Introduction
Guangxi Bama minipigs have been used for a long time in biomedical research in China, due to their anatomical and physiological similarities to humans (Li et al., Reference Li, Liu, Zhang, Wei and Yang2006). Although the birth of Guangxi Bama miniature pigs achieved from somatic cell nuclear transfer (SCNT) has been reported previously (Liu et al., Reference Liu, Lv, Yang, Qin, Pi, Lu, Lu, Lu and Li2010), the efficiency was very low, and various questions and problems still remain concerning the development of SCNT embryos. For instance, abnormal epigenetic remodelling of the donor nuclei leads to further reprogramming of the nucleus to a totipotent state after SCNT (Eilertsen et al., Reference Eilertsen, Power, Harkins and Misica2007). Epigenetic modifications such as DNA methylation and histone modifications play a key role in regulation of chromatin structure and transcriptional activity (Enright et al., Reference Enright, Jeong, Yang and Tian2003). Incomplete reprogramming of epigenetic modifications is widely postulated to be the main stumbling block to improved efficiency of SCNT (Couldrey & Lee, Reference Couldrey and Lee2010).
DNA methylation is known to be a major epigenetic modification of the genome, which regulates important aspects of genome function (Kang et al., Reference Kang, Lee and Han2003), methylation generally occurs on cytosine residues in CpG dinucleotides. Following fertilization, zygotes have a demethylated paternal genome, in which active demethylation, and then de novo DNA methylation occur during preimplantation development. In porcine embryos, new methylation patterns can be examined at the blastocyst stage (Bonk et al., Reference Bonk, Li, Lai, Hao, Liu, Samuel, Fergason, Whitworth, Murphy, Antoniou and Prather2008). However, embryos produced by SCNT do not exhibit active demethylation activity, which would affect the formation of new methylation patterns. Many reports have indicated that DNA methylation of cloned embryos remains much higher than that of fertilized embryos (Bonk et al., Reference Bonk, Li, Lai, Hao, Liu, Samuel, Fergason, Whitworth, Murphy, Antoniou and Prather2008; Niemann et al., Reference Niemann, Carnwath, Herrmann, Wieczorek, Lemme, Lucas-Hahn and Olek2010; Beaujean et al., Reference Beaujean, Taylor, Gardner, Wilmut, Meehan and Young2004); the DNA methylation pattern in early SCNT embryos was more like to be differentiated cells than fertilized embryos (Chen et al., Reference Chen, Zhang, Jiang, Liu, Schatten, Chen and Sun2004; Bonk et al., Reference Bonk, Cheong, Li, Lai, Hao, Liu, Samuel, Fergason, Whitworth, Murphy, Antoniou and Prather2007). There is now accumulating evidence that suggests that aberrant DNA methylation is conducive to the inefficiency of SCNT (Ohgane et al., Reference Ohgane, Wakayama, Senda, Yamazaki, Inoue, Ogura, Marh, Tanaka, Yanagimachi and Shiota2004; Wrenzycki et al., Reference Wrenzycki, Herrmann, Gebert, Carnwath and Niemann2006). As the DNA methylation pattern is established and maintained by DNA methyltransferases, the level of DNA methylation can be decreased easily by the use of the DNA methyltransferase inhibitor, 5-aza-dC. However, at high concentrations, 5-aza-dC is considered to be toxic for donor cells and preimplantation embryos (Enright et al., Reference Enright, Sung, Chang, Yang and Tian2005; Mohana Kumar et al., Reference Mohana Kumar, Jin, Kim, Song, Hong, Balasubramanian, Choe and Rho2006; Tsuji et al., Reference Tsuji, Kato and Tsunoda2009). In the current study, we investigated the treatment of donor cells with lower concentrations (0.01 μmol/l) of 5-aza-dC to avoid the cytotoxic effect.
In addition, it has been shown that TSA, a histone deacetylase inhibitor (HDACi), can enhance the levels of histone acetylation, which are associated with decreased DNA methylation levels (Kishigami et al., Reference Kishigami, Bui, Wakayama, Tokunaga, Van Thuan, Hikichi, Mizutani, Ohta, Suetsugu, Sata and Wakayama2006; Wu et al., Reference Wu, Li, Li, Yang, Yue, Wang, Li, Xin, Bou and Yu2008), and there are many reports that indicate that TSA has a beneficial effect on the development of SCNT embryos. TSA treatment also significantly improved blastocyst formation rates in porcine cloned embryos (Zhang et al., Reference Zhang, Li, Villemoes, Pedersen, Purup and Vajta2007; Li et al., Reference Li, Svarcova, Villemoes, Kragh, Schmidt, Bogh, Zhang, Du, Lin, Purup, Xue, Bolund, Yang, Maddox-Hyttel and Vajta2008; Cervera et al., Reference Cervera, Marti-Gutierrez, Escorihuela, Moreno and Stojkovic2009; Himaki et al., Reference Himaki, Yokomine, Sato, Takao, Miyoshi and Yoshida2010). Furthermore, the adult ICR mouse, an outbred stain that had been regarded as ‘unclonable’, was cloned successfully only when TSA was used (Kishigami et al., Reference Kishigami, Mizutani, Ohta, Hikichi, Thuan, Wakayama, Bui and Wakayama2007) and, recently, it has been reported that bovine reconstructed embryos and donor cells that were treated with a combination of TSA and 5-aza-dC showed improved histone acetylation, reduced DNA methylation and enhanced blastocyst development (Ding et al., Reference Ding, Wang, Zhang, Wang, Guo and Zhang2008; Wang et al., Reference Wang, Xiong, An, Wang, Liu, Quan, Hua and Zhang2011b). These results, therefore, suggested that TSA may work in concert with 5-aza-dC (Ding et al., Reference Ding, Wang, Zhang, Wang, Guo and Zhang2008).
The objectives of the present study were to ascertain preimplantation development potential of Guangxi Bama minipig cloned embryos after donor cells were treated with TSA, 5-aza-dC, or both in combination, as there is currently no information about the effect of the combination of TSA and 5-aza-dC on epigenetic status during preimplantation in porcine SCNT. Donor cells and SCNT blastocysts were analysed for the level of DNA methylation via immunofluorescence techniques and gene expression profiles in donor cells. The relative abundance of mRNA transcripts that are known to have a key role in DNA methylation (DNMT1 and DNMT3a), histone deacetylation (HDAC1), growth-promoting mitogen (IGF2) was determined by quantitative real-time polymerase chain reaction (qRT-PCR).
Materials and methods
All chemicals were purchased from the Sigma Chemical Company (St. Louis, MO, USA) unless otherwise stated. At least three replicates were used for each experiment.
Somatic cell culture and treatment
Porcine kidney fibroblast cells were derived from a newborn Guangxi Bama minipig. Procedures for somatic cell culture were described previously (Liu et al., Reference Liu, Lv, He, Yang, Qin, Pan, Huang, Huang, Lu, Lu, Li and Lu2009). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories Inc., Logan, UT, USA) at 37°C and in 5% CO2 in humidified air. When cells achieved 70–80% confluency, they were divided randomly into four different groups: (1) culture with 0.05 μmol/L TSA for 24 h; (2) 0.01 μmol/L 5-aza-dC for 72 h; (3) 0.05 μmol/L TSA for 24 h + 0.01 μmol/L 5-aza-dC for 72 h; and (4) untreated control donor cells. In this study, both 0.05 μmol/L TSA (24 h) and 0.01 μmol/L 5-aza-dC (72 h) reagents were selected to treat donor cells. The concentration and duration of treatment were chosen based on previous studies (Ding et al., Reference Ding, Wang, Zhang, Wang, Guo and Zhang2008; Enright et al., Reference Enright, Sung, Chang, Yang and Tian2005) and our preliminary studies (unpublished). These cells were then used for SCNT, their DNA methylation levels were analysed by flow cytometry and for gene expression by qRT-PCR.
RNA isolation and reverse transcription (RT)
Total RNA was isolated from four groups of donor cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and their quantities and qualities determined by spectrophotometry and electrophoresis. A total of 1.0 μg RNA was used for reverse transcription, which was carried out using oligo dT18 primers and M-MLV reverse transcriptase (Promega, Madison, WI, USA) in a 20-μl reaction. The cDNA was completed under the following conditions: 25°C for 10 min, 37°C for 60 min, and 95°C for 5 min. First-strand cDNA synthesis in RT was performed essentially as described previously (Jiang et al., Reference Jiang, Jobst, Lai, Samuel, Ayares, Prather and Tian2007).
Quantitative real-time RT-PCR
The sequences of PCR primers used for the amplification of H2A, HDAC1, DNMT1, DNMT3a and IGF2 are listed in Table 1. qRT-PCR was performed on the MJ AL079721 real-time PCR system (MJ Research, Inc., Waltham, MA, USA) using SYBRR Premix Ex Taq™ (TaKaRa, Dalin, China). The reaction mixture of total 20-μl volume consisted of 10 μl SYBRR Premix Ex Taq™ (2×), 0.8 μL of forward and reverse primers (10 μmol/l), l 2 μl of cDNA, and 6.4 μl double-distilled water. The reaction parameters were as follows: initial denaturing step at 94°C for 5 min, followed by 40 denaturing cycles at 94°C for 20 s, annealing at 57°C for 20 s, and extension at 72°C for 20 s. After each PCR run, a melting curve analysis was performed for each sample to verify that a single specific product was generated. Melting curves were obtained by increasing the temperature stepwise from 65 to 95°C. Quantification of target gene mRNA was performed by applying the 2–ΔΔCt method as previously described (Livak & Schmittgen, Reference Livak and Schmittgen2001). Briefly, a standard curve was constructed for each gene by amplifying a 10-fold dilution series of plasmid. Quantification of each gene was normalized to the housekeeping gene H2A mRNA.
Table 1 Primers for gene amplification

Analysis of cell cycle and DNA methylation levels by flow cytometry
Total levels of cellular DNA methylation were quantified with flow cytometry by measurement of cell fluorescence after immunolabelling with anti-5-methylcytosine antibodies. The procedures for flow cytometry were as described previously (Giraldo et al., Reference Giraldo, Hylan, Ballard, Purpera, Vaught, Lynn, Godke and Bondioli2008). Briefly, cells were trypsinized and resuspended in Dulbecco's phosphate-buffered saline (DPBS; Ca2+ and Mg2+ free) and then fixed in 4% paraformaldehyde for 30 min at room temperature. After permeabilization with 0.5% Triton X-100 for 15 min at room temperature, cells were treated with 2 N HCl for 30 min, followed by incubation in 100 mM Tris–HCl buffer (pH 8.5) for 10 min. To block non-specific binding sites, the cells were resuspended in 2% bovine serum albumin (BSA) in DPBS for 1 h. Cells were incubated with mouse anti-5-methylcytosine antibody (Calbiochem, Darmstadt, Germany; 1:200 dilution) overnight at 4°C, followed by labelling with a goat anti-mouse IgG FITC-conjugated antibody (Calbiochem; 1:200 dilution) for 1 h at room temperature. For DNA staining, cells were incubated in DPBS that contained 30 μg/ml propidium iodide (PI) and 100 μg/ml RNase A for 30 min at room temperature. To eliminate multicell aggregates, cells were filtered through a 30-μm nylon mesh. Finally, cells were resuspended in DPBS for flow cytometric analysis. The percentage of cells at each cell-cycle stage was determined by their DNA content. Determination of the relative contents of DNA and methylated DNA was performed with a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). In each experiment, at least 10,000 cells were used for the analysis.
Nuclear transfer and embryos culture
Porcine ovaries were collected from a local abattoir. Cumulus–oocyte complexes (COCs) were obtained from 2 to 6 mm diameter follicles using a 12-gauge needle connected to a 10-ml disposable syringe. The resulting COCs were cultured in group of 50–70 in a 400 μl drop of TCM199 (Gibco) supplemented with 10% (v/v) porcine follicular fluid, 0.57 mM cysteine, 3.05 mM d-glucose, 0.91 mM Na pyruvate, 75 μg/ml penicillin, 50 μg/ml streptomycin, 10 ng/ml epidermal growth factor, 1 μg/ml FSH, and 1 μg/ml LH. After 21–22 h of maturation culture, COCs were transferred to 400-μl droplets of maturation medium without hormone supplementation and incubated for an additional 21–22 h.
SCNT from Bama minipig kidney fibroblast cells was performed as described previously (Liu et al., Reference Liu, Lv, He, Yang, Qin, Pan, Huang, Huang, Lu, Lu, Li and Lu2009, Reference Liu, Lv, He, Yang, Qin, Pan, Huang, Huang, Lu, Lu, Li and Lu2010). After culture of the oocytes for 42–44 h, the COCs were stripped of cumulus cells by treatment with 0.2% (w/v) hyaluronidase in modified TL-HEPES-PVA medium. Then oocytes were enucleated and injected individually with a donor cell via a glass micropipette with a 20–25-μm outer diameter. After nuclear transfer, the reconstructed oocytes were placed in activation/fusion medium (0.25 M mannitol solution supplemented with 0.01% polyvinyl alcohol, 0.5 mM HEPES, 0.1 mM CaCl2.H2O, and 0.1 mM MgCl2.6H2O with pH 7.2–7.4). Electrofusion were performed with the oocyte-cell couplet sandwiched between a pair of self-made platinum electrodes (150 μm in diameter) connected to the micromanipulator. The distance between the electrodes was approximately 150 μm. Three direct pulses of 100 v/mm for 30 μs on a BTX Electro-cell Manipulator 2001 (BTX, San Diego, CA, USA) were applied.
In all experiments, the reconstructed embryos were transferred immediately after fusion into a microdrop of North Carolina State University 23 (NCSU23) medium that contained 0.4% (w/v) BSA supplemented with 7.5 μg/ml cytochalasin B (CB) for 3 h, and then cultured in CB-free embryo culture medium at 39°C and in 5% CO2 in humidified air. Embryonic cleavage was evaluated under a stereomicroscope on day 2 and blastocyst formation was examined on days 5–7. The blastocysts were fixed in 4% paraformaldehyde and used for analysis of DNA methylation levels by confocal microscopy.
Analysis of DNA methylation levels by confocal microscopy
DNA methylation levels were analysed as described previously (Enright et al., Reference Enright, Sung, Chang, Yang and Tian2005). Fixed blastocysts were permeabilized with 1% Triton X-100 for 6 h at room temperature. Embryos were then treated with 2 N HCl for 30 min, followed by incubation in 100 mM Tris–HCl buffer (pH 8.5) for 10 min. Blastocysts were blocked for 1 h in DPBS that contained 2% BSA. Embryos were then incubated in anti-5-methycytosine primary antibody (Calbiochem; 1:200 dilution) overnight at 4°C, followed by labelling with FITC-conjugated secondary antibody (Calbiochem; 1:200 dilution) for 1 h at room temperature. For DNA staining, embryos were incubated in DPBS that contained 10 μg/ml PI for 10 min, and observed under a confocal laser-scanning microscope (Nikon, Tokyo, Japan). Images were acquired by sequential excitation with 488 nm and 543 nm laser lines. Digital images of the DNA methylation were captured using the same contrast, brightness, and exposure settings for all embryos. To make relative comparisons, fluorescence images were subjected to densitometric analysis using Nikon NIS-Elements AR 3.0. Appropriate controls for autofluorescence and non-specific binding by the secondary antibody were included.
Statistical analysis
Statistical analysis was performed using SPSS software (version 13.0 for Windows). One-way analysis of variance (ANOVA) was used to detect differences in gene expression. The test was also used to detect differences in methylation levels of donor cells and embryos. Data are expressed as mean ± standard deviation (SD). Cell-cycle stages of donor cells, and the percentage of embryo development were analysed using the chi-squared test. A value of P < 0.05 was considered to represent a statistically significant difference.
Results
Transcript levels for DNMT1, DNMT3a, HDAC1, and IGF2 in donor cells
The relative abundance of the gene transcripts studied is shown in Fig. 1. Compared with the untreated control group, 0.05 μmol/L TSA (24 h) significantly increased the expression level of DNMT3a (P < 0.05). Donor cells treated with 0.01 μmol/L 5-aza-dC (72 h) resulted in a significantly decreased expression of DNMT1, and a significantly increased expression of IGF2 genes (P < 0.05). Transcription levels of HDAC1 were decreased significantly after treatment with a combination of TSA and 5-aza-dC, along with a significantly increased level of IGF2 (P < 0.05).

Figure 1 Effect of TSA, 5-aza-dC or both on the relative abundance of transcript levels of DNMT1, DNMT3a, HDAC1, and IGF2 in donor cells. Bars with different superscripts within each gene transcript indicate statistically significant differences (P < 0.05).
Cell-cycle analysis of donor cells
As show in Table 2, more than 85% of treated cells were at the G0/G1 phase of the cell cycle. A similar result was observed with the control cells. In this study, changes in the proportions of cells at the G0/G1 phase, G2/M phase, or S phase were negligible among all groups.
Table 2 Cell cycle stages of Bama minipig kidney fibroblast cells treated with 5-aza-dc, TSA or both
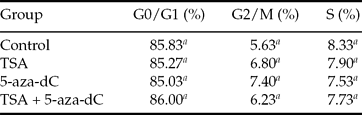
a Values in columns with different superscripts differ significantly (P < 0.05).
DNA methylation levels in donor cells and SCNT blastocysts
When donor cells were treated with a combination of TSA and 5-aza-dC, the DNA methylation levels in the G0/G1 and G2/M phases were lower than those in the control group (P < 0.05, Fig. 2). For SCNT blastocysts, embryos cloned from donor cells treated with both TSA and 5-aza-dC had reduced levels of methylation compared with NT embryos cloned from untreated cells (1180.39 ± 247.11 vs. 1532.95 ± 204.84, P < 0.05). However, there were no significant differences for other treatment groups (Figs. 3 and 4).

Figure 2 Relative levels of DNA methylation in Bama minipig's kidney fibroblast cells treated with TSA, 5-aza-dC or both. Bars with different superscripts at a given cell cycle stages indicate statistically significant differences (P < 0.05).

Figure 3 Confocal micrographs of day 7 SCNT blastocysts from untreated control donor cells, and cloned from donor cells treated with TSA, 5-aza-dC or both. The embryos were labelled for 5-methylcytosine (green) and DNA (red). The merged images of labelled 5-methylcytosine and DNA appear yellow. Images were magnified ×200. A colour version of this article is available online.

Figure 4 DNA methylation status of day 7 SCNT blastocysts after donor cells were treated with TSA, 5-aza-dC or both. At least 12 embryos per groups were analysed. Bars with different superscripts indicate statistically significant differences (P < 0.05).
Development of NT embryos from donor cells treated with TSA and 5-aza-dC
As shown in Table 3, there was no significant difference in the fusion rate and cleavage rate of the reconstructed embryos among control and treated groups (P > 0.05). However, when donor cells were treated with both TSA and 5-aza-dC, the rate of embryo development to blastocysts was significantly higher than in the untreated control group (25.6% vs. 16.0%, P < 0.05).
Table 3 Developmental potential of SCNT embryos derived from donor cells treated with TSA, 5-aza-dC or both

Fusion rate: no. of fused embryos/no. of oocytes.
Cleaved embryos rate: no. of cleaved embryos (day 2)/no. of fused embryos.
Blastocyst rate: no. of blastocysts (day 7)/no. of fused embryos.
a,b Values within columns with different superscripts differ significantly (P < 0.05).
Discussion
After completing the process of SCNT a series of epigenetic events follow. For this process to be successful, nuclear reprogramming of a differentiated somatic nucleus must be restored to a totipotent embryonic state (Eilertsen et al., Reference Eilertsen, Power, Harkins and Misica2007). Recently, various methods have been tested to modify the global epigenetic markers in donor cells. These methods include treatment of donor cells with TSA or 5-aza-dC to enhance developmental potential of cloned embryos by altering the epigenetic marks of the nucleus.
In the present study, we determined the influence of TSA and 5-aza-dC, both separately and in combination on the expression level of DNMT1, DNMT3a, HDAC1, and IGF2. Of the DNA (cytosine-5)-methyltransferases (DNMTs), DNMT1 is expressed constitutively and is responsible for maintenance of global methylation following DNA replication (Bosak et al., Reference Bosak, Fujisaki, Kiuchi, Hiraiwa and Yasue2003), whereas DNMT3a and DNMT3b are required for establishment of de novo methylation patterns (Pradhan & Esteve, Reference Pradhan and Esteve2003). Deletion of both DNMT3a and DNMT3b or DNMT1 alone results in embryonic lethality (Okano et al., Reference Okano, Bell, Haber and Li1999). Reversible histone acetylation is a dynamic process regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDAC1 is a member of the histone deacetylases, and participates in removal of acetyl moieties from histone tails (Murko et al., Reference Murko, Lagger, Steiner, Seiser, Schoefer and Pusch2010). Insulin-like growth factor 2 (IGF2) is a classic imprinted gene that plays a crucial role in prenatal growth and placental development (Gebert et al., Reference Gebert, Wrenzycki, Herrmann, Groger, Reinhardt, Hajkova, Lucas-Hahn, Carnwath, Lehrach and Niemann2006). In the present study, we observed a dramatic increase in IGF2 levels after donor cells were treated with 5-aza-dC, along with a significantly decreased level of DNMT1. These data are consistent with previous work describing porcine fetal fibroblasts treated with 5-aza-dC (Mohana Kumar et al., Reference Mohana Kumar, Jin, Kim, Song, Hong, Balasubramanian, Choe and Rho2006). HDAC1 transcription, which was previously reported to be decreased significantly after porcine fetal fibroblasts cultured with sodium butyrate (NaBu, a histone deacetylase inhibitor) (Mohana Kumar et al., Reference Mohana Kumar, Song, Cho, Balasubramanian, Choe and Rho2007), was not detected in the present study. NaBu has a potent HDACi activity and has substantially stronger inhibitory effects at higher concentrations, whereas TSA binds reversibly to HDAC (Sambucetti et al., Reference Sambucetti, Fischer, Zabludoff, Kwon, Chamberlin, Trogani, Xu and Cohen1999). It is important to note that the exposure timing and concentration of HDAC inhibitor were different from those used in the above-mentioned study (Mohana Kumar et al., Reference Mohana Kumar, Song, Cho, Balasubramanian, Choe and Rho2007).
The pattern of DNA methylation is quite different in porcine blastocysts produced either in vitro and in vivo (Bonk et al., Reference Bonk, Li, Lai, Hao, Liu, Samuel, Fergason, Whitworth, Murphy, Antoniou and Prather2008), and abnormal DNA hypermethylation is believed to be associated with the low success rate of SCNT (Simonsson & Gurdon, Reference Simonsson and Gurdon2004). Thus, accurate regulation of DNA methylation during embryonic development might be essential for normal development (Yamagata, Reference Yamagata2008). Recently, it was found that the DNA methylation state in donor cells can be altered by treatment with TSA (Wu et al., Reference Wu, Li, Li, Yang, Yue, Wang, Li, Xin, Bou and Yu2008) or 5-aza-dC (Mohana Kumar et al., Reference Mohana Kumar, Jin, Kim, Song, Hong, Balasubramanian, Choe and Rho2006), and in the present study we evaluated the levels of DNA methylation in donor cells and SCNT-produced blastocysts by immunofluorescence analysis. We observed that neither TSA nor 5-aza-dC alone had any effect on DNA methylation status, at least at the levels tested, but 5-aza-dC plus TSA produced a significantly greater inhibition of global DNA methylation. These results indicated that there is synergistic interaction between 5-aza-dC and TSA, and findings are similar to those of previous studies (Primeau et al., Reference Primeau, Gagnon and Momparler2003; Shaker et al., Reference Shaker, Bernstein, Momparler and Momparler2003; Ding et al., Reference Ding, Wang, Zhang, Wang, Guo and Zhang2008), which showed a positive interaction for 5-aza-dC in combination with an HDAC inhibitor.
To improve the success rate of SCNT, various approaches have been devised to modify the SCNT procedure, and these include pretreatment of donor cells or embryos with 5-aza-dC and TSA. TSA is the most widely used HDAC inhibitor, and the effects of TSA on cloning efficiency are controversial. It has been demonstrated that TSA can improve cloning efficiency, including live births, in mouse (Kishigami et al., Reference Kishigami, Bui, Wakayama, Tokunaga, Van Thuan, Hikichi, Mizutani, Ohta, Suetsugu, Sata and Wakayama2006; Costa-Borges et al., Reference Costa-Borges, Santalo and Ibanez2010), but it has also been reported to have no positive effect on the in vitro and in vivo developmental capacity of cloning embryos in rabbits and pigs (Meng et al., Reference Meng, Polgar, Liu and Dinnyes2009; Martinez-Diaz et al., Reference Martinez-Diaz, Che, Albornoz, Seneda, Collis, Coutinho, El-Beirouthi, Laurin, Zhao and Bordignon2010). This apparent inconsistency may be due to different species or the use of different concentrations of TSA, which makes comparison with previous research difficult. Treatment of donor cells with 50 nmol/l TSA for 24 h did not have a significant effect on subsequent in vitro development of cloned porcine embryos. When the concentration was more than 50 nmol/L, TSA is considered too toxic for porcine adult fibroblasts (Bo et al., Reference Bo, Di, Qing-Chang, Liang, Hong, Liang, Zhen-Hua and Zhong-Qiu2010). Favourable effects of TSA on porcine in vitro and in vivo SCNT embryo development were obtained with 50 nmol/L TSA applied for 10 h after activation (Zhao et al., Reference Zhao, Hao, Ross, Spate, Walters, Samuel, Rieke, Murphy and Prather2010). Kishigami et al. (Reference Kishigami, Bui, Wakayama, Tokunaga, Van Thuan, Hikichi, Mizutani, Ohta, Suetsugu, Sata and Wakayama2006) demonstrated that treatment with 5–50 nmol/l TSA for 10 h following oocyte activation resulted in much improvement in mouse somatic cloning, and TSA became effective at concentrations greater than 5 nmol/l, but showed toxicity at 500 nmol/l. 5-Aza-dC, a derivative of the nucleoside cytidine (Eilertsen et al., Reference Eilertsen, Power, Harkins and Misica2007), is the classic DNA methyltransferase inhibitor. Enright et al. (Reference Enright, Sung, Chang, Yang and Tian2005) reported that there was a trend toward reduced cloned bovine embryo development when the dose of 5-aza-dC was greater than 10 nmol/l. Tsuji et al. (Reference Tsuji, Kato and Tsunoda2009) demonstrated that the potential of cloned mouse embryos treated with 100 nmol/L 5-aza-dC (48 h) to develop into morulae was significantly lower than that of control embryos, whereas lower doses of 5-aza-dC (10 nmol/L) had no deleterious effect on the morulae formation rates. In the present study, donor cells that had been pretreated with either TSA or 5-aza-dC showed no improvement in blastocyst formation rate. However, when both chemicals were used together, the rate of blastocyst formation were clearly higher to that of the untreated control group, thus confirming a synergistic relationship between TSA and 5-aza-dC on the in vitro development of SCNT embryos reported previously (Ding et al., Reference Ding, Wang, Zhang, Wang, Guo and Zhang2008; Wang et al., Reference Wang, Su, Wang, Xu, Quan, Liu and Zhang2011a). Furthermore, these improved in vitro development rates in the combined treatment group were accompanied by decreased levels of DNA methylation and the methylation status of donor cells was correlated to blastocyst rates following SCNT. Thus, we inferred that appropriate alteration of DNA methylation levels may result in improved porcine cloning efficiency. These results are consistent with those of previous studies (Bonk et al., Reference Bonk, Cheong, Li, Lai, Hao, Liu, Samuel, Fergason, Whitworth, Murphy, Antoniou and Prather2007; Wee et al., Reference Wee, Shim, Koo, Chae, Lee and Han2007).
In summary, this study demonstrated that, in combination with TSA, lower than previously used concentrations of 5-aza-dC may still produce a potent demethylating activity, and lead to a significantly enhanced blastocyst development percentage of Bama minipig SCNT embryos. Further studies should be performed to ascertain the effect of TSA and 5-aza-dC on full-term development after minipig SCNT.
Acknowledgements
We gratefully thank Dr Bernard A. Goodman for language revision of the manuscript. This work was supported by the National Natural Science Foundation of China (No. 30860039), the Key Project of Guangxi Natural Science Foundation (2010GXNSFD013021) and the Plan for Construction of Scientific Topnotch and Innovation Team in Guangxi University, China.