INTRODUCTION
Malaria remains the most important parasitic disease in the world, with a global strategy for control and elimination as outlined in a 2016–2025 roadmap (Abdulla et al. Reference Abdulla, Alonso, Binka, Graves, Greenwood, Leke, Malik, Marsh, Meek, Mendis, Schapira, Slutsker, Tanner, Valecha, White, Bosman, Cibulskis, D'Souza, Lynch, MacDonald, Mintcheva, Mnzava, Newman, Ringwald, Szilagyi and Wongsrichanalai2013). Local actions against malaria are typically tailored with its epidemiology; where resources permit, interventions are coordinated attacking vulnerable points in the parasite's life cycle, e.g. mosquito control, or involve strengthening the health system at all levels in disease management (Wernsdorfer, Reference Wernsdorfer2012). Concerning the latter, there has been significant scale-up in the use of first point-of-contact (POC) tests in screening either symptomatic or asymptomatic patients within the health periphery, either in remote clinics or in surrounding populations directly by community health workers (Bell et al. Reference Bell, Wongsrichanalai and Barnwell2006; Mouatcho and Goldring, Reference Mouatcho and Goldring2013).
In sub-Saharan Africa where the burden of malaria is greatest, diagnostic scale-up has been justified to ensure optimal, cost-effective deployment of new front-line antimalarials such as artemisinin-based combination therapies (ACTs) and better dovetailing within integrated management of childhood illness (IMCI) guidelines (WHO, 2013). To consolidate progress and harmonize general reporting of malaria cases for effective epidemiological surveillance, in 2012 the WHO announced a new initiative within the global strategy coined the ‘3Ts: test, treat and track’ (WHO, 2012). Here every suspected malaria case should be confirmed by microscopy or by rapid diagnostic test (RDT), ensuring access to quality-assured diagnostic tools, prior to any antimalarial treatment being administered. The intention is that microscopy and RDTs do not directly compete or replace each other but rather are used appropriately to strengthen the management of (non-)febrile illnesses (WHO, 2012).
Dating back to Laveran in 1880, the history of malaria microscopy is long and today light microscopy remains the ‘gold standard’ for it can visualize, quantify and speciate Plasmodium. UK diagnostic guidelines continue to recognize the importance of inspection of Giemsa or Field's stained thick blood films, even with readily available RDTs, fluorescent microscopy and DNA-based assays (Bailey et al. Reference Bailey, Williams, Bain, Parker-Williams and Chiodini2013). This is also affirmed internationally by WHO guidelines (WHO, 2010) alongside quality control and quality assurance measures to embed light microscopy firmly within the health system (WHO, 2009). In sub-Saharan Africa, however, there are well-known operational and logistical challenges at the health periphery, where access to reliable electricity and running water is not always guaranteed. More specifically, the quality and upkeep of laboratory equipment, viz. microscope(s), and diagnostic reagents is variable, as is also often the motivation, competence and workload of associated diagnostic staff (Hanscheid, Reference Hanscheid1999; Mundy et al. Reference Mundy, Ngwira, Kadewele, Bates, Squire and Gilks2000; Opoku-Okrah et al. Reference Opoku-Okrah, Rumble, Bedu-Addo and Bates2000; Bates et al. Reference Bates, Bekoe and Asamoa-Adu2004; Bell et al. Reference Bell, Wongsrichanalai and Barnwell2006; Wernsdorfer, Reference Wernsdorfer2009).
A key bottleneck at the POC setting is simply access to microscopes themselves which are often in short supply (Dunning and Stothard, Reference Dunning and Stothard2007). In Uganda, for example, few Level I health centres and associated community outposts are equipped with light microscopes, largely due to their high initial investment expenditure (approximately £750) and the need for on-site electricity (albeit today from grid, generator or solar sources). Since the binocular table-top microscope is rather bulky, cumbersome and fragile, light microscopes are not particularly amenable for use by mobile field-teams (Dunning and Stothard, Reference Dunning and Stothard2007). Some of these constraints were realized several decades ago by John McArthur which led him to develop the McArthur inverted compound microscope, first in metal (as shown in Fig. 1A), then later in the 1980s in moulded plastic, which was portable, handheld, battery operated and robust (Jones et al. Reference Jones, Nyalwidhe, Tetley and Barrett2007). Owing to unfavourable production costs and generally low commercial uptake, the McArthur microscope failed to penetrate and feature within the diagnostic repertoire in sub-Saharan Africa (McArthur, Reference McArthur1984; Jones et al. Reference Jones, Nyalwidhe, Tetley and Barrett2007). Early pioneers that used the McArthur microscope, however, developed novel staining protocols in miniature, clearly seeing a role for this instrument with a stand-alone diagnostic tool-kit, for use at the patient bedside, peripheral clinics and mobile field-teams (Collier and Longmore, Reference Collier and Longmore1983; Longmore, Reference Longmore1983, Reference Longmore1986).

Fig. 1. Familiarization and training of Ugandan Ministry of Health staff in the use of inverted microscopes. (A) An original metal McArthur microscope being assessed; (B) Basic training using the Newton Nm1 microscope on tripod; (C) Digital image of a Giemsa-stained thick blood film (with several malaria parasites indicated by arrows) as taken with an iPhone attached to the Newton Nm1 at ×1000 (oil) magnification.
Seeking to again realize McArthur's vision of having an affordable, functioning light microscope to be deployed in tropical health clinics in need of them, Newton Microscopes, UK, received a Translation Award from the Wellcome Trust, UK in 2008. This led to the design and subsidized commercial production of a compact monocular inverted microscope, the Newton Nm1 (Fig. 1B), which was launched in 2013 with a product demonstration at the headquarters of the World Health Organization (WHO) in Geneva. Specifically tailored for use in stand-alone malaria microscopy (Fig. 1C), the Newton Nm1 is battery operated (×3 AAs) with an LED white light source (low energy consumption), x–y slide indexing and magnifications at ×100, ×400 and ×1000 (oil). By way of benchmarking its performance against the conventional table-top microscope, a blinded trial (using Giemsa-stained slides) was conducted where four experienced Ugandan technicians each reported on diagnostic sensitivity (SS), specificity (SP), positive predictive value (PPV) and negative predictive value (NPV), recording inter-reader agreement.
MATERIALS AND METHODS
Study design and procedures
An archive of field-prepared and field-read Giemsa-stained thick blood films is maintained in Kampala as part of the ‘schistosomiasis in mothers and infants’ (SIMI) project, where enrolled mothers and children were tested and treated for malaria by a combination of RDT, light microscopy and qPCR (Betson et al. Reference Betson, Sousa-Figueiredo, Atuhaire, Arinaitwe, Adriko, Mwesigwa, Nabonge, Kabatereine, Sutherland and Stothard2014). The slides were prepared under typical field conditions and originate from endemic communities where the prevalence of Plasmodium infection in adults is ~25%, while in children it can be much higher and approach 75%. The mean parasite count across positive slides is ~1464 μL−1 of blood (median 520, maximum 3800) for adults and 9928 μL−1 of blood (median 1080, maximum 55 000) for children (Sousa-Figueiredo et al. Reference Sousa-Figueiredo, Oguttu, Adriko, Besigye, Nankasi, Arinaitwe, Namukuta, Betson, Kabatereine and Stothard2010).
From this SIMI slide archive, 50 thick blood films were initially selected and then verified by inspection at ×1000 (oil) by BN using a binocular compound microscope (Olympus CX22), to conform to 30 positive slides with a correspondence range of parasitaemia as found in the field, and 20 slides where no parasites could be seen despite exhaustive searching of 500 high-power fields of view. Thus with a mean prevalence of 60%, and sample size of 50, any estimate of diagnostic precision at 95% confidence intervals level would be ±15% (Harper and Reeves, Reference Harper and Reeves1999). A total of 50 slides was chosen as being a typical daily workload for a microscope technician to process with an accurate estimate of parasitaemia, rather than a qualitative examination. The Giemsa-stained slides were then labelled with a specific master code and then ‘blinded’ by JRS with randomization and relabelling against a new code A1–A50.
Four competent malaria microscopists presently working at Vector Control Division, Kampala who are tasked weekly with ensuring quality control of malaria blood films were selected and assigned reader 1–4 status. In July 2012, each reader was given a training afternoon using the Newton Nm1 microscope to ensure familiarization with its controls and mounting the slide in an inverted orientation. The relabelled slides A1–A50 were then given as a group to each of the four readers who then separately, and in turn, examined the slides at ×1000 (oil) with an Olympus CX22 microscope. They each recorded the number of malaria parasites seen as tabulated against 200 white blood cells (WBCs). At the end of the procedure the slides were then ‘re-blinded’ by JRS and re-labelled B1–B50. The slides were then given as a group to each slide reader who then viewed them with a Newton Nm1 microscope at ×1000 (oil). They each recorded the number of malaria parasites seen as tabulated against 200 WBCs, which later allows expressing of number of parasites viewed per μL of blood (WHO, 2010). At no time were the readers able to confer amongst themselves with the results of their slide reads, nor were they aware of the status of the slides given to them. At the end of the 2-week study period, the slide codes were broken and then tabulated for each reader against the master list (i.e. ‘gold standard’). Readers were asked to write a short report and list the advantages and disadvantages of using the Newton Nm1 against the Olympus CX22 during the trial and also how the Newton Nm1 might be used in a POC setting.
Statistical analysis
Data were collected from each of the four readers using pro-forma data sheets for slides A1–50 and B1–50, which were then entered using Microsoft Excel™ and reconciled against the master list or ‘gold standard’. The data thus collated were analysed using R statistical package® v 2.10.1. For each reported variable, 95% confidence intervals (95% CI) were estimated using the binomial exact method (Armitage and Berry, Reference Armitage and Berry1994), for prevalence values and the various point estimates of diagnostic measures: SS, SP, NPV and PPV (Harper and Reeves, Reference Harper and Reeves1999).
Correlations between slide reads performed on the Newton Nm1 and Olympus CX22 were calculated and plotted as bivariate scattergrams. Additionally, and to ascertain not only the correlation but also the agreement between Newton Nm1 and Olympus CX22 reads, a Bland-Altman plot was performed (Bland and Altman, Reference Bland and Altman1986). The Bland-Altman plot is a tool for comparing two different methods of measuring the same value, when the true value being measured is not known precisely. The purpose of a Bland-Altman plot is to try and determine whether the Newton Nm1 was ‘better’ than Olympus CX22 using a hypothesis testing approach. In these analyses, the parasitaemia inferred by both microscopes were log transformed to approach normality.
Ethics statement
As part of a test and treat strategy, thick blood films were prepared from all participants enrolled in the SIMI cohort, and a Giemsa-stained slide archive was maintained in Kampala to facilitate later cross-validation of malaria tests (Sousa-Figueiredo et al. Reference Sousa-Figueiredo, Oguttu, Adriko, Besigye, Nankasi, Arinaitwe, Namukuta, Betson, Kabatereine and Stothard2010; Betson et al. Reference Betson, Sousa-Figueiredo, Atuhaire, Arinaitwe, Adriko, Mwesigwa, Nabonge, Kabatereine, Sutherland and Stothard2014). Ethical approval was provided by the London School of Hygiene and Tropical Medicine (LSHTM 5538.09) and the Ugandan National Council of Science and Technology.
RESULTS
Slide trial
It typically took each reader a single day to complete their examination of 50 slides on the Newton Nm1 and Olympus CX22, respectively. Slightly longer time was needed per slide on the Newton Nm1 (8–9 min) than on the Olympus CX22 (6–7 min) due to the movement control of the x–y stage on the Newton Nm1 being at eye-level, rather than below the slide stage, thus handling of the WBCs and parasite tally counters was more cumbersome thereby slowing the recording process.
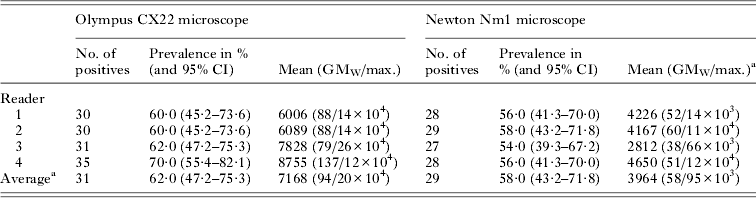
As might be expected there was some inter-reader disagreement, which is shown in Table 1, when using the Olympus CX22. Since the ‘true’ number of slides deemed positive was 30, readers 3 and 4 have over-estimated this value by 1 and 5, respectively. This discrepancy was statistically insignificant, however, as shown by the 95% CI values. Nonetheless reader 4 appears to over-estimate the prevalence and intensity of Plasmodium within this set of slides. When using the Newton Nm1, it is clear that all readers incurred a systematic error, downwardly biased in estimating the number of positive slides and thus prevalence, but again this is not significantly different from the true 60·0% with an average value of 58·0% (95% CI 43·2–71·8%). It is apparent that there is also a systematic downward bias in estimation of the intensity of infection being approximately half of that as measured by the Olympus CX22.
Table 1. Parasitaemia within the 50 slides according to each reader and each microscope

a Average was created to compile all results from all four readers; GMw stands for geometric mean of Williams.
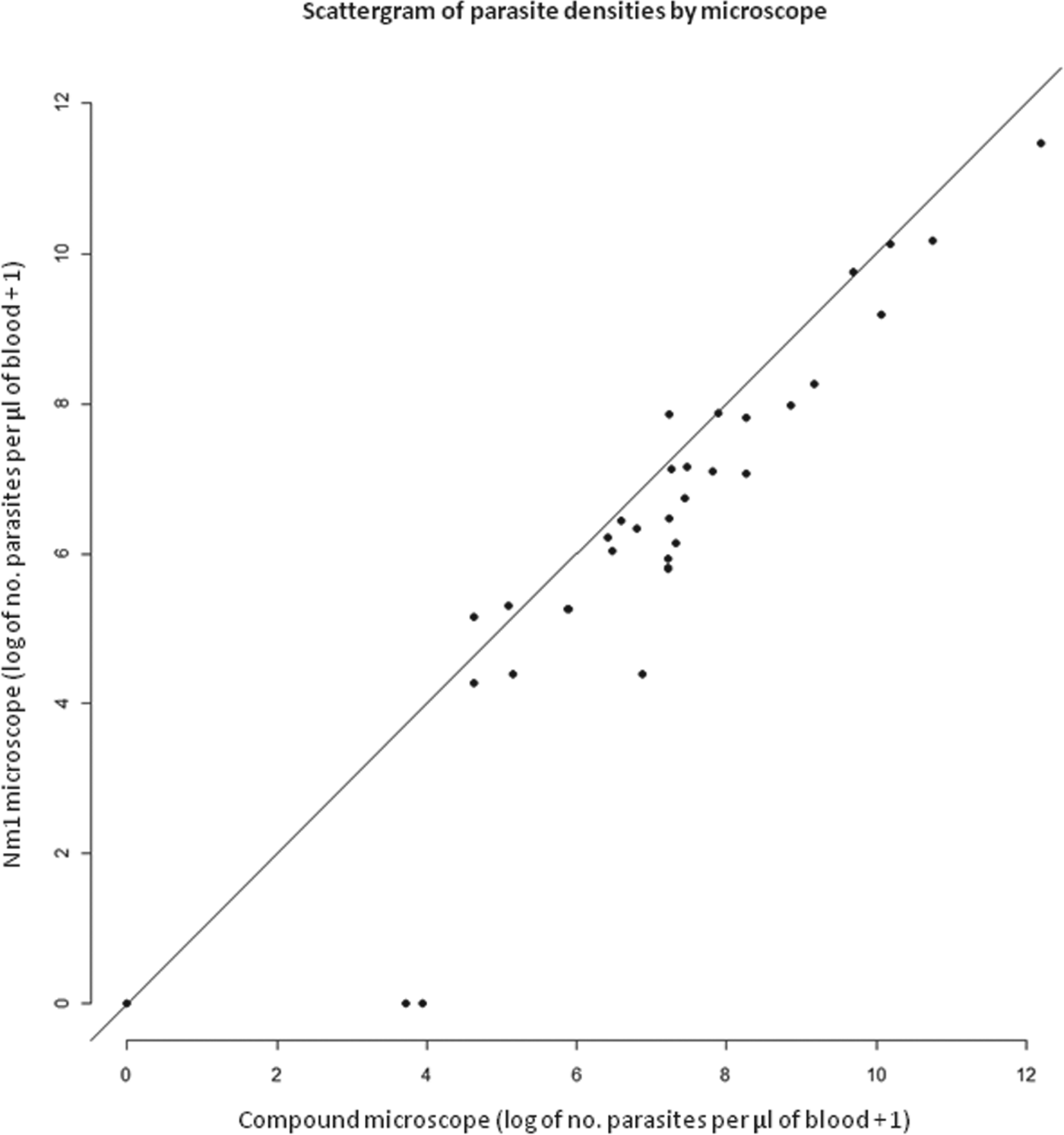
This downward bias in the estimate of parasitaemia can be clearly seen in Fig. 2, as the majority of data points are placed right of the mid-line, thus a higher parasitaemia is seen with the Olympus CX22. Moreover, this bias appears broadly linear in values >4 and <12, with too few points plotted in lower parasitaemia to infer any visual trends. In consideration of Fig. 3, the Bland–Altman plot shows that the level of disagreement can be considered non-significant in those points labelled in green. The three points in red, however, are not and can be considered a significant disagreement where the Newton Nm1 is incorrect, where the reported parasitaemia is much lower than by chance alone.

Fig. 2. Bivariate scattergram demonstrating a positive correlation between Olympus CX22 and Newton Nm1 microscopes (average taken across all four readers).

Fig. 3. Bland–Altman difference plot comparing Newton Nm1 and Olmpus CX22 by plotting difference between microscopes the y-axis, and the mean of the two observations on the x-axis. The confidence bounds are plotted as dotted red lines, and all points within the confidence bounds are coloured green, all points outside the confidence bounds in red (sigma = 1·5). The region of agreement is the area within the confidence bounds. The blue dotted line is the ‘difference = zero line’ (optimal agreement) and the red solid line indicates the ‘average difference’.
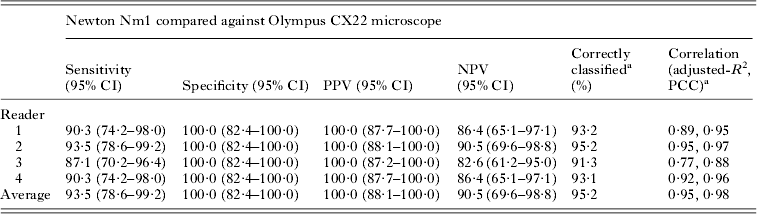
The diagnostic performance of the Newton Nm1 for each slide reader is shown more formally in Table 3. Both SS and NPV vary by reader with an average value of 93·5 and 90·5%, respectively. On the other hand, both SP and PPV are 100%, even though some of the slides have been incorrectly classified, i.e. a positive slide by Olympus CX22 is judged negative by Newton Nm1. The positive linear association between parasitaemia in Fig. 2 is corroborated by the correlations (adjusted R 2 and Pearson's correlation) reported in Table 2. Generally, any discordance was largely due to a systematic error underestimating general parasitaemia by ~45%; blood films with low parasite numbers (i.e. <100 μL−1 of blood) could be overlooked when counting against 200 WBCs.
Table 2. Diagnostic performance (%) of Newton Nm1 according to individual reader (and average) against ‘gold’ standard as determined by Olympus CX22

a Correctly classified is the average of the sum between PPV and NPV; PCC stands for Pearson's correlation coefficient.
Table 3. Summary of the advantages and disadvantages reported by the four readers in use of the Newton Nm1 microscope

Upon soliciting the opinions and discussions with the four readers, their views of the Newton NM1 are summarized in Table 3. All found that using the tripod could obtain a comfortable reading position although the monocular viewing with these numbers of slides was considered a strain in comparison to the binocular microscope. To preserve battery life the Newton Nm1 has an auto-shut off at 10 min; this feature was not considered desirable during the slide trial evaluation.
DISCUSSION
This blinded slide trial conducted over a 2-week period in Kampala showed that the Newton Nm1 performed favourably in a manner which was not significantly different from classic compound microscopy. While there is a standard framework to evaluate new diagnostics, such as RDTs, with reference diagnostic material and associated sample sizes (Banoo et al. Reference Banoo, Bell, Bossuyt, Herring, Mabey, Poole, Smith, Sriram, Wongsrichanalai, Linke, O'Brien, Perkins, Cunningham, Matsoso, Nathanson, Olliaro, Peeling and Ramsay2010), our study has certain features that are at odds with this generic design and is typical of malaria (Hanscheid, Reference Hanscheid1999; Bell et al. Reference Bell, Wongsrichanalai and Barnwell2006; Bailey et al. Reference Bailey, Williams, Bain, Parker-Williams and Chiodini2013). This is inherently due to a more complicated diagnostic algorithm for Plasmodium, so we attempted to benchmark the diagnostic performance of the Newton Nm1 against a conventional table-top compound light microscope in an equivalence study; conditions that approach those that might be expected within a POC setting in Uganda. For example, the Giemsa-stained slides were prepared directly in the field in a typical community where malaria is endemic, the daily workload for reading the blood smears was broadly similar to that expected of a government employee, and the local malaria microscopists who are employed in the Uganda Ministry of Health were of a qualified level to perform routine quality control for reading parasites.
Following Harper and Reeves (Reference Harper and Reeves1999) a general sample size of 50 slides with a mean prevalence of 60% would typically give a level of diagnostic precision of ±15%, which also matches a typical daily workload for a single technician in a moderate-to-high local level of malaria. Of course, in areas where malaria is much lower <10%, the time taken to examine slides would be much longer and likely use counts against 500 WBCs; this might alter the diagnostic scores presented in Tables 1 and 2, certainly increasing the level of eyestrain needed to more closely examine blood-films in greater detail. Thus with a novel microscope, and perhaps unlike an RDT, diagnostic scores are a subtle blend of both competence of the user, the counting criteria to be applied and the quality of equipment being used. The Newton Nm1 has also been evaluated for diagnosis of helminthiasis by examination of faecal smears and urine filtrates that require much lower magnifications at ×100 or ×400; Bogoch et al. (Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'Goran and Utzinger2014) documented good diagnostic scores for Schistosoma and Trichuris trichiura infections. They went on to conclude that portable microscopy could enable greater diagnostic coverage in several clinical and epidemiological settings.
By contrast, light microscopy for malaria diagnostic testing is more demanding owing to the higher magnification needed. It is encouraging that the average diagnostic performance of the Newton Nm1 across the four readers was: SS = 93·5% (95% CI 78·6–99·2%), SP = 100·0% (95% CI 82·4–100·0%), PPV = 100·0% (95% CI 88·1–100·0%) and NPV = 90·5% (95% CI 69·6–98·8%) which can also be considered favourable. It must be remembered that the overriding intention of using a Newton Nm1, rather than supplanting an existing compound microscope, is to help the expansion of microscopy into areas where there are no or too few microscopes present. This would fit well within the WHO's 3Ts strategy to bring diagnostic testing and surveillance to areas where there is a paucity of such options (WHO, 2012). This then raises an interesting set of related questions – what exactly is needed at the health periphery to reform itself if light microscopy is to be expanded and how can this gap be best fulfilled in the near and distant future?
With the previous uptake failure of the McArthur, despite having proven satisfactory technical performance (Collier and Longmore, Reference Collier and Longmore1983; Longmore, Reference Longmore1983, Reference Longmore1986), it is clear that there is more to solving these problems than technical innovation and good diagnostic performance can provide. The economics and cost-effective calculations of expanding use of the Newton Nm1 might therefore be more convincing, e.g. in terms of cost per treatment saved or cost per death averted by malaria microscopy, but this falls outside the present remit of this paper. Nonetheless, initial unit pricing of the Newton Nm1 is likely a major factor. The intention has always been to retail the unit at a much higher price in the developed world where its use is predominately recreational and educational, to later subsidize and even donate for use in the African health sector (Dunning and Stothard, Reference Dunning and Stothard2007).
An insight into the answer might be best explored in the following scenario: the Newton Nm1 is proven satisfactory for diagnosis of several tropical diseases and has desirable features that make its use in remote clinics pragmatic but it is presently donated gratis to Ministries of Health that request it. The question maybe thus be posed, what evidence or information is needed to allow a Ministry of Health make such a request or commit to a nominal purchase payment? Without a convincing answer, it is unlikely that diagnostic reform at the health periphery with light microscopy is possible without significant external pressure from either philanthropic agencies or international health stakeholders. The compound light microscope also provides a solid platform for the diagnosis of several other diseases yet coordinated advocacy for this multi-disease diagnostic testing is presently minimal. On the other hand, it is clear that the international commitment and momentum underlying the manufacture, supply and delivery of malaria RDTs to the health periphery seems to utilize a very different and more persuasive economic model. It remains to be seen how the long-term balance between malaria microscopy and use of RDTs will be struck in fulfilment of the WHO's 3Ts (WHO, 2012).
ACKNOWLEDGEMENTS
We thank Newton Microscopes, UK for the provision and donation of the Newton Nm1 to Vector Control Division, Kampala. We are grateful for the help of Prossy Kabubu, Annet Enzaru, Andrina Namakuta and Moses Adriko for their diligence during the microscope reads. Funding was gratefully received from the Wellcome Trust, UK and encouragement from Ted Bianco, Wendi Bailey and Malcolm Guy.
DECLARATION OF INTERESTS
JRS is a non-paid trustee of the Millennium Health Microscope Foundation (www.millennium-microscope.org), a registered charity that acts as the donation arm of Newton Microscopes, UK to provide microscopes at low cost to those that request them.