Published online by Cambridge University Press: 19 April 2005
Since the discovery of the Lyme disease spirochete in North America in 1982 and in Europe in 1983, a plethora of studies on this unique group of spirochetes that comprise Borrelia burgdorferi sensu lato has been accumulated. In an attempt to compare and contrast Lyme borreliosis in Europe and North America we have reviewed the biology of the aetiologic agents, as well as the clinical aspects, diagnosis and treatment of this disease on both continents. Moreover, we have detailed the ecology of the Ixodes ticks that transmit this infection and the reservoir hosts that maintain the spirochete cycle in nature. Finally, we have examined the transmission dynamics of the spirochete on both continents, as well as the available prevention strategies. Although it has been over two decades since the discovery of the Lyme disease spirochete, Lyme borreliosis is an expanding public health problem that has defied our attempts to control it. By comparing the accumulated experience of investigators in North America and Europe, where the disease is most frequently reported, we hope to advance the cause of developing novel approaches to combat Lyme borreliosis.
Arthropod-borne spirochetes have long caused human suffering and disease. Louse-borne relapsing fever (LBRF), caused by Borrelia recurrentis and transmitted by the human body louse (Pediculus humanus), was once widespread in the extensive areas where human body lice were found. Today, LBRF is reported mainly from northeastern and central Africa including the countries of Ethiopia, Somalia and Sudan, in discrete foci where human body lice remain prevalent (Porcella et al. 2000). Tick-borne relapsing fever (TBRF) was first described in Africa where the argasid tick, or soft tick Ornithodoros moubata, was found to transmit Borrelia duttoni (see historical review by Burgdorfer, 2001). Isolated endemic cycles of TBRF caused by individual species of relapsing fever spirochetes and their matching argasid vector species have been subsequently described in Asia, Europe, and the Americas (Felsenfeld, 1979). Recent reports detailing the epidemiology and biology of relapsing fever include studies in Tanzania, where Borrelia duttoni frequently causes human disease (Melkert & Stel, 1991; Fukunaga et al. 2001), as well as studies in North America where Borrelia hermsii is the primary aetiologic agent of relapsing fever (Dworkin et al. 2002). Although Borrelia were known to cause human disease in isolated pockets, scant attention was directed toward the study of these organisms in the latter half of the 20th century until an epidemic of arthritis was described in Lyme, Connecticut (Steere et al. 1977b). In sequential fashion, this condition was associated with a typical rash previously described in Europe as erythema chronicum migrans (later shortened to erythema migrans or EM) and the bite of the blacklegged tick, Ixodes scapularis (Steere, Broderick & Malawista, 1978; Steere & Malawista, 1979). A significant breakthrough occurred in 1982 when Burgdorfer et al. (1982) reported the discovery of a spirochete in Ixodes scapularis, and a few months later in Ixodes ricinus (Burgdorfer et al. 1983), that proved to be the aetiologic agent of Lyme disease (LD) or Lyme borreliosis (LB). This spirochete was subsequently named Borrelia burgdorferi (Johnson et al. 1984). It seems appropriate to review at this time (two decades following the discovery of B. burgdorferi), the large body of knowledge accumulated concerning the ecology, entomology, epidemiology, microbiology and prevention of LB in the 2 areas of the world where the most human cases have been described: Europe and North America.
By necessity, this review must focus solely on Lyme borreliosis in Europe and North America since the topic is extensive and the literature vast on this subject alone. The subject of Lyme borreliosis in Asia, where I. persulcatus is the primary vector, has recently been reviewed by Miyamoto & Masuzawa (2002), as well as Korenberg, Gorelova & Kovalevskii (2002). Moreover, the focus of this review is placed on the aspects of Lyme borreliosis that principally affect human health. An extensive review of Lyme borreliosis in livestock, companion animals and wildlife is beyond the scope of the current review. In the veterinary literature, the most comprehensive body of knowledge for disease in animals has been developed through the use of a canine model (Appel et al. 1993). Initial studies on developing an equine model of infection have also been reported (Chang et al. 2000).
In Europe, B. burgdorferi sensu lato (sl) has been reported from 26 countries from Italy to Iceland and from Portugal to Russia (Hubálek & Halouzka, 1997). The reported mean rates of B. burgdorferi in unfed I. ricinus ticks vary from 0 to 11 (mean 1·9) for larvae, from 2 to 43 (mean 10·8) for nymphs and from 3 to 58 (mean 17·4) for adults (Hubálek & Halouzka, 1998). Occasionally, higher infection rates have been reported, mainly using PCR, as for example in a study in Portugal where B. burgdorferi DNA was detected in 75 of I. ricinus ticks (de Michelis et al. 2000).
Five Borrelia genospecies have been found associated with I. ricinus: B. burgdorferi sensu stricto (ss) (Johnson et al. 1984), B. garinii (Baranton et al. 1992), B. afzelii (Canica et al. 1993), B. valaisiana (Wang et al. 1997) and B. lusitaniae (Le Fleche et al. 1997) (Fig. 1). In addition, two other genospecies have been obtained from patient tissues: B. bissettii, a species present in North America, has been isolated from patients in Slovenia (Picken et al. 1996; Strle et al. 1997), and a novel B. burgdorferi sl genospecies has been cultured from an erythema migrans biopsy of a patient who contracted the disease in the Netherlands (Wang, Van Dam & Dankert, 1999). The European vector ticks and natural hosts of these two genospecies have not been identified as yet. Recently, a single I. ricinus from Slovakia was found to be reactive with probes specific for B. bissettii (Hanincová et al. 2003b); the fact that this tick was also reactive with probes for two other genospecies of B. burgdorferi complicated the specific identification of the spirochetes present in this tick.

Fig. 1. Transmission cycle of B. burgdorferi in Europe. a) Cycle involving I. hexagonus and hedgehogs. b) Cycles involving I. ricinus, various genospecies of B. burgdorferi sl as well as birds and rodents.
Very early after the discovery of B. burgdorferi, phenotyping of Borrelia isolates showed that the protein profiles of B. burgdorferi sl isolates are heterogeneous (Barbour, Heiland & Howe, 1985). A few years later, outer surface protein A (OspA) and outer surface protein C (OspC) serotyping of isolates was established using sets of monoclonal antibodies (Wilske et al. 1993, 1995, 1996). Eight OspA serotypes of B. burgdorferi sl have been defined (Wilske et al. 1993, 1996). These serotypes correlated well with the delineated three most frequent genospecies: serotype 1 corresponds to B. burgdorferi ss, serotype 2 to B. afzelii and serotypes 3 to 8 correspond to B. garinii. The heterogeneity among B. garinii isolates was confirmed on a genetic basis (Will et al. 1995). Strikingly, B. garinii serotype 4 isolates have been cultivated from cerebrospinal fluid (CSF) from patients from Germany, the Netherlands, Denmark and Slovenia and have been more frequently cultivated from CSF than other serotypes (Wilske et al. 1993, 1996; Van Dam et al. 1997) but were only recently shown to be transmitted by I. ricinus ticks (Hu et al. 2001).
Although there is much that is not yet known about the distribution of the various genospecies in Europe, current knowledge suggests that B. garinii and B. afzelii are the most frequent and most widely distributed species whereas B. burgdorferi ss is present mainly in western areas of Europe and has been rarely described in Eastern parts of Europe.
Data from these last five years suggest that B. valaisiana and B. lusitaniae are more frequent than previously thought. B. valaisiana was first described in Switzerland (Peter & Bretz, 1992; Peter, Bretz & Bee, 1995; Humair et al. 1998), the Netherlands (Rijpkema et al. 1995), Great Britain (Cutler, Williams & Wright, 1989), Ireland (Kirstein et al. 1997) and Croatia (Rijpkema et al. 1996). Later reports on B. valaisiana extended to Germany (Liebisch, Sihns & Bautsch, 1998b; Kurtenbach et al. 2001), Spain (Escudero et al. 2000; Barral et al. 2002), Italy (Cinco et al. 1998), Slovakia (Gern et al. 1999; Kurtenbach et al. 2001), Portugal and Latvia (Kurtenbach et al. 2001) and Russia (Alekseev et al. 2001). Concerning B. lusitaniae, this species was first isolated from I. ricinus ticks in Portugal (Nuncio et al. 1993) and has subsequently been reported in the Czech Republic, Moldavia, Ukraine (Postic et al. 1997), Slovakia (Gern et al. 1999), Tunisia (Zhioua et al. 1999), Morocco (Gern et al. 2002), Poland (Mizak & Krol, 2000), Spain (Escudero et al. 2000; Barral et al. 2002) and Switzerland (Jouda et al. 2003 and unpublished data). Interestingly, in Portugal (de Michelis et al. 2000), in Tunisia (Younsi et al. 2001; Gern et al. 2002) and in Morocco (Gern et al. 2002) B. lusitaniae is very frequent and greatly exceeds the other genospecies in I. ricinus ticks whereas B. lusitaniae is only sporadically reported in ticks from other areas. De Michelis et al. (2000) even hypothesized that B. lusitaniae has a narrow ecological niche that involves host species restricted to the Mediterranean Basin and that are highly competent reservoirs for this genospecies. However, the recent report on the presence of this species in countries located outside the Mediterranean Basin, in Poland (Mizak & Krol, 2000), in France (Richter, Schlee & Matuschka, 2003) and in Switzerland (Jouda et al. 2003 and unpublished data) demonstrates that B. lusitaniae can be found outside of its well defined foci in southern Europe. Nevertheless, the fact that B. lusitaniae is by far the dominant species in I. ricinus ticks in Portugal (de Michelis et al. 2000), Tunisia (Zhioua et al. 1999; Younsi et al. 2001) and Morocco (Gern et al. 2002; Sarih et al. 2003) indicates that the genospecies diversity of B. burgdorferi sl decreases towards the southern margin of its European distribution. If B. lusitaniae appears clearly to dominate in southern Europe, data from northern Europe report a dominance of B. afzelii (Jenkins et al. 2001; Junttila et al. 1999; Schouls et al. 1999).
Since many Borrelia species may circulate in an endemic area, mixed infection in ticks can be observed. Such mixed infections are reported less frequently than single infections and are often detected by PCR methods. Detection of mixed infections in ticks using cultivation might be more difficult because one genospecies may overgrow another as recently observed for B. afzelii and B. garinii OspA serotype 4 (Hu et al. 2001). Mixed infections in ticks may result from the feeding of ticks on a host infected by multiple Borrelia species or from infected ticks feeding simultaneously on a host and exchanging the Borrelia species through co-feeding transmission (Gern & Rais, 1996; Randolph, Gern & Nuttall, 1996). Moreover, ticks may acquire various Borrelia species through their successive blood meals on various hosts and maintain the infection to the subsequent stage via trans-stadial transmission. Infections by multiple B. burgdorferi sl genospecies have been observed in ticks in many parts of Europe, including the Netherlands (Rijpkema et al. 1995), Croatia (Rijpkema et al. 1996), Switzerland (Leuba-Garcia et al. 1994; Jouda et al. 2003 and unpublished observations), France (Pichon et al. 1995), Austria (Stunzner et al. 1998), Belgium (Misonne, Van Impe & Hoet, 1998), Estonia, Kirghizia, Moldavia, Russia and Ukraine (Postic et al. 1997), Ireland (Kirstein et al. 1997), Italy (Cinco et al. 1998), Germany (Liebisch et al. 1998b; Hu et al. 2001; Kurtenbach et al. 2001), Latvia, United Kingdom and Slovakia (Kurtenbach et al. 2001), Norway (Jenkins et al. 2001), Finland (Junttila et al. 1999), Czech Republic (Basta et al. 1999) and Poland (Stanczak et al. 2000). Different combinations of mixed infection with two or three genospecies have been detected in I. ricinus. Borrelia garinii and B. valaisiana constitute the majority of mixed infections followed by mixed infections with B. garinii and B. afzelii.
The diversity of spirochetes was thought to be much greater in Europe than in North America until close examination of spirochete populations across the Atlantic was initiated during the 1990s. A landmark study involved molecular characterization of a total of 186 strains from throughout the United States (Mathiesen et al. 1997). These strains fell into 2 major groups: a fairly uniform B31 division and a more heterologous division from more moderate climates, resembling the well characterized 25015 strain. A smaller group included several isolates from Ixodes dentatus ticks in Missouri. Mathiesen et al. (1997) also noted that all the strains they examined that were human-derived fell within the B31 group.
Three formal genospecies have now been well defined in North America. The predominant one is the former B31 division, corresponding to the genospecies B. burgdorferi ss according to molecular criteria (Baranton et al. 1992; Postic et al. 1994). This is the only genospecies that has been demonstrated to infect humans in North America and it is ubiquitous in Ixodes scapularis ticks in the hyperendemic regions of the Northeastern United States (Seinost et al. 1999). The 25015 division (also called the DN127 group) was defined as a unique genospecies named B. bissettii by Postic et al. (1998). Several B. bissettii strains were described from I. pacificus ticks collected in California in this original description. In addition, a large number of strains isolated from an enzootic cycle involving woodrats and I. spinipalpis ticks in Colorado were found to be B. bissettii (Norris et al. 1999; Schneider et al. 2000) (Fig. 2). B. bissettii has also been isolated from a variety of rodents and ticks in the southern United States (Lin, Oliver & Gao, 2002), as well as from rodents in the metropolitan Chicago area (Picken & Picken, 2000). The third recognized genospecies in North America has been isolated from rabbits and from a tick associated with rabbits, I. dentatus. These spirochetes were formally described as a new genospecies (B. andersonii) by Marconi, Liveris & Schwartz (1995) (Fig. 2). Spirochetes that appear to fit the definition of B. burgdorferi sl but that are distinctly different from any well described genospecies have also been detected in California (Postic et al. 1998) and Florida (Lin et al. 2002). Although the known diversity of B. burgdorferi sl in North America is likely to expand, it must be stressed that human-derived culture confirmed isolates have all been B. burgdorferi ss. Additional attempts to make human-derived Borrelia isolates in culture medium from various geographic locations in North America are urgently needed.

Fig. 2. Transmission cycle of B. burgdorferi in North America. a) Cycles involving B. andersonii, I. dentatus and rabbits, as well as B. bissettii, I. spinipalpis and rodents. b) Cycle involving I. scapularis or I. pacificus, B. burgdorferi ss, as well as birds and rodents.
A group of spirochetes quite separate and distinct from B. burgdorferi sl have been reported to infect hard ticks in North America (see Telford & Goethert in this Supplement). These include B. lonestari from the lone star tick Amblyomma americanum (Barbour et al. 1996), ‘Novel Borrelia-MP2000’ from I. scapularis (Scoles et al. 2001), and B. theileri from Boophilus microplus and Rhipicephalus spp. (Rich et al. 2001). Attempts to culture all three of these Borrelia have failed. Based on molecular analysis they are more closely related to relapsing fever spirochetes than to B. burgdorferi sl. They have been informally called ‘hard-tick relapsing fever spirochetes’. Moreover, they are very closely related to a spirochete that has been successfully isolated from I. persulcatus in Japan: B. miyamotoi (Fukunaga et al. 1995), as well as a spirochete detected from I. ricinus in Sweden (Fraenkel, Garpmo & Berglund, 2002), and in Germany and France (Richter et al. 2003). Although B. lonestari DNA has been detected in an erythema migrans lesion and an associated A. americanum (James et al. 2001), the pathogenic potential of all of these hard tick relapsing fever spirochetes is still undetermined. A prime importance of these spirochetes is the confusion they cause when surveying tick populations for B. burgdorferi sl. Specific tools to differentiate the hard tick relapsing fever spirochetes from B. burgdorferi sl must be developed before generic molecular tools can be used to survey ticks for Borrelia in North America. In both North America and Europe, special care will be required to distinguish spirochetes infecting unfed larvae in estimates of transovarial transmission rates, due to the possibility these hard tick relapsing fever spirochetes are transovarially transmitted, as suggested by Rich et al. (2001).
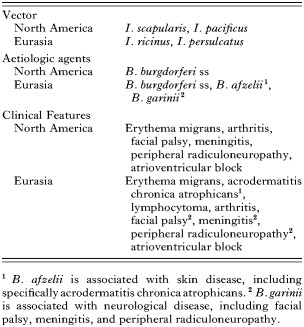
The first clinical case of what is now known as Lyme borreliosis was reported in Europe at the end of the 19th century (Buchwald, 1883). In the following years, Erythema migrans (EM), Lymphadenosis benigna cutis, Acrodermatitis chronica atrophicans (ACA) and meningopolyneuritis were described (Weber & Pfister, 1993).
In Europe, the clinical case definition described by the European Union Concerted Action on Risk Assessment (Stanek et al. 1996) can serve as a guideline for clinical diagnosis of the disease. Two different aspects can be distinguished in the development of the infection: the localized infection and the disseminated infection.
EM is the hallmark of Lyme borreliosis. The erythema begins as a red macule or papule, often with central clearing at the site of the tick bite approximately a few days to one month after the tick bite. At that stage the infection is localized.
The disseminated form of the disease appears a few days or weeks after the tick bite. Manifestations of early neurological involvement including meningitis, unilateral facial palsy, other cranial neuritis and radiculitis may occur. Chronic involvement of the central nervous system includes encephalomyelitis and chronic meningitis. But these manifestations are very rare. Lyme arthritis includes brief attacks of joint swelling with occasional persistence of synovitis. Cardiac involvement appears as an acute onset of disturbances in the atrio-ventricular conduction. Endomyocarditis, pericarditis and rhythm disturbances have also been reported (Stanek et al. 1996).
The EM is similar in the whole geographic distribution of Lyme borreliosis whereas there appear to be differences in the manifestations of the disease as well as the frequency and severity of the disease. This may be related to the geographical distribution of the various pathogenic genospecies and their prevalences. In Europe, where more pathogenic genospecies have been described than in North America, the disease expresses itself under a wider range of manifestations (Stanek et al. 1996, 2002). It is believed that this may reflect regional differences in the distribution and frequency of the different Borrelia genospecies. Currently, only three Borrelia species, B. burgdorferi ss, B. garinii and B. afzelii, have been isolated from patients suffering from Lyme borreliosis. B. valaisiana's status as a pathogen has yet to be confirmed (Wang et al. 1999) as well as B. lusitaniae's status although this species has recently been reported to be pathogenic for laboratory mice (Zeidner et al. 2001).
Various studies have suggested an association between clinical manifestations and Borrelia species in Europe. The three pathogenic species, B. burgdorferi ss, B. garinii and B. afzelii, have a different organotropism and preferentially cause different clinical manifestations (Assous et al. 1993; Van Dam et al. 1993; Dressler, Ackermann & Steere, 1994; Balmelli & Piffaretti, 1995; Busch et al. 1996a,b; Eiffert et al. 1998; Picken et al. 1998; Jaulhac et al. 2000) (Table 1). Borrelia afzelii is predominant among human skin isolates and B. garinii among CSF isolates (Wilske et al. 1993, 1996) whereas a considerable heterogeneity has been described in Borrelia species detected in synovial fluid (Vasiliu et al. 1998). Currently, the situation appears to be more complicated than that. Recent studies reported that a few groups of Borrelia within the 3 pathogenic species are responsible for the disseminated form of the disease (Seinost et al. 1999; Baranton et al. 2001). In fact, the authors of these studies showed that 58 OspC groups could be defined within the 3 pathogenic species based on OspC sequence analysis of various Borrelia isolates obtained from ticks and patients, and that all isolates from patients with disseminated forms of LB were contained in only 10 groups. All tick and EM isolates were included in the other groups.
This suggests that the OspC gene is involved in invasiveness of strains leading to either localized infections due to non-invasive clones, or to the disseminated form of the disease due to a few clones that are invasive. The geographic distribution and frequency of these various OspC groups are unknown.
Stanek et al. (1996), in their paper describing clinical manifestations of LB in Europe, also reported on laboratory evidence that is essential or which supports the clinical findings. Diagnosis of Lyme borreliosis by serological testing is difficult and is even complicated in Europe by the presence of at least 3 different pathogenic species (Dressler et al. 1994; Hauser et al. 1998). A European multicentre study on immunoblotting showed that it would be very difficult to have a standardized immunoblotting method because it would require agreement on the strains used as antigens (Robertson et al. 2000). Moreover, this approach appears as unlikely due to the local distribution of species and strains of B. burgdorferi sl and the heterogeneity within the strains. A new test developed in the USA (Liang et al. 1999) (see next section) based on the vslE protein of B. burgdorferi may help in the future to improve serological testing in Europe. However, it is clear from all accumulated studies on Lyme borreliosis serology that serological testing should be used as a support of clinical diagnosis rather than a confirmation.
Treatment practices in Europe and North America are fairly similar. Treatment practices are described in detail in the section that follows.
Lyme disease in North America was first described as a distinct clinical entity in Lyme, Connecticut among a population of children believed to have juvenile rheumatoid arthritis (Steere et al. 1977b). In rapid succession, the skin (Steere et al. 1977a), neurological (Reik et al. 1979) and cardiac (Steere et al. 1980) manifestations of Lyme disease were brilliantly elucidated. The clinical manifestations of Lyme disease in North America can be broken down into an acute and chronic phase. The earliest stage of the disease, often called localized early infection, usually starts as a macule or papule at the site of a tick bite, 3 to 32 days following exposure. This spreads into a large annular lesion, most often with a bright red border and partial central clearing (Steere, 1994). This so-called bull's eye or target lesion was originally called erythema chronicum migrans (ECM), as per the older literature in Europe. This was shortened to erythema migrans (EM), in part because these lesions proved not to be chronic in North America as they can be in European patients. A large-scale multi-centre study that examined 10936 participants described 118 patients with microbiologically confirmed erythema migrans; curiously, most of these patients had fairly homogeneous EM lesions, with only 9 demonstrating classical bull's eye lesions with central clearing (Smith et al. 2002). The reason for the lack of bull's eye rashes was thought to be the short duration between onset of symptoms and presentation at the clinics (mean=3 days) for diagnosis and treatment when compared to previous studies (Nadelman & Wormser, 2002).
The next stage of the disease has been called early disseminated infection. This stage follows the original EM lesion and may include systemic symptoms e.g. severe headache, mild neck stiffness, fever, chills, migratory musculoskeletal pain, arthralgias, and profound malaise and fatigue (Steere, 1994). Another key characteristic of this stage is the presence of secondary EM lesions at sites remote from the original lesion. These lesions may reflect the haematogenous spread of spirochetes from the original tick bite site. Interestingly, in a study of patients in a clinic in Westchester County, New York, patient-derived isolates of B. burgdorferi ss fell into 3 distinct genetic subtypes based on restriction fragment length polymorphism (RFLP type 1, 2, 3); RFLP Type 1 strains were found in the blood of patients with disseminated disease, as compared to skin lesion biopsies from patients with localized disease (predominantly Type 2 and 3) (Wormser et al. 1999). In an elegant series of studies, Seinost et al. (1999) and Qiu et al. (2002) demonstrated that at least 21 major clonal groups of B. burgdorferi ss (as defined by OspA and OspC haplotypes) have been isolated from I. scapularis ticks along the eastern seaboard; 15 of these groups have been isolated from primary EM lesions. However, only 4 of the clonal groups (A, B, I, K) have been isolated from secondary sites of infection (e.g. blood and cerebral spinal fluid-CSF). Groups A, B, and K are 3 of the most common haplotypes and the type strain of B. burgdorferi ss (B31) is a group A strain. The proportion of Lyme disease patients that present with an EM lesion has been estimated to be between 80 (Steere, 2001) and 90 (Nadelman & Wormser, 2002). Early symptoms seem to disappear within several weeks.
Prior to the association of Lyme disease with a specific bacterial aetiology, many cases of Lyme disease in North America were not treated with antibiotics. This permitted the natural course of the disease to evolve in patients and for observation by physicians. Several months after the acute disease, approximately 60 of patients begin to have intermittent attacks of joint swelling and pain, particularly in the large joints; the knee is the joint most commonly affected site, but not exclusively so (Steere, 1989, 1994). This pattern can best be described as an oligoarticular arthritis. Although the arthritis can move from joint to joint, in a small number of patients the lesions in one or both knees may become chronic with actual erosion of cartilage and bone. These types of severe chronic arthritic lesions are not seen very often in North America today due to prompt recognition and treatment of the disease in its early stages.
Several weeks after the onset of illness, about 5 of untreated patients in North America develop cardiac involvement (Steere, 2001). The most common cardiac abnormality is an atrioventricular block of fluctuating degrees. In some cases, more diffuse cardiac involvement occurs, including acute myopericarditis, left ventricular dysfunction, cardiomegaly or pancarditis (Steere, 1994). Symptoms may include lightheadedness, palpitations, and chest pains.
The most complex manifestations of Lyme disease involve neurological disease. This occurs in about 15 of untreated patients in North America (Steere, 1989, 1994). Neurological abnormalities include meningitis, subtle encephalitic signs, cranial neuritis, bilateral facial palsy, motor or sensory radiculoneuropathy, mononeuritis multiplex, chorea or myelitis. The usual pattern is fluctuating symptoms of meningitis accompanied by facial palsy and peripheral radiculoneuropathy. CSF may show a lymphocytic pleocytosis at this point of about 100 cells per ml. Although these symptoms may resolve even in untreated patients and respond well to treatment, a small minority of patients in North America may develop a late neurological syndrome called ‘Lyme encephalopathy’ manifested by subtle cognitive disturbances (Halperin et al. 1989; Logigian, Kaplan & Steere, 1990). The frequency and severity of these cognitive disturbances appear to be the source of much controversy in the United States. Severe neurological consequences of Lyme disease in the United States are rare in the present day, but, at least one case of permanent bilateral blindness in a child due to increased cranial pressure has been reported (Rothermel, Hedges & Steere, 2001). The comparative clinical aspects of Lyme disease in United States and Europe (Table 1) have been succinctly reviewed by Steere (2001).
Like many bacterial diseases, the ideal basis for diagnosis of Lyme disease is isolation of the aetiologic agent, namely Borrelia burgdorferi. The standard culture media is called Barbour-Stoenner-Kelly media or BSK. Tissue samples are generally surface disinfected, minced, placed in BSK and incubated at 33–34 °C. Successful culture of frank EM lesions has been achieved on a routine basis in research settings in highly endemic regions of the United States through biopsy and culture of the skin at the affected site (Berger et al. 1992; Schwartz et al. 1992). Recently, quantitative PCR techniques have proved quite successful in the detection of spirochetes in EM lesions, with detection of Borrelia DNA in up to 80 of the lesions tested (Nowakowski et al. 2001; Liveris et al. 2002). Large volume blood cultures yielded positives in 44 of early Lyme disease patients (Wormser et al. 2001); the yield of spirochetes or DNA in late stage Lyme disease from blood, CSF or synovial fluid is, however, much less successful (Nocton et al. 1994; Steere, 2001). Unfortunately, in the United States Lyme disease has become a potential diagnosis in an extremely large number of patients lacking a frank EM and presenting with a complex of symptoms including fatigue and vague feelings of ill health. Although diagnosis of Lyme disease is fundamentally based on a clinical evaluation of the patient, in practice diagnosis is often based upon serology. In fact, a market analysis predicted that approximately 2·8 million serological tests for Lyme disease were performed in the United States during 1995 (Johnson et al. 1996). The majority of those tested do not have Lyme disease. Thus, serological diagnosis of Lyme disease in the United States has become an area of current controversy. In general, a 2-tiered testing regime that involves an ELISA screening test and a confirmatory western blot test can produce reliable results (Dressler et al. 1993; CDC, 1995; Johnson et al. 1996). Due to the large number of serological samples that are tested each year, however, the specificity of this testing regime is not robust enough to completely eliminate the problem of false positive results. This is a particularly acute problem with IgM blots conducted after the first month of illness. Thus, only IgG results should be used to support the diagnosis of Lyme disease after the first month of infection (Steere, 2001). A new test, based on a recombinant protein of a portion (C6) of the vlsE protein of B. burgdorferi shows promise for improving the sensitivity and specificity of the serological diagnosis of Lyme disease in the future (Liang et al. 1999; Philipp et al. 2001).
Practice guidelines for the treatment of Lyme disease were issued by the Infectious Diseases Society of America (Wormser et al. 2000). Under these definitive guidelines, adults with early Lyme disease should be treated with doxycycline (100 mg twice daily) or amoxicillin (500 mg 3 times daily) for 14–21 days. Cefuroxime axetil (500 mg orally twice daily) should be reserved for patients who cannot take doxycycline or amoxicillin. Children should be treated with amoxicillin (50 mg/kg/d, maximum of 500 mg/dose) divided into 3 doses per day, or doxycycline for those [ges ]8 years of age at a dose of 1–2 mg/kg twice daily (maximum of 100 mg/dose). Cefuroxime axetil can be used as a suitable alternative in children. The use of ceftriaxone (2 g once daily i.v. for 14–28 days) should be reserved for those with early Lyme disease who are also suffering from meningitis or radiculopathy; in addition, ceftriaxone is useful in early Lyme disease patients with third-degree atrioventricular heart block. Lyme arthritis can usually be treated with doxycycline or amoxicillin at the same doses mentioned above but for a duration of 28 days. In patients with late neurological disease affecting the CNS or peripheral nervous system, treatment with ceftriaxone (2 g once a day i.v. for 2–4 weeks) is recommended. Clinical trials that have attempted to enrol patients with ‘chronic Lyme disease’ or ‘post-Lyme disease syndrome’ have generally been terminated early due to a lack of enrolees with objective evidence of Lyme disease. In these limited trials, however, no benefit of continued antibiotic treatment was found (Klempner et al. 2001).
The practice of prophylactic treatment of tick bite in Lyme disease endemic areas has generated much public discussion. A cost–benefit analysis concluded that prophylactic treatment should only be considered in areas with an extremely high risk of Lyme disease transmission (Magid et al. 1992). In a recent clinical trial, Nadelman et al. (2001) found that prophylactic treatment of bites by partially fed nymphal I. scapularis with a single dose of doxycycline in Westchester County, NY was 87 effective in preventing Lyme disease. Experimental work with rodents has demonstrated that transmission of B. burgdorferi ss becomes efficient after nymphal I. scapularis are attached for >48 hours (des Vignes et al. 2001). Prophylactic treatment of only those patients exposed to infected I. scapularis that have fed for more than 2 days would be ideal. It remains to be seen whether this ideal plan can be put into widespread clinical practice, since it would involve rapid testing of ticks for infection and estimation of the duration of attachment based on a scutal index of tick engorgement.
In Europe, three tick species have been described as being vectors of B. burgdorferi sl: I. ricinus, I. hexagonus and I. uriae. These three species have very different ecologies, but they all are three-host ticks with each parasitic stage (larva, nymph and adult female) feeding on different hosts. Although adult male Ixodes sometimes ingest fluids from hosts, they do not ingest significant amounts of blood and their role as vectors is probably insignificant but has not been thoroughly evaluated. These three species, however, have a rather different host range and different biology. Vector biology is an important factor since it dictates much of the epidemiology of the diseases. According to their habitats, the three recognized European vectors of B. burgdorferi sl can be divided into non-nidiculous ticks, I. ricinus, and nidiculous ticks, I. uriae and I. hexagonus. Non-nidiculous ticks occupy open habitats whereas nidiculous ticks live in caves, burrows or nests of their hosts. The differences concerning behaviour and physiology between nidiculous and non-nidiculous ticks are enormous especially in their host-finding behaviours. The non-nidiculous tick I. ricinus awaits a host on the vegetation. The nidiculous tick species, like I. uriae and I. hexagonus, have closer contact with their host by living in their nests or in their very close environment. This implies that contacts between non-nidiculous ticks and humans are more frequent than between nidiculous ticks and humans and shows that vector biology is an important factor dictating much of the epidemiology of Lyme disease.
The common European tick species, I. ricinus, is the main vector of B. burgdorferi sl. This tick species has a very wide geographical distribution throughout Europe. It has been described within the latitudes 65° and 39° and from Portugal to Russia (Gern & Humair, 2002) and also in North Africa (Tunisia, Algeria and Morocco) (Gern et al. 2002). This wide geographical distribution of I. ricinus implies that this tick survives under various environmental conditions. I. ricinus prefers deciduous woodlands and mixed forests. High humidity is a prerequisite for tick survival since they are susceptible to desiccation when questing for hosts on vegetation. High humidity will be found at the base of vegetation in the leaf litter where ticks periodically return to uptake atmospheric water. Therefore I. ricinus will survive only where relative humidity in its micro-environment is higher than 80 (Kahl & Knülle, 1988; Randolph et al. 2000). If saturation deficit (measurement of the drying power of the air) is above c. 4 mmHg (calculated according to Randolph & Storey, 1999), I. ricinus shows positive geotropism (McLeod, 1935). In nature, abrupt declines in questing tick density have been reported to coincide with abrupt increases in saturation deficit (Perret et al. 2000; Randolph et al. 2002). Temperature, which is known to have an effect on tick questing activity and on tick development rates, varies throughout the geographical distribution of I. ricinus. Specific dynamics of seasonal activity have been demonstrated under different climatic conditions (Steele & Randolph, 1985; Tälleklint & Jaenson, 1996; Korenberg, 2000; Perret et al. 2000; Randolph et al. 2002). The seasonal activity pattern is either unimodal or bimodal. Data and model predictions from Randolph et al. (2002) suggest a simple life cycle for I. ricinus with a single cohort of each stage starting in the autumn and not two separate cohorts as previously thought (Lees & Milne, 1951; Donnelly, 1976; Gray, 1982, 1985, 1991; Walker, 2001). The height at which I. ricinus ticks quest on vegetation depends on each stage and on the vegetation structure, and it influences host encounters (Mejlon & Jaenson, 1997). I. ricinus feeds on an extraordinarily broad array of hosts, from small, medium and large-sized mammals to birds and reptiles (Anderson, 1991) and is the tick species which most frequently bites humans in Europe.
Ixodes hexagonus is an endophilous nidicole tick and is one of the most widespread tick species in Europe (Morel, 1965). It has been reported from Northern Europe (Jaenson et al. 1994) to the North of Africa (Bailly-Choumara, Morel & Rageau, 1974). This tick species lives in the nest and burrow of its hosts, an environment which provides a suitable micro-climate for the tick survival and therefore the geographical distribution of I. hexagonus is less dependent on meso- and micro-climatic conditions than that of I. ricinus. Nevertheless, temperature also influences duration of I. hexagonus development as reported by Toutoungi, Aeschlimann & Gern (1993). This suggests that duration of tick development may be longer during the winter months than in spring or summer. In view of its habitats, I. hexagonus rarely comes in contact with humans. Nevertheless, humans can be bitten occasionally, particularly when they handle nests of hedgehogs when gardening – a frequent host of I. hexagonus – which have surface nests and are frequent in gardens in Europe. I. hexagonus parasitizes primarily Mustelidae (e.g. Meles meles – European badger, Martes fouina – beach marten, Mustela putorius – European polecat, Mustela ermina – Ermine) and hedgehogs (Erinaceus europaeus). In Switzerland, it was collected from 15 animal species, especially from foxes and Mustelidae, but also from domestic animals like dogs and cats (Toutoungi et al. 1991). I. hexagonus may also occasionally infest birds (Pica pica, Falco tinnunculus) and deer (Capreolus capreolus) (Hubbard, Baker & Cann, 1998; Toutoungi et al. 1991). An additional tick species that is widely distributed throughout Europe and may serve a secondary role as an enzootic vector of B. burgdorferi is I. trianguliceps, a tick that feeds predominantly on rodents and has been found to be infected with B. burgdorferi (Gorelova et al. 1996).
The third known vector of B. burgdorferi sl in Europe, I. uriae, is a tick species parasitizing seabirds which has a three-stage life cycle usually corresponding to one stage each year. Each stage attaches to the host for a single, long blood meal and then returns to the host nesting substrate to overwinter (Eveleigh & Threlfall, 1974). It has been reported that the prevalence and abundance of I. uriae are autocorrelated in both space and time at the scale of the host-breeding cliff (Danchin, Boulinier & Massot, 1998; McCoy et al. 1999). The seabird tick, I. uriae, has a distribution area covering coasts situated at high latitudes both in northern and southern hemispheres. In Europe, this includes coast in Ireland, Iceland, Norway, Sweden, Denmark, UK and France (Olsen et al. 1995a; Hillyard, 1996). I. uriae infests principally seabirds, but it has occasionally been reported on mammals such as seals, river otters and humans (Olsen, 1995).
The two principal vectors of Lyme disease in North America are the blacklegged tick (Ixodes scapularis) in the eastern half of the continent and the western blacklegged tick (Ixodes pacificus) in the western half of the continent (Fig. 2). The vast majority of Lyme disease infections in North America are acquired through the bites of I. scapularis. Both biotic and abiotic factors control the distribution of these fascinating and nefarious ticks. One key component that controls I. scapularis distribution is humidity. These ticks are very susceptible to desiccation (Stafford, 1994). In fact, on a local level in Westchester County, New York researchers found that the distribution of I. scapularis was positively associated with a remotely sensed greenness–wetness index (Dister et al. 1997). In the north-central United States, the presence of I. scapularis was positively associated with deciduous, dry to mesic forests and alfisol-type soils of sandy or loam-sand textures overlying sedimentary rock; tick absence was associated with grasslands, wet to wet/mesic forests, conifer forests, acidic soils of low fertility and a clay soil texture, and Precambrian bedrock (Guerra et al. 2002). In other words, these ticks were found in moist soils, but not in poorly drained soils where standing water occurred.
There seems little doubt that I. scapularis ticks are forest inhabitants. An analysis of I. scapularis populations in suburban landscapes in Westchester County, New York demonstrated that these tick populations were highest in the woods, intermediate in ecotonal vegetation and sparse in ornamental planting and lawns (Maupin et al. 1991). In Long Point (Ontario, Canada), I. scapularis were most abundant in maple forests, followed by oak savannah; these ticks were rare in white pine forest and cottonwood dunes (Lindsay et al. 1999a,b). In general, these ticks are found in hardwood forests where abundant leaf litter provides ample cover from desiccation and protective cover during snowfall. The importance of leaf litter was demonstrated in an experiment where leaf litter was actually removed from a forested plot; I. scapularis populations decreased by 72–100 as a result of litter removal (Schulze, Jordan & Hung, 1995). Although populations of I. scapularis are mainly associated with mature oak–maple forests in the northeastern United States, these ticks have the flexibility to inhabit diverse habitats. In coastal regions and islands, these ticks can be found in extremely dense shrub-like habitat that contains bayberry, rose and scrub oak as predominant vegetation (Piesman & Spielman, 1979). Some of these coastal areas have been affected by intense deer browsing that promotes non-native plant species like Japanese barberry and honeysuckle. The cycle of intense deer browse and selected understory may add to the suitability of the habitat for tick populations (P. Rand, personal communication). Another interesting observation has been the influence of masting in oak trees (wherein a massive crop of acorns are produced every 2–5 years) on the density of ticks within forests and the annual variation in populations of I. scapularis (Jones et al. 1998). Although populations of I. scapularis are mainly associated with hardwood forests they can also be found in pine forests that are surrounded by hardwoods (Schulze, Jordan & Hung, 1998). The minimal amount of ground cover and hardwood leaf litter that will allow I. scapularis to thrive in a conifer dominated forest has not been established.
In eastern North America, I. scapularis essentially takes 2 years to go through its life cycle (Fig. 3). Setting year 0 as the first year of active questing, larvae generally feed in August–September (Piesman & Spielman, 1979). The majority of larvae moult to nymphs that overwinter as flat nymphs. Nymphs feed the following year (year 1) in May–July. These nymphs then moult to adults that begin questing that same year in the fall. Adults quest from October of year 1 until April of the next year (year 2). In areas with cold winter temperatures, adult feeding ceases in the middle of winter, but in southern areas adult questing may actually peak in February (Goddard, 1992). In year 2, these replete females lay eggs in May and June; larvae hatch by August and begin questing thus completing the 2-year life cycle. This standard life cycle has been elegantly described by Yuval & Spielman (1990). Interestingly, in some colder climates this idealized life cycle may not take place. Lindsay et al. (1995) discovered that locations in northern Ontario that lacked sufficient degree-days >11 °C, larvae did not emerge from eggs laid in May or June during that same year. Although some eggs could survive the winter and hatch the next year, the overall survivability of these eggs was quite low in this extreme environment. The key factor was not just latitude, since one area (Kenora) that was north and west of other areas had sufficient degree days, while other areas to the south and east did not have sufficient degree-days during most years (Kapsuskasing, Geraldton, Thunder Bay). Sufficient thermal warming during the summer months may be required for I. scapularis to efficiently go through its life cycle. This key factor may be much more important than the mean or minimum winter temperatures, when the ticks may be insulated by snow cover and well protected. Thus, there may be thermal limits on the areas where I. scapularis can efficiently go through its life cycle in North America.
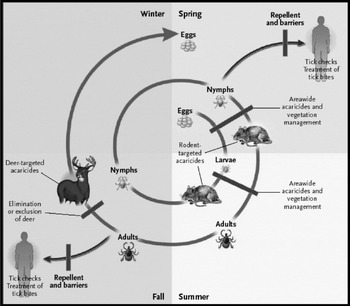
Fig. 3. Life cycle of B. burgdorferi in the United States, highlighting various points for intervention strategies to prevent Lyme disease. (Reprinted with permission from the Massachusetts Medical Society; Hayes & Piesman, 2003.)
The distribution of I. scapularis in the United States was surveyed in 1991, 1994, and 1997 via questionnaires delivered to entomologists and public health officials. A county-by-county map was produced showing records of I. scapularis in 952 of the 3141 counties within the United States (Dennis et al. 1998). This tick is distributed along the eastern seaboard from Florida to Maine and as far west as central Texas. Interestingly, the distribution is not continuous, with a ‘hole’ in the distribution seen in central states (e.g. West Virginia, Ohio, Kentucky, Tennessee). The possibility that I. scapularis was once extant over the entire eastern United States, then reduced to a few refugia due to human encroachment and host reduction and now slowly but surely regaining its former range is intriguing; this hypothesis will be difficult to test.
There has been debate concerning the species status of I. scapularis. Spielman et al. (1979) proposed that this species be divided into a southern species (I. scapularis) and northern species (I. dammini). In contrast, Oliver et al. (1993b) challenged the validity of I. dammini as a species separate and distinct from I. scapularis. Molecular studies on the mitochondrial genes 16S and 12S suggested that 2 distinct clades of I. scapularis could be defined: one clade, called the southern clade, stretched from Florida to North Carolina, while the northern or All-American clade stretched from Massachusetts to Mississippi (Rich et al. 1995; Norris et al. 1996). The southern clade was considered the basal group of I. scapularis by Norris et al. (1996). A recent phylogenetic analysis of I. scapularis from South Carolina to Massachusetts confirmed that 2 mitochondrial clades of I. scapularis exist (Qiu et al. 2002). These researchers found that the northern clade (Clade A) had a low within-population sequence divergence (0·2), while the southern clade (Clade B) had a much higher diversity (1·5). Qiu et al. 2002 suggested that the northern and southern clades have separate and different evolutionary histories, perhaps influenced by separation during the last glacial maximum 18000 years ago. The theory that the northern clade of I. scapularis results from several refugia established during the last ice age and that this population is now expanding across the eastern United States, with these ticks being the principal ticks responsible for transmitting Lyme disease in North America is worthy of further objective study. But the complexity and diversity of host (see Reservoir Hosts section below) populations present in different geographical areas may play a predominant role in determining local risk of Lyme disease transmission, in addition to the genetic makeup of local I. scapularis populations.
The western blacklegged tick I. pacificus is found along the Pacific Coast from British Columbia to Baja California Norte, Mexico (Kain, Sperling & Lane, 1997). Although the populations of I. pacificus are principally coastal, isolated inland populations have been described in such arid overall climates as Mohave County, Arizona (Olson et al. 1992) and the southwestern corner of Utah (Kain et al. 1997). There is no evidence of genetic isolation among these diverse populations of I. pacificus (Kain et al. 1999). Habitats where adult I. pacificus can be collected are extremely varied, from redwood or Douglas fir forest, to more open habitats such as chaparral and open grasslands. When collecting adult I. pacificus along trails established by people or animals, several researchers have found that the majority of these ticks can be found on the uphill side of the trail. Like I. scapularis, I. pacificus has been collected mainly at lower altitudes, but I. pacificus has been collected, in at least one instance, at an altitude of 2345 m (Olson et al. 1992). The habitats where questing nymphal I. pacificus can be collected in abundance are more restricted than comparable adult habitat. Areas of high nymphal abundance are mainly characterized by the presence of mature trees and abundant leaf litter (Tälleklint-Eisen & Lane, 1999; Li, Peavey & Lane, 2000).
Among all of the main tick vectors of B. burgdorferi sl, I. ricinus is the one which feeds on the largest variety of vertebrate hosts (>300 vertebrate species, Anderson, 1991). However, only a few dozen of these vertebrate hosts have been currently identified as reservoir hosts for B. burgdorferi sl in Europe (Gern et al. 1998). Thus, little information is available on the real significance of most animal hosts as sources for infecting ticks with B. burgdorferi sl. From the few tick hosts which have been studied up to now some of them act as reservoirs whereas others appear to be refractory to infection. A distinction must be made between animals that serve as hosts for ticks and are occasionally found to be infected with Borrelia burgdorferi, vs. true reservoirs of infection that infect a significant proportion of the immature ticks that feed on them. A careful analysis of the term reservoir capacity was recently published by Kahl et al. (2002).
The enzootic cycle involves larval and nymphal ticks becoming infected with B. burgdorferi while feeding on their hosts. Small mammals are frequent hosts of these developmental stages and are certainly the group that has been the most extensively investigated up to now in Europe and North America. Currently several species of mice, voles, rats and shrews have been shown to be competent reservoirs of B. burgdorferi sl in Europe (Gern et al. 1998). In particular, evidence that the mice Apodemus flavicollis, A. sylvaticus, A. agrarius and the vole, Clethrionomys glareolus, act as reservoirs for B. burgdorferi sl has been obtained in many European countries (Aeschlimann et al. 1986; Matuschka et al. 1992; de Boer et al. 1993; Humair et al. 1993a; Gern et al. 1994; Kurtenbach et al. 1994, 1995, 1998b; Tälleklint & Jaenson, 1994; Randolph & Craine, 1995; Hu et al. 1997; Humair, Rais & Gern, 1999; Richter et al. 1999; Hanincová et al. 2003a). Apodemus, once infected, persistently remain infectious for ticks (Gern et al. 1994); and since small rodents are frequently parasitized by nymphal and larval I. ricinus (Humair et al. 1993a; Randolph et al. 2000) they are potent reservoir hosts. However, a study highlighted different transmission patterns in nature between Apodemus and ticks and Clethrionomys and ticks (Humair et al. 1999). The authors of this study observed that each host species seems to have developed different strategies towards tick infestation and Borrelia infection. Borrelia infection in Apodemus is rarely detected by Borrelia isolation; this may be related to the fact that Apodemus appear to maintain low level of Borrelia infection through their immune system (Kurtenbach et al. 1994). On the other hand, Borrelia is efficiently transmitted from Apodemus to ticks (Humair et al. 1999). In contrast, in Clethrionomys, Borrelia infection is easily detectable by isolation and spirochetes are easily transmitted to ticks but most ticks do not feed completely or do not moult (Humair et al. 1999). This is in line with the observation that Clethrionomys develop an immune response to ticks that prevent ticks from engorging and moulting successfully (Kurtenbach et al. 1994; Dizij & Kurtenbach, 1995). Consequently, the reservoir competence of Apodemus and Clethrionomys is modulated by their immune response towards the pathogen and towards the tick.
More limited information has been obtained on the implications of other small mammals in the maintenance cycles of Borrelia in nature. Nevertheless, another species of vole, Microtus agrestis in Sweden (Tälleklint & Jaenson, 1994), and black rats (Rattus rattus) and Norway rats (R. norvegicus) in urbanized environments in Germany (Matuschka et al. 1996, 1997) and in Madeira (Matuschka et al. 1994a) may serve to infect I. ricinus ticks. Similarly, only a few studies mentioned B. burgdorferi sl in shrews or in ticks attached on them: Sorex minutus and S. araneus (Humair et al. 1993a; Tälleklint & Jaenson, 1994) and Neomys foediens (Tälleklint & Jaenson, 1994).
Observations in endemic areas in Germany and in France showed that edible dormice (Glis glis) (Matuschka et al. 1994b) and garden dormice (Eliomys quercinus) (Matuschka et al. 1999) are reservoir hosts for Borrelia. In Germany, the edible dormice were frequently parasitized by subadult ticks and infected around 95 of larvae (Matuschka et al. 1994b).
Additional rodents, like grey squirrels (Sciurus carolinensis) in the UK (Craine et al. 1997) and red squirrels (S. vulgaris) in Switzerland (Humair & Gern, 1998), also contribute to the amplification of Borrelia in the tick population. Observations made on infestations of squirrels by ticks indicated that red and grey squirrels were heavily infested with ticks and one study reported a high prevalence of infection (69) in ticks feeding on red squirrels (Humair & Gern, 1998).
Several researchers demonstrated that the European hedgehog (Erinaceus europaeus) also perpetuates B. burgdorferi sl in Ireland (Gray et al. 1994), Germany (Liebisch, Finkbeiner-Weber & Liebisch, 1996) and Switzerland (Gern et al. 1997) (Fig. 1). In Switzerland, an enzootic transmission cycle of B. burgdorferi sl involving hedgehogs and another tick vector, I. hexagonus, has been described in an urban environment (Gern et al. 1997).
Another group of animals, lagomorphs, play a role in the support of the enzootic cycle of B. burgdorferi sl. In Sweden in habitats where hares coexist with small mammals (Tälleklint & Jaenson, 1993, 1994), as well as on islands where hares are the only terrestrial mammal species permanently present, lagomorphs like the brown hare (Lepus europaeus) and the varying hare (L. timidus) contribute to the maintenance of B. burgdorferi sl in nature (Jaenson & Tälleklint, 1996). An alternative candidate reservoir host among lagomorphs includes the European rabbit (Oryctolagus cuniculus) (Matuschka et al. 2000). However, the European rabbit appears to be poorly competent, since only 1/7 rabbits (14) in this study were infective to ticks.
Among larger mammals, the red fox is implicated in the maintenance of Borrelia in nature as described in two studies in Germany (Kahl & Geue, 1998; Liebisch et al. 1998a). These animals did not appear to be very potent reservoirs since spirochetes were poorly transmitted to ticks.
Not all tick-hosts are competent to serve as reservoirs. This is the case for cervids in general which act primarily as sources of blood for ticks. In fact, studies on roe deer (Capreolus capreolus) (Jaenson & Tälleklint, 1992), moose (Alces alces) (Tälleklint & Jaenson, 1994), red deer (Cervus elaphus) (Gray et al. 1995), and fallow deer (Dama dama) (Gray et al. 1992) suggest that these species do not infect feeding ticks with B. burgdorferi. Interestingly, sheep have been found to be reservoirs of Borrelia burgdorferi in areas of the UK, but the principal mechanism by which they infect I. ricinus is cofeeding (Ogden, Nuttall & Randolph, 1997). This is a phenomenon in which the host does not necessarily become infected, but neighbouring ticks serve to infect each other while feeding on the host.
After a long period of controversy, the role of birds in the maintenance of B. burgdorferi sl in endemic areas is currently recognized (Humair, 2002). The first report in Europe of B. burgdorferi sl in I. ricinus ticks feeding on birds dates back to 1993 (Humair et al. 1993b). The same year, Olsen et al. (1993) demonstrated the existence of a transmission cycle of B. burgdorferi sl in seabird colonies among razorbills (Alca torda) and I. uriae on a Swedish Island. Later, spirochetes were reported in I. ricinus ticks collected from migratory birds in Sweden (Olsen, Jaenson & Bergström, 1995b) and in ticks feeding on birds captured in endemic areas in the Czech Republic (Hubálek et al. 1996) and in the UK (Craine et al. 1997). In 1998, two studies clearly defined the reservoir role of birds, one on a passerine bird, the blackbird (Turdus merula) (Humair et al. 1998), the other one on a gallinaceous bird species, the pheasant (Phasianus colchicus) (Kurtenbach et al. 1998a). Both studies demonstrated the reservoir role of these bird species using xenodiagnosis, obtaining the evidence that birds contribute to the circulation of Borrelia in endemic areas. The involvement of seabirds and I. uriae in the marine environment was also confirmed by additional studies in both the northern and the southern hemispheres (Olsen et al. 1995a; Gylfe et al. 1999).
In endemic areas in Europe, at least 5 Borrelia genospecies may circulate between vertebrate hosts and ticks. The first findings on host specificity of Borrelia species came from a study conducted in Switzerland (Humair et al. 1995). In this study, it was shown that Borrelia species isolated from Apodemus spp. captured in two different endemic sites all belonged to B. afzelii, whereas genospecies diversity in ticks collected by flagging vegetation in these sites displayed heterogeneity. Later, it was shown that small rodents of the genus Apodemus, such as woodmice (A. sylvaticus) and yellow-necked mice (A. flavicollis) and of the genus Clethrionomys as well as red (Sciurus vulgaris) and grey squirrels (S. carolinensis) are usually infected by B. afzelii and less frequently by B. burgdorferi ss; moreover, these hosts transmit these Borrelia species to ticks feeding on them (Craine et al. 1997; Hu et al. 1997; Humair et al. 1999; Kurtenbach et al. 1998b). On the other hand, an increasing body of evidence first showed that B. garinii was mostly associated with migratory birds (Olsen et al. 1995b), and later that B. garinii and B. valaisiana were associated with blackbirds and pheasants (Humair et al. 1998; Kurtenbach et al. 1998a,b). B. garinii was also described as the Borrelia species involved in marine environments, in seabird colonies located on the northern and southern hemispheres (Olsen et al. 1995a; Gylfe et al. 1999). This gives us the opportunity to reiterate that Olsen and colleagues, in their 1995a study, detected the presence of B. garinii DNA in North America (Alaska). In fact, amplified flagellin gene fragments from positive I. uriae ticks collected from fork-tailed storm petrels on Egg Island (Alaska) subjected to DNA sequencing showed that they were closely related to the fla gene of B. garinii suggesting the presence of B. garinii in North America at least in a very specific area (marine enzootic cycles).
B. garinii has also occasionally been described associated with rodents in Austria, Germany and Russia (Khanakah et al. 1994; Gorelova et al. 1995; Richter et al. 1999). From these studies, it is unknown whether the incriminated B. garinii belonged to one serotype or another. In view, however, of recent findings showing that laboratory mice challenged with nymphs collected in nature were able to transmit B. garinii OspA serotype 4 to xenodiagnostic ticks (Hu et al. 2001) and that Apodemus captured in Switzerland transmitted B. garinii OspA serotype 4 to xenodiagnostic ticks (Huegli et al. 2002), rodents, at least in some well specified areas, are also reservoir hosts for B. garinii (Huegli et al. 2002). In summary, rodents are mainly associated with B. afzelii, but also with B. burgdorferi ss and B. garinii OspA serotype 4 whereas other B. garinii serotypes are associated with birds (Kurtenbach et al. 2002b); B. valaisiana has been currently described only in birds and never in rodents and therefore appears to be very specific to birds (Gylfe et al. 2000; Humair et al. 1998; Kurtenbach et al. 1998b; Olsen, 1995; Olsen et al. 1993, 1995a,b) (Fig. 1).
Concerning the fifth Borrelia species infecting I. ricinus, B. lusitaniae, although this species may be very frequent in I. ricinus ticks in some areas of North Africa (Younsi et al. 2001; Gern et al. 2002) and Portugal (de Michelis et al. 2000) and usually infects ticks with large numbers of spirochetes (unpublished data), its reservoir hosts have not yet been identified.
The host complement system appears to be a major determinant of host-specificity of B. burgdorferi sl in Europe (Kurtenbach et al. 1998c, 2002a). It was demonstrated in vitro that the various Borrelia species show different patterns of resistance or sensitivity to serum according to host species, which corresponds to the host specificity observed in nature (Kurtenbach et al. 1998c, 2002a). The specificity of complement lysis of genospecies of B. burgdorferi is apparently mediated through the expression of the erp gene loci. These gene loci encode for the so-called CRASPs (complement regulatory-acquiring surface proteins) which bind differentially to complement inhibitors (e.g. factor H). Intense research is ongoing into the specific receptors involved in this interaction between host and pathogen (Kraiczy et al. 2001; Stevenson et al. 2002). The evolutionary consequences of this system are an intriguing subject for further study.
There is little doubt that white-tailed deer (Odocoileus virginianus) play a key role in the Lyme disease enzootic cycle in North America because they serve as the principal hosts for the adult stage of these ticks. Early studies demonstrated that white-tailed deer support large numbers of adult I. scapularis (Piesman et al. 1979, Main et al. 1981; Spielman et al. 1985). In addition, populations of white-tailed deer have exploded during the latter half of the 20th century, exactly coinciding with the time period of the Lyme disease epidemic in North America. Observations on islands or parks, with and without deer, added to the impression that these tick populations were dependent on the presence of deer (Wilson, Adler & Spielman, 1985; Anderson et al. 1987; Duffy et al. 1994). Although other animals such as medium-sized mammals (Fish & Dowler, 1989), dogs and cats are often infested with adult I. scapularis, these hosts generally do not support the large numbers needed to support populations of I. scapularis. Black bears (Ursus americanus) can be infested with large numbers of I. scapularis, but these large animals are few in number (Kazmierczak, Amundson & Burgess, 1988).
Despite the importance of white-tailed deer as hosts for the adult stage of the principal vector of Lyme disease spirochetes in North America, these hosts are not an important reservoir of B. burgdorferi due to the lytic properties of the complement contained in deer sera. Telford et al. (1988) demonstrated that larval I. scapularis dropping off deer carcasses were not infected with B. burgdorferi in an area of Massachusetts highly endemic for this aetiologic agent. European researchers have now demonstrated that complement contained in deer sera is highly lytic to a wide variety of B. burgdorferi (Kurtenbach et al. 2002a) and this observation has been confirmed using a North American strain of B. burgdorferi ss (Nelson et al. 2000). Thus, white-tailed deer are a doubled-edged sword in the maintenance of the Lyme disease spirochete enzootic cycle in eastern North America; deer are needed to support large populations of vector ticks, but these hosts also serve to decrease the proportion of the tick population that becomes infected with spirochetes. Columbian black-tailed deer (O. hemionus columbianus), and other so-called ‘mule deer’ play a similar role in support of I. pacificus populations (Westrom, Lane & Anderson, 1985) in western North America.
Another group of animals that serve as important hosts for I. scapularis and I. pacificus but apparently do not serve as reservoirs for spirochetal infection are lizards. Spielman et al. (1985) originally coined the phrase ‘zooprophylaxis’ for hosts that fail to infect the ticks that infest them. Lizards appear to be important zooprophylactic hosts, serving to drive down the B. burgdorferi infection rates of questing ticks in key areas. In the southern United States, lizards serve as hosts to the majority of immature I. scapularis (Apperson et al. 1993; Oliver, Cummins & Joiner, 1993a). Similarly, lizards also serve as important hosts for immature I. pacificus (Lane & Loye, 1989). Several researchers speculated that lizards were incompetent hosts for B. burgdorferi and that nymphs and adult ticks that had previously fed on these hosts were not infected with B. burgdorferi (Spielman, 1988). Recently, Kuo, Lane & Gicias (2000) demonstrated that complement from Sceloporus and Elgaria lizards were highly lytic for B. burgdorferi ss, thus supporting the rationale that lizards were zooprophylactic hosts. A note of caution must be sounded, however, based on the observations of Levin et al. (1996). These researchers found that lizards in the genus Eumeces and Anolis could serve to infect I. scapularis with spirochetes under experimental conditions. The activity of the complement of a wide range of lizards that serve as hosts for Ixodes ticks should be studied before general conclusions are made about the reservoir competence of reptilian hosts.
Birds play two roles in the support of the enzootic cycle of B. burgdorferi in North America. Migrating birds may serve to move immature I. scapularis and I. pacificus into new locations (Spielman, 1988), and some birds may serve as reservoir hosts infecting the ticks that feed on them (Fig. 2). The fact that a variety of birds serve as hosts for immature I. scapularis has been documented numerous times (Anderson & Magnarelli, 1984; Battaly & Fish, 1993; Stafford, Bladen & Magnarelli, 1995). In general, ground-feeding and ground-nesting birds are the most heavily infested birds (Weisbrod & Johnson, 1989). Numerous isolates of B. burgdorferi have been obtained from birds in North America (Anderson, Magnarelli & Stafford, 1990; McLean et al. 1993), but the reservoir competence or ability of birds to infect larval I. scapularis feeding on them has been controversial. The fact that larval I. scapularis removed from many species of birds in the wild were infected with B. burgdorferi ss was repeatedly documented (Weisbrod & Johnson, 1989; Stafford et al. 1995). The first experiments attempting to infect xenodiagnostic larval I. scapularis by feeding them on grey catbirds (Dumatella carolinensis) indicated that these birds could not serve as reservoirs of B. burgdorferi (Mather et al. 1989a). In contrast, American robins (Turdus migratorius) efficiently infected larval I. scapularis that fed upon them (Richter et al. 2000). The degree to which various bird species serve to infect I. scapularis with B. burgdorferi in the eastern United States needs further research. In addition, the relationship between birds and I. pacificus seems to vary from site to site, with birds in some western regions carrying extremely light burdens of ticks (Manweiler et al. 1990), and birds in other regions heavily infested (Wright et al. 2000). The observation that unidentified spirochetes were found in blood smears from birds in Placer County, California (Wright et al. 2000) needs further evaluation.
Rodents are clearly the primary reservoir hosts of B. burgdorferi ss in the regions most highly endemic for Lyme disease in North America (Fig. 2). Initial studies on Nantucket Island, Massachusetts pointed toward the white-footed mouse (Peromyscus leucopus) as the primary host for larval and nymphal I. scapularis. Spielman, Levine & Wilson (1984) estimated that 91 of larval and nymphal I. scapularis fed on P. leucopus and these hosts could infect as many as 76 of larval ticks feeding on them (Mather et al. 1989a). Clearly, the contribution of P. leucopus as a reservoir host serving to infect ticks with B. burgdorferi ss in coastal New England is substantial. A study on Monhegan Island (Maine) demonstrated that on an island where P. leucopus is absent, other rodents such as Norway rats (Rattus novegicus) could serve efficiently as substitute reservoir hosts for B. burgdorferi (Smith et al. 1993). Short-tailed shrews (Blarina brevicauda) have also been mentioned as efficient reservoirs of B. burgdorferi (Telford et al. 1990). Interestingly, the reservoir potential of eastern chipmunks appears to differ from region to region. In coastal Massachusetts, white-footed mice were found to infect many more immature I. scapularis with B. burgdorferi compared to chipmunks (Mather et al. 1989b), but in the midwestern state of Illinois, evidence suggested that chipmunks may be more important than white-footed mice as reservoirs of B. burgdorferi (Slajchert et al. 1997). Local variation in the importance of various reservoir hosts in the enzootic cycle of B. burgdorferi in eastern North America is an important factor that must be taken into account when designing control strategies for Lyme disease spirochetes transmitted by I. scapularis.
The importance of rodents as reservoirs of B. burgdorferi in areas of western North America, where I. pacificus is the principal vector is a complex subject. A study in Oregon demonstrated that rodents such as Neotoma fuscipes, Peromyscus maniculatus, and Peromyscus boylii were infected with B. burgdorferi and infested with both I. pacificus and I. spinipalpis (Burkot et al. 1999). In northern California, various rodents were infected with B. burgdorferi and infested with I. spinipalpis (Peavey, Lane & Kleinjan, 1997). A possible scenario exists, wherein I. spinipalpis serves as the principal enzootic vector of B. burgdorferi in western North America, transmitting the pathogen from rodent to rodent; people only are at risk of acquiring these rodent-derived strains when I. pacificus acquires infection from rodents and subsequently transmits these spirochetes to humans. This hypothesis warrants further investigation.
B. burgdorferi sl is transmitted orally while ticks are feeding on hosts. Indeed, it is currently well established for North American and European tick vectors that B. burgdorferi sl is transmitted to the host via infected saliva during the blood meal. Only a few studies in Europe investigated the transmission dynamic of B. burgdorferi sl by I. ricinus. However, it is currently known that, in the majority of infected unfed I. ricinus nymphs and adults, spirochetes are present in the midgut and migrate during blood feeding to the salivary glands from which they are transmitted to the host via saliva (Gern, Zhu & Aeschlimann, 1990; Gern, Lebet & Moret, 1996; Zhu, 1998). However, microscopic examination of unfed nymphal and adult I. ricinus collected in endemic areas in Switzerland demonstrated that spirochetes may infect salivary glands even before any blood uptake (Leuba-Garcia et al. 1994; Lebet & Gern, 1994; Zhu, 1998). These systemic or generalized infections may occur rather frequently compared to what has been described for I. scapularis in North America. When unfed I. ricinus attaches to a vertebrate host Borrelia transmission does not occur at the beginning of the blood uptake but later and transmission efficiency increases with the duration of the blood-meal (Kahl et al. 1998). In a laboratory study, an early transmission of borreliae with high efficiency was described for I. ricinus. In fact, Kahl et al. (1998) reported that 50 of laboratory animals were infected by B. burgdorferi sl after only 16.7 h of tick attachment. The observations of high infection rates in salivary glands of unfed I. ricinus suggest that systemically infected ticks may transmit Borrelia early after attachment to hosts (Leuba-Garcia et al. 1994; Lebet & Gern, 1994) and this might be a factor which influences delay of transmission after attachment of the ticks to the hosts. It was recently reported that this delay may also be influenced by the Borrelia species infecting the ticks (Crippa, Rais & Gern, 2002). In fact, earlier transmission by I. ricinus when ticks were infected by B. afzelii rather than by B. burgdorferi ss may occur. Crippa et al. (2002) noted that during the first 48 h of attachment to the host, B. burgdorferi ss-infected ticks did not infect the 18 exposed mice whereas B. afzelii-infected ticks transmitted infection to 33 of mice. This study showed that I. ricinus transmits B. afzelii earlier than B. burgdorferi ss, and also that I. ricinus is a more efficient vector for B. afzelii than for B. burgdorferi ss.
It is well known from studies on I. scapularis, that spirochetes express outer surface protein A in the tick midgut and that during blood feeding, OspA synthesis is repressed and OspC synthesis is induced (Schwan & Piesman, 2002). In I. ricinus, very few studies addressed this point. Leuba-Garcia, Martinez & Gern (1998) observed that B. afzelii spirochetes expressing OspA and OspC were present in the midgut of unfed ticks and that spirochetes expressing OspA were not detected in ticks attached to the host for more than 24 h. In salivary glands of engorged ticks B. afzelii spirochetes expressed OspC. This study also reported that in the skin of mice infected by B. afzelii-infected nymphs, borreliae expressed OspC. Later Fingerle et al. (2002), using different B. afzelii and B. garinii strains, demonstrated that in capillary-infected I. ricinus ticks OspA was expressed in the tick midgut and that the proportion of OspC-positive borreliae was usually greater when the borreliae reached the salivary glands. In this study, a B. afzelii strain unable to produce OspC was unable to disseminate and to induce infection in salivary glands, showing the role of OspC in Borrelia dissemination in I. ricinus. The degree of strain specificity on the dynamics of Osp expression and the dissemination of spirochetes in the vector is an interesting topic. The interactions of the various Borrelia species and strains with I. ricinus are clearly extremely complex.
A recent review by Schwan & Piesman (2002) summarized the intricate relationship between Borrelia and their tick vectors. One of the systems that have received the most intense research attention is the B. burgdorferi–I. scapularis interaction during spirochete transmission. Transmission by nymphal I. scapularis, in particular, has received close scrutiny since the vast majority of Lyme disease cases in North America acquire infection from nymphal ticks (Piesman et al. 1987a; Piesman, 1989; Falco et al. 1999). A key factor in the transmission dynamics of B. burgdorferi is the duration of attachment required for efficient transmission of spirochetes to the host. Several animal studies with laboratory infected and/or field collected nymphal I. scapularis have demonstrated that nymphs must be attached to hosts for >48 h in order for B. burgdorferi ss to be efficiently transmitted (Piesman et al. 1987b; des Vignes et al. 2001). Observations with patients in Lyme disease endemic regions also support the concept that only those ticks feeding for >48 h transmit an infectious dose of spirochetes (Sood et al. 1997; Nadelman et al. 2001). Although the smaller nymphs often escape detection and feed for a sufficient interval to transmit spirochetes, the larger adult female I. scapularis is more routinely detected and removed before feeding long enough to transmit spirochetes (Piesman et al. 1991; Falco, Fish & Piesman, 1996) (Fig. 4). Thus, Lyme disease cases occur in North America virtually exclusively when nymphal I. scapularis are active (May–July) as opposed to when adults are active (October–April).

Fig. 4. The principal ticks found commonly biting people in eastern North America, including the principal vector of Lyme disease, Ixodes scapularis. (Reprinted with permission from the Massachusetts Medical Society; Hayes & Piesman, 2003.)
The underlying reasons for the delay between tick attachment and spirochete transmission, have been described by Ohnishi, Piesman & de Silva (2001), as well as Schwan & Piesman (2002). Spirochetes in flat nymphs are restricted mainly to the tick midgut; the vast majority of these spirochetes express OspA. When feeding commences, rapid multiplication of the spirochetes occurs and OspA is down-regulated and a proportion of the population now express OspC. The down-regulation of OspA may allow the spirochetes to leave the midgut since OspA apparently binds to uncharacterized tick midgut proteins (Pal et al. 2000). Spirochete populations increase in tick salivary glands during the feeding process and are eventually transmitted to the skin adjacent to the feeding site (Piesman, Schneider & Zeidner, 2001; Ohnishi et al. 2001). This process occurs in I. scapularis infected with B. burgdorferi ss. Transmission dynamics of other genospecies of B. burgdorferi may differ dramatically (Crippa et al. 2002). The requirement for ticks to be attached for a period >48 h in order to transmit B. burgdorferi ss efficiently also seems to hold true for I. pacificus (Peavey & Lane, 1995).
The first line of defense against Lyme disease in North America and in Europe is clearly personal protection. This involves avoidance of tick-infested habitat, wearing protective clothing, the prudent use of tick repellents and daily tick checks to detect and promptly remove attached ticks. In order to be effective, personal protection requires that residents of highly endemic regions have a fairly sophisticated knowledge of the enzootic cycle of Lyme disease and the biology of infected ticks.
In North America, the knowledge, attitudes and practice of Lyme disease prevention differs from region to region (Herrington et al. 1997). Certainly, people living in the endemic regions of the northeastern United States have an in-depth understanding of the biology of Lyme disease; however, prevention practices are not routinely employed even in a place like Nantucket Island, Massachusetts that has been dealing with the Lyme disease epidemic for decades (Phillips et al. 2001). Intense education campaigns are currently underway in select communities in Massachusetts, Connecticut, New York and New Jersey to see if the community education approach to Lyme disease prevention can be optimized.
Tick control methods are also an essential part of Lyme disease prevention in North America (Fig. 3). A single well-timed area-wide application of acaricides such as carbaryl, cyflutherin or deltamethrin to vegetation can reduce populations of questing nymphs by 68–100 (Stafford, 1991a; Curran, Fish & Piesman, 1993; Schulze et al. 1994, 2001). Although many home-owners do allow licensed pest control operators to apply acaricides to their properties in endemic areas (Stafford, 1997), the majority of home-owners have reservations about using this approach due to concerns about the effect of these chemicals on non-target species and human toxicity. It is therefore incumbent upon the public health community to present home-owners with alternative means for tick control.
Vegetation management is one alternative means of tick control. Burning (Stafford, Ward & Magnarelli, 1998), brush removal (Wilson, 1986), leaf litter removal (Schulze et al. 1995) and the establishment of wood-chip barriers have all been tested as means of reducing contact with ticks. In addition, treatment of vegetation with soaps and desiccants holds promise for tick reduction (Allan & Patrican, 1995). The use of biological control agents such as parasitoid wasps, parasitic nematodes and fungi is under evaluation as well (Stafford, Denicola & Magnarelli, 1996; Zhioua et al. 1995, 1997; Benjamin, Zhioua & Ostfeld, 2002; see also chapter by Samish et al. in this Supplement). Vegetation management and biological control agents are well received by the community due to their reputation as non-toxic environmentally-sound control methodologies. In general, however, these ‘bio-friendly’ methods are less consistently effective than chemical acaricidal agents.
Host-targeted control methods have also been tested for their efficacy in reducing populations of I. scapularis. Some of these methods aim at overall population reduction and others aim at specifically reducing the number of questing nymphs infected with B. burgdorferi. Due to their role as primary hosts for the adult stage of I. scapularis, white tailed deer have been targeted for various intervention efforts to control ticks. These include deer eradication (Wilson et al. 1988), deer reduction (Deblinger et al. 1993), fencing (Stafford, 1993; Daniels & Fish, 1995), and the application of acaricides to deer via bait stations charged with chemicals on paint roller delivery systems. This latter method is called the ‘4-poster’ method; it has been shown to be effective against Amblyomma americanum (Pound, Miller & George, 2000) and is currently under evaluation for the control of I. scapularis. Although deer-target methods for the control of I. scapularis hold great promise for the future, they have yet to be put into common practice in Lyme disease endemic regions in North America, with the possible exception of fencing of individual properties.
Rodents have also been the target of tick control intervention efforts in North America. A commercial product that utilizes permethrin-treated cotton balls collected specifically by white-footed mice has been developed. It appears to have worked to reduce the population of questing I. scapularis in some trials, but not in others (Mather, Ribeiro & Spielman, 1987; Deblinger & Rimmer, 1991; Stafford, 1991b, 1992; Daniels, Fish & Falco, 1991). One of the reasons suggested for its varied success was the importance of white-footed mice vs. other reservoir hosts in different locations. Permethrin-treated cotton balls were also ineffective when tested against I. pacificus (Leprince & Lane, 1996). Other rodent-targeted methods involve the use of bait stations for applying acaricides to various rodent species (Sonenshine & Haines, 1985; Gage et al. 1997; Lane et al. 1998). Trials are currently being conducted using bait boxes treated with fipronil, a promising new topical acaricide (Davey et al. 1998), for the control of I. scapularis infected with B. burgdorferi. Significant decreases in ticks on rodents and questing nymphs have been observed where these bait boxes are undergoing field trials. Systemic treatment of deer and rodents with ivermectin or closely related compounds is also a future avenue for research.
The current status of tick control methods is in a rapid state of change. No one single method holds promise as the ‘magic bullet’ for tick control. Certainly, an integrated pest management approach (IPM) that utilizes various methods in diverse situations will be the most effective response to prevent Lyme disease in the future.
Although much research on control strategies against I. scapularis in North America has been carried out, much less has been done in Europe to control I. ricinus. This is probably essentially due to different risk exposures. In the highly endemic areas of the northeastern United States, people have close contact with I. scapularis and residential exposure dominates. In Europe, the majority of residential properties do not have borders with woodlands or forests and most people contract Lyme borreliosis while visiting tick-endemic areas. Tick bites are primarily due to occupational or recreational exposure.
A study undertaken in Sweden evaluated permethrin-treated rodent nesting materials similar to those used in North America (Mejlon, Jaenson & Mather, 1995). The authors concluded that application of these methods in natural ecosystems would not represent a practical and economic method to reduce risk of humans to acquire Lyme borreliosis mainly because host reservoirs for B. burgdorferi in Europe are diverse and are not restricted to rodents. A review of various potential methods to reduce I. ricinus in Sweden similarly concluded that most methods were not appropriate to reduce Lyme borreliosis cases (Tälleklint & Jaenson, 1995). Therefore, in Europe personal protection is favoured. Simple measures include use of tick repellents before entering a tick-infested area, avoidance of, or minimization of, exposure to tick-infested areas and thorough examination of cloths and skin after exposure. Protective clothing, particularly boots, long trousers tucked into the boots and a shirt tucked into the trousers are recommended. Light-coloured clothes will favour detection of ticks crawling on humans before they find a suitable place to attach to skin. Attached ticks should be removed as soon as possible because I. ricinus may transmit B. burgdorferi sl quite early after tick attachment (Kahl et al. 1998; Crippa et al. 2002). Therefore, regular checks for ticks on clothes and the body immediately when one leaves the area where ticks are present is highly recommended.
A survey conducted in various countries of Europe on Lyme borreliosis awareness showed that awareness was greater in countries showing a prevalence of Lyme borreliosis like Germany, Russia, Sweden and the Czech Republic whereas it was lower in countries with low prevalence such as UK and Ireland (Gray et al. 1998). Interestingly, the media were the main source of information for most people.
In the United States, a Lyme disease vaccine based on a recombinant OspA protein was tested (Steere et al. 1998; Sigal et al. 1998) and licensed for use. Despite the fact that it was reasonably efficacious, and an adverse events registry reported only minor side effects associated with the vaccine (Lathrop et al. 2002), the vaccine was withdrawn from the market essentially due to lack of demand. Problems associated with the vaccine also included the need for a series of three shots and an undetermined need to get booster injections in the future, high cost and a theoretical but widespread concern that the vaccine could cause serious autoimmune reactions (Gross et al. 1998).
In Europe, where at least three pathogenic species are infecting I. ricinus ticks, the variability in OspA expression among B. burgdorferi sl isolates was often used as an argument against a development of an OspA vaccine. However, a study showed that a vaccine compatible with human use, containing multiple OspA antigens, was very efficient at protecting mice against the three causative agents of Lyme borreliosis in Europe, B. burgdorferi ss, B. garinii and B. afzelii, using the natural mode of transmission (Gern et al. 1997). However, after the licensed vaccine in North America was withdrawn from the market, the development programme of this OspA vaccine for Europe has been stopped.
As discussed in the ‘Treatment’ section of this review, prophylactic treatment of tick bites in highly endemic regions of North America remains an option (Nadelman et al. 2001). In Europe, prophylactic treatment after a tick bite is usually not recommended (Stanek & Kahl, 1999). In the end, the most effective prevention campaign against Lyme disease may involve education of the community to practise personal protection measures, IPM campaigns for tick control and an alert and dedicated public health infrastructure to combat Lyme disease.

Fig. 1. Transmission cycle of B. burgdorferi in Europe. a) Cycle involving I. hexagonus and hedgehogs. b) Cycles involving I. ricinus, various genospecies of B. burgdorferi sl as well as birds and rodents.

Fig. 2. Transmission cycle of B. burgdorferi in North America. a) Cycles involving B. andersonii, I. dentatus and rabbits, as well as B. bissettii, I. spinipalpis and rodents. b) Cycle involving I. scapularis or I. pacificus, B. burgdorferi ss, as well as birds and rodents.

Table 1. Clinical features of Lyme borreliosis in North America and Eurasia

Fig. 3. Life cycle of B. burgdorferi in the United States, highlighting various points for intervention strategies to prevent Lyme disease. (Reprinted with permission from the Massachusetts Medical Society; Hayes & Piesman, 2003.)

Fig. 4. The principal ticks found commonly biting people in eastern North America, including the principal vector of Lyme disease, Ixodes scapularis. (Reprinted with permission from the Massachusetts Medical Society; Hayes & Piesman, 2003.)