INTRODUCTION
The protozoan parasite Giardia duodenalis (syn. G. intestinalis and G. lamblia) infects man but is also one of the more common parasites of domestic and production animals worldwide (Bowman and Lucio-Forster, Reference Bowman and Lucio-Forster2010; Geurden et al. Reference Geurden, Vercruysse and Claerebout2010; Plutzer et al. Reference Plutzer, Ongerth and Karanis2010). Although most infections remain asymptomatic, intermittent diarrhoea is the most relevant clinical sign among the broad spectrum of symptoms. Host- and parasite-related factors are recognized to be associated with this clinical variability (Muller and von Allmen, Reference Muller and von Allmen2005), although no specific virulence factors have been identified yet. Treatment of giardiasis relies on different drug classes with specific action mechanisms: nitro-imidazoles (metronidazole, tinidazole), nitrofurans (furazolidone) and benzimidazoles (albendazole) (Escobedo and Cimerman, Reference Escobedo and Cimerman2007). These drugs are generally effective, although treatment failure and drug resistance have already been reported (Escobedo and Cimerman, Reference Escobedo and Cimerman2007). Strain- or assemblage-dependent drug susceptibility may contribute to treatment failures; however, other reasons for drug failure such as ‘cure followed by re-infection’ and poor patient compliance must also be taken into account (Boreham et al. Reference Boreham, Phillips and Shepherd1987; Farbey et al. Reference Farbey, Reynoldson and Thompson1995).
Currently, isolates of G. duodenalis are grouped into 7 defined genotypes (assemblages), based on analysis of conserved genetic loci. All human isolates characterized to date belong to assemblages A and B, which have also been recovered from livestock, cats, dogs, beavers and guinea-pigs. The other assemblages are linked to a specific host: assemblages C and D to dogs, assemblage E to livestock, assemblage F to cats and assemblage G to rats. By sequence analysis of the glutamate dehydrogenase (gdh) locus, isolates have been further subdivided into the subgroups AI–III, BIII–IV and EI–XI (Feng et al. Reference Feng, Ortega, Cama, Terrel and Xiao2008; Read et al. Reference Read, Monis and Thompson2004). Although subtypes AI and AII are both identified in man and animals, subtype AI seems to predominate in animals whereas humans are mostly infected with subtype AII. Subtype AIII is restricted to wild hoofed stock (Caccio et al. Reference Caccio, Beck, Lalle, Marinculic and Pozio2008).
Culture media improvements and development of in vitro and in vivo excystation techniques enabled the establishment of a large number of human and animal field isolates (Kasprzak and Majewska, Reference Kasprzak and Majewska1985; Karanis and Ey, Reference Karanis and Ey1998), for which a large heterogeneity was demonstrated not only at the molecular level but also with regard to in vitro and in vivo growth (Binz et al. Reference Binz, Thompson, Lymbery and Hobbs1992), drug susceptibility (Arguello-Garcia et al. Reference Arguello-Garcia, Cruz-Soto, Romero-Montoya and Ortega-Pierres2004), infectivity and virulence (Cevallos et al. Reference Cevallos, Carnaby, James and Farthing1995) and disease outcome (Sahagun et al. Reference Sahagun, Clavel, Goni, Seral, Llorente, Castillo, Capilla, Arias and Gomez-Lus2008). Unfortunately, most studies were conducted using non-clonal isolates or without characterization of the assemblage (Karanis and Ey, Reference Karanis and Ey1998; Mohamadnezhad et al. Reference Mohamadnezhad, Ghaffarifar and Dalinmi2008) and included laboratory strains that had been maintained for more than 20 years and were well adapted to in vitro cultivation. In vitro studies generally use the human assemblage A strains P-1 (ATCC 30888) or WB (ATCC 30957) (Cruz et al. Reference Cruz, Sousa, Azeredo, Leite, Figueiredo de Sousa and Cabral2003; Arguello-Garcia et al. Reference Arguello-Garcia, Cruz-Soto, Romero-Montoya and Ortega-Pierres2004). In addition, studied field isolates mostly belonged to assemblage A and to a lesser extent to assemblage B. Except for the host restriction, no comparable information is available for the assemblages C, D, E and F. As such, a large genetic and phenotypic diversity among Giardia isolates exists, but without a clear relationship between genetic markers, biological characteristics and clinical outcome (Cevallos et al. Reference Cevallos, McHugh, Carnaby and Farthing1991). Isoenzyme analysis and genome sequencing have been used to identify genes that may be responsible for the observed biological variability, but only for WB (assemblage AI), GS/M-83-H7 (assemblage B) and P15 (assemblage EIII) (Franzen et al. Reference Franzen, Jerlstrom-Hultqvist, Castro, Sherwood, Ankarklev, Reiner, Palm, Andersson, Andersson and Svard2009; Jerlstrom-Hultqvist et al. Reference Jerlstrom-Hultqvist, Franzen, Ankarklev, Xu, Nohynkova, Andersson, Svard and Andersson2010). The genetic differences between both were similar in magnitude to the differences between G. duodenalis and G. muris (Franzen et al. Reference Franzen, Jerlstrom-Hultqvist, Castro, Sherwood, Ankarklev, Reiner, Palm, Andersson, Andersson and Svard2009). Furthermore, isoenzyme electrophoresis revealed different migration patterns of key metabolic enzymes in assemblage A and B (Meloni et al. Reference Meloni, Lymbery and Thompson1988; Mayrhofer et al. Reference Mayrhofer, Andrews, Ey and Chilton1995), strengthening the assumption that both should be considered as 2 different Giardia species.
In the present series of in vitro and in vivo experiments, we focused on 2 aspects (1) in vitro growth characteristics of 3 laboratory and 6 field strains from assemblages A, B and E and (2) assemblage-linked differences in drug susceptibility to the current anti-giardia reference drugs.
MATERIALS AND METHODS
Axenic culture of trophozoites
Nine strains of G. duodenalis were selected on the following criteria: genotype (assemblages A, B and E), host (human (h) or cattle (c)) and cultivation history (laboratory or field strains). The WB (ATCC 30957; assemblage A) and GS/M-83-H7 (ATCC 50581; assemblage B) strain and an assemblage A laboratory strain (G1) were obtained from Reto Brun (Swiss Tropical and Public Health Institute, Basel, Switzerland) and Norbert Mueller (Institute of Parasitology, Berne, Switzerland). Four field strains of assemblage A and 2 of assemblage E were recently established as in vitro trophozoite cultures (Bénéré et al. Reference Benere, Geurden, Robertson, Van Assche, Cos and Maes2010). All field isolates were cloned and characterized by sequence analysis of 3 genes: the gdh (Read et al. Reference Read, Monis and Thompson2004), the β-giardin (Lalle et al. Reference Lalle, Jimenez-Cardosa, Caccio and Pozio2005) and the triosephosphate isomerase (tpi) gene (Geurden et al. Reference Geurden, Geldhof, Levecke, Martens, Berkvens, Casaert, Vercruysse and Claerebout2008, Reference Geurden, Levecke, Caccio, Visser, De, Casaert, Vercruysse and Claerebout2009) (Table 1).
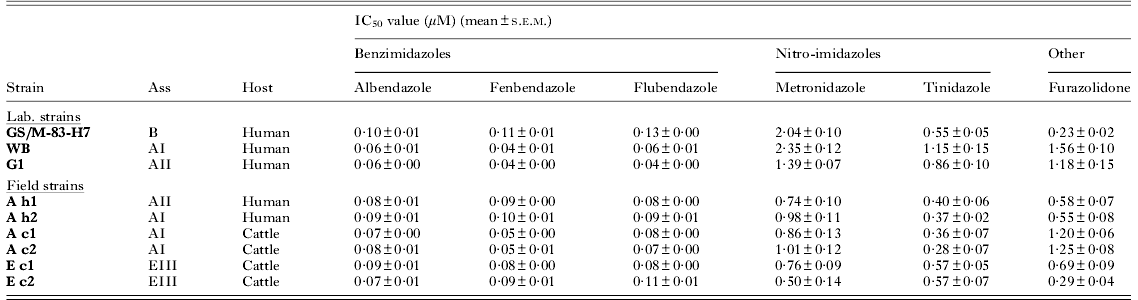
Table 1. In vitro susceptibility (IC50) against a panel of laboratory and field strains of assemblages A, B and E to different anti-giardia reference drugs
Trophozoites were routinely maintained in TYI-S-33 medium (Keister, Reference Keister1983) at pH 6·8, supplemented with 10% heat-inactivated bovine serum (BSi) (Gibco, Merelbeke, Belgium), 10 g/L yeast (Becton Dickinson, Aalst, Belgium), 0·023 g/L ammonium ferrous citrate (Sigma, Bornem, Belgium) and 1 μl/ml of fresh bovine bile obtained from a local slaughterhouse and stored in small aliquots at −20°C until use. To grow assemblage B trophozoites, the TYI-S-33 medium was supplemented with 0·5 g/L powdered bovine bile (Sigma, Bornem, Belgium) and 10% heat-inactivated fresh human serum (Bénéré et al. Reference Benere, Geurden, Robertson, Van Assche, Cos and Maes2010). Cultivation was performed in 10 ml screw-cap culture vials (Nunc, Langenselbold, Germany) filled to 95% of total volume capacity and incubated at 37°C. Subcultures were made twice weekly. Detachment of trophozoites for preparation of inocula was achieved by chilling the cultures on ice for 20 min. The number of trophozoites was determined using 0·4% Trypan blue (w/v) (Sigma, Bornem, Belgium) and 0·04% formol in a KOVA Glasstic® counting slide (Bayer, Brussels, Belgium).
Determination of generation time
Adherent trophozoites of monolayer cultures were used to initiate growth-rate experiments. Culture tubes were filled with fresh medium, inoculated at 104 trophozoites/ml and incubated at 37°C. Trophozoite growth was determined daily for 4 consecutive days. For enumerating trophozoite density, aliquots of 2 chilled trophozoite cultures were ½ diluted in 0·04% formol and counted in a Cell Lab QuantaTM SC flow cytometer (Analis, Ghent, Belgium). The generation time (g) is calculated in the exponential growth phase using a modified formula of Visvesvara (Reference Visvesvara1980):
with n0=number of trophozoites at the beginning of the experiment (t0); nx=number of trophozoites at 24 h, 48 h, 72 h or 96 h (tx).
Since chilling on ice may influence subsequent growth, it would be inappropriate to follow trophozoite growth in the same culture tube, so separate culture tubes were used daily. Each test was repeated on 4 occasions and all culture tubes were monitored on a daily basis by microscopic examination.
In vitro drug susceptibility assay
A protocol for growth in microtitre plates and in vitro drug susceptibility testing of Giardia trophozoites was previously established (Bénéré et al. Reference Benere, da Luz, Vermeersch, Cos and Maes2007). To obtain optimal growth, near-total well volume (300 μl) and air-tight sealing of the plate was required to fulfil the low oxygen tension requirements of trophozoites. For assemblage A trophozoites, the amount of yeast (20 g/L), BSi (11·2%), bovine bile (0·56 g/L) and ammonium ferrous citrate (0·026 g/L) was increased compared to the standard TYI-S-33 culture medium. Assemblage B and E trophozoites do not require these supplementations, although human serum must substitute the bovine serum to obtain more acceptable growth. Dependent on the growth rate of the strain, an inoculum achieving confluence within 72 h of incubation was selected (5×104 to 105 trophozoites/well). Stock solutions (20 mm) of albendazole (ABZ), fenbendazole (FEN), flubendazole (FLU), metronidazole (MET), tinidazole (TIN) and furazolidone (FUR) were prepared in 100% dimethylsulphoxide (DMSO). All compounds were obtained from Sigma and/or Gibco. The highest in-test drug concentration was 64 μ m containing only 0·4% DMSO. Four replicate wells with the pre-diluted test compound, the non-treated control (100% growth) and the culture medium control (0% growth) were included on each plate. Growth was assessed after 3 days upon removal of the medium and addition of resazurin (33 μ m) on the adherent fraction. Fluorescence is measured (λ ex 550 nm, λ em 590 nm) after 20 h incubation at 37°C. Each test was repeated on at least 3 separate occasions. Probit analysis was used to calculate the concentration of drug that inhibited trophozoite viability in vitro by 50% (StatView® statistical software, V.5.0.1).
In vivo drug susceptibility evaluation
Four-week-old male SPF gerbils (Meriones unguiculatus) (BW∼30 g) were purchased from Janvier (Le Genest, St Isle, France) and housed individually with food and water ad libitum. They were randomly allocated to the different experimental groups after an acclimatization period of 1 week during which prior exposure to Giardia was excluded by microscopic examination of feces collected over 3 consecutive days. The animals were fasted overnight before oral inoculation with 106 trophozoites in 500 μl of PBS. MET, ABZ and FLU were retained for further evaluation in vivo and formulated in PEG400 (dosing volume of 200 μl/50 g BW). The drug formulations were administrated by gavage as a single daily oral dose for 3 consecutive days starting on day 7 post-infection (p.i.). A dose-titration against the WB strain was performed in groups of 6 animals at 5, 10, 20, 40 and 80 mg/kg for MET and at 2, 4, 6, 8 and 10 mg/kg for ABZ and FLU to determine the dose required to reduce the number of trophozoites by 50% and 90% (ED50 and ED90) compared to vehicle-treated infected controls. Next, the isolates of the assemblages A, B and E were tested at the ED50–70 to check variability in intrinsic drug susceptibility. Since only limited information is available in the gerbil model, the assemblage A and B laboratory strains (WB, GS/M-83-H7) were included to allow comparison with available literature data. Gerbils were fasted for about 48 h and euthanized with CO2 36–48 h after the last dosing (=11 days p.i.). The entire small intestine was collected, opened longitudinally and suspended in PBS. The test tube was kept on ice for 35 min to detach trophozoites and, after removal of the intestinal segment, centrifuged at 800 g for 10 min at 4°C to determine the number of recovered intestinal trophozoites. Percentage reduction compared to vehicle-treated infected control animals is used as a measure for drug activity. All data represent a minimum of 3 animals for each data point with experiments carried out on at least 2 separate occasions. All animal experiments were approved by the ethical committee of the University of Antwerp.
Statistics
In all protocols, in-test and inter-test replicates were included. Comparison of values of generation times, in vitro and in vivo drug susceptibility assays were carried out by one-way analysis of variance (ANOVA) with Bonferoni post-hoc. Results are expressed as mean±s.e.m.
RESULTS
Generation time
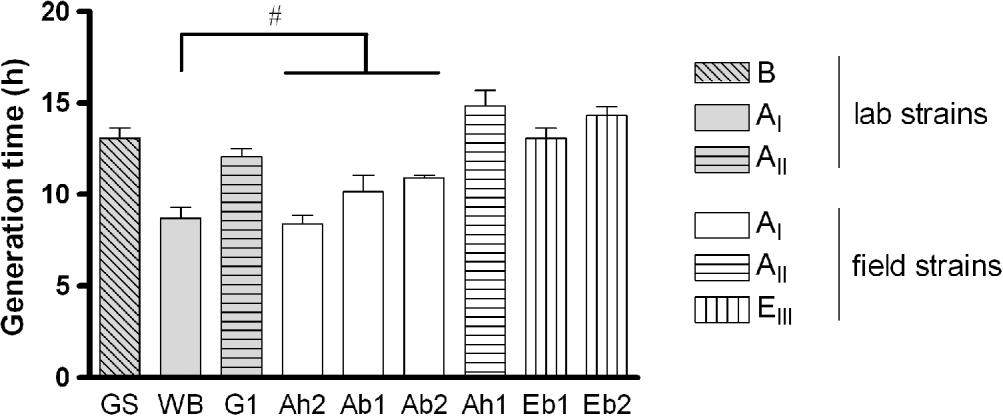
The in vitro growth of the 9 isolates of G. duodenalis showed a comparable pattern, although differences in maximum trophozoite numbers and generation times were observed. Trophozoites steadily increased in number until day 3 when, after pellet formation, clumping and signs predictive for decreasing trophozoite viability started to appear. Logarithmic growth was observed until day 3 for assemblage AI (laboratory and field strains) and assemblage EIII (field strains), and until day 4 for assemblage AII (laboratory and field strain) and assemblage B (data not shown). The generation times within the different assemblage subtypes (AI: Ah2, Ac1, Ac2 and EIII: Ec1, Ec2) were not significantly different, irrespective of host origin (Fig. 1). Since the laboratory strains were already adapted to in vitro cultivation, their generation time may have been changed. Nevertheless, the AI laboratory (WB) (8·7±0·6 h) versus AI field (Ah2, Ac1 and Ac2) (9·8±0·4 h) isolates and the AII laboratory (G1) (12·1±0·4 h) versus AII field (Ah1) (14·8±0·9 h) isolates showed comparable growth (Fig. 1). The major observation was that all assemblages/subtypes displayed similar generation times (overall mean 13·4±0·6 h), with the exception of assemblage subtype AI that grew significantly faster (mean 9·3±0·5 h) (Fig. 1).
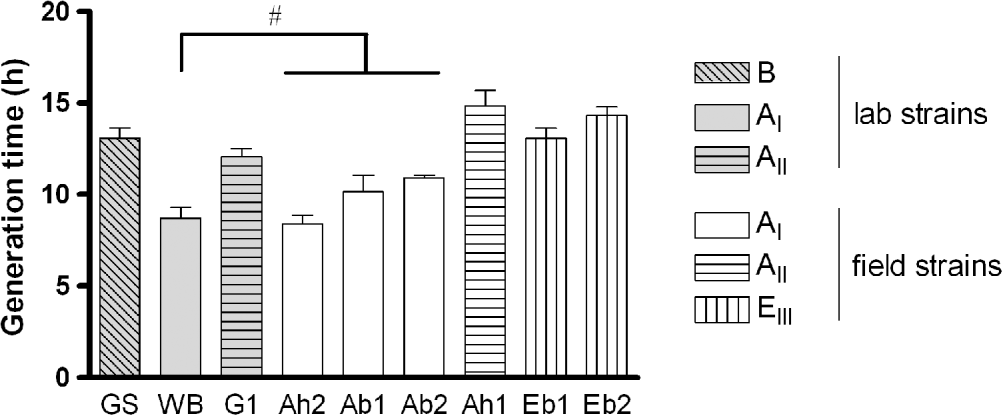
Fig. 1. In vitro generation times for the selected laboratory and field strains of assemblages A, B and E. (#: Not significantly different amongst each other, but significantly different compared to the other strains, P<0·05.) Data from 4 independent replicates: means±s.e.m.)
In vitro drug susceptibility
The drug susceptibility of assemblage A field isolates originating from man diverged significantly from those obtained from bovines for FEN and FUR. For all other drugs, there were no differences within and between the different assemblage subtypes (AI: Ah2, Ac1, Ac2 and EIII: Ec1, Ec2) (Table 1). The AI (WB) and AII (G1) laboratory strains displayed altered susceptibilities to all drugs compared to their assemblage-matching field strains. The benzimidazoles ABZ, FEN and FLU displayed a similar susceptibility pattern across the different laboratory and field isolates, with assemblage B isolate showing a decreased susceptibility (0·10 to 0·13 μ m) (Table 1). For the nitro-imidazoles, TIN (IC50 0·28 to 1·15 μ m) was about 1·1 to 3·7 times more potent than MET (IC50 0·50 to 2·35 μ m) (Table 1). A comparable pattern of susceptibility was obtained across all isolates with assemblage AI and AII laboratory strains showing a decreased susceptibility to both nitro-imidazoles (P<0·05), whereas the assemblage B strain was less susceptible only to MET (Table 1). For FUR, IC50 values varied from 0·23 to 1·56 μ m across all strains without significant differences between the field strains except for the subtypes AI and EIII, but this difference is driven by the AI cattle isolates. In addition, the AI and AII laboratory strains showed a decreased susceptibility compared to the field strains and the assemblage B lab strain (Table 1).
In vivo drug susceptibility
The timing of drug administration and autopsy was based on the course of infection of G. duodenalis in the gerbil. Trophozoites steadily increased in number (day 7 and 11 p.i.) and remained high until day 14 p.i. Peak trophozoite numbers depend on the isolate and attain 3×106/gerbil to 1·7×107/gerbil. Trophozoite numbers start to decline from day 14 onwards, and most trophozoites are cleared from the gastrointestinal tract by day 21 p.i. (data not shown).
For the WB strain, total cure was obtained at 80 mg/kg MET, 10 mg/kg FLU and 10 mg/kg ABZ (data not shown). Treatment at the projected EC50–70 dose levels (MET: 20 mg/kg, ABZ: 6 mg/kg, FLU: 5 mg/kg) reduced WB infection by 67·9±5·6%, 51·2±12·9% and 67·9±14·0% respectively. Consistent to the in vitro results, MET was the least active compound in vivo since 20 mg/kg was required to obtain 68% reduction of a G. duodenalis assemblage A infection, while only 5–6 mg/kg of the benzimidazoles was required to obtain similar activities (51% to 68%). A comparable pattern of susceptibility was observed for ABZ and FLU against all isolates. In contrast to the in vitro activity results, assemblage B was the most sensitive (79·9±7·2% (ABZ) to 97·8±1·2% (FLU)) and assemblage A the least sensitive (51·2±12·9% (ABZ) to 67·9±14·0% (FLU)). With regard to drug-specific susceptibility, assemblage E (Ec1) (90·9±2·8%) was significantly more susceptible than assemblage A (51·2±12·9%) to ABZ, while both assemblages were equally susceptible to FLU (68·8±6·1% (Ec1), 67·9±14·0% (WB)). For MET, no significant differences in activity between assemblages A, B and E trophozoites were observed, which is in contrast to the in vitro activity results. Treatment with 20 mg/kg MET reduced the WB, GS/M-83-H7 and Ec1 infection by 67·9±5·6%, 89·1±9·3% and 77·1±8·7% respectively.
DISCUSSION
Detailed information on the biological characteristics of strains from different assemblage subtypes of Giardia is still rather scarce in the literature and quite fragmented. In vitro studies have shown variation in susceptibility to anti-giardia drugs but the importance of this finding in explaining treatment failures and the relation to genotype remains to be established. To our knowledge, this is the first study in which the characteristics of growth and drug susceptibility among cloned trophozoite cultures of laboratory and field strains of assemblages A, B and E are directly compared both in vitro and in vivo.
Even though laboratory strains have been adapted to in vitro cultivation over a long period of time, a comparable growth rate was observed for the AI and AII laboratory strains versus the AI and AII field strains, suggesting that growth rate remains quite stable during cultivation and that available laboratory strains are representative for the in vitro growth characteristics of their matching genotype/subtype field isolates. This is in agreement with the generation times reported in the literature for laboratory and field strains of assemblage AI (Boreham et al. Reference Boreham, Phillips and Shepherd1984). In our study, assemblage AII, B and EIII trophozoites showed comparable generation times with only AI subtype isolates growing slightly faster. These observations are in accordance with previous reports in which no significant differences were observed in the generation time of 2 AI strains and their clones (Boreham et al. Reference Boreham, Phillips and Shepherd1987), while 3 AI isolates showed a significantly faster growth compared to AII isolates (Boreham et al. Reference Boreham, Phillips and Shepherd1984). The slower growth of assemblage B compared to assemblage AI is also consistent with the literature (Karanis and Ey, Reference Karanis and Ey1998), which was also the case for the EIII isolates in the present study. It is clear that the AI subtype can be linked to enhanced in vitro growth rate.
Although the laboratory strains displayed similar growth characteristics compared to their matching field isolates, in vitro drug susceptibilities were less comparable. For the field assemblage subtypes AI, AII and EIII, no significant differences were noted in susceptibility to any of the drugs. However, assemblage-linked differences in drug susceptibility were shown between the assemblage A and B laboratory strains, which is in accordance with previous reports on the genetic and biological differences of the genotypes infecting man (Farbey et al. Reference Farbey, Reynoldson and Thompson1995; Cruz et al. Reference Cruz, Sousa, Azeredo, Leite, Figueiredo de Sousa and Cabral2003; Franzen et al. Reference Franzen, Jerlstrom-Hultqvist, Castro, Sherwood, Ankarklev, Reiner, Palm, Andersson, Andersson and Svard2009). Comparable in vitro drug susceptibilities were observed for the assemblage A field isolates of human origin (Ah1 and Ah2) and cattle origin (Ac1 and Ac2, Ec1 and Ec2), despite their different subtype. This suggests that drug susceptibility in recently established isolates does not depend on the host origin. Surprisingly, assemblage A field isolates of human origin differed from those of cattle origin in their response to FEN and FUR, which could be related to different previous drug exposure patterns as part of different treatment regimens in man and cattle (Geurden et al. Reference Geurden, Claerebout, Dursin, Deflandre, Bernay, Kaltsatos and Vercruysse2006; Escobedo and Cimerman, Reference Escobedo and Cimerman2007). The IC50 values of the WB strain were within the same range as those found by other researchers using slightly different in vitro drug susceptibility assays (Katiyar et al. Reference Katiyar, Gordon, McLaughlin and Edlind1994; Cruz et al. Reference Cruz, Sousa, Azeredo, Leite, Figueiredo de Sousa and Cabral2003). Benzimidazoles clearly show the highest intrinsic in vitro potency (Morgan et al. Reference Morgan, Reynoldson and Thompson1993) and TIN proves to be more potent than MET (Ellis et al. Reference Ellis, Wingfield, Cole, Boreham and Lloyd1993), which has been linked to its hydrophilic/lipophilic properties (Jokipii and Jokipii, Reference Jokipii and Jokipii1987; Raether and Hanel, Reference Raether and Hanel2003).
With respect to variability, furazolidone exhibited the largest variation in drug susceptibility and similar potencies between the isolates were obtained against the benzimidazoles. These results are in agreement with previous reports on G. duodenalis isolates of human origin (Upcroft and Upcroft, Reference Upcroft and Upcroft2001;Mohamadnezhad et al. Reference Mohamadnezhad, Ghaffarifar and Dalinmi2008). Benzimidazoles interfere with the polymerization of tubulin and also bind to giardins. Both cytoskeletal proteins are specific for Giardia (Cevallos et al. Reference Cevallos, McHugh, Carnaby and Farthing1991) and interference with these proteins may explain the low variability amongst them. The nitro-imidazoles MET, TIN and the nitrofuran FUR are in fact pro-drugs that need reductive activation of the nitro-group (R-NO2) to form a highly toxic nitro-radical anion (R-NO2•−) (Raether and Hanel, Reference Raether and Hanel2003), essential for activity. Specific enzymes of the anaerobic metabolic pathway of Giardia are involved in the reduction of the nitro-group and include pyruvate:ferredoxin oxidoreductase (PFOR) and ferredoxin in the case of metronidazole and NADH oxidase as well as PFOR in the case of FUR (Moreno et al. Reference Moreno, Mason and Docampo1984; Brown et al. Reference Brown, Upcroft and Upcroft1995). In addition, a nitroreductase (GlNR1) has recently been identified in G. duodenalis, which carries unknown but essential functional activities in the metabolism of Giardia, and is suggested to participate in the activation of nitro-drugs (Nillius et al. Reference Nillius, Muller and Muller2011). For the nitro-imidazoles and FUR, the assemblage A laboratory strains were less susceptible than their matching field isolates, suggesting a correlation between the long history of in vitro cultivation and decreased susceptibility to the toxic nitro-radicals or a decreased activation of the nitro-group (e.g. reduced GlNR1expression (Nillius et al. Reference Nillius, Muller and Muller2011) or diminished PFOR/ferredoxin activity (Townson et al. Reference Townson, Upcroft and Upcroft1996; Upcroft and Upcroft, Reference Upcroft and Upcroft2001; Muller et al. Reference Muller, Sterk, Hemphill and Muller2007)).
Since very few studies aim to define a relationship between in vitro drug susceptibility and drug activity in vivo, the different genotypes were evaluated in gerbils treated at the ED50–70 of ABZ, FBZ and MET for 3 days. No significant assemblage-linked differences in susceptibility against the benzimidazoles or metronidazole could be observed in the gerbil model, which may be related to differences in drug uptake and/or the effect of drug metabolism (Boreham et al. Reference Boreham, Phillips and Shepherd1986). The levels of efficacy of MET and ABZ against the WB strain in our study are comparable to ED50 values obtained for non-resistant human clinical isolates (Boreham et al. Reference Boreham, Phillips and Shepherd1986; Lemee et al. Reference Lemee, Zaharia, Nevez, Rabodonirina, Brasseur, Ballet and Favennec2000). Several studies in dog, cattle and man support the efficacy of ABZ and FEN in giardiasis (O'Handley et al. Reference O'Handley, Olson, McAllister, Morck, Jelinski, Royan and Cheng1997; Zajac et al. Reference Zajac, LaBranche, Donoghue and Chu1998; Solaymani-Mohammadi et al. Reference Solaymani-Mohammadi, Genkinger, Loffredo and Singer2010); however, no information is yet available on the clinical potential of FBZ for the treatment of giardiasis, although potent activity can be expected based on its common mode of action and pharmacokinetic properties (Gottschall et al. Reference Gottschall, Theodorides and Wang1990; Sweetman, Reference Sweetman2007). Combined with its high potency in vitro (Katiyar et al. Reference Katiyar, Gordon, McLaughlin and Edlind1994), a strong proof of concept is provided for the potential use of FBZ against giardiasis in the dog and cattle, particularly since it is available on the market for treatment of gastro-intestinal helminths (Vanparijs et al. Reference Vanparijs, Hermans and Van der Flaes1985).
To conclude, there is little variation in the in vitro growth characteristics of G. duodenalis, except for AI subtypes. In vitro growth may be related to the assemblage subtype although a role of the cultivation conditions (e.g. particular deficiencies in culture medium, pH) cannot be ruled out (Binz et al. Reference Binz, Thompson, Lymbery and Hobbs1992; Karanis and Ey, Reference Karanis and Ey1998). Slightly different in vitro drug susceptibility patterns were demonstrated, at least among assemblage A and B laboratory strains, while resistance to any of the drugs was not detected. These findings are relevant for anyone working in the field of anti-giardia chemotherapy and suggest that the variation in drug responsiveness and the emergence of drug-resistance problems in clinical giardiasis infections may not be associated with the genetic diversity of the parasite. However, to consolidate these findings, a larger number of assemblages A, B and E isolates will need to be evaluated, including advanced genetic studies and genome sequencing.
ACKNOWLEDGEMENTS
We gratefully thank Desmet Margot for her excellent technical laboratory support.
FINANCIAL SUPPORT
The post-doctoral researcher Paul Cos and the Ph.D. student Ely Bénéré are both supported by the Fund for Scientific Research (FWO-Flanders, Belgium). This project was also supported by a grant of the Research Council of the University of Antwerp (KP BOF UA to T.V.A.).