Introduction
Cambrian Konservat-Lagerstätten (‘Lagerstätten’ hereafter) provide exceptional insights into early metazoan evolution, not least because of an abundance of lightly sclerotized and soft-bodied taxa (Hagadorn, Reference Hagadorn, Bottjer, Etter, Hagadorn and Tang2002). Laurentian examples include the iconic Burgess Shale in British Columbia (Canada) as well as a series of important deposits in Utah (Spence Shale, Wheeler Formation, Marjum Formation; e.g., Muscente et al., Reference Muscente2017; Fig. 1). By contrast, the Weeks Formation (Miaolingian), exposed near Notch Peak, Utah, only more recently has yielded an important Burgess Shale–type fauna (Hesselbo, Reference Hesselbo1989; Lerosey-Aubril et al., Reference Lerosey-Aubril, Ortega-Hernández, Kier and Bonino2013, Reference Lerosey-Aubril, Hegna, Babcock, Bonino and Kier2014, Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018; Lerosey-Aubril, Reference Lerosey-Aubril2015; Ortega-Hernández et al., Reference Ortega-Hernández, Lerosey-Aubril, Kier and Bonino2015; Robison et al., Reference Robison, Babcock and Gunther2015). Not only is this latter assemblage important in extending our knowledge of Cambrian life, but also its chronological position close to the Miaolingian–Furongian boundary fills a significant gap in the fossil record of non-biomineralizing animals and apparently corresponds to the onset of major biotic changes (Lerosey-Aubril et al., Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018).
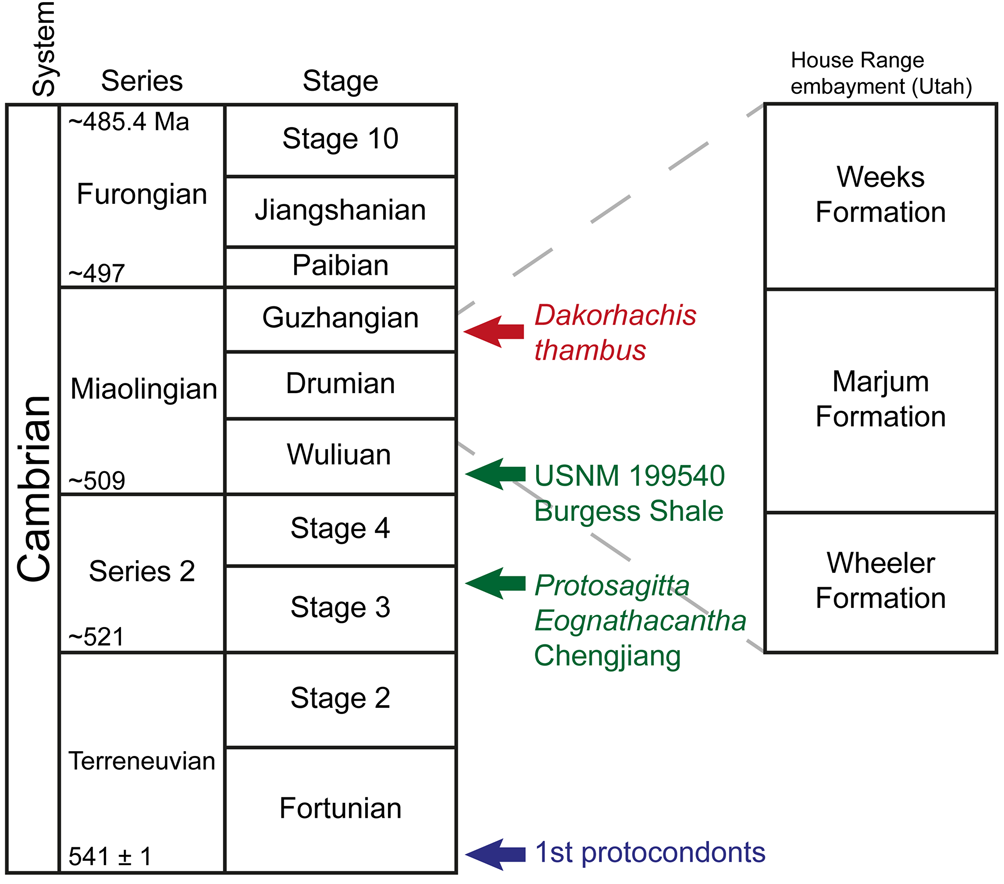
Figure 1. Stratigraphic occurrences of Dakorhachis thambus (in color version, red) and the oldest chaetognaths (in color version, green) and protoconodonts (in color version, blue). Cambrian chaetognaths Eognathacantha, Protosagitta and USNM 199540.
To the first approximation, Burgess Shale–type faunas (e.g., Briggs et al., Reference Briggs, Erwin and Collier1994; Hou et al., Reference Hou, Aldridge, Bergstrom, Siveter, Siveter and Feng2004) have a well-established identity with a predominance of arthropods (both trilobites, including agnostoids, and lightly skeletonized taxa), priapulids (and related scalidophorans), and sponges. Somewhat more occasional there occur such groups as the annelids, vetulicolians, wiwaxiids, and other sclerite-bearing taxa. Such faunas remain a focus of attention not only on account of their sheer diversity but also because a number of hitherto problematic taxa appear to belong to stem groups that in principle are instrumental in our understanding of the origin of phyla.
Not all such taxa, however, can be accommodated in this fashion, and in one way or another, a number of them retain their enigmatic status. Broadly, these can be divided into three categories, although the boundaries that separate them are by no means absolute. Some, such as the vetulicolians, form a relatively diverse clade but their wider relationships within the deuterostomes continue to be controversial (e.g., Ou et al., Reference Ou, Conway Morris, Han, Zhang, Liu, Chen, Zhang and Shu2012; García-Bellido et al., Reference García-Bellido, Lee, Edgecombe, Jago, Gehling and Paterson2014). Others, such as Nectocaris, have deeply polarized opinion, in this case as to whether this animal is an early cephalopod (e.g., Smith and Caron, Reference Smith and Caron2010; Kroger et al., Reference Kröger, Vinther and Fuchs2011; Smith, Reference Smith2013). Finally, there are singletons that for all intents and purposes remain in taxonomic limbo, and it is to this last category we add a remarkable new taxon, Dakorhachis thambus n. gen. n. sp. (Fig. 1). These three categories also have the heuristic value of providing a crude metric of relative phylogenetic ignorance, although in each case new fossil finds ultimately will ensure more secure placement within the metazoan tree. Moreover, properly interpreted, these enigmatic taxa may help to throw crucial light on key transitions between major groups. At this juncture, we are unable to assign D. thambus with confidence to any known group, but it is evidently a member of the Bilateria. In our opinion, this taxon is more likely to fall within the Spiralia (rather than the deuterostomes or ecdysozoans). In the following, we tentatively suggest that D. thambus might represent a stem-group gnathiferan.
Geological setting
The general setting of this Lagerstätte has been reviewed by Lerosey-Aubril et al. (Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018). In brief, the Weeks Formation (Miaolingian, Guzhangian) is a relatively deepwater deposit, apart from the upper section (70 m) that records a substantial shallowing of the depositional environment associated with the end of basinal accumulation in the so-called House Range Embayment. Below this transitional interval, lithologies are alternating micrites and calcareous claystones. These are indicative of a low-energy, distal ramp environment, which was periodically disturbed by storm-induced gravity flows and episodes of oxygen depletion. Unlike the Burgess Shale, where much of the biota was introduced into a toxic environment by small turbidity flows (e.g., Conway Morris, Reference Conway Morris1986), transport in this Lagerstätte was evidently minimal. The exceptional preservation in the Weeks Formation is restricted to a 25 m interval about 205 m below the top of the unit. This interval has yielded a diverse fauna (~73 species), which according to agnostoids (Proagnostus gibbus Zone) and trilobites (Cedaria Zone) is of mid-Guzhangian age.
Preservation
The fossils described here show the same style of preservation as most of the non-biomineralizing taxa of the fauna (Lerosey-Aubril et al., Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018, fig. 3b, c). This is the result of a series of chemical and physical alterations that occurred mostly at a late stage of diagenesis. Such is very much a hallmark of the Weeks Formation fauna where evidence of diagenetic phosphatization is associated with strong taxonomic and histological controls. Indeed, all known instances of secondary phosphatization concern organs rich in phosphorus (e.g., arthropod guts) or tissues underneath primary phosphatic structures, such as aglaspidid cuticle or palaeoscolecid plates (Lerosey-Aubril, Reference Lerosey-Aubril2015; Lerosey-Aubril et al., Reference Lerosey-Aubril, Hegna, Kier, Bonino, Habersetzer and Carré2012, Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018).
In the case of D. thambus n. gen. n. sp., these postmortem changes include the initial flattening of the carcasses and, much later, the replacement of the presumably carbonaceous material with pyrite and subsequent coating of this pyritic layer (now as oxidized pseudomorphs) by chlorite (in a fan-like arrangement) (Fig. 2). This strong diagenetic imprint is related to major igneous intrusions as well as more recent intense weathering. Scanning electron micrographs of specimens of D. thambus suggest that the trunk is composed chiefly of iron oxides and chlorite (Fig. 2), and this is consistent with compositional (energy-dispersive X-ray spectroscopy [EDS]) analyses (Supplemental Fig. 1).
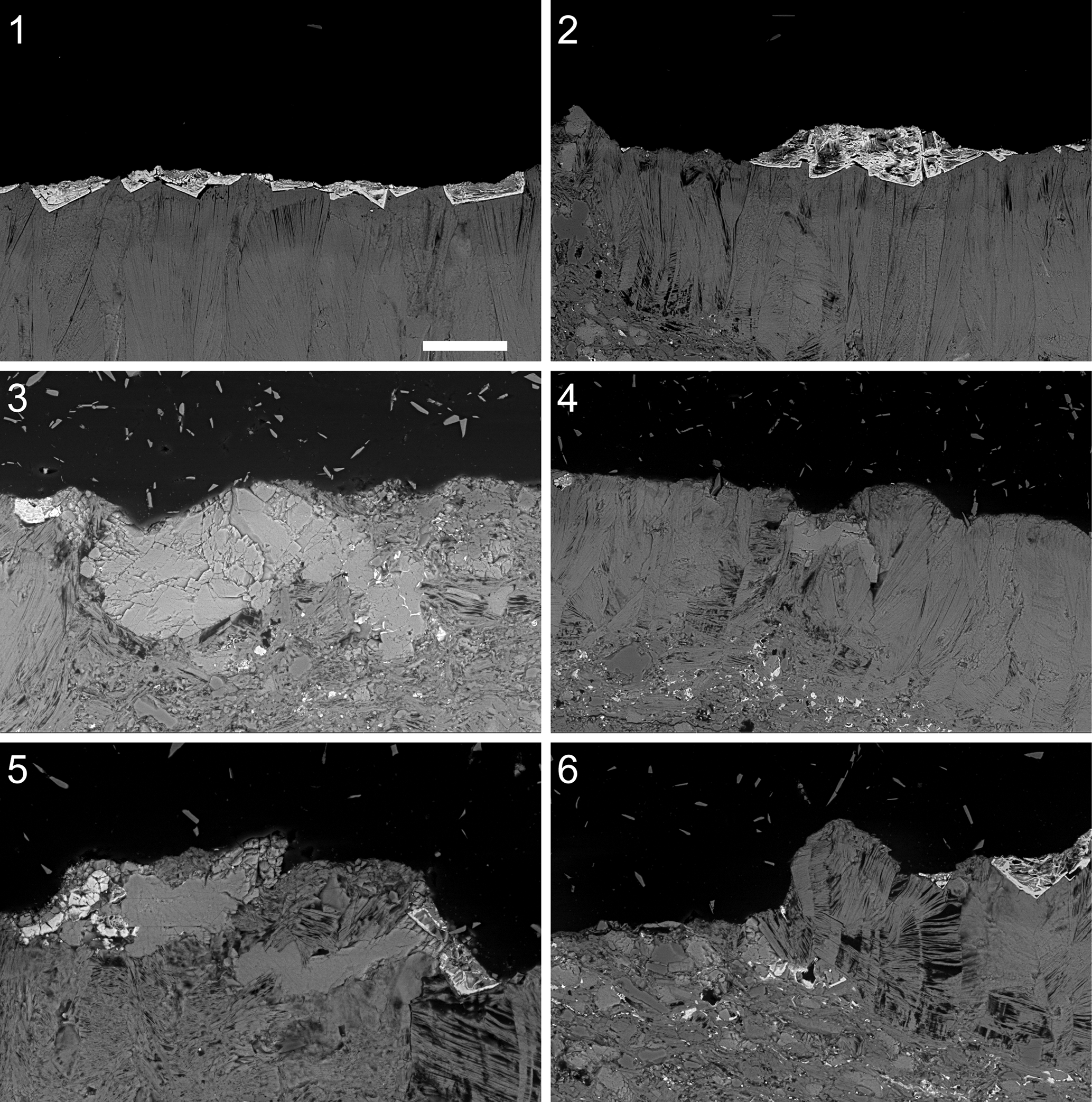
Figure 2. Dakorhachis thambus n.gen. n.sp. from the Weeks Formation (Miaolingian, Guzhangian), Utah, USA. Scanning electron micrographs in backscatter mode of polished sections. (1, 2) UU15101.07: (1) fossil body composed of radiating fans of a chloritic mineral with pseudomorphs of pyrite across upper surface; (2) detail of fossil body and pseudomorphs. (3–6) UU15101.08: (3) tooth, composed of calcite; (4) tooth and surrounding fossil body; (5) two teeth and surrounding fossil body, including pyrite pseudomorphs; (6) fossil body with stacked chloritic mineral. Scale bar = 50 μm.
The translucent teeth differ in composition from the trunk and appear to have a predominantly calcitic composition (Fig. 2.3–2.5). As discussed in the following, while an original composition cannot be excluded, it seems as likely that the calcite is also diagenetic. Micro-CT shows moderate three-dimensional (3D) preservation of the teeth at the specimen surface. However, due to the mode of fossil preservation (low-density contrast composition and compression), no further (e.g., internal or subsurface) 3D information was recovered.
Materials and methods
The material consists of nineteen specimens preserved flattened parallel to bedding. One slab bears two specimens (UU.15101.05, 15101.06), two slabs have three specimens each (UU15101.02, 15101.03, 15101.04 and 15101.12, 15101.13, 15104.14), while another slab has five superimposed specimens (UU15101.07, 15101.08, 15101.09, 15101.10, 15101.11); other specimens are isolated. This material was examined under a binocular microscope with a drawing tube employed to prepare camera-lucida interpretative drawings. Specimens UU17122.03, 18056.27, and 18056.28 were photographed immersed in dilute ethanol using a Leica IC80 HD camera mounted on a Leica M80 microscope. Specimen UU17122.03 was studied uncoated (low vacuum mode) using a scanning electron microscope (SEM) JEOL JSM-6010LV equipped with an EDS module JEOL EX-94410T1L11 at the University of New England. Similar SEM and EDS investigations were performed on both entire specimens (UU15101.01, UU15101.07) and polished sections using a QEMSCAN 650F SEM at the University of Cambridge. Last, computed tomography (CT) scans of specimen UU15101.01 (holotype) were obtained using a Nikon XTH225 ST CT scanner at the Cambridge Biotomography Centre.
Repositories and institutional abbreviations
Types, figures, and other specimens (including petrographic sections) examined in this study are deposited in the Department of Geology and Geophysics (Research Collections), University of Utah, USA (UU,) and Back to the Past Museum, Cancún, Mexico (BPM).
Systematic paleontology
?Superphylum Spiralia
?Gnathifera-Chaetognatha
Family Dakorhachiidae new family
Type genus (by monotypy)
Dakorhachis n. gen. from the Miaolingian (Guzhangian) Weeks Formation of the House Range, Utah, USA.
Diagnosis
Vermiform, segmented body anteriorly bearing prominent ?calcitic teeth.
Remarks
Chaetognatha is currently treated as a distinct phylum, and recent molecular evidence (Fröbius and Funch, Reference Fröbius and Funch2017; Marlétaz et al., Reference Marlétaz, Peijnenburg, Goto, Satoh and Rokhsar2019) links them to the Gnathifera, whose component phyla are Gnathostomulida, Micrognathozoa, and Rotifera (with parasitic Acanthocephala). Phylum status denotes their morphological distinctiveness, but all these phyla are united by the possession of an anterior basket of chitinous teeth. As discussed in the following, D. thambus n. gen. n. sp. is tentatively interpreted as a stem-group representative of a Gnathifera-Chaetognatha clade (we suggest the colloquial moniker chaetognathiferans).
Genus Dakorhachis new genus
Type species
Dakorhachis thambus n. gen. n. sp. (by monotypy).
Diagnosis
As for type species by monotypy.
Etymology
A combination of dakos (Greek), a biter, and rachis (Greek), ridge.
Remarks
A new genus that appears to have no known equivalents elsewhere.
Dakorhachis thambus new species
Figures 3–7, Supplemental Figure 2
- Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018
‘Enigmatic organism’ Lerosey-Aubril et al., fig. 3a–c.

Figure 3. Dakorhachis thambus n. gen. n. sp. from the Weeks Formation (Miaolingian, Guzhangian), Utah, USA. (1) UU15101.02 (upper) and UU15101.03 (lower); (2) UU15101.04; (3) UU15101.05 (upper) and UU15101.06 (lower); (4) BPM1090; (5) UU15101.01 (holotype); (6) UU18056.27; (7) UU17122.03; (8) UU18056.28. (1–5) Specimens photographed dry. (6–8) Specimens immersed in dilute ethanol. (1, 5) Scale bars = 5 mm; (2–4, 6–8) scale bars = 2 mm.

Figure 4. (1, 3) Feeding apparatus of Dakorhachis thambus n. gen. n. sp.: (1) UU15101.01 (holotype; CT images, Fig. 5); (3) UU15101.02. (2, 4) Corresponding camera lucida drawings. Body (blue/light gray), teeth exterior view (red/very dark gray), interior view (pink/fairly dark gray), V-shaped units (green/dark gray), rods (yellow/very pale gray), adhesive (grey/darkish gray), oxides (hatched), sediment (white). Scale bars = 1 mm.

Figure 5. Holotype (UU15101.01) of Dakorhachis thambus n. gen. n. sp. (1) micro-CT volume rendering; false color represents specimen density. (2) Rotated view showing 3D transverse banding on the trunk, perpendicular to the long axis. (3) Detail of teeth. (4) Simplified reconstruction. Scale bar = 5 mm.

Figure 6. Electron micrographs of the feeding apparatus of the holotype (UU15101.01) of Dakorhachis thambus n. gen. n. sp. (1) Overview; (2) detail showing the hollow tooth interior and fibrous microstructure. Scale bar = 500 μm.

Figure 7. SEM of the body trunk surface of Dakorhachis thambus n. gen. n. sp. specimen UU15101.01 showing iron oxides layer (black arrow) and the imprints of pseudomorphs of iron oxides after pyrite on the segmented chloritic surface (white arrows). Scale bar = 0.5 mm.
Holotype
Complete specimen (UU15101.01), Department of Geology and Geophysics (Research Collections), University of Utah, Salt Lake City, Utah.
Diagnosis
Elongate and robust body. Feeding apparatus comprising at least six hollow teeth, characterized by gently convex outer side with prominent central ridge and concave inner side with narrow ridge-like margins, and in posterior direction associated skeletal elements in form of hook-like elements, inverse V-shaped sclerites, and elongate rods. Trunk composed of 30 segments, gently tapering posteriorly, terminating in blunt tip.
Occurrence
Exposures in North Canyon, adjacent to Notch Peak, House Range, Utah. Weeks Formation (Cambrian Series 3, Guzhangian).
Description
Apart from minor preservational variants, the material is united in showing a body consisting of a relatively elongate trunk (Figs. 3, 5, Supplemental Fig. 3) that, at its anterior, bears a prominent feeding apparatus (Figs. 4, 5.3). Total length can reach 28 mm, and maximum width of trunk is 7 mm (Supplemental Fig. 3). The feeding apparatus bears at least six prominent teeth, of which about half are exposed in outer aspect and the remainder in inner aspect, suggesting that, originally, they formed a circum-oral circlet (Fig. 4.1, 4.3). Each tooth (~3 mm long) has a narrow triangular form and in outer aspect is gently convex and bears a prominent and relatively narrow longitudinal ridge. In inner aspect, the tooth is concave, but the margins are defined by very narrow ridges. The teeth have a fibrous texture, while the broken margin of one tooth shows what may be a hollow interior (Fig. 6). Elemental analyses indicate that the teeth have a predominantly calcitic composition (see the preceding and Dryad file). Posterior to the teeth are three other skeletal components, evidently with a composition similar to the teeth (Fig. 4.2, 4.4). Immediately to the posterior of the teeth are small hook-like structures, and behind them is a series of inverse V-shaped units. Most likely these units also formed circlets. Finally, adjacent to, or superimposed on, the anteriormost trunk are rod-like structures, usually straight but occasionally with a sinuous shape.
The trunk is relatively featureless and lacks appendages or other extensions. In some specimens, the configuration is somewhat sinuous (Fig. 3.3; see also Lerosey-Aubril et al., Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018, fig. 3a), suggesting an original degree of flexibility (also Fig. 3.7). The width is more or less uniform, and although most specimens have a rounded termination, it occasionally appears to be acute. Broad transverse folds (~0.8 mm) may be surficial annulations but here are interpreted as segments (Figs. 3.1, 3.2, 5.1, 5.2, 7). In life these would have totaled about 30. That these structures are original rather than postmortem (or tectonic) is supported by three lines of evidence. First, these transverse bands are evidently three dimensional (Supplemental Fig. 2) and sometimes match a corrugated body margin. In addition, associated specimens with different orientations have folds transverse to their respective bodies rather than parallel to any rock fabric (Supplemental Fig. 3).
Etymology
From thambos (Greek), an astonishment.
Materials
Specimens UU15101.02–15101.15, 17122.03, 18056.27, 18056.28; BPM-1090.
Remarks
A new species that has no close counterpart among other Cambrian taxa.
Discussion
Paleoecology and mode of life
D. thambus n. gen. n. sp. lacks fins or other anatomical features consistent with a pelagic mode of life and therefore is interpreted as benthic. Co-association of specimens indicates a gregarious habit, although the case of parallel stacking (UU15101.07–15101.11) is most likely postmortem. Locomotory organs are not evident, but presumably this animal could have moved across or within the seafloor by peristaltic contractions. Given, however, that the arrangement of the teeth is in the form of a sort of basket, it may have captured its prey as an ambusher, and as such the animal may have been semi-sessile and partially concealed in the seafloor. The attitude of the teeth varies from parallel to an anterior convergence, but in life they presumably opened wider to tackle larger prey. The function of the skeletal elements posterior to the teeth is more conjectural. One suggestion is that they served for insertion of muscles associated with protrusion and subsequent closure of the teeth.
Phylogenetic affinities
The wider relationships of D. thambus are necessarily problematic given its lack of close identity to any known group. Such evidence as there is must look to the feeding apparatus. A potentially important clue might be the calcitic composition of the teeth, although as noted this may well be diagenetic. Certainly among metazoans, calcitic teeth are unusual, with the most notable instances being in the echinoids (e.g., Wang et al., Reference Wang, Addadi and Weiner1997; Stock et al., Reference Stock, Ignatiev, Lee and Almer2014) and extinct ophiocistioids (e.g., Reich et al., Reference Reich, Stegemann, Hausmann, Roden and Nützel2018). Moreover, in the former group, the teeth can on occasion show a fibrous microstructure (Reich and Smith, Reference Reich and Smith2009, text-fig. 9C, D). There is, however, no other feature of Dakorhachis that would indicate an affinity to either the echinoids or any other echinoderm, especially if the principal teeth totaled six, an obvious departure from the characteristic pentaradial symmetry of this phylum.
In passing, it is worth noting that D. thambus shows some broad similarities in overall shape to the unusual sponge Takakkawia lineata Walcott, Reference Walcott1920 from the Burgess Shale, which has marginal ‘fins’ extending from a conical body (Botting, Reference Botting2012). However, numerous detailed differences in morphology indicate that the resemblance between these taxa is superficial. Specifically, there are differences in the size, shape, and annulation of the body in D. thambus (which averages 2.1 cm long, is vermiform, and has transverse annulations) versus T. lineata (which is longer [Botting, Reference Botting2012, fig. 1], more vasiform, and displays diagnostic lengthwise lineations). Furthermore, there are major differences in the shape and organization of the teeth of Dakorhachis (which are elongate, sharply pointed, and restricted to the anterior body margin) versus the ‘fins’ of T. lineata (which are wider, flat topped, and accompanied by broad spicules extending down the length of the body). While there is no other reason to interpret D. thambus as any sort of sponge, the potential complexities of assigning Cambrian taxa to particular groups and the consequent phylogenetic implications are apparent from Botting and Muir's (Reference Botting and Muir2018) proposed linkage of Takakkawia to the putative ctenophore Thaumactena. That said, there is no evidence for comparing D. thambus with any of the Cambrian ctenophores (e.g., Ou et al., Reference Ou, Xiao, Han, Sun, Zhang, Zhang and Shu2015).
Notwithstanding such comparisons, D. thambus is evidently a bilaterian rather than a representative of the diploblasts (let alone a sponge). There appears to be no particular similarity to either the deuterostomes or ecdysozoans. Although, in the latter case, it is true that the priapulids and related scalidophorans typically have an introvert equipped with circlets of teeth, these and associated structures show a complex zonation and diversity of forms (e.g., Smith et al., Reference Smith, Harvey and Butterfield2015) that find no counterpart in the array of teeth seen in D. thambus or in its ancillary skeletal structures. Most likely, D. thambus is a member of the Spiralia.
Among the spiralians, the most fruitful comparisons may possibly lie with the Gnathifera. This monophyletic group (e.g., Laumer et al., Reference Laumer2015) comprises the gnathostomulids (e.g., Herlyn and Ehlers, Reference Herlyn and Ehlers1997; Sørensen et al., Reference Sørensen, Sterrer and Giribet2006), its sister group the micrognathozoans (e.g., Bekkouche et al., Reference Bekkouche, Kristensen, Hejnol, Sørensen and Worsaae2014; Bekkouche and Worsaae, Reference Bekkouche and Worsaae2016), and the syndermatans (the group encompassing the rotifers and endoparasitic acanthocephalans; e.g., Rieger and Tyler, Reference Rieger and Tyler1995; Sørensen, Reference Sørensen2002a; Wulfken and Ahlrichs, Reference Wulfken and Ahlrichs2012). Gnathiferans are millimetric and typically meiofaunal, but despite this, all possess intricate jaw apparatuses that reach an apogee in the complex array found in the micrognathozoans (e.g., Kristensen and Funch, Reference Kristensen and Funch2000; De Smet, Reference De Smet2002; Sørensen, Reference Sørensen2003). Current phylogenetic schemes place the gnathiferans as sister to all other spiralians (e.g., Laumer et al., Reference Laumer2015; Bekkouche and Worsaae, Reference Bekkouche and Worsaae2016), which in turn are broadly divided into the ‘platyozoans’ and the more securely identified lophotrochozoans.
The disparity of extant gnathiferans, combined with an almost nonexistent fossil record (e.g., Poinar and Ricci, Reference Poinar and Ricci1992; Waggoner and Poinar, Reference Waggoner and Poinar1993; Jha et al., Reference Jha, Kumar, Aggarwal, Bhattacharyya and Pande2011), and their still poorly resolved systematic position within the bilaterians pose a series of evolutionary questions. Among the most problematic is the visualization of a stem-group form and its corresponding recognition in the fossil record. This question may be further exacerbated if the millimetric size of the extant gnathiferans is the result of secondary miniaturization from macroscopic predecessors rather than a primitive state.
Intriguingly, there is also phylogenomic evidence for a link between the gnathiferans and chaetognaths (Fröbius and Funch, Reference Fröbius and Funch2017; Marlétaz et al., Reference Marlétaz, Peijnenburg, Goto, Satoh and Rokhsar2019). The latter are equipped with a formidable feeding apparatus consisting of prominent grasping spines and associated teeth (e.g., Bone et al., Reference Bone, Kapp and Pierrot-Bults1991), although at first sight there is no obvious macroscopic connection to any of the considerably more complex gnathiferan jaws. The phylogenetic position of the chaetognaths has long been regarded as basal among the bilaterians (Perez et al., Reference Perez, Rieger, Martin, Müller and Harzsch2013) but with conflicting views suggesting either a place among the most primitive protostomes (e.g., Marlétaz et al., Reference Marlétaz, Martin, Perez, Papillon and Caubit2006; Marlétaz and Le Parco, Reference Marlétaz and Le Parco2008; Shen et al., Reference Shen, Sun, Zhao, Zhang, Tian, Tsang, Wang and Chu2016) as opposed to a position among the basal lophotrochozoans (e.g., Matus et al., Reference Matus, Halanych and Martindale2007; Dunn et al., Reference Dunn2008; Bernt et al., Reference Bernt2013).
The contribution of the Cambrian fossil record to the early evolution of the chaetognaths and gnathiferans to date has focused almost entirely on the former group. Here the protoconodonts, which apart from occasional fused clusters are effectively dispersed as small shelly fossils (Szaniawski, Reference Szaniawski1982, Reference Szaniawski2002), are complemented by several soft-bodied taxa similar to extant chaetognaths (Chen and Huang, Reference Chen and Huang2002; Hu et al., Reference Hu, Steiner, Zhu, Erdtmann, Luo, Chen and Weber2007; Vannier et al., Reference Vannier, Steiner, Renvoisé, Hu and Casanova2007; Shu et al., Reference Shu, Conway Morris, Han, Cuthill, Zhang, Cheng and Huang2017) and what appear to be two more primitive representatives (Ankalodus sericus Shu et al., Reference Shu, Conway Morris, Han, Cuthill, Zhang, Cheng and Huang2017 and Capinatator praetermissus Briggs and Caron, Reference Briggs and Caron2017) characterized by supernumerary teeth (Briggs and Caron, Reference Briggs and Caron2017) or a multi-jawed morphology (Shu et al., Reference Shu, Conway Morris, Han, Cuthill, Zhang, Cheng and Huang2017) (Supplemental Fig. 1). It is now clear, however, that the hitherto enigmatic Amiskwia (Conway Morris, Reference Conway Morris1977) possesses a jaw apparatus that supports some sort of connection to the gnathiferans and/or chaetognaths (Caron and Cheung, Reference Caron and Cheung2019; Vinther and Parry, Reference Vinther and Parry2019).
Although the record of relevant soft-bodied taxa (Amiskwia, Ankalodous, Capinatator) is meager, as potential stem-group chaetognathiferans they hint at both morphological disparity and a range of ecologies from swimming to benthic. To this roster we tentatively propose to add D. thambus. As is the case with a number of other controversial Cambrian groups, a convincing phylogenetic analysis is frustrated by the paucity of available character-states and the added possibility that those available for tabulation in reality are convergent. Our assignment relies on a tentative interpretation of the feeding apparatus of D. thambus as a precursor to the much more complex jaws seen in extant gnathiferans as well as the possible equivalent in the chaetognaths. Here, therefore, we sketch a possible set of transitions (Fig. 8) that might link the feeding apparatus of Dakorhachis n. gen to those of the gnathiferans and chaetognaths.
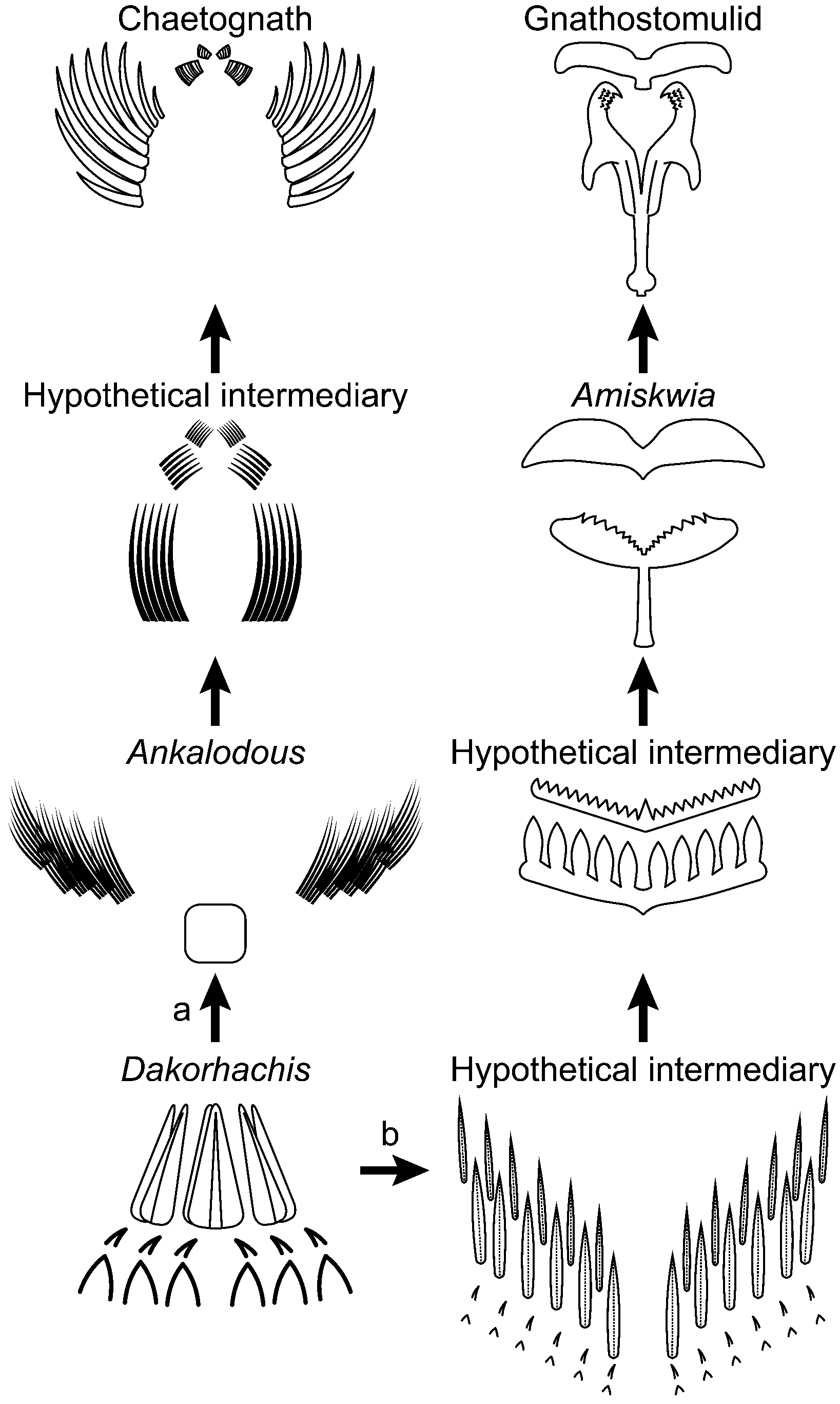
Figure 8. Hypothetical transitions between the jaw apparatus of Dakorhachis thambus n. gen. n. sp. and (a) those of the chaetognaths (and protoconodonts) via forms similar to Ankalodous sericus and (b) the gnathiferans (as represented by the gnathostomulids) via forms similar to Amiskwia sagittiformis Walcott, Reference Walcott1911.
There is agreement that some of the elements of gnathiferan apparatuses are homologous (e.g., Sørensen, Reference Sørensen2002b; Sørensen et al., Reference Sørensen, Sterrer and Giribet2006), but nevertheless collectively the clade shows a wide diversity of forms. Interestingly, the more basal gnathostomulids possess a somewhat less elaborate jaw (e.g., Riedl and Rieger, Reference Riedl and Rieger1972), and within this group there are a number of trends that can be traced from what appears to be the most primitive arrangement (e.g., Sterrer, Reference Sterrer1972; Sørensen, Reference Sørensen2002b). Thus, despite various elaborations, the basic configuration of the jaw is as a forceps-like unit joined to a proximal base and a basal plate. Derivation of this arrangement from something similar to D. thambus via an amiskwiid (Caron and Cheung, Reference Caron and Cheung2019) would, in principle, involve a shift from an effectively radial symmetry to a bilateral configuration, reduction from six teeth to three (along with substantial miniaturization), and possibly incorporation of the more proximal skeletal elements in D. thambus into the jaw apparatus.
The likely phylogenetic relationship between chaetognaths and gnathiferans (Fröbius and Funch, Reference Fröbius and Funch2017; Marlétaz et al., Reference Marlétaz, Peijnenburg, Goto, Satoh and Rokhsar2019) may also find some support in the morphology exhibited by D. thambus. While there is little obvious similarity between the jaw configurations of the gnathiferans and chaetognaths, in both cases the principal composition is chitinous (e.g., Bone et al., Reference Bone, Ryan and Pulsford1983; Sørensen and Sterrer, Reference Sørensen and Sterrer2002). The distinctive rod-like microstructures of most gnathiferan teeth (e.g., Riemann and Ahlrichs, Reference Riemann and Ahlrichs2008) is presumably a synapomorphy of the group, but in D. thambus the fibrous microstructure and possible hollow interior find a possible counterpart in the protoconodonts (e.g., Szianiawski, Reference Szaniawski2002). If there is an evolutionary connection between D. thambus and the chaetognaths, then in parallel to the gnathiferans this would involve a transition from the apparently radial configuration of the teeth in the former taxon to the bilateral arrangement on the chaetognaths. Although very different to the trajectory of the gnathiferans that led toward a meiofaunal existence, this proposed evolutionary path would also be a consequence of a major ecological shift, from a perhaps semi-sessile benthic lifestyle to a more motile pelagic one.
It is worth pointing out that while the fused clusters of protoconodonts (e.g., Szaniawski, Reference Szaniawski1982, Reference Szaniawski2002) are convincingly compared to the bundles of feeding spines in the chaetognaths, by contrast most protoconodont taxa are never recovered as fused clusters. While this disaggregation may be the consequence of standard processing of samples by acid digestion, it seems equally possible that in such taxa the arrangement of the feeding apparatus was more open and/or arranged as multiple series (Shu et al., Reference Shu, Conway Morris, Han, Cuthill, Zhang, Cheng and Huang2017). An alternative option might be that some of these feeding spines actually belonged to animals closer to D. thambus, where the teeth were not clustered but radially organized around a terminal mouth. In terms of similarities of the teeth of D. thambus and supposed protoconodonts, two possible candidates are some specimens of Protohertzina robusta Qian, Reference Qian1977 (Pyle et al., Reference Pyle, Narbonne, Nowlan, Xiao and James2006, fig. 6.8) and an unnamed taxon described by Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015, fig. 53M, their ‘undetermined form 4’). Our knowledge of early chaetognath evolution may also be incomplete. Thus, the otherwise distinctive coelocerodonts (Szaniawski, Reference Szaniawski2015) have a chaetognath-like arrangement of the teeth, while the possible protoconodont Huayuanodontus has a tooth histology distinct from other taxa (Dong, Reference Dong2007).
If we are correct in regarding D. thambus as a sister taxon of the clade gnathiferans-chaetognaths, this suggests that their common ancestor was macroscopic, semi-sessile, and segmented. Thus, the miniaturization and largely meiofaunal existence would have been secondarily acquired in the evolutionary history of gnathiferans, in contrast to the general assumption that it is a plesiomorphic condition for the group (e.g., Laumer et al., Reference Laumer2015). As to the chaetognaths, our discovery cannot resolve more precisely their position relative to other early bilaterians (e.g., Marlétaz and Le Parco, Reference Marlétaz and Le Parco2008; Shen et al., Reference Shen, Sun, Zhao, Zhang, Tian, Tsang, Wang and Chu2016; Marlétaz et al., Reference Marlétaz, Peijnenburg, Goto, Satoh and Rokhsar2019). It supports, however, the idea that, notwithstanding subsequent loss and redeployment (Blair, Reference Blair2008), segmentation among the bilaterians is primitive. Moreover, in extant chaetognaths, the progenitor neural cells of the trunk not only are highly organized but also form 30–35 rows (Perez et al., Reference Perez, Rieger, Martin, Müller and Harzsch2013), comparable to the segment total in D. thambus. Primitive chaetognaths such as Ankalodous (Shu et al., Reference Shu, Conway Morris, Han, Cuthill, Zhang, Cheng and Huang2017) may have also had relatively limited motility, but overall there was evidently a shift to a much more active mode of life (e.g., Vannier et al., Reference Vannier, Steiner, Renvoisé, Hu and Casanova2007). Evidence for a migration to a pelagic mode of life (Hu et al., Reference Hu, Steiner, Zhu, Erdtmann, Luo, Chen and Weber2007; Vannier et al., Reference Vannier, Steiner, Renvoisé, Hu and Casanova2007; Casenove et al., Reference Casenove, Oji and Goto2011) is supported by both the evolution of chaetognath musculature (Casanova and Duvert, Reference Casanova and Duvert2002) and molecular data (Papillon et al., Reference Papillon, Perez, Caubit and Le Parco2006). Significantly, this shift may have been via benthoplanktonic intermediates, although the few truly benthic chaetognaths extant are very derived (Casanova and Duvert, Reference Casanova and Duvert1996) and show no significant similarities to D. thambus. This transition to the pelagic realm would also have been marked by the separation of the teeth into two separate grasping bundles (along with smaller teeth adjacent to the mouth), changes in the patterns of their replacement (Moreno and Kapp, Reference Moreno and Kapp2003), and loss of mineralization to assist buoyancy. This would have been combined with extensive reorganization of the head musculature. Further changes would have included narrowing of the body, reduction to an oligomeric (tripartite) segmentation (Balavoine and Adoutte, Reference Balavoine and Adoutte2003), and the development of prominent fins and complex eyes.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.p5hqbzkkz.
Acknowledgments
We thank V. Brown for extensive editorial assistance, I. Buisman, G. Lampronti, and R. Asher for access to analytical facilities, and N.J. Butterfield and J. Ortega-Hernández for critical reviews. Three anonymous reviewers made many helpful suggestions. Financial support from the Harry and Dorothy Whittington Fund and Templeton World Charity Foundation (TWCF) to SCM, and from the National Geographic Society (grant number 9567-14) to RL-A is gratefully acknowledged.










