Introduction
Our understanding of where and how growth occurs in lichen thalli is limited. Previously, lichen growth was assumed to be restricted to the margins and apices of thalli (Hill Reference Hill1981; Hale Reference Hale1983), consistent with the notion that the component fungal hyphae grow exclusively at their tips (but see Voisey Reference Voisey2010). In crustose lichens, and foliose taxa that are closely attached to the substratum, expansion may indeed be limited mainly to a marginal zone (Hale Reference Hale1970; Armstrong Reference Armstrong1974; Fisher & Proctor Reference Fisher and Proctor1978). In foliose lichens with less restrictive attachment, however, much of the thallus may contribute to expansion, as sequential images (Larsson & Gauslaa Reference Larsson and Gauslaa2009) and growth rates proportional to size suggest (e.g. Rhoades Reference Rhoades1977), at least during early stages of development. In the few fruticose lichens studied, all of which are long pendulous taxa, growth occurs diffusely throughout the thallus, while morphogenetic and histogenetic processes are localized at branch apices (Sanders Reference Sanders1989, Reference Sanders1992; Rolstad & Rolstad Reference Rolstad and Rolstad2008; Sanders & de los Ríos Reference Sanders and de los Ríos2012; Sanders & Tokamov Reference Sanders and Tokamov2015). However, because lichen thalli are so diverse, many more growth studies are needed before we can assess what generalizations, if any, are warranted.
Particularly intriguing in this regard are the umbilicate lichens, whose laminar, dorsiventral thalli attach to their rock substratum by a central holdfast. In the opinion of Honegger (Reference Honegger2008), their growth patterns are the least understood. Based on carbon fixation rates that varied irregularly throughout individual thalli of Umbilicaria, Larson (Reference Larson1983) inferred that thallus growth is most likely diffuse. Anatomical study of the umbilicate genus Lasallia led Valladares & Ascaso (Reference Valladares and Ascaso1994) to postulate that growth occurs mainly in a broad intermediate zone between the umbilicus and the margin. Hestmark (1997 Reference Hestmarka ) measured the displacement of thallus pustules in L. pustulata over a four-year period, reporting a diffuse pattern of growth consistent with the inferences of Larson (Reference Larson1983) and Valladares & Ascaso (Reference Valladares and Ascaso1994). His description of pustules arising near the central umbilicus and moving radially outwards over time suggested a pattern of morphogenesis quite different from any proposed previously in macrolichens, where morphogenetic processes are typically localized at apices and margins (e.g. Sanders Reference Sanders1992, Reference Sanders1993). However, no sequential photographs were provided. While geometric increases in length over time were observed in the fruticose Usnea longissima (Rolstad & Rolstad Reference Rolstad and Rolstad2008), Hestmark (1997 Reference Hestmarkb ) found no correlation between thallus size and diameter increments over a four-year period in L. pustulata. To better understand the structural basis of growth in this lichen, we explore features of tissue behaviour and cell proliferation that would be expected to occur under conditions of diffuse expansion as reported by Hestmark (1997 Reference Hestmarka ).
Lasallia pustulata has a stratified (heteromerous) thallus with several distinct tissue layers. A cortex of moderately thick-walled, approximately isodiametric cells covers upper and lower surfaces. At its exterior, especially on the upper surface, dead cortical cells accumulate as a thick epinecral layer (Fig. 1), reportedly serving to reflect high light intensity and/or increase water absorption (Büdel Reference Büdel1990; Valladares Reference Valladares1994; Dietz et al. Reference Dietz, Büdel, Lange and Bilger2000). Dead cells are sloughed off at the surface and resupplied by division of the living cortical cells below. If the living tissue layer is also increasing diffusely in surface area, the epinecral accumulations above should develop ruptures in regular succession because dead cells cannot grow or divide to keep pace with the expanding tissue beneath. Furthermore, one would expect the ruptured sectors of epinecral tissue to have a tapered form, narrower in the upper portions and widening towards the base, due to the ever-increasing surface area of the tissue contributed from below. The anatomical basis of growth may also be evident in the pattern of cortical cell divisions. Diffuse growth would likely require isodiametric cortical cells to proliferate laterally within the plane of expansion (new anticlinal cross walls) to increase the number of cells in the layer, in addition to the vertical divisions (new periclinal cross walls) that supply the overlying epinecral tissue. Such division patterns would directly contradict the long-standing paradigm that vegetative tissues in fungi and lichens are never parenchymatous (Jahns Reference Jahns1973, Reference Jahns1988; Moore Reference Moore1998). However, a recent study demonstrated that parenchymatous divisions do indeed occur routinely in the cortices of at least some familiar lichens (Sanders & de los Ríos Reference Sanders and de los Ríos2017). That study used TEM to distinguish walls of septal origin and show that new septa adjoin them, which is central to the concept of parenchymatous division (Schwendener Reference Schwendener1873; Fritsch Reference Fritsch1935). In the present work, we examine anatomical and ultrastructural features in L. pustulata to determine whether their development is consistent with predictions based on the diffuse growth model (Larson Reference Larson1983; Valladares & Ascaso Reference Valladares and Ascaso1994; Hestmark 1997 Reference Hestmarka ).
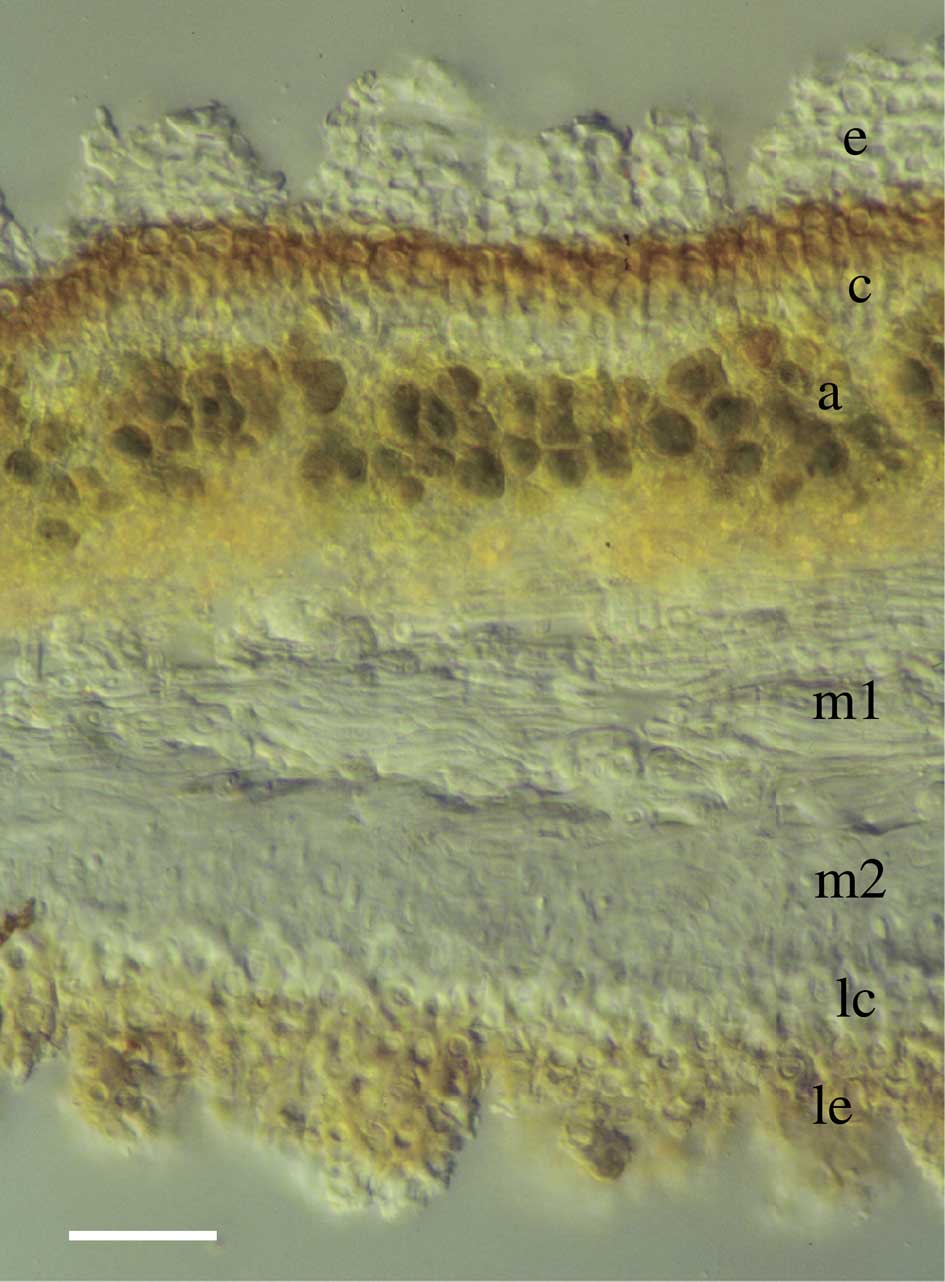
Fig. 1 Lasallia pustulata. TS thallus (LM). Abbreviations: e, epicortex; c, upper cortex; a, algal layer; m1, lax medulla; m2, dense medulla; lc, lower cortex; le, epinecral accumulations of lower surface. Scale=20 µm.
Materials and Methods
Thalli of Lasallia pustulata were collected air-dry near El Escorial and Pinilla del Buitrago, Madrid, Spain, in June 2017. Specimens processed for TEM were first hydrated with distilled water using a spray mister and maintained in covered Petri dishes for 24 h. Moist thalli were then hand-sectioned with a razor blade to a thickness of c. 0·25 mm to facilitate chemical penetration. Tissue was chosen from a pustular region roughly equidistant from the margin and the umbilicus. Sections were placed immediately in fixative solution (2·5% v/v glutaraldehyde in phosphate buffer), followed by postfixation in 1% w/v OsO4 solution, dehydration in an ethanol series, infiltration with Spurr’s low-viscosity resin and subsequent polymerization, according to de los Ríos & Ascaso (Reference de los Ríos and Ascaso2002). Ultrathin sections were cut c. 79 nm thick using a Leica EM UC-6 ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany). Specimens were oriented so as to section the upper cortex paradermally (i.e. parallel to the surface, the plane in which thallus expansion takes place). Sections were post-stained with lead citrate (de los Ríos & Ascaso Reference de los Ríos and Ascaso2002) then examined in a JEM-1011 transmission electron microscope (JEOL, Tokyo, Japan). For SEM imaging, air-dried specimen fragments were sputter-coated with gold and observed with an FEI Inspect scanning electron microscope (Thermo Fisher Scientific, Waltham, Massachusetts, USA), using the secondary electron imaging mode.
Results
The upper surface of the L. pustulata thallus was uniformly covered by adjacent, spine-like, roughly cone-shaped epinecral accumulations with somewhat angular surfaces. They were distinctly tapered from their broad base, which contacts those of adjacent spines, to their narrowing, irregular apex (Fig. 2). Empty compartments of dead cells were visible with SEM at higher magnification (Figs 2B & 3). One could distinguish the basal outlines of what were formerly individual tufts of epinecral tissue, now subdivided by further tissue rupture (arrows in Fig. 3). On the lower surface, epinecral accumulations were less extensive and cell walls thicker but a comparable pattern of tissue rupture was observed (Fig. 4).

Fig. 2 Lasallia pustulata. Upper surface (SEM). Scales: A=100 µm; B=50 µm.

Fig. 3 Lasallia pustulata. Upper surface (SEM). Arrows are placed at perimeter of an epinecral tuft, while arrow direction indicates fissures which subsequently subdivided the structure. Scales: A–C=25 µm; D=20 µm.
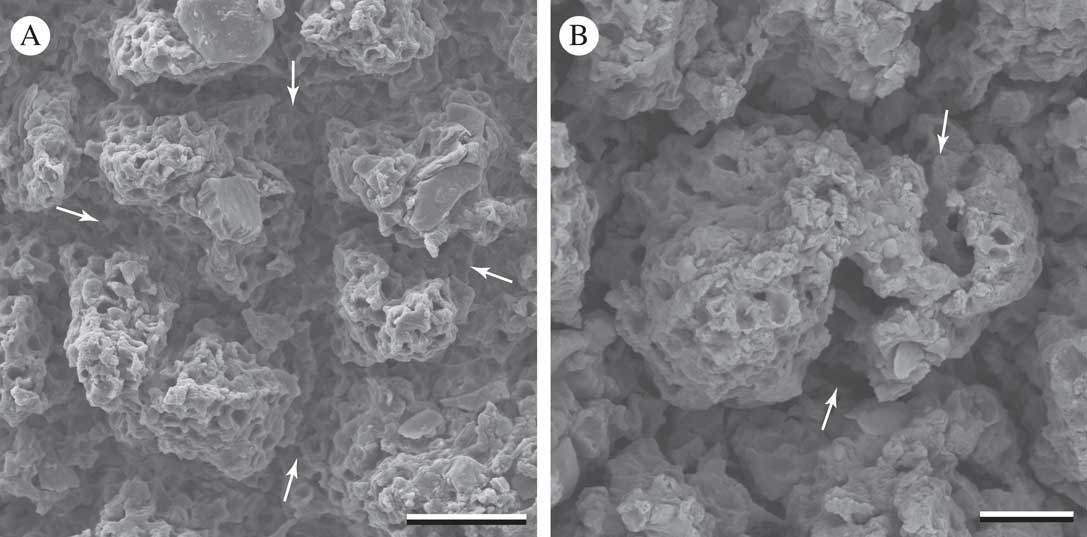
Fig. 4 Lasallia pustulata. Lower surface (SEM). Arrows indicate fissures forming within epicortical accumulations. Scales: A=20 µm; B=10 µm.
TEM images of paradermal sections showed anticlinal divisions of cells within the plane of the thallus surface. An evanescent but still recognizable median layer allowed septal walls to be distinguished. New septa were clearly adjoined to previous septa (Fig. 5). At these junctures, the sequence of wall formation was evident: the newer septum adjoined to the outer wall layers of a previous septum, and therefore its median layer did not make contact with that of the older wall (arrowheads in Fig. 5).
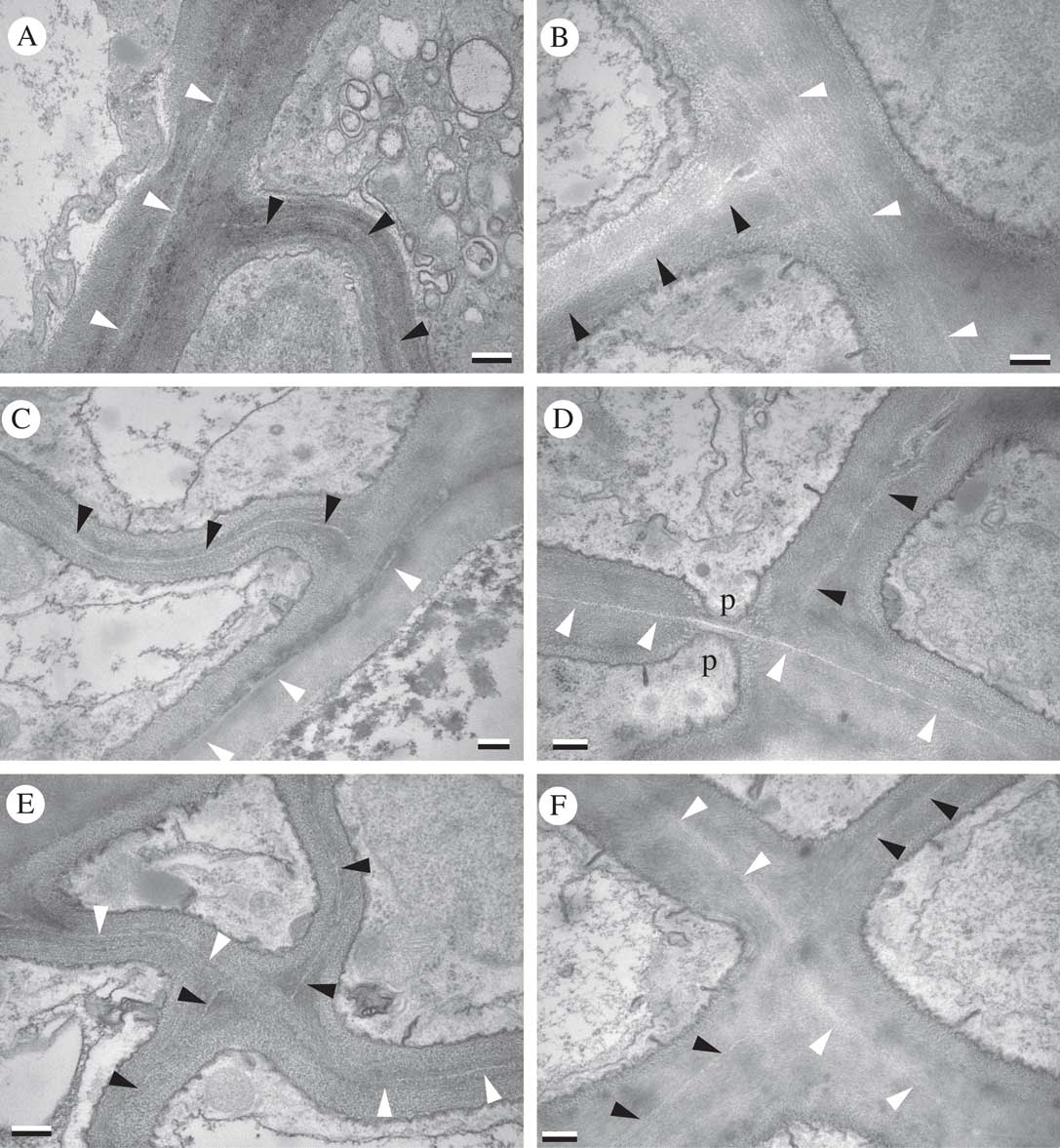
Fig. 5 Lasallia pustulata. Paradermal sections through upper cortex (TEM). Black arrowheads indicate median layer of newer septa; white arrowheads indicate median layer of previous septa to which newer septa adjoin; p=septal pore. Scales: A–F=0·25 µm.
Discussion
A number of published studies have explored the anatomy of the Umbilicariaceae from structural, functional or biosystematic perspectives (Frey Reference Frey1929; Scott & Larson Reference Scott and Larson1983; Krog & Swinscow Reference Krog and Swinscow1986; Sancho & Balaguer Reference Sancho and Balaguer1989; Valladares & Ascaso Reference Valladares and Ascaso1994: Schroeter & Scheidegger Reference Schroeter and Scheidegger1995; Valladares et al. Reference Valladares, Wierzchos and Ascaso1993, Reference Valladares, Sancho and Ascaso1998; Valladares & Sancho Reference Valladares and Sancho1995; de los Ríos et al. Reference de los Ríos, Ascaso and Wierzchos1999). The “areolate-echinate” shape of the epicortical accumulations in Lasallia (Sancho & Crespo Reference Sancho and Crespo1989) was noted and the ecophysiological significance of the fissures that delimit them was discussed (Valladares Reference Valladares1994). However, the developmental context of these structural features does not appear to have been considered previously. The broadening bases of the epinecral accumulations and their repeated sub-fragmentation through tissue rupture are predictable results of the underlying tissue’s continued expansion in surface area (Figs 2–4). Evidence of cortical septa adjoining perpendicularly to each other within the plane of the thallus surface (Fig. 5) indicates growth processes at the cellular level that have not been proposed previously for umbilicate lichens. These results are consistent with Hestmark’s (1997 Reference Hestmarka ) report of diffuse growth in Lasallia and may also be applicable to other members of the family that show a variety of surface tissue rupture patterns (Frey Reference Frey1929; Scott & Larson Reference Scott and Larson1983). Other foliose and squamulose lichen thalli with fissured epinecral layers and/or thorn-like tapered stacks of cortical material (e.g. Timdal Reference Timdal1984; Vogel Reference Vogel1955; Heiđmarsson Reference Heiđmarsson1996) might also show diffuse growth patterns and merit further investigation from a developmental perspective.
The basal broadening and fissuring of epinecral accumulations can be compared in a general way to the formation of bark by cambial activity in dicotyledonous plants. In both cases, layers of dead tissue are augmented continuously by divisions of living tissue beneath, whose growth in surface area causes repeated ruptures in the mature and moribund tissues accumulating above them. In the fruticose lichen Usnea longissima, diffuse growth of thallus axes (Rolstad & Rolstad Reference Rolstad and Rolstad2008) involves the continued elongation of the medullary cord tissue which provides the thallus with mechanical support. Here too, diffuse elongation disrupts overlying tissue, in this case the living cortex and photobiont layers, from whose fragments the lateral branches or fibrils emerge (Sanders & de los Ríos Reference Sanders and de los Ríos2012). Tissue rupture may be further augmented by the regular cycles of hydration and dehydration to which the poikilohydric thallus is continually subjected. The other fruticose lichens known to have diffuse growth are long pendulous species of Ramalina (Sanders Reference Sanders1989; Sanders & Tokamov Reference Sanders and Tokamov2015); their expanding structural tissues are cortical, with no epinecral layers produced.
Diffuse growth of lichen tissue challenges our understanding of how fungal growth occurs at a cellular level. In Ramalina menziesii, R. usnea and U. longissima, supportive (mechanical) tissue consists of prosoplectenchyma oriented longitudinally along the expanding thallus axes; hyphal tip growth cannot account for diffuse elongation of such tissue. In micrographs of these lichen tissues, cell walls appear to be continuously disrupted by tissue expansion and replaced by new wall layers from within. These massive accumulations of wall material form a kind of intercellular matrix, but the concentric layers evident in transverse section can be attributed to the individual cells that produced them (Sanders & Ascaso Reference Sanders and Ascaso1995; Sanders & de los Ríos Reference Sanders and de los Ríos2012). Lichen cortices with isodiametric cells, by contrast, may accommodate diffuse growth by proliferating via parenchymatous divisions (Sanders & de los Ríos Reference Sanders and de los Ríos2017), as is evident here in Lasallia pustulata (Fig. 5).
Our results suggest that portions of lichen thalli might transition to parenchymatous division patterns while other thallus layers presumably remain plectenchymatous. Vuillemin (Reference Vuillemin1912) proposed the term “merenchyma” for tissue arising (secondarily?) via three-dimensional divisions in fungi, as opposed to synenchyma, arising by cell fusion. Significantly, he pointed out that de Bary’s application of the term “pseudo-tissue” to fungal structures of filamentous origin inconveniently restricts the concept of tissue itself to those originating from parenchymatous division. We would strongly argue that the term tissue should maintain its broad sense, free of any developmental implications. Without such a concept there is no way to refer to organized arrangements of cells without making unwarranted assumptions about development when no such data are available. Such implicit assumptions are probably the main reason why parenchymatous divisions in lichen “plectenchyma” have remained unacknowledged for so long.
Whether parenchymatous divisions in the cortex of L. pustulata occur uniformly or in a locally variable pattern remains to be determined. Certainly, the protruding morphology of pustules suggests that tissue expansion in those structures might be greater or faster than that of the interpustular regions, at least at some point in their development. A separate but equally interesting question is how the remaining thallus tissues, including both lax and prosoplectenchymatous medullary layers, are themselves accommodating diffuse growth at the cellular level. The potential diversity of thallus and tissue growth patterns among lichens is of considerable mycological interest as the few studies carried out so far have revealed patterns of cell behaviour strikingly different from those characteristic of typical fungal hyphae.
Funding was provided by a Spanish Ministry of Economy and Competitiveness grant (CTM2015-64728-C2-2-R to A. de los Ríos). We thank Cristina Patiño (Centro Nacional de Biotecnología, Universidad Autónoma, Madrid) for accommodating the TEM work and Javier Bueno Chamorro for preparing the ultrathin sections with great skill and patience. Alberto Jorge García (MNCN Microscopy Service) kindly provided technical assistance with the SEM. The manuscript benefited from critical review by G. Hestmark, C. Scheidegger and an anonymous referee.







