Introduction
The nematode Angiostrongylus costaricensis causes abdominal angiostrongyliasis (AA), a zoonotic disease with reported occurrences extending from North America to subtropical regions of South America (Morera et al., Reference Morera, Lazo, Urquizo and Llaguno1983; Rambo et al., Reference Rambo, Agostini and Graeff-Teixeira1997; Kramer et al., Reference Kramer, Greer and Quinonez1998; Incani et al., Reference Incani, Caleiras, Martín and González2007; Rodriguez et al., Reference Rodriguez, Dequi, Peruzzo, Mesquita, Garcia and Fornari2008). Its intermediate hosts are terrestrial molluscs and its definitive hosts are rodents, while humans may be accidental hosts (Morera & Céspedes, Reference Morera and Céspedes1971; Graeff-Teixeira et al., Reference Graeff-Teixeira, Camillo-Coura and Lenzi1991). The pre-patent period of this nematode lasts for about 24 days and the females of the parasite begin to perform oviposition around the 15th day after infection (Mota & Lenzi, Reference Mota and Lenzi2005).
The definitive diagnosis of AA is based on identification of worms, eggs or larvae through histopathological evaluation (Graeff-Teixeira et al., Reference Graeff-Teixeira, Camillo-Coura and Lenzi1991). In addition, haematological tests serve as an aid in the diagnosis, through findings of leukocytosis, eosinophilia and anaemia (Rodriguez et al., Reference Rodriguez, Dequi, Peruzzo, Mesquita, Garcia and Fornari2008; Romero-Alegría et al., Reference Romero-Alegría, Belhassen-García, Velasco-Tirado, Garcia-Mingo, Alvela-Suárez, Pardo-Lledias and Sánchez2014). Characteristic histopathological findings of the disease include acute pancreatitis as an important alteration. This is a form of inflammation that affects the exocrine system of the pancreatic parenchyma, and it may range in severity from mild to severe (Singh et al., Reference Singh, Jain and Kiran2016; Hermes et al., Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2020).
Parasitic infections generally give rise to increased eosinophil counts and modulate a T-helper 2 response in which immunoglobulin (mainly immunoglobulin E [IgE]) and pro-inflammatory cytokines are secreted (Moreau & Chauvin, Reference Moreau and Chauvin2010). These play an important role in infection control, because they orchestrate the innate immune response (Paim et al., Reference Paim, Duarte and Costa2011). This consists especially of interleukins 6 and 12 (IL-6 and IL-12) (Fernando et al., Reference Fernando, Reyes, Iannuzzi, Leung and McKay2014), tumour necrosis factor alpha (TNF-α) (Körner et al., Reference Körner, McMorran, Schlüter and Fromm2010) and interferon gamma (IFN-γ) (Green et al., Reference Green, DiFazio and Flynn2013; Inoue et al., Reference Inoue, Niikura, Mineo and Kobayashi2013). Another characteristic of inflammatory processes is an increase in cortisol levels: this parameter is used to ascertain stress levels in response to stressful agents (María et al., Reference María, Villarroel, Chacón and Gebresenbet2004). Higher cortisol levels facilitate proliferation of parasites in their hosts (Maswoswe et al., Reference Maswoswe, Peters and Warhurst1985; Escobedo et al., Reference Escobedo, Roberts, Carrero and Morales-Montor2005).
Although macroscopic and microscopic lesions due to experimental infection by A. costaricensis in mice have already been described, there are no reports on the effects of these lesions on blood count, cortisol and cytokine parameters. Therefore, our objective was to evaluate the relationship between histopathological lesions and the cytokine, cortisol and blood count levels in infections caused by A. costaricensis.
Materials and methods
Animals
Twenty-eight male Swiss mice, from the Animal Laboratory of the Institute of Biological Sciences of the University of Passo Fundo (UPF), located in the city of Passo Fundo, in the state of Rio Grande do Sul (RS), Brazil, were used in this study. The animals were kept in special cages where they received water and commercial food (Nuvilab®, Canguiri, Colombo-PR, Brazil, www.quimtia.com), ad libitum, under ideal temperature (23 ± 2°C) and light conditions (light/dark cycles of 12 h each). This study followed the ethical standards of the relevant national and institutional guidelines for the care and use of laboratory animals and this research protocol was approved by the UPF Committee on Animal Research Ethics (034/2016).
Isolation of A. costaricensis larvae
Isolates of A. costaricensis were obtained from Biomphalaria glabrata snails from the Parasitology Laboratory of the Pontifical Catholic University of RS, Brazil. Soon after obtaining third-stage larvae (L3), the cycle was maintained at the Animal Laboratory of the Institute of Biological Sciences, UPF, using Swiss mice as definitive hosts and terrestrial molluscs (Phyllocaulis sp.) as intermediate hosts. The L3 were obtained using the methodology described by Bender et al. (Reference Bender, Maurer, da Silva, Bem, Terraciano, da Silva and Graeff-Teixeira2003), through euthanasia and digestion of molluscs in 0.03% pepsin solution and 0.07% hydrochloric acid.
Experimental design
The 28 animals were separated into two homogenous groups (G1 and G2; n = 14 each), in which each group had eight infected animals and six control animals (non-infected). The experimental infection was performed by means of oral gavage, and an infecting dose of ten L3 per animal was used. The animals were euthanized at 14 days post infection (dpi) (G1) and 24 dpi (G2), and samples were collected at the time of euthanasia.
Samples
Blood samples were collected in the mornings, at the same time (starting at 07:30), by means of intracardiac puncture, with the animals under sedation with isoflurane (Isoforine®, Itapira, São Paulo, Brazil, https://cristalia.com.br/). In order to perform haematological analyses, blood was collected in tubes containing ethylenediamine tetra-acetic acid. On the other hand, the cortisol and cytokine levels were analysed using serum obtained from total blood collected in 1.5 ml tubes that was centrifuged at 2500 rpm for five minutes. The samples were stored at −70°C until use.
Blood count
A blood aliquot was taken using a microcapillary tube in order to perform a microhematocrit test and make a blood smear for a leukocyte differential count. The microcapillary tubes were sealed by means of heat, centrifuged at 10,000 rpm for 5 min and read using a reader card. The erythrocyte, leukocyte and platelet counts, and the haemoglobin determination were done in an automated haematological analyser (pocH-100iV Diff®, Sysmex do Brasil Indústria e Comércio Ltda, São Paulo, Brazil, www.sysmex.com.br). The erythrocyte indices (mean corpuscular volume (MCV); and mean corpuscular haemoglobin concentration (MCHC)) were calculated as described by Jain (Reference Jain, Weiss and Wardrop1986).
Cortisol determination
Cortisol concentration was determined with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EIAgen™Cortisol Test, https://www.arborassays.com/product/k003-h-cortisol-eia-kit/; BioChem ImmunoSystems, LEAC Lab, LEAC Produtos para Pesquisa Científica LTDA, Brazil, www.leaclab.com.br) following the manufacturer's instructions. Plates were read at 450 nm using a Synergy HI plate reader (Bio-Tek, USA, https://www.biotek.com/). The range and detection limits for the cortisol assay was 0 to 60 μg/dl.
Histopathology
A necropsy was performed. All organs (lungs, heart, kidneys, liver, spleen and digestive tract) were fixed in 10% buffered formalin for 24 h, to prevent decomposition, after which they were embedded in paraffin and sectioned in a microtome (at a thickness of 5 μm) for microscopic analysis. The slides were stained with haematoxylin and eosin, and analysed under an optical microscope (Junqueira & Carneiro, Reference Junqueira and Carneiro1995). The following aspects were investigated: infarction; presence of parasites (larvae, adults and eggs); mild (focal), moderate (multifocal) or severe (diffuse) eosinophilic infiltrate; mild (focal), moderate (multifocal) or severe (diffuse) granuloma formation; mild (focal), moderate (multifocal) or severe (diffuse) vasculitis; thrombosis; pancreatitis; and bronchopneumonia. The lesions were subjectively evaluated by an observer who was blinded to their intensity. The eosinophilic infiltrate, granulomatous reaction and vasculitis were graded according to intensity, as follows (Fan et al., Reference Fan, Lin, Du and Su2003): absent (0); mild/focal (1+); moderate/multifocal (2+); or severe/diffuse (3+).
Cytokine detection
To understand whether the infection process was capable of modulating the release of proinflammatory cytokines, a flow cytometric assay for cytokine determination was performed. The proinflammatory cytokine profile (IL-6, IL-12p70, IFN-γ and TNF-α) was determined using the BD™ Cytometric Bead Array (CBA) kit for mouse inflammation (BD Biosciences, San Jose, CA, USA, https://www.bdbiosciences.com/en-us). The dilution of serum samples, as well as the cytometer setup and data evaluation, were performed following the manufacturer's instructions.
Statistical analysis
To compare the groups (non-infected vs. G1; non-infected vs. G2), the normality of distribution and homoscedasticity of the variables haematocrit, total erythrocytes, haemoglobin, MCV, MCHC and total plasma protein were confirmed through the Shapiro–Wilk and Levene tests, respectively. Thus, these variables were analysed through the t-test for independent samples. The other variables, which did not present normal distribution (TNF-α, IFN-γ and IL-6), were analysed using the Mann–Whitney U test. The qualitative variables were organized into contingency tables, and the relative and/or absolute frequencies were obtained from descriptive statistics. To explain the effects of lesions and cytokine levels on cortisol, neutrophil and monocyte values (14 and 24 dpi), generalized linear models (GLMs) were used and the normality of the response variables was analysed. Thus, at 14 dpi, the influence of predictor variables on cortisol, neutrophil and monocyte levels was proposed from a GLM with gamma distribution for each response variable. At 24 dpi, the influence of predictor variables on cortisol and monocyte levels was proposed from a GLM with gamma distribution, while for neutrophils, a GLM with linear distribution was proposed.
Given the large number of explanatory variables in each model, they were selected through their t-values if these values presented significant differences and if the model with this variable was significantly better than the model without it. Thus, to select the best models, they were subjected to model simplification using the Akaike's Information Criterion corrected for small sample size (AICc) (Hurvich & Tsai, Reference Hurvich and Tsai1989; Burnham et al., Reference Burnham, Anderson and Huyvaert2011; Symonds & Moussalli, Reference Symonds and Moussalli2011). The data were considered significantly different with a probability (P) of less than 5%. The R statistical package (R Core Team, Reference R Core Team2014) was used for the statistical analyses and the GraphPad Prism software, version 7.01 (https://www.graphpad.com/scientific-software/prism/), was used to make the graphs.
Results
Macroscopic and microscopic findings
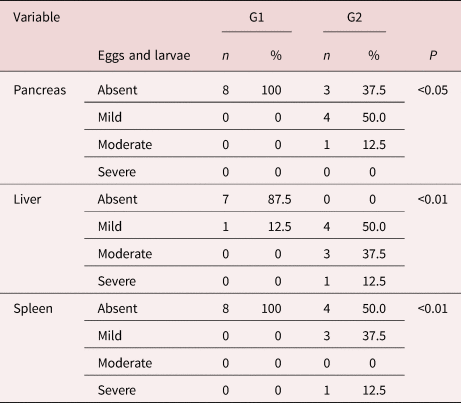
At necropsy, we found macroscopic changes in the liver and spleen. The infected group at 24 dpi presented spleen infarction (37.5%) and hepatic nodules were identified in both infected groups (28% at 14 dpi and 62.5% at 24 dpi). Changes in the pancreas such as pancreatitis were observed both times, through the kinetics of the infection (37.5% at 14 dpi and 75% at 24 dpi). At 24 dpi, 62.5% of the mice had eosinophilic infiltrate, in addition to granuloma, vasculitis, thrombi and necrosis. All animals in G2 (24 dpi) had eggs and larvae in different organs (table 1) indicating the patent infection.
Table 1. Eggs and larvae in pancreas, liver and spleen in mice experimentally infected with Angiostrongylus costaricensis (14 dpi and 24 dpi).
Cytokines
Significantly higher plasma IL-6 levels were observed in the animals infected with A. costaricensis than in the control animals (fig. 1a). The infected animals at 14 dpi presented an IL-6 level of 23.2 pg/ml; and at 24 dpi, 10.6 pg/ml. These levels were 20 times higher than the IL-6 levels of the control group: at 14 dpi, 2.6 pg/ml; and at 24 dpi, 0.4 pg/ml. All the infected animals had higher TNF-α levels than those of the non-infected animals at 14 dpi (infected: 13.5 pg/ml; control: 7.3 pg/ml). On the contrary, and curiously, at 24 dpi the TNF-α levels found in the control animals were significantly higher than those in the infected animals (infected: 4.2 pg/ml; control: 9.4 pg/ml) (fig. 1b). The levels of IFN-γ (fig. 1c) detected in infected animals (G1: 0.2 pg /ml; G2: 0.5 pg/ml) were two to three times higher than the ones found in the control animals (G1: 0.1 pg/ml; G2: 0.1 pg/ml). Lastly, there was no differences in the levels of IL-12 between infected and non-infected animals (fig. 1d).

Fig. 1. Proinflammatory cytokine profile induced by Angiostrongylus costaricensis in mice. Serum concentrations of IL-6 (A), TNF-α (B), IFN-γ (C) and IL-12p70 (D) were assessed in mice experimentally infected with A. costaricensis. The data are expressed as mean ± standard deviation. Asterisks indicate statistically significant differences (*P < 0.05 and **P < 0.01). The red dotted line indicates the minimum quantifiable levels of IL-6 (5 pg/ml), IL-12p70 (10.7 pg/ml), IFN-γ (2.5 pg/ml) and TNF-α (7.3 pg/ml) based on standard curves of the BD CBA Mouse Inflammatory Kit.
Haematological parameters
Considering the haematocrit, haemoglobin and total erythrocyte in the infected animals of G2 (24 dpi), we observed a decrease in the numbers when comparing to the control groups (fig. 2a–c). The same was observed for CHCM and total plasmatic protein (fig. 2e–f). There was no difference in the MVC of haemoglobin among groups (fig. 2d).

Fig. 2. Haematocrit analysis (A), haemoglobin (B), total erythrocyte count (C), mean corpuscular volume (D), mean corpuscular haemoglobin concentration (E) and total plasma protein (F) in mice experimentally infected with Angiostrongylus costaricensis, expressed as mean ± standard deviation. Asterisks indicate statistically significant differences (*P < 0.05 and **P < 0.01).
Cortisol parameters
The cortisol analysis was included in this study because this hormone can interfere in the normal activity of the immune system. The serological quantification of cortisol showed a significant increase of this molecule in G1 and G2 animals when compared to their control groups (fig. 3).

Fig. 3. Cortisol analysis in mice experimentally infected with Angiostrongylus costaricensis (14 dpi and 24 dpi).
The specificity of the test was evaluated by examining the extent of the parallelism between the standard curve for human cortisol concentrations and the curve of a series of dilutions of the plasma samples in phosphate buffered saline (PBS) (pH 7.4). The results of linear-regression testing indicated the existence of a high positive correlation (R2 = 0.9818) between the curves, with inter- and intra-assay coefficients of variation that ranged from 9% to 12% and 6% to 9%, respectively.
Predictive models for cortisol, neutrophil and monocyte levels
For day 14, the model that was proposed for explaining the changes in neutrophil values (AICc = 189.2; difference in AICc values of the best model (dAICc) = 0.00; model weight = 0.47) was able to explain 49% (Nagelkerke R-square). In this, the predictor variables of intestinal vasculitis and IL-6 positively corroborated the increase in neutrophil level (table 2). Intestinal vasculitis promoted an increase of 735.50 neutrophils/μl. Moreover, an increase in IL-6 of 1 pg/ml promoted an increase of 44.18 neutrophils/μl. Also, the model proposed for explaining the changes in monocyte values (AICc = 147.9; dAICc = 0.00; model weight = 0.72) was able to explain 53% (Nagelkerke R-square). The predictor variable of pancreatitis had a positive influence on the model, since the presence of pancreatitis promoted an increase of 538.10 monocytes/μl (table 3). Lastly, changes in cortisol levels (AICc = 110.2; dAICc = 0.00; model weight = 0.58) were able to explain 62% (Nagelkerke R-square) in the proposed model. The presence of intestinal vasculitis was responsible for the addition of 15.97 ng/ml of cortisol, in the same way that the presence of eosinophilic infiltrates in the intestine provided an increase in cortisol of 14.08 ng/ml (table 4).
Table 2. Result of generalized linear models (GLMs) with gamma (14 dpi) and linear (24 dpi) distribution in relation to the predictor variables that influenced the levels of neutrophils in mice infected by Angiostrongylus costaricensis.

IL-6, interleukin-6.
Table 3. Result of the generalized linear models (GLMs) with gamma (14 dpi) and linear (24 dpi) distribution in relation to the predictor variables that influenced the monocyte levels in mice infected with Angiostrongylus costaricensis.

Table 4. Result of generalized linear models (GLMs) with gamma distribution (14 dpi and 24 dpi) in relation to the predictor variables that influenced cortisol levels in mice infected with Angiostrongylus costaricensis.

On day 24, the explanation model proposed for the changes in neutrophil values (AICc = 209.6; dAICc = 0.00; model weight = 0.86) was able to explain 92% (Nagelkerke R-square). The predictor variables of bronchopneumonia and pancreatitis positively influenced the increase in neutrophil levels. The presence of bronchopneumonia gave rise to an increase of 790.50 neutrophils/μl, while the presence of pancreatitis gave rise to an increase of 1638.10 neutrophils/μl (table 2). The model proposed for changes in monocyte values (AICc = 176.5; dAICc = 0.00; model weight = 0.39) was able to explain 45% (Nagelkerke R-square). The presence of bronchopneumonia gave rise to an increase of 219.03 monocytes/μl (table 3). Lastly, the model proposed for changes in cortisol levels (AICc = 86.7; dAICc = 0.40; model weight = 0.42) was able to explain 74% (Nagelkerke R-square). The presence of pancreatic necrosis gave rise to an increase in cortisol level of 12.75 ng/ml. Likewise, the presence of vasculitis provided an increase in cortisol level of 8.62 ng/ml (table 4).
Discussion
Based on our experimental design, it was possible to evaluate the influence of cytokines and histopathological lesions on cortisol levels and leukogram parameters in animals with experimentally induced AA. We highlight the importance of this research, as it has provided the first description of these parameters at 24 dpi, the length of time taken by the parasite to complete its life cycle (Mota & Lenzi, Reference Mota and Lenzi2005). These authors have described at 11 dpi larvae in lymphatic vessels of the stomach, intestines, mesentery and pancreas, with peripheral inflammatory reaction represented by the presence of macrophages, eosinophils and neutrophils. In our experiment, this was also observed in the intestines and liver at 14 dpi.
Among the main microscopic findings of the infection described by Graeff-Teixeira (Reference Graeff-Teixeira, Camillo-Coura and Lenzi1991), eosinophilic infiltration in the intestinal wall, granulomatous reaction and eosinophilic vasculitis stood out. These were also found in the present study, mainly in the intestine, liver and spleen, with increased severity of these lesions at 24 dpi. Pancreatitis is related to infections due to other microorganisms: approximately 10% of cases are caused by viruses, bacteria or parasites (Economou & Zissis, Reference Economou and Zissis2000), especially the nematode Ascaris lumbricoides (Khuroo et al., Reference Khuroo, Zargar, Yattoo, Koul, Khan, Dar and Alai1992) and, more rarely, plathelminths of the genus Taenia (Liu et al., Reference Liu, Bair, Chang, Lin and Chan2005).
At 24 dpi, we found a considerable increase in eosinophilic infiltrate, along with presence of granuloma, vasculitis, thrombi and necrosis. Another feature of this experimental design that drew attention was pancreatitis, which affected 75% of the mice. This corroborated the results of Hermes et al. (Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2020), who found that 87.5% of the infected animals presented this infectious condition.
In relation to haematocrit in the infected animals, a condition of anaemia that had already been found in rodents (Ishih & Nishimura, Reference Ishih and Nishimura1997), dogs (Alfaro-Alarcón et al., Reference Alfaro-Alarcón, Veneziano and Galiero2015) and humans (Romero-Alegría et al., Reference Romero-Alegría, Belhassen-García, Velasco-Tirado, Garcia-Mingo, Alvela-Suárez, Pardo-Lledias and Sánchez2014) was detected. However, in our study, it was possible to characterize it as hypochromic normocytic anaemia (Chulilla et al., Reference Chulilla, Colás and Martín2009). This type of anaemia indicates iron deficiency, possibly associated with blood loss (Burkhard et al., Reference Burkhard, Brown and McGrath2001). Our hypothesis is that the lesions caused by the parasite in the intestinal vessels can cause blood loss in the same way as in Angiostrongylus vasorum infection in dogs (Schnyder et al., Reference Schnyder, Fahrion, Riond, Ossent, Webster, Kranjc, Glaus and Deplazes2010; de Vasconcelos et al., Reference de Vasconcelos, Mota and Pelajo-Machado2017). We believe that this finding at 24 dpi may have occurred because the parasite was already in the organs, causing inflammatory reactions and continuing its reproductive cycle (Mota & Lenzi, Reference Mota and Lenzi2005; Spratt, Reference Spratt2015). In a novel study, Mota & Lenzi (Reference Mota and Lenzi2005) made a new proposal for the cycle of A. costaricensis and, based on this information, we believe that our findings of anaemia at 24 dpi may have been related to inflammatory reactions and intestinal lesions during the migratory process.
In analysing the leukogram, we found neutrophilia and monocytosis, due to the infectious and inflammatory process that the individuals affected by A. costaricensis experienced. In this, not only neutrophils but also macrophages are activated and recruited to the target tissues that have been invaded by helminths (Anthony et al., Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007). At 14 dpi, our model was able to explain 49% of the effect between the presence of intestinal vasculitis and increased IL-6 levels and increased neutrophil levels. Regarding monocytosis, the model was able to explain 53% of the relationship with the presence of pancreatitis.
At 14 dpi, our model was able to explain 62% of the effect between the presence of vasculitis and eosinophilic infiltrate in the intestine and increased cortisol levels. At 24 dpi, vasculitis and pancreatic necrosis were seen to positively influence serum cortisol changes, and our model was able to explain 74% of this change. Cortisol levels increase because of the stress caused by the infection. This occurs regardless of the period of the infection cycle, either through morbid actions of the host organism or through clinical signs of pain caused by pancreatitis and inflammation of other organs in infected mice (Rebello et al., Reference Rebello, Barros, Mota, Carvalho, Perales, Lenzi and Neves-Ferreira2011; Alfaro-Alarcón et al., Reference Alfaro-Alarcón, Veneziano and Galiero2015; Törnhage & Alfvén, Reference Törnhage and Alfvén2015; Gülen et al., Reference Gülen, Dur, Serinken, Karcioğlu and Sönmez2016). It is noteworthy that during the inflammatory reaction, the release of cytokines can also activate a stress response (Calcagni, Reference Calcagni2006).
A long and intense inflammatory process, such as that observed in helminth infections, triggers several immunological responses in the hosts. These responses consist of orchestrated soluble cytokines that activate effector cells of the innate and adaptive immune systems (Geiger et al., Reference Geiger, Abrahams-Sandi, Soboslay, Hoffmann, Pfaff, Graeff-Teixeira and Schulz-Key2001; Anthony et al., Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007). Mice with a major histocompatibility complex (MHC)-II deficiency were found to shed larger numbers of first-stage larvae (L1) in their stools than mice with a fully functional immune system, though L1 shedding levels did not correlate with length of survival in these mice (Geiger et al., Reference Geiger, Hoffmann, Soboslay, Pfaff, Graeff-Teixeira and Schulz-Key2003). The pattern of inflammatory cytokines found in the infected animals of the present study made it possible to characterize the infection process at 14 dpi as an acute inflammatory condition, with elevated concentrations of TNF-α and IL-6 in comparison with control mice.
TNF-α triggers a series of events, such as vasodilation and oedema, cell recruitment to the site of infection and activation of coagulation factors (Zelová & Hošek, Reference Zelová and Hošek2013). This explains our findings of presence of eosinophils at sites of inflammation, and of thrombus formation and necrosis in the liver, pancreas and intestines of the mice. The decreased serum TNF-α levels in the infected animals at 24 dpi may have been related to increased cortisol levels at this stage of infection, since high levels of this parameter can cause suppression of cytokines, relating to the inflammatory response (Castro et al., Reference Castro, Zou, Secombes and Martin2011).
The increase in IFN-γ levels in the infected animals, in relation to those of the control group, was an expected change, considering the inflammatory response triggered by A. costaricensis. This cytokine plays an important role in macrophage activation and modulation of the adaptive T helper one (Th1) immune response (Hu & Ivashkiv, Reference Hu and Ivashkiv2009). On the other hand, IFN-γ cannot stimulate a T helper two (Th2) response, which would be the most effective modulation towards elimination of the parasite (Cortes et al., Reference Cortes, Sotillo, Muñoz-Antoli, Fried, Esteban and Toledo2014). It is important to mention that the levels of INF detected were lower than the last point of the standard quantification curve for this cytokine (fig. 1c). As recommended by the manufacturer of the kit used (BD CBA Mouse Inflammatory Kit), it is up to the researcher to decide the best method for calculating values for unknown samples using this assay; consequently, we trust that the differences found are due to the infection, and not a limitation of detection of the assay. Interestingly, the levels of IL-12p70 were low and also below the standard curve of the kit (fig. 1d). IL-12 induces IFN-γ production by T cells (Vignali & Kuchroo, Reference Vignali and Kuchroo2012), and the overall low levels of INF-γ observed can perhaps be associated to the low levels of IL-12p70 induced by A. costaricensis during the infection.
In conclusion, we demonstrated the relationship that histopathological lesions in mice experimentally infected by A. costaricensis have with increased levels of cortisol, neutrophil and monocytes, along with the relationships with IL-6 and TNF-α. We characterized the presence of normocytic and hypochromic anaemia in the infected groups.
Acknowledgements
The authors thank the University of Passo Fundo, Brazil, for supporting this study, which was carried out in its laboratory animal facility; and also the Institute of Pathology of Passo Fundo and the Parasitology Laboratory of the Pontifical Catholic University of Rio Grande do Sul, Brazil.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES; finance code 001).
Conflicts of interest
None.









