Introduction
Conservation of germplasm and maintenance of genetic diversity are important considerations for the survival of forest trees, in particular the endemics, in the face of global forest decline and climate change. The four redwoods discussed in this study are endemics in their native countries: coast redwood (Sequoia sempervirens (D. Don) Endl.) and giant sequoia or Sierra redwood (Sequoiadendron giganteum (Lindl.) Buchholz), in the USA (Olson et al., Reference Olson, Roy, Walters, Burns and Honkala1990; Weatherspoon, Reference Weatherspoon, Burns and Honkala1990); dawn redwood (Metasequoia glyptostroboides Hu & Cheng) in China (Chu and Cooper, Reference Chu and Cooper1999); and alerce or South American redwood (Fitzroya cupressoides (Mol.) Johnst.) in Chile/Argentina (Allnutt et al., Reference Allnutt, Newton, Lara, Premoli, Armesto, Vergara and Gardner1999). All four relectual genera are long-lived conifers and belong to the family Cupressaceae. These redwood genera are monospecific, share a number of common phenotypic traits and have the same basic chromosome number of x = 11 (Gadek et al., Reference Gadek, Alpers, Heslewood and Quinn2000; Ahuja, Reference Ahuja2009). Although polyploidy is rare in conifers, two of the four redwood genera are polyploids (Ahuja, Reference Ahuja2005, Reference Ahuja2009). Sequoia is a hexaploid (2n = 6x = 66) and in fact the only hexaploid conifer (Saylor and Simons, Reference Saylor and Simons1970; Ahuja and Neale, Reference Ahuja and Neale2002), while Fitzroya is a tetraploid (2n = 4x = 44) (Hair, Reference Hair1968). On the other hand, both Sequoiadendron and Metasequoia are diploids (2n = 22) (Schlarbaum and Tsuchiya, Reference Schlarbaum and Tsuchiya1984).
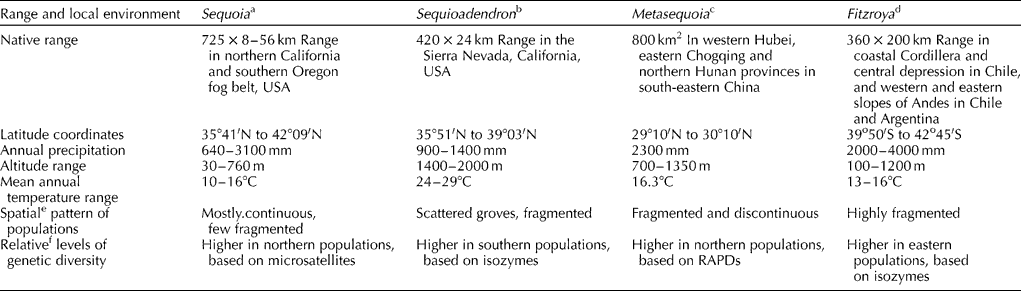
The native ranges, local environments and spatial patterns of the four redwoods are shown in Table 1. Sequoia extends from south-western corner of Oregon (latitude 42°09′N) to Santa Lucia Mountains of southern Monterey County (latitude 35°41′N) in California, USA. The native redwood forests are confined to a narrow coastal fog belt that is 725 km long and 8–56 km wide. The mean annual temperatures vary between 10 and 16°C, while the mean annual maximum and minimum temperatures range between 12 and − 1°C in the Sequoia region. The Sequoia forests remain frost-free from 6 to 11 months. Most stands of Sequoia are found between 30 and 760 m, and this region receives an annual precipitation between 640 and 3100 mm/year (Olson et al., Reference Olson, Roy, Walters, Burns and Honkala1990). Sequoia has undergone both expansions and contractions in its range in the past 10,000 years, and currently it appears that Sequoia is retreating from its southern range and expanding northwards (Sawyer et al., Reference Sawyer, Gray, West, Thorburgh, Noss, Engbeck, Marcot, Raymond and Noss2000).
Table 1 Native ranges, local environments and spatial patterns of redwoods
a Olson et al. (Reference Olson, Roy, Walters, Burns and Honkala1990); Rogers (Reference Rogers1997); Brinegar et al. (Reference Brinegar, Bruno, Kirkbride, Glavas and Udransky2007).
b Hartesveldt et al. (Reference Hartesveldt, Harry, Schellhammer and Stecker1975); Fins and Libby (Reference Fins and Libby1982); Weatherspoon (Reference Weatherspoon, Burns and Honkala1990).
c Li et al. (Reference Li, Chen, Zhang, Wu, Lu and Cai2005); Leng et al. (Reference Leng, Fan, Wang and Yang2007).
d Allnutt et al. (Reference Allnutt, Newton, Lara, Premoli, Armesto, Vergara and Gardner1999); Premoli et al. (Reference Premoli, Kitzberger and Veblen2000).
e Impacted by human activity and climate change.
f Sequoia (Brinegar et al., Reference Brinegar, Bruno, Kirkbride, Glavas and Udransky2007); Sequoiadendron (Fins and Libby, Reference Fins and Libby1982); Metasequoia (Li et al., Reference Li, Chen, Zhang, Wu, Lu and Cai2005); Fitzroya (Premoli et al., Reference Premoli, Kitzberger and Veblen2000).
The natural range of Sequoiadendron consists of 75 groves scattered over 420 km long and 24 km wide belt extending from Tulare to Placer counties on the western slopes, within an altitude range of 1400–2000 m, in the Sierra Nevada region in central California, USA. The annual precipitation varies between 900 and 1400 mm, and the annual mean temperatures vary between 24 and 29°C, while the annual maximum and minimum vary between 1 and − 6°C (Hartesveldt et al., Reference Hartesveldt, Harry, Schellhammer and Stecker1975; Weatherspoon, Reference Weatherspoon, Burns and Honkala1990). The third redwood Metasequoia is now confined to its native range of ~800 km2, within an altitude range of 700–1350 m, in western Hubei, eastern Chogqing and northern Hunan provinces (latitudes 29°10′N to 30°10′N) in south-central China. The annual precipitation is ~2360 mm, and the moderate temperature mean is 16.3°C in the Metasequoia region (Leng et al., Reference Leng, Fan, Wang and Yang2007). The fourth redwood Fitzroya is also an endemic to the temperate rain forests, within an altitude range of 100–1200 m, in southern South America. It grows in discontinuous populations within an area of ~360 × 200 km in the coastal Cordilleras and central depression in Chile, and on the western slopes of Andes in Chile and Argentina from latitudes 39°50′S to 42°45′S. The annual mean precipitation varies between 2000 and 4000 mm, and the temperature is between 13 and 16°C in the native habitats of Fitzroya (Allnutt et al., Reference Allnutt, Newton, Lara, Premoli, Armesto, Vergara and Gardner1999; Premoli et al., Reference Premoli, Kitzberger and Veblen2000).
Although the spatial pattern of Sequoia is largely continuous in most of its native range (Table 1), it also has fragmented populations in certain parts of its range (Olson et al., Reference Olson, Roy, Walters, Burns and Honkala1990; Sawyer et al., Reference Sawyer, Gray, West, Thorburgh, Noss, Engbeck, Marcot, Raymond and Noss2000). Sequoiadendron, by contrast, has mainly scattered groves in its entire range (Weatherspoon, Reference Weatherspoon, Burns and Honkala1990). Metasequoia populations are fragmented and discontinuous (Leng et al., Reference Leng, Fan, Wang and Yang2007), while Fitzroya forests are highly fragmented and discontinuous (Table 1) (Premoli et al., Reference Premoli, Kitzberger and Veblen2000). Fragmentation is disadvantageous in outcrossed conifers, as it leads to isolation and inbreeding and a substantial loss of heterozygosity. Common symptoms of inbreeding depression in conifers are abortive embryos, reduced seed set, reduced vigour, growth and survival in the inbreds (Wright, Reference Wright1976; White et al., Reference White, Adams and Neale2007). Inbreeding depression has been investigated in Sequoia (a hexaploid) and Metasequoia (a diploid). Both redwoods exhibited reduced growth rates and survival in the inbred progenies compared with outcrossed trees (Libby et al., Reference Libby, McCutchan and Millar1981; Kuser, Reference Kuser1983). Species with polyploid genomes, particularly allopolyploids, often do not experience severe inbreeding depression (Stebbins, Reference Stebbins1957). Even though Sequoia is a hexaploid, it did not exhibit any buffering effect against inbreeding depression. It is not clear whether the nature of polyploidy in Sequoia (Ahuja and Neale, Reference Ahuja and Neale2002), which may be either an autoallohexaploid (AAAABB) or a segmental allohexaploid (A1A1A2A2A2A2 or A1A1A2A2A3A3), but not a strict allohexaploid (AABBCC), might have been responsible for the lack of sheltering effect against inbreeding depression.
All four genera are threatened in their native ranges, due to human activity and a changing climate. According to the International Union for Conservation of Nature and Natural Resources (IUCN) categories of threat, Sequoia and Sequoiadendron are classified as vulnerable, while Metasequoia is listed as critically endangered and Fitzroya as endangered species (IUCN, 2010). In this study, we review strategies and current practices for conservation of germplasm in the endemic redwoods in the face of climate change.
Conservation of germplasm
Genetic diversity is essential for the survival and conservation of a species in a changing environment. Greater the level of genetic diversity in a species, better are the chances for its survival and deployment over a wide range of environments (Ledig, Reference Ledig1988). Appropriate levels of genetic variation must be maintained in the species and populations for conservation planning (Ledig, Reference Ledig1986, Reference Ledig and Elliot1987). This should be based on an understanding of genetic architecture and how genetic variation is organized and distributed within and among populations. Initially, those populations with higher levels of genetic diversity would be worth for germplasm conservation. In addition, selection of genetic variation for traits related to adaptation to climate change, for example time of bud flush, growth rates, drought and cold hardiness, and timing of initiation and cessation of growth, would be more relevant to conservation of genetics resources of redwoods. In other words, the conservation of adaptive gene complexes would be important for the future survival of the redwoods. Molecular markers have been widely applied to characterize patterns of genetic variation within and among populations of a species. These patterns provide baseline information for determining the appropriate levels of genetic diversity within selected populations for gene conservation. However, molecular genetic markers, including isozymes, random amplification of polymorphic DNAs (RAPDs), restriction fragment length polymorphisms (RFLPs), simple sequence repeats (SSRs), and amplified fragment length polymorphisms (AFLPs), which are derived from non-coding DNA sequences, are selectively neutral markers and may not often be predictive of adaptive genetic diversity (Karhu et al., Reference Karhu, Hurme, Karjalainen, Karvonen, Kärkkäinen and Neale1996; González-Martinez et al., Reference González-Martinez, Krutovsky and Neale2006; Holderegger et al., Reference Holderegger, Kamm and Gugerli2006). Although in recent years, much emphasis has been placed on the utility of adaptive genetic diversity in population genetics and gene conservation (Krutovsky and Neale, Reference Krutovsky, Neale, Geburek and Turok2005; González-Martinez et al., Reference González-Martinez, Krutovsky and Neale2006; Hidalgo et al., Reference Hidalgo, González-Martinez, Lexer, Heinze, Jansson, Bhalerao and Groover2010), very little is known about the molecular genetic control (number of loci involved) involved in the adaptive genetic diversity. Furthermore, if adaptive traits are under a very strong selection pressure and the numbers of gene loci are a few, much of the genetic variance would be lost.
In spite of the controversy regarding the utility of molecular makers in gene conservation, molecular markers have many applications with regard to conservation of genetic resources in forest trees. Molecular markers are useful in the characterization of a number of evolutionary forces that impact the maintenance of genetic diversity, mating systems, gene flow and genetic drift. Molecular markers can also reveal whether a small isolated population is experiencing a bottleneck (Ledig et al., Reference Ledig, Hodgekiss and Jacob-Cervantes2002) and thus is particularly susceptible to accelerated decline due to inbreeding depression and genetic drift. Most molecular markers, including isozymes, have provided useful estimates of the levels of genetic variation in a tree species (Hamrick et al., Reference Hamrick, Godt and Sherman-Boyles1992; Millar and Westfall, Reference Millar and Westfall1992; Hidalgo et al., Reference Hidalgo, González-Martinez, Lexer, Heinze, Jansson, Bhalerao and Groover2010). Studies on these markers have revealed that conifers exhibit greater levels of genetic diversity within populations as compared to between different populations.
Molecular genetic markers (isozymes) have revealed that hexaploid Sequoia seems to have a relatively higher level of genetic diversity (Libby et al., Reference Libby, Anekonda, Kuser and Leblanc1996; Rogers, Reference Rogers1997, Reference Rogers2000) than Fitzroya (a tetraploid) and Metasequoia and Sequoiadendron (both diploids) (Kuser et al., Reference Kuser, Sheely and Hendricks1997; Rogers, Reference Rogers2000; Premoli et al., Reference Premoli, Kitzberger and Veblen2000; Chen et al., Reference Chen, Li, Wu, Zhang and Lu2003; Ahuja, Reference Ahuja2009). Preliminary studies using molecular genetic markers (microsatellites) have indicated relatively higher levels of genetic diversity (Table 1) in the northern populations of Sequoia (Brinegar et al., Reference Brinegar, Bruno, Kirkbride, Glavas and Udransky2007) and Metasequoia (using RAPDs) (Li et al., Reference Li, Chen, Zhang, Wu, Lu and Cai2005). Higher levels of genetic diversity were detected in the southern populations of Sequoiadendron (employing isozymes) (Fins and Libby, Reference Fins and Libby1982) and eastern populations of Fitzroya (using isozymes) (Premoli et al., Reference Premoli, Kitzberger and Veblen2000). Although, these data are not entirely comparable as different population sizes and molecular markers were employed, they provide, at least, preliminary baseline data on genetic diversity for these redwoods. Again, both Sequoia and Fitzroya are polyploids, and yet Sequoia, a complex hexaploid, either an autoallohexaploid (AAAABB) or a segmental allohexaploid (A1A1A2A2A2A2 or A1A1A2A2A3A3), seemingly has a relatively higher genetic diversity compared with Fitzroya, a putative autotetraploid (AAAA), as estimated by isozyme analyses (Rogers, Reference Rogers2000; Premoli et al., Reference Premoli, Kitzberger and Veblen2000). The differences may be due to the type and nature of polyploidy.
Although molecular markers have provided reasonable estimates of genetic diversity in the redwood populations, the molecular genetic basis of adaptive genetic diversity still remains to be investigated. Structural and functional genomic information is still lacking in these redwoods. Linkage maps have not been constructed in the redwoods. Because of the large genome sizes in these redwoods, ranging from 10,000 MB in Sequoiadendron and Metasequoia to 31,500 MB in Sequoia (Ahuja and Neale, Reference Ahuja and Neale2005), genome sequencing would be a very difficult and a challenging problem in the redwoods. Initially, gene discovery based on identifying expressed sequence tags would be an alternative to genome sequencing in the redwoods. Therefore, presently, the conservation of germplasm would rely on the availability of molecular marker-based genetic information that would be relevant to the genetic diversity in the redwood populations.
In order to evaluate the plans for conservation of germplasm, it is important to recognize threats to genetic diversity (human activity and climate change) and to have adequate knowledge on geographic variation of adaptive traits, so that, selected populations may be prioritized relative to the threat. In this context, a better understanding of geographical variation in adaptive traits and its relationship to the endemic environment would be helpful in determining which populations of redwoods are most threatened (for example, northern or southern, coastal or inland, low or high altitude, fragmented or continuous, sensitive to frost or drought) and should be given priority for germplasm conservation. However, for global conservation of redwood germplasm, selected genotypes with high levels of diversity (both neutral and adaptive) from the entire species range should be included in the face of uncertainty regarding the climate change.
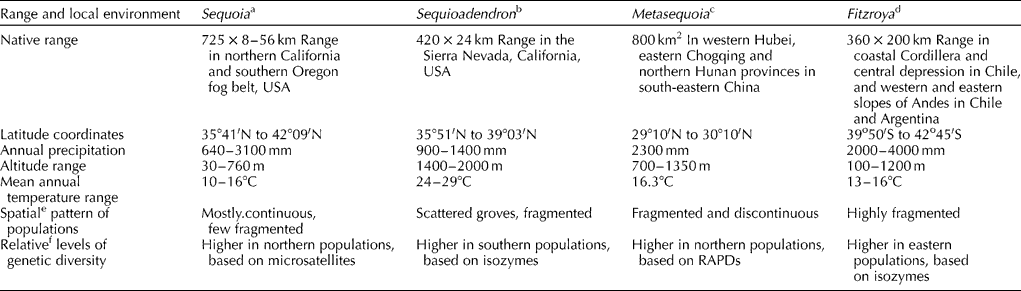
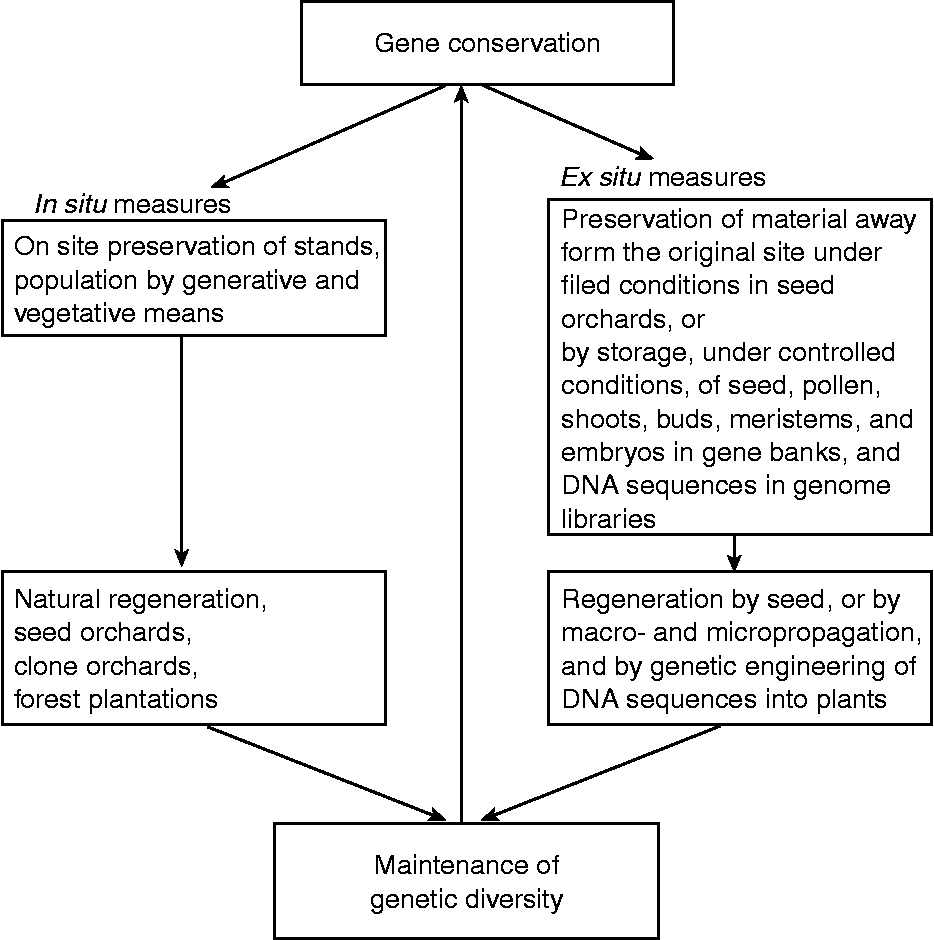
Conservation of germplasm can be accomplished by in situ (on site, within the original ecosystem) and ex situ (outside the natural habitat) methods, which take into account the maintenance of genetic diversity (Fig. 1). The conservation strategy has to be holistic, which is based on the entire gene pool of a species. One of the objectives of gene conservation is to ensure that functionally adaptive alleles will be available in the future for the breeding programmes and evolution of the species (Ledig, Reference Ledig1986; Hattemer, Reference Hattemer1995). In order to hedge against uncertainty regarding the level of climate change in the future, it would be desirable to maximize genetic diversity by selecting individuals heterozygous for a number of genes that exhibit hybrid vigour (Ledig and Kitzmiller, Reference Ledig and Kitzmiller1992; Hamrick, Reference Hamrick2004; Geburek and Konrad, Reference Geburek and Konrad2007). Since climate change is unlikely to stabilize for a long time in the foreseeable future, the maintenance of both neutral and adaptive genetic diversity (Volis and Blecher, Reference Volis and Blecher2010) will be absolutely essential for the survival of the forest trees. One of the best strategies might be to deploy intimate mixture of seed from the selected genotypes from disparate regions and environments for future in situ and ex situ forest tree plantations in the face of uncertain climate change (Ledig and Kitzmiller, Reference Ledig and Kitzmiller1992). Finally, it would be desirable to use molecular markers (Tikader et al., Reference Tikader, Vijayan and Kamble2009; Hidalgo et al., Reference Hidalgo, González-Martinez, Lexer, Heinze, Jansson, Bhalerao and Groover2010) to monitor the genetic fidelity of populations/clones during the conservation and management of redwood germplasm in the in situ and ex situ programmes.

Fig. 1 Conservation of forest tree genetic resources by in situ and ex situ strategies. Gene conservation approaches should aim at maintaining genetic diversity in the forest tree species.
In situ conservation
In situ preservation of a forest tree includes conservation of stands and populations via regeneration by generative and vegetative methods within the area of natural occurrence (Fig. 1). Although this type of conservation has been in practice in the national parks and nature reserves, it is not necessarily based on genetic criteria. However, a better approach to conservation of genetic resources in the redwoods in the USA, China, Chile and Argentina should be based on gene-ecological approaches that ensure not only the conservation of the redwoods but also the associated plant and animal species in the ecosystem (Parker and Donoso, Reference Parker and Donoso1993; Li, Reference Li1999; Noss et al., Reference Noss, Strittholt, Heilman, Frost, Sorensen and Noss2000; Evarts and Popper, Reference Evarts, Popper, Evarts and Popper2001; Premoli et al., Reference Premoli, Vergara, Souto, Lara and Newton2003). The regeneration of populations is essential, and new generation of trees should originate from the controlled hybridizations within the conserved but diverse populations to enhance performance (hybrid vigour) that would be advantageous for an overall conservation of genetic diversity in the face of climate change. However, the potential consequences of genetic swamping should be curtailed in these gene conservation management programmes. Furthermore, the number of genotypes, both for vegetative and for generative progeny, should be large enough to preserve the common alleles and the adaptive gene complexes. Thus, a gene conservation strategy seeks not only to preserve germplasm in the old growths and secondary growth populations, but also to maintain sufficient genetic variability to allow adaptation in the new environment.
In the context of in situ conservation of redwoods, it is important to recognize the species' composition, their abundant pattern, genetic architecture, ecological dynamics, genetic diversity and ecosystem protection and maintenance (Libby et al., Reference Libby, Anekonda, Kuser and Leblanc1996; Noss et al., Reference Noss, Strittholt, Heilman, Frost, Sorensen and Noss2000). Each redwood species has a unique endemic environmental niche in its native country (Table 1). An additional understanding of the soil and nutritional requirements, and companion forest and other vegetation can also provide the direction for the maintenance and conservation of the redwoods in the present and future ecosystems in the face of climate change. The contingency planning for the conservation of genetic resources of future redwood forests would be to move their genetically diverse populations in protected areas and reserves outside of their native habitats before the anticipated rapid climate changes impact them. That implies that ex situ strategies for the conservation of redwood germplasm should be fully explored at this stage.
Ex situ conservation
The ex situ measures conserve the genetic resources outside the natural habitat of a species (Fig. 1). These include (1) seed and clonal orchards under nursery and field conditions, and (2) biotechnological approaches for preservation of germplasm. We examine in the following sections the status of these ex situ preservation methods in redwoods. Implementing ex situ programmes can be time consuming and expensive. Among other things, ex situ conservation has a drawback in that the plant material is subjected to a selection pressure that may be quite alien to that in nature under which the original populations evolved (Ledig, Reference Ledig1986). However, these problems can be partially circumvented by regularly monitoring genetic fidelity and variation in the genotypes by employing molecular markers.
Seed and clonal orchards
Seed and clonal orchards under nursery and field conditions are a routine for the ex situ conservation of forest tree species (Melchior et al., Reference Melchior, Muhs and Stephan1986; Ledig, Reference Ledig1986, Reference Ledig1988; Millar, Reference Millar, Ahuja and Libby1993; Hattemer, Reference Hattemer1995; Behm et al., Reference Behm, Becker and Dorflinger1997). Ex situ plantations of redwoods have also been tested for their performance within the USA and in a number of countries. Sequoia has been successfully grown out of its fog belt for more than 100 years in Placerville and in the foothills of Sierra Nevada in California, Seattle, Washington, Hawkinsville, Georgia, USA; and Victoria, British Columbia, Canada (Kuser, Reference Kuser1981). It is now known that Sequoia can be grown in many parts of the world: Western Europe, Turkey, Crimea, New Zealand, Chile, South Africa and Tasmania (Kuser et al., Reference Kuser, Bailly, Franclet, Libby, Martin, Reydelius, Schoenike and Vagle1995). A range-wide international provenance trial, which included 180 clones from 90 locations throughout the natural range of Sequoia, was carried out in four plantation sites in the USA, two in France and one each in Spain, England and New Zealand (Kuser et al., Reference Kuser, Bailly, Franclet, Libby, Martin, Reydelius, Schoenike and Vagle1995). Early results from this study suggest that provenances from the north end of the Sequoia range survive well in South Carolina and suffer less frost damage in northern France. Provenances from Humboldt County (middle of the Sequoia range) do survive well in Lafayette, California, and Entacon, France (Kuser et al., Reference Kuser, Bailly, Franclet, Libby, Martin, Reydelius, Schoenike and Vagle1995). This implies that the survival of redwoods outside their native range depended on the comparable climate/soil conditions in the distant locations. Therefore, differences in the origin of the material are important consideration for ex situ plantations of Sequoia.
Single trees or stands of Sequoiadendron were also planted more than 100 years ago in a number of countries of Europe (Hartesveldt, Reference Hartesveldt1969). In several countries, solitary trees in Arboretums/Parks or stands of Sequoiadendron have survived (France, Hungary, Greece, Belgium, Netherlands, Denmark, Norway and Germany), while in other countries (Yugoslavia and Romania), Sequoiadendron has not performed well (Libby, Reference Libby1981). Although healthy Sequoiadendron trees/stands still exist in Europe, the species is sensitive to frost and disease damage. In Germany, Sequoiadendron has performed well in southwest Germany, but not in northern Germany. One study tested frost tolerance in Sequoiadendron at the seedling stage (Guinon et al., Reference Guinon, Larson and Spethmann1982), and the second investigated the survival of 14-year-old trees under field conditions in Germany (Melchior and Hermann, Reference Melchior and Hermann1987). Significant differences were observed in the degree of frost tolerance, as measured by the freezing test conditions (temperatures ranging from − 5 to − 14°C), in the 2-year-old seedlings from 22 provenances (representing the entire natural range of Sequoiadendron in California) in Germany (Guinon et al., Reference Guinon, Larson and Spethmann1982). Significant differences were also observed in the survival and growth performance of 14-year-old Sequoiadendron from four provinces at three different locations in Germany (Melchior and Hermann, Reference Melchior and Hermann1987). In the northern location at Grosshansdorf, Germany, Sequoiadendron was badly damaged by frost and infection by Armillaria mellea. The authors recommended the use of frost-tolerant genotypes of Sequoiadendron for suitable locations in Germany (Melchior and Hermann, Reference Melchior and Hermann1987).
Metasequoia has also been planted outside China and in different countries of the world as single trees in Arboretums/Parks or stands (Satoh, Reference Satoh1999). Metasequoia seems to be more resilient to environmental extremes, for example, it has survived the heavy snows in the botanical gardens of Hamilton and Montreal, Canada, sizzling summer temperatures of Adelaide, Australia (Satoh, Reference Satoh1999), and perhaps in other regions of the world with similar climates. In the USA, Metasequoia was introduced more than 50 years ago and showed good growth in the eastern and western USA (Kuser, Reference Kuser1999). Even though Metasequoia is an endemic, it has a moderate reservoir of genetic (Kuser et al., Reference Kuser, Sheely and Hendricks1997) and phenotypic and ecotypic diversity (Li, Reference Li1999) for ex situ plantations (Li et al., Reference Li, Chen, Zhang, Wu, Lu and Cai2005) worldwide.
Although single trees may have been planted in the botanical gardens/parks in some countries, only small experimental stands of Fitzroya have been mainly restricted to Chile and Argentina (C. Donoso, pers. commun.). Of all the endemic redwoods, perhaps Fitzroya is the most endangered species and must be conserved in ex situ plantations and by other ex situ approaches for the conservation of its genetic resources. Genetic diversity in Fitzroya is lower than other conifers (Premoli et al., Reference Premoli, Kitzberger and Veblen2000), but still has a moderate level of diversity, similar to Metasequoia and Sequoiadendron (Fins and Libby, Reference Fins and Libby1982; Kuser et al., Reference Kuser, Sheely and Hendricks1997), and should be able to adapt to different environments. Therefore, it is high time that, like other three redwoods, ex situ plantations of Fitzroya are also established in suitable locations in Chile/Argentina and other countries for testing their adaptability and survival.
Biotechnological approaches for preservation of germplasm
Biotechnological approaches for preservation of germplasm include (1) in vitro storage of tissues at non-frozen temperatures and (2) storage of germplasm (seed, tissues, pollen and DNA) at sub-zero and ultra-low temperatures (cryopreservation).
In vitro storage of tissues
Tissues (meristems and shoots) can be used as a resource for clonal propagation and conservation of germplasm, since these can be maintained in culture at 4–25°C over a long period of time (Aitkin-Christie and Singh, Reference Aitkin-Christie, Singh, Bonga and Durzan1987; Ahuja, Reference Ahuja, Pardos, Ahuja and Rosello1994, Reference Ahuja, Edwards and Naithani1999). The potential of tissue culture for differentiation and organogenesis has been investigated in Sequoia for more than 50 years. We have come a long way from the early studies with callus cultures (Ball, Reference Ball1950) to differentiation of plantlets from shoot cultures of juvenile and mature trees, up to 90 years of age, in Sequoia (Boulay, Reference Boulay1997; Arnaud et al., Reference Arnaud, Franclet, Tranvan and Jacques1993; Bon et al., Reference Bon, Riccari and Monteuuis1994; Liu et al., Reference Liu, Xia, Yin, Huang and Zhou2006). In general, stump shoots from the base of mature trees are more responsive than shoots from the crown of the same mature tree to in vitro differentiation and organogenesis. We had employed tissue culture using bud meristems from four frost-tolerant Sequoia trees (23 years old) from a plantation near Cologne, Germany, to clonally propagate them in 1986 at the Institute of Forest Genetics, Grosshansdorf, Germany (Ahuja, Reference Ahuja and LeBlanc1996). More than 1000 clones were produced by tissue culture, and several hundred clones were tested under field conditions for their overwintering capacity. Frost tolerance capacity of Sequoia clones from the frost-tolerant donor trees was tested for many years in Grosshansdorf and Trenthorst, in northern Germany. The Sequoia clones grown in the Grosshansdorf nursery seemed to be frost-tolerant and have survived in the winters in Germany. However, only those frost-tolerant Sequoia clones survived in the field trial in Trenthorst were sheltered by the tree canopy during early growth (Ahuja, Reference Ahuja and LeBlanc1996). Therefore, it would be necessary to shelter the putative frost-tolerant Sequoia clones for several years of early growth for their survival in climates with harsh winters.
Tissues (bud meristems) from juvenile and mature trees (up to 100 years old) of Sequoiadendron have been cultured in vitro, and plants have been regenerated from such cultures (Monteuuis, Reference Monteuuis1987, Reference Monteuuis1991; Bon and Monteuuis, Reference Bon and Monteuuis1991; Monteuuis et al., Reference Monteuuis, Doulbeau and Verdeil2008). Tissue culture offers opportunities for clonal propagation from selected genotypes of redwoods, including frost-tolerant genotypes in Sequoia, for ex situ plantations. To my knowledge, tissue culture studies for clonal propagation have not been reported in Metasequoia and Fitzroya.
Storage of germplasm under sub-zero and ultra-low temperatures
Storage of germplasm (seeds, dormant buds, meristems, embryos, cells, pollen and DNA) under sub-zero temperatures (0 to − 80°C) and cryopreservation ( − 196°C) offers opportunities for conservation of germplasm (Table 1) in forest trees (Sakai, Reference Sakai and Bajaj1986; Ahuja, Reference Ahuja and Dhawan1989, Reference Ahuja, Pardos, Ahuja and Rosello1994; Engelmann, Reference Engelmann2004; Suszka et al., Reference Suszka, Chmielarz and Walkenhorst2005). Seeds of redwoods have been stored at low temperatures for various lengths of time. Sequoia seeds containing 6–10% moisture in airtight sealed bottles stored at 5°C for 3 years retained 14% viability, but seed viability dropped to 0% after 16 years of storage (Schubert, Reference Schubert1952). Sequoia seeds stored at − 2 to − 4°C retained viability for 1 year but lost viability rapidly after removal from cold storage (Metcalf, Reference Metcalf1924). On the other hand, after storage at − 16°C for 7 years, Sequoia seeds retained 12–15% viability (Boe, Reference Boe and Schopmeyer1974). Sequoiadendron seeds, which had an 18% germination capacity, dropped their viability to 8% after storage at 5°C for 14 years (Schubert, Reference Schubert1952). Dry seeds of Metasequoia have been satisfactorily stored in airtight bottles at 2–4°C (Johnson, Reference Johnson and Schopmeyer1974).
Seeds, buds, meristems and cells have been successfully cryopreserved in a number of forest tree species (Stanwood, Reference Stanwood and Kartha1985; Ahuja, Reference Ahuja1986, Reference Ahuja and Dhawan1989, Reference Ahuja, Edwards and Naithani1999; Bonner, Reference Bonner1990; Ryynänen, Reference Ryynänen1996). In addition, conservation of DNA at − 20 to − 80°C in genebanks offers prospects for the application of genomics to germplasm conservation (Adams, Reference Adams, Callow, Ford-Lloyd and Newbury1997; Rice et al., Reference Rice, Cordeiro, Shepherd, Bundock, Bradburry, Pacey-Miller, Futado and Harry2006). Cryopreservation of germplasm has not been researched in the redwoods so far, but this avenue offers new options for storage of germplasm of the redwoods for future exploitation.
Future of redwoods
The redwoods are threatened in their native endemic locations in the face of climate change, and, therefore, the conservation of their germplasm is essential. Although these redwoods are protected in the national parks, reserves and in privately owned forests in their habitats, we have to consider their conservation in view of global warming and climate change. It would be desirable to conserve them (1) in situ on the model conservation sites (Noss et al., Reference Noss, Strittholt, Heilman, Frost, Sorensen and Noss2000) that have comparable environmental conditions to their endemic climate regions (Table 1) and (2) in ex situ locations, similar to endemic conditions and also different environments to challenge their genotypes, and by other biotechnological ex situ strategies for conservation of their germplasm in genebanks.
Although there is an uncertainty about the actual amount of global warming, recent estimates predict an increase in global mean temperature, as a result of human activity, by 2.4–6.4°C (IPCC, 2007), and significant changes in the rainfall cycles (Trenberth et al., Reference Trenberth, Dai, Rasmussen and Pearson2003) by the end of the current century. Global climate change is impacting species distributions and functioning and their terrestrial ecosystems (Parmesan, Reference Parmesan2006; Thuiller et al., Reference Thuiller, Albert and Araújo2008). There is substantial evidence to suggest that the species ranges are shifting (Parmesan and Yohe, Reference Parmesan and Yohe2003; Root et al., Reference Root, MacMynowski, Mastrandrea and Schneider2005; McKenney et al., Reference McKenney, Pedlar, Lawrence, Campbell and Hutchison2007; Kelly and Goulden, Reference Kelly and Goulden2008), and some species are facing extinction risks, whereas others have become extinct (Thomas et al., Reference Thomas, Cameron and Green2004; Schwartz et al., Reference Schwartz, Iverson, Prasad, Matthews and O'Connor2006). Different models have been used to predict plant migration patterns to potentially suitable habitats under the future climate change scenarios. Many North American tree species will likely shift their ranges at a rate of 10–100 km/100 years to keep pace with the predicted climate changes in this century (Davis and Zabinski, Reference Davis, Zabinski, Peters and Lovejoy1992; Iverson and Prasad, Reference Iverson and Prasad2002; Iverson et al., Reference Iverson, Schwartz and Prasad2004, Reference Iverson, Prasad and Schwartz2005). In a recent study, Iverson et al. (Reference Iverson, Prasad, Matthews and Peters2008) have examined the potential response of 134 tree species in the USA under different climate change scenario in this century. Depending on the climate change scenario, they predicted that more than a quarter of the species could shift their ranges more than 400 km northwards, and in the hottest climate change scenario, most of the species would advance up to 800 km northwards (Iverson et al., Reference Iverson, Prasad, Matthews and Peters2008). However, the use of molecular markers as indicators of potential migration capacity based on two North American tree species (Fagus grandifolia and Acer rubrum) under rapid climate change scenario has provided lower estimates of less than 10 km/century, which seem to be consistent with their life history and dispersal capacity (McLachlan et al., Reference McLachlan, Clark and Manos2005). It would appear that the potential migration rates of forest tree species in the face of rapid climate change, based on different models and methodologies, have resulted in widely different estimates of range shift (Pearson, Reference Pearson2006).
Based on the dispersal capacity, we speculate that if the climate changes slowly raising the global temperature by 1–2°C and the southern areas in the northern hemisphere start becoming hot and dry, the tree species from such climatic zones may have to move only a few km north to colonize areas that are suitable habitats for their survival. The reverse would be the case in species in the southern hemisphere, where the ranges would move further south. However, if the earth warms 2–6°C in this century, the climate niches of the tree species may have to move, depending upon the species, 10–100 km or even more in this century. Tree species whose seeds are dispersed by birds may be able to colonize new areas in that range. But, species whose seeds are dispersed by wind (unless strong winds) may not be able to spread more than a few hundred metres from their stands. Though redwoods have winged seeds, their dispersal is usually very close to their plantations. Therefore, rapid climate change would pose a challenging problem for colonization of the redwoods into new habitats. And then there are human-created impediments (industry and new settlements) that would become barriers to dispersal and migration. What will be the fate of redwoods in a rapid future climate change scenario? Although the extinction of endemic redwoods seems unlikely in the foreseeable future because of human conservation intervention (parks and reserves), they are still vulnerable to climate change in their native ranges.
A recent study has shown that there is a 33% reduction in the summer fog in the coast redwood (Sequoia) region along the California coast during the past century, and this climate change may impact recruitment of new coast redwood trees in the forest (Johnstone and Dawson, Reference Johnstone and Dawson2010). Anticipated changes in temperature and precipitation cycles in the ‘climate envelops’ of other redwoods (Sequoiadendron, Metasequoia and Fitzroya) may also affect their growth and survival. If the retraction of Sequoia in its southern range and expansion in the northern range is any indication of the threat from climate change or other causes to the species (Sawyer et al., Reference Sawyer, Gray, West, Thorburgh, Noss, Engbeck, Marcot, Raymond and Noss2000), then a similar phenomenon might be happening to the other three redwoods in their native ranges. That would imply that Sequoiadendron and Metasequoia may also be contracting in the southern ranges and expanding northwards, while Fitzroya may be retracting in the northern range and expanding southwards. Since California has varied terrains, anticipated migration of endemics, depending on the magnitude of emissions, may be more complex and, as a result, species may migrate in different (altitude and latitude) directions, thus disrupting the present endemic floras (Loarie et al., Reference Loarie, Carter, Hayhoe, McMahon, Moe, Knight and Ackerly2008). And Sequoiadendron, which is already vulnerable endemic in California, may have an unpredictable fate in the face of climate change. The redwoods ranges have temperate temperatures (10–29°C zones) and mild winters (Table 1), but the redwoods are sensitive to frost. Therefore, development/isolation of frost-tolerant genotypes in redwoods may offer excellent opportunities for future ex situ reserves in colder climates.
Faced with a threat of climate change, it might be useful to deploy an intimate mixture of seeds from widely divergent populations from different environments (Ledig and Kitzmiller, Reference Ledig and Kitzmiller1992) as a resource for seed orchards and planting material for potential future climatic conditions. In addition, conservation planning in a changing climate calls for strategies that locate, configures and maintains areas that are managed to promote biodiversity and ecosystem stability (Hannah et al., Reference Hannah, Midgley, Andelman, Araújo, Hughes, Martinez-Meyer, Pearson and Williams2007; Pressey et al., Reference Pressey, Cabeza, Watts, Cowling and Wilson2007; Thuiller et al., Reference Thuiller, Albert and Araújo2008). In the face of uncertainty regarding climate change, it would be prudent to pursue flexible approaches that include adaptive strategies (actions that promote and maintain genetic diversity) and mitigation approaches (actions that sequester carbon and reduce overall greenhouse gas emissions) for forests of the future (Millar et al., Reference Millar, Stephenson and Stephens2007). In any event, it is imperative that germplasm conservation of redwoods must proceed unabatedly, with more emphasis on ex situ strategies to ‘Save the Redwoods’.
Acknowledgements
I am thankful to the Institute of Forest Genetics, USDA, Placerville, and Davis, California, for research and library facilities for this review. I thank David Neale for interesting discussions, and anonymous reviewers for comments on the manuscript.