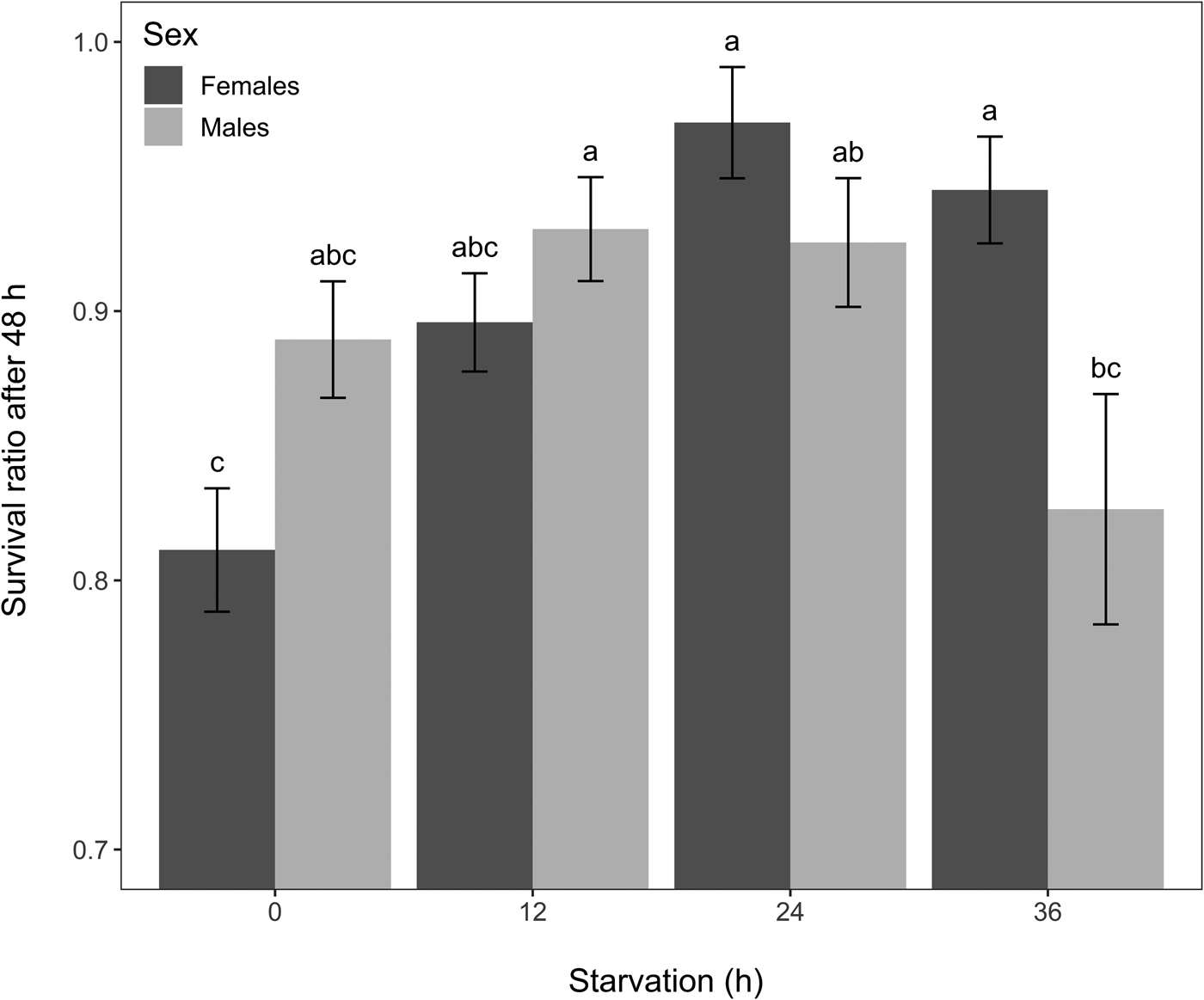
Starvation globally affected chronic cold tolerance (GLM, χ2 = 33.91, df = 3, P < 0.001), and overall survival differed between sexes (GLM, χ2 = 4.79, df = 1, P = 0.028). Females that were starved for 24 or 36 h had significantly higher chronic cold survival than control females (EMMs, P < 0.05 for all treatments) (fig. 4). In males, starvation for 12 and 24 h increased survival, but these changes were not significant, and a 36 h starvation caused lower survival than a 12 h starvation (EMMs, P = 0.038) (fig. 4). Fasting for 36 h resulted in more females surviving than males (EMMs, P = 0.008), but such sex difference was not found in the other starvation treatments or the control group (fig. 4). These differences between males and females were highlighted by a significant ‘sex by starvation’ interaction (GLM, χ2 = 25.68, df = 3, P < 0.001).
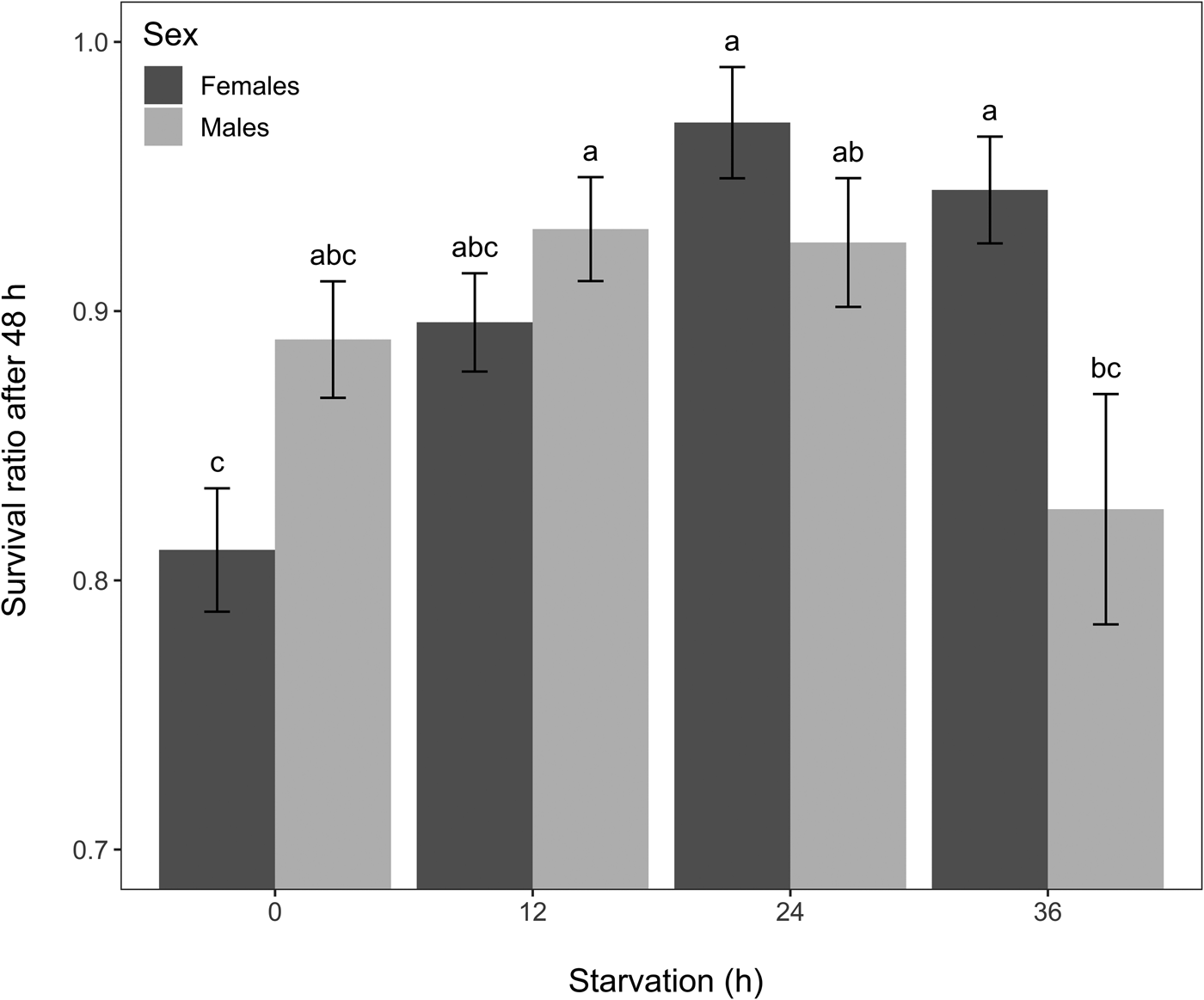
Figure 4. Mean survival ratio of Drosophila suzukii 48 h after a chronic cold exposure (8 h at 0°C) according to sex and starvation group (n = 200 individuals for each bar). Error bars represent standard errors of the means. Bars with different letters indicate differences among all the treatment combinations (i.e. significant ‘sex by starvation’ interaction followed by EMMs post-hoc tests, P ≤ 0.05).
Gravimetric measurements
Fresh weight was affected by starvation (Kruskal–Wallis, χ2 = 167.61, df = 7, P < 0.001). Among the different starvation treatments, only 36 h of fasting distinctly reduced male and female fresh weight (Dunn tests, all P values < 0.05) (table 1). The same response was observed for the dry mass (Kruskal–Wallis, χ2 = 166.85, df = 7, P < 0.001) and lean dry mass (Welch's ANOVA, F (7, 94.4) = 89.98, P < 0.001). Body weight measurements, i.e. fresh, dry and lean dry mass, indicated that females were on average heavier than males in all treatment groups, as expected (P < 0.001 for all pairwise comparisons of fresh and dry mass (Dunn post-hoc tests), and lean dry mass (Games–Howell post-hoc tests)).
Table 1. Body weight, water and lipid content of non-starved and starved male and female Drosophila suzukii adults

1Number of tested individuals (replicates).
Means (±SEM) or median within a column followed by the same letter are not significantly different (P > 0.05).
Post-hoc P values were determined with Dunn's tests (for fresh mass, dry mass), Games–Howell tests (for lean dry mass, water content) or EMMs tests (for lipid content).
Water content was significantly affected by starvation (Welch's ANOVA, F (7, 94.3) = 17.06, P < 0.001). The water content of females that starved for 24 h or more was slightly higher than that of fed females (Games–Howell, all P < 0.001). Furthermore, with the exception of the 12 h starvation group, both starved and non-starved males contained significantly more water than females (i.e. 72 vs. 70%) (Games–Howell, P values < 0.05) (table 1).
For the total lipid content, the parametric ANOVA indicated that all factors had a significant effect (F 3, 221 = 12.46, P < 0.001; F 1, 221 = 165.66, P < 0.001; F 3221 = 3.06, P = 0.029 for starvation, sex and their interaction, respectively). Starvation for 24 and 36 h resulted in females having lower body lipid content than controls (EMMs, P ≤ 0.01) (table 1). Males that fasted for 24 or 36 h contained less lipids compared to 12 h starved males (EMMs, P < 0.05 for both pairwise comparisons), but not to controls (EMMs, P values > 0.05) (table 1). In all treatments, females had a markedly higher lipid content than males (EMMs, all P values < 0.001) (table 1).
Triglyceride and glycerol quantification
Starvation and sex affected TAG quantity, but there was a significant interaction between these two factors (ANOVA, starvation: F 3, 53 = 29.81, P < 0.001; sex: F 1, 53 = 190.03, P < 0.001; sex by starvation: F 3, 53 = 1.04, P = 0.380). The longer the starvation period, the lower the TAG level (EMMs, P < 0.05 for both males and females) (table 2). Triglycerides were detected in significantly higher amounts in females than in males, and this for both starved and non-starved flies (EMMs, all P values < 0.001). Females contained between 43.7 and 72.1% more TAG compared to males (table 2).
Table 2. Amounts of triglycerides, glycerol, glucose and soluble proteins of non-starved and starved Drosophila suzukii adults
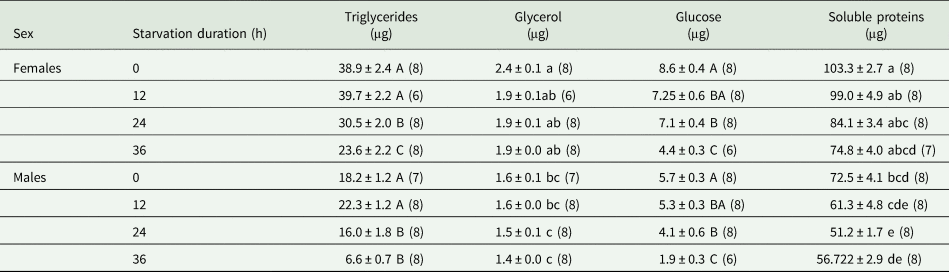
The number of tested replicates, each containing five pooled individuals, is shown in parentheses.
Within a column, means (±SEM) or median followed by the same letters are not significantly different (P > 0.05). Lowercase letters correspond to comparisons among all conditions (Kruskal–Wallis tests followed by Dunn's post-hoc tests for glycerol and proteins). Uppercase letters correspond to comparisons among the four different starvation durations only (ANOVA followed by EMMs post-hoc tests for triglycerides and glucose).
For glycerol, although Kruskal–Wallis test showed significant differences among all the combined groups (Kruskal–Wallis, χ2 = 44.824, df = 7, P < 0.001), the amount of glycerol in males and females was not noticeably impacted by increased starvation time within each sex group (Dunn tests, P > 0.05 for all pairwise comparisons) (table 2). This difference was due to the females that typically contained more glycerol than males (table 2).
Glucose and protein quantification
Glucose content was significantly influenced by sex and starvation, but not by their interaction (ANOVA, sex: F 1, 55 = 69.29, P < 0.001; starvation: F 3, 55 = 26.10, P < 0.001; starvation × sex: F 3, 52 = 0.734, P = 0.537). Starvation led to decreased glucose contents in both sexes. This inverse relationship was significant for flies starved for 24 or 36 h (EMMs, all P < 0.05) (table 2). In general, glucose fractions differed markedly between males and females, with the latter having a 26.4–57.6% higher glucose content than males (EMMs, all P values < 0.001) (table 2).
Fasting also affected the protein content of D. suzukii (Kruskal–Wallis, χ2 = 46.25, df = 7, P < 0.001). Fasting for 24 h caused males to lose a distinct quantity of soluble proteins compared to controls (Dunn test, P = 0.027), but no such loss was detected in females (table 2). Although no other significant differences were found between treatments in both sexes, the mean soluble protein content tended to diminish gradually with increasing starvation periods (table 2). Females contained considerably more soluble protein than males (Dunn tests, all P values < 0.05), except for those starved for 36 h (Dunn test, P = 0.056) (table 2).
Discussion
In the present study, we assessed the effect of food deprivation on subsequent cold tolerance of D. suzukii adults. Starvation resulted in decreased body weights in both males and females, demonstrating clearly that flies had experienced a nutritional shortage. Similarly, lipid and glucose stores dropped because of starvation, and 12 h of fasting were already sufficient to significantly reduce glucose and glyceride levels. It is well known that a mixture of different energy resources, mainly carbohydrates and lipids, and occasionally proteins, are metabolized during starvation in Drosophila flies (Oudman et al., Reference Oudman, Van Delden, Kamping and Bijlsma1994; Marron et al., Reference Marron, Markow, Kain and Gibbs2003; Schwasinger-Schmidt et al., Reference Schwasinger-Schmidt, Kachman and Harshman2012). Starvation in Drosophila melanogaster flies initially leads to the catabolism of non-lipid reserves (Lee and Jang, Reference Lee and Jang2014). This may change after approximately 36–48 h (depending on the diet) when body fat resources become the primary energy source (Lee and Jang, Reference Lee and Jang2014). Starved flies may ultimately die due to the complete depletion of their lipid reserves (Rion and Kawecki, Reference Rion and Kawecki2007; Lee and Jang, Reference Lee and Jang2014). In our study, we starved adults for various periods to generate mild stress pre-treatments before the cold stress exposures; yet, the starvation periods chosen were short enough to avoid significant mortality. Since previous studies have shown that fasting has a robust hormetic effect on subsequent survival to cold in many D. melanogaster genotypes (Le Bourg, Reference Le Bourg2013; Le Bourg and Massou, Reference Le Bourg and Massou2015), we speculated it might also be the case for D. suzukii. From our data, it was clear that flies had their physiological status and their energy stores altered by starvation, and we thus expected this pre-treatment to affect cold tolerance in some positive ways (cross-tolerance and hormesis), as reported in other Drosophila studies (e.g. Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015; Pathak et al., Reference Pathak, Munjal and Parkash2018). Genetic associations between resistance to cold and starvation have been investigated in laboratory-selected strains for different stressors in D. melanogaster to find cross-tolerance effects (Bubliy and Loeschcke, Reference Bubliy and Loeschcke2005).
Our data showed that starvation affected cold tolerance in D. suzukii but these effects were metric-dependent, as we observed positive, negative or no effect of starvation according to the assay. For instance, in starved adults, chill coma occurred at slightly higher temperatures than in fed individuals (5.3 vs. 4.9°C). Some studies have focused on the effect of starvation on the cold tolerance of insects but a few have used CTmin as a metric for low-temperature performance. Nyamukondiwa and Terblanche (Reference Nyamukondiwa and Terblanche2009) assessed the influence of feeding status on the thermal activity thresholds of two tephritid fruit flies and found similar results as in our study: CTmin of both species increased as a result of a 48 h starvation period, and no major sex-related variation was detected. This slight increase in CTmin values could be due to the depletion of energy reserves because of nutrient restriction. Genetic experiments on lines of D. melanogaster, which were selected either for increased starvation tolerance or for decreased CCRT, revealed that starvation and cold resistance were negatively correlated (Hoffmann et al., Reference Hoffmann, Hallas, Anderson and Telonis-Scott2005). This could be due to the competitive use of lipid storage. Moreover, tests performed on the tsetse fly Glossina pallidipes showed that individuals with the lowest CTmin had the highest body lipid and water content, suggesting an inverse relationship between energy resources and knockdown temperature thresholds (Terblanche et al., Reference Terblanche, Clusella-Trullas, Deere and Chown2008). This could, however, not be fully confirmed in our study, as the lowest CTmin values were observed in ad libitum fed D. suzukii and increased already after a 12 h starvation. On the other hand, total lipids and body water mass only dropped markedly after 36 h of starvation. Thus, in our case, D. suzukii adults with the highest lipid and water levels did not necessarily have the lowest critical thermal minima. The slight increase in CTmin of <1°C probably has a very limited ecological impact on stress resistance in the field.
Fasting affected CCRT; however, patterns were rather erratic and no clear pattern was observed among the different starvation treatments. This cold tolerance metric is a highly variable trait at the individual level (David et al., Reference David, Gibert, Pla, Petavy, Karan and Moreteau1998), as noted in the present study. There is relatively little information in the literature on the influence of feeding or starvation on CCRT in insects but positive, negative or no effects were reported. Fed locusts (Locusta migratoria) had an increased CCRT compared to fasted counterparts (Andersen et al., Reference Andersen, Findsen and Overgaard2013). Similarly, in a study on Drosophila immigrans, a beneficial effect of starvation on the cold tolerance was found (Pathak et al., Reference Pathak, Munjal and Parkash2018). In that study, flies that have fasted for 48 h had greater cold tolerance than their fed counterparts (differences in CCRT and survival after cold-shock), thus suggesting again a possible cross-tolerance between starvation and cold tolerance. On the contrary, red flour beetles (Tribolium castaneum) starved for 48 h recovered slower from chill coma than fed beetles, although this detrimental effect could also be attributed to the combined effect of starvation and desiccation because no water source was available during the starvation treatment (Scharf et al., Reference Scharf, Wexler, MacMillan, Presman, Simson and Rosenstein2016). Research on Ceratitis capitata adults revealed no change in CCRT between ad libitum fed flies and those deprived of food for 72 h (Mitchell et al., Reference Mitchell, Boardman, Clusella-Trullas and Terblanche2017). Likewise, a 21-day fasting period did not significantly affect CCRT of Myrmeleon hyalinus (Neuroptera: Myrmeleontidae) or Vermileo sp. (Diptera: Vermileonidae), two ambush insect predators (Scharf et al., Reference Scharf, Daniel, MacMillan and Katz2017). It thus appears that the effects of starvation on non-lethal measures of cold tolerance are not universal and context-dependent. Here, we found that starvation affected CCRT and CTmin differently, which is not so surprising as these metrics have different underlying mechanisms, despite both being related to chill coma (David et al., Reference David, Gibert, Moreteau, Gilchrist and Huey2003; MacMillan and Sinclair, Reference MacMillan and Sinclair2011; Ransberry et al., Reference Ransberry, MacMillan and Sinclair2011; Andersen et al., Reference Andersen, Manenti, Sørensen, MacMillan, Loeschcke and Overgaard2015). Accordingly, in a study assessing different measures of cold tolerance as predictors of the cold distribution limits of 14 drosophilid species, no significant correlation was detected between CTmin and CCRT (Andersen et al., Reference Andersen, Manenti, Sørensen, MacMillan, Loeschcke and Overgaard2015).
We conclusively found that starved males and females had higher survival rates than well-fed flies when they were subjected to acute cold stress. Yet, this positive effect of short-term starvation on acute cold tolerance occurred faster in males than in females. These observations support the notion that starvation can promote cold tolerance, particularly survival-related traits (Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015; Pathak et al., Reference Pathak, Munjal and Parkash2018). Generally, males were more likely than females to survive acute cold exposure, albeit significant differences between both sexes were merely found after 12 and 24 h of fasting. Recent research on the basal heat and low-temperature survival of D. suzukii also reported males to be less cold susceptible than females when exposed to acute cold stress (Enriquez and Colinet, Reference Enriquez and Colinet2017). Stephens (Reference Stephens2015), on the contrary, observed females to be more cold tolerant than males when subjected to −5°C for 2 h. Again, such inconsistencies between studies may be due to uncontrolled factors such as age, genotype, larval density or even microbiota (Colinet et al., Reference Colinet, Siaussat, Bozzolan and Bowler2013; Gerken et al., Reference Gerken, Eller, Hahn and Morgan2015; Henry and Colinet, Reference Henry and Colinet2018; Henry et al., Reference Henry, Renault and Colinet2018, Reference Henry, Overgaard and Colinet2020). In general, it remains unclear if females are more cold tolerant than males because results depend on the cold-tolerance metrics, as observed in Toxopeus et al. (Reference Toxopeus, Jakobs, Ferguson, Gariepy and Sinclair2016), as well as in the present study.
Just as found in the acute cold survival assays, chronic cold tolerance assays also revealed that females starved for 24 or 36 h had higher survival than fed flies. Furthermore, a 36 h starvation period seemed to have an adverse effect on the chronic cold survival of males, although this was statistically detectable only when compared to the 12 h starvation group. After 36 h of fasting, survival of females was also higher than that of males. This could indicate that, unlike for females, a 36 h starvation period might be a stress too severe for males. Females of D. melanogaster have higher starvation resistance than males (Kubrak et al., Reference Kubrak, Nylin, Flatt, Nässel and Leimar2017). The effect of food deprivation on the resistance to long-term cold stress has also been described in D. melanogaster (Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015). In these three studies, 1-week-old adults were starved for 24 h before being exposed to 0°C for 16–48 h, and resulting data showed similar responses as those observed here. Fasting increased the chronic cold tolerance of young D. melanogaster females but had no effect or a deleterious effect on young males (Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015). In addition, a 2–6 h delay between starvation and cold exposure could further enhance the survival of young flies, and this was the case for both sexes (Le Bourg, Reference Le Bourg2013; Le Bourg, Reference Le Bourg2015; Le Bourg and Massou, Reference Le Bourg and Massou2015). Age and timing thus also seem to play a role in the magnitude of the beneficial effect of starvation on cold survival traits.
Overall, both acute and chronic cold survival of D. suzukii were promoted by a starvation pre-treatment, though males and females reacted slightly differently. The other performance-related traits, such as knockdown and recovery, were affected by starvation in some complex way that can hardly be conclusively linked to starvation pretreatment. The mechanisms responsible for the better tolerance to acute and chronic cold survival of fasting flies are still unknown. Using RNA interference, Le Bourg and Massou (Reference Le Bourg and Massou2015) tested different genes involved in cold resistance (Frost), the innate immune system (Dif1) and the metabolic pathways at play during starvation (autophagy (Atg7) and the insulin/insulin-like growth factor 1 pathway (dFOXO)). However, none of them could explain the augmented chronic cold tolerance of starved D. melanogaster. The positive effect of starvation on cold survival (chronic and acute) may result from a reduced gut content. During exposure to sub-zero temperatures, ingested food materials may act as ice nucleating agents. An empty gut could prevent this and, hence, decrease the cold susceptibility of freeze-intolerant insects to a certain extent (Salt, Reference Salt1953; Danks, Reference Danks1996; Chapman, Reference Chapman2013). For this reason, the ingestion of food has been linked to higher supercooling points in various taxa (Baust and Morrissey, Reference Baust and Morrissey1975; Sømme and Conradi-Larsen, Reference Sømme and Conradi-Larsen1977; Sømme, Reference Sømme1982; Sømme and Block, Reference Sømme and Block1982; Leather et al., Reference Leather, Walters and Bale1993; Salin et al., Reference Salin, Renault, Vannier and Vernon2000). Nevertheless, this principle could neither explain the enhanced chronic cold survival, nor the enhanced acute cold survival because spontaneous freezing occurs at much lower temperatures than −5°C in D. suzukii (Toxopeus et al., Reference Toxopeus, Jakobs, Ferguson, Gariepy and Sinclair2016). In our case, we believe that the beneficial effect of starvation pre-treatment on acute and chronic cold tolerance of flies may be related to changes in haemolymph osmolality and altered ions or water balance, as observed in starved migratory locusts (Andersen et al., Reference Andersen, Findsen and Overgaard2013). Indeed, the maintenance of hydric and ionic homeostasis is directly related to the cold tolerance of insects (Overgaard and MacMillan, Reference Overgaard and MacMillan2017).
In conclusion, we found that fasting D. suzukii adults had lower body mass and energy reserves, especially when starvation periods were longer than 12 h. Short-term starvation pre-treatment led to an increased acute and chronic survival. Chill knockdown-related metrics were either slightly affected. Our study suggests that metrics of acute and chronic survival may rely on different physiological responses than those responsible for chill coma onset and recovery. The underlying mechanisms responsible for the beneficial impact of short starvation on acute and chronic survival warrant further investigation. Our results suggest that the absence of food during short periods can promote the cold survival of D. suzukii females. Such food scarcity conditions may occur in the field for instance in late fall, winter and early spring. As our study was conducted under standardized laboratory conditions, further research is required to elucidate the ecological significance of these results in natural situations.
Acknowledgements
We are grateful to EcoChimie Platform (EcoChim) from UMS OSUR 3343 for access to facilities and support.
Financial support
This study was supported by SUZUKILL project (The French National Research Agency): ANR-20-CE02-0011-01 and Austrian Science Fund (FWF): I 2604-B25.
Conflict of interest
None.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.