Introduction
White matter pathology in patients with schizophrenia and its impact on brain connectivity has been widely reported (Bora et al. Reference Bora, Fornito, Radua, Walterfang, Seal, Wood, Yücel, Velakoulis and Pantelis2011; Samartzis et al. Reference Samartzis, Dima, Fusar-Poli and Kyriakopoulos2014), but whether these deficits remain stable, deteriorate, or improve between illness stages is unclear. Most diffusion imaging studies in schizophrenia have been undertaken in chronic patients, and have found that illness is associated with reduced fractional anisotropy (FA), a measure of myelination, membrane permeability, and fiber density (Song et al. Reference Song, Sun, Ramsbottom, Chang, Russell and Cross2002; Song et al. Reference Song, Sun, Ju, Lin, Cross and Neufeld2003; Sampaio-Baptista et al. Reference Sampaio-Baptista, Khrapitchev, Foxley, Schlagheck, Scholz, Jbabdi, DeLuca, Miller, Taylor, Thomas, Kleim, Sibson, Bannerman and Johansen-Berg2013). Reduced FA has been most consistently localized to the superior longitudinal fasciculus, uncinate fasciculus, cingulum bundle, and corpus callosum (Abdul-Rahman et al. Reference Abdul-Rahman, Qiu and Sim2011; Fitzsimmons et al. Reference Fitzsimmons, Kubicki and Shenton2013; Liu et al. Reference Liu, Lai, Wang, Hao, Chen, Zhou, Yu and Hong2013; Fujino et al. Reference Fujino, Takahashi, Miyata, Sugihara, Kubota, Sasamoto, Fujiwara, Aso, Fukuyama and Murai2014; Holleran et al. Reference Holleran, Ahmed, Anderson-Schmidt, McFarland, Emsell, Leemans, Scanlon, Dockery, McCarthy, Barker, McDonald and Cannon2014). Automated fiber tracking, or tractography, is a more direct approach to investigate white matter connectivity with diffusion imaging (Kim et al. Reference Kim, Kim, Park, Lee, Kim, Kim and Park2008). This is particularly important in psychiatric disorders such as schizophrenia, where symptoms are thought to result from altered connectivity between distributed brain regions (Friston & Frith, Reference Friston and Frith1995; Friston, Reference Friston1998; Stephan et al. Reference Stephan, Baldeweg and Friston2006; Stephan et al. Reference Stephan, Friston and Frith2009). Fiber tracking studies conducted in patients with chronic schizophrenia have reported widespread disruptions in brain connectivity, reflecting disintegrated network architecture (Van Den Heuvel et al. Reference Van Den Heuvel, Mandl, Stam, Kahn and Hulshoff Pol2010; Zalesky et al. Reference Zalesky, Fornito, Seal, Cocchi, Westin, Bullmore, Egan and Pantelis2011; Collin et al. Reference Collin, Kahn, de Reus, Cahn and Van Den Heuvel2014), whereas others suggest a subtle randomization of network organization (Rubinov et al. Reference Rubinov, Knock, Stam, Micheloyannis, Harris, Williams and Breakspear2009).
Diffusion imaging studies in early stages of schizophrenia are scarcer, yet they can provide crucial leads into the primary origins of white matter pathology, since confounding epiphenomena owing to medication, illness chronicity, and normal brain degeneration are minimized. Several neuroimaging studies have reported white matter pathology following the initial onset of psychosis, most commonly in frontal, frontotemporal, and frontolimbic connections (Samartzis et al. Reference Samartzis, Dima, Fusar-Poli and Kyriakopoulos2014). However, findings are less consistent in early illness stages, with some studies reporting significant FA reductions (Cheung et al. Reference Cheung, Cheung, McAlonan, Deng, Wong, Yip, Tai, Khong, Sham and Chua2008; Szeszko et al. Reference Szeszko, Robinson, Ashtari, Vogel, Betensky, Sevy, Ardekani, Lencz, Malhotra, McCormack, Miller, Lim, Gunduz-Bruce, Kane and Bilder2008; Rathi et al. Reference Rathi, Kubicki, Bouix, Westin, Goldstein, Seidman, Mesholam-Gately, McCarley and Shenton2011; Lee et al. Reference Lee, Kubicki, Asami, Seidman, Goldstein, Mesholam-Gately, McCarley and Shenton2013; Yao et al. Reference Yao, Lui, Liao, Du, Hu, Thomas and Gong2013; Zhang et al. Reference Zhang, Wei, Kang, Zalesky, Li, Xu, Li, Wang, Zheng, Wang, Zhao, Zhang and Huang2014) and other studies reporting no between-group differences (Price et al. Reference Price, Bagary, Cercignani, Altmann and Ron2005; Peters et al. Reference Peters, de Haan, Dekker, Blaas, Becker, Dingemans, Akkerman, Majoie, van Amelsvoort, den Heeten and Linszen2008). These inconsistencies might reflect methodological discrepancies related to image acquisition and postprocessing, limitations of FA as a measure of white matter integrity as well as heterogeneity in the sample population, or reflect different points in illness trajectories (Pantelis et al. Reference Pantelis, Yücel, Bora, Fornito, Testa, Brewer, Velakoulis and Wood2009; Cropley & Pantelis, Reference Cropley and Pantelis2014).
FA has been evaluated in both recently diagnosed and chronic schizophrenia cohorts to investigate potential deterioration in white matter integrity over the illness (Friedman et al. Reference Friedman, Tang, Carpenter, Buchsbaum, Schmeidler, Flanagan, Golembo, Kanellopoulou, Ng, Hof, Harvey, Tsopelas, Stewart and Davis2008; Kong et al. Reference Kong, Ouyang, Tao, Liu, Li, Zhao, Xue, Wang, Jiang, Shan and Liu2011; White et al. Reference White, Magnotta, Bockholt, Williams, Wallace, Ehrlich, Mueller, Ho, Jung, Clark, Lauriello, Bustillo, Schulz, Gollub, Andreasen, Calhoun and Lim2011; Wu et al. Reference Wu, Hwang, Chen, Hsu, Lo, Liu, Hwu, Liu, Hsieh, Chien, Chen and Isaac Tseng2015). These studies have also yielded inconsistent results. For example, Wu et al. (Reference Wu, Hwang, Chen, Hsu, Lo, Liu, Hwu, Liu, Hsieh, Chien, Chen and Isaac Tseng2015a ) found that FA did not markedly differ between first-episode and chronic patients, except within callosal fibers interconnecting the dorsolateral prefrontal cortex bilaterally. They concluded that white matter pathology remained largely static after illness onset. In contrast, earlier studies identified widespread FA reductions in chronic illness, while less severe (Friedman et al. Reference Friedman, Tang, Carpenter, Buchsbaum, Schmeidler, Flanagan, Golembo, Kanellopoulou, Ng, Hof, Harvey, Tsopelas, Stewart and Davis2008) or no alterations (Kong et al. Reference Kong, Ouyang, Tao, Liu, Li, Zhao, Xue, Wang, Jiang, Shan and Liu2011; White et al. Reference White, Magnotta, Bockholt, Williams, Wallace, Ehrlich, Mueller, Ho, Jung, Clark, Lauriello, Bustillo, Schulz, Gollub, Andreasen, Calhoun and Lim2011) in FA were observed at illness onset. As such, the extent and overlap of connectivity deficits between early and chronic illness remains unclear.
We addressed this by investigating white matter connectivity between recent-onset and chronic patients with schizophrenia, comparing them to separate, age-matched controls. Using cross-sectional neuroimaging data, we investigated whether connectivity disruptions in recently diagnosed patients were either comparable in extent and magnitude to chronic patients or significantly more confined and subtle. Tractography was performed to map a connectome for each individual and quantify the strength of white matter connectivity between all pairs of cortical and subcortical regions. Firstly, all mapped connections were tested for a significant streamline count reduction in recent-onset and chronic groups, relative to separate age-matched control groups, and permutation tests were then used to test whether any reductions significantly overlapped between the two patient groups (overlap model). We hypothesized that connectivity disruptions in recent-onset patients would form a circumscribed subset of a more widespread network of connectivity disruptions found in chronic patients. Secondly, a linear decrease in streamline count as a function of illness stage was tested, with connectivity strongest in controls, weakest in chronic patients, and midway between these two extremities in recent-onset patients (linear decline model).
Methods
Participants
This study was approved by the Melbourne Health (2012.066, 2012.069) and Austin Health (H2012/04525) Human Research Ethics Committees, consistent with the Helsinki Declaration of 1975, as revised in 2008. Participants provided written informed consent prior to participation. A total of 151 participants were recruited, which comprised 45 patients with chronic schizophrenia-spectrum disorder (illness duration > 10 years), 19 recent-onset patients (within 2 years of a first episode of psychosis), and 87 healthy controls. Controls consisted of two groups that were age-matched to recent-onset and chronic patients: young controls (n = 27, age: 21.8 ± 1.8) and older controls (n = 60, age: 39.7 ± 10.4). This enabled accurate age matching and provided an independent control group for each patient group.
Eligible patients were diagnosed with a schizophrenia-spectrum disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (First & Gibbon, Reference First, Gibbon, Hilsenroth and Segal2004). Diagnoses were confirmed using two instruments: the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First & Gibbon, Reference First, Gibbon, Hilsenroth and Segal2004) or the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998).
Healthy control participants were recruited using online advertisements from the same geographical location as patients. Healthy controls were interviewed to confirm they had no history of psychopathology using the SCID or MINI.
Exclusion criteria for all participants included a history of head injury or seizures, diagnosis of a neurological disorder, pregnancy, and contraindication to MRI scanning. Additional exclusion criteria for healthy controls were personal and first-degree relatives with a history of mental illness and alcohol or drug dependence.
Clinical measures
The following scales were employed within 2 weeks of the MRI scan: the SCID (First & Gibbon, Reference First, Gibbon, Hilsenroth and Segal2004) or MINI (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) to confirm diagnoses and assess exclusion criteria; the Wechsler Test of Adult Reading (Wechsler, Reference Wechsler2001) to determine premorbid IQ; the Expanded Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, Reference Overall and Gorham1962) or the Positive and Negative Syndrome Scale (PANSS; Kay et al. Reference Kay, Flszbein and Opfer1987) to provide a rating for general psychopathology and positive symptoms and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1983) to measure negative symptoms. PANSS scores were converted to BPRS scores (Leucht et al. Reference Leucht, Rothe, Davis and Engel2013), in order to provide a measure of general psychopathology and positive symptoms.
MRI acquisition
Sixty diffusion-weighted volumes and 10 diffusion-unweighted (b0) volumes were obtained with an echo planar imaging sequence (see online Supplement 1 for acquisition parameters, preprocessing, and tractography pipeline). An overview of the preprocessing pipeline and statistical analysis is shown in Fig. 1.

Fig. 1. Schematic of connectome mapping and analysis methods. (a) Automated white matter fiber tracking was used to map whole-brain connectivity matrices for recent-onset (RO) and chronic (CH) patients with schizophrenia as well as age-matched healthy controls. Each cell of an individual's connectivity matrix quantified the number of streamlines identified between a distinct pair of gray-matter regions and provided a measure of inter-regional white matter connectivity strength. Two statistical models were used to investigate the extent of overlap in connectivity disruptions between early and chronic stages of illness. (b) The overlap model tested whether connections with a reduced streamline count in RO patients were significantly confined within the network of disrupted connections identified in CH patients. The linear decline model tested whether streamline counts satisfied a linear trajectory of decline between the three groups of individuals [black trajectory, (c)]. Note that the linear model was also mildly sensitive to the non-linear pattern of decline [red trajectories, (c)].
Network construction
White matter connectivity was modeled as an undirected network (connectome) for each individual. Network nodes were based on the 116 cortical, subcortical, and cerebellar regions comprising the automated anatomical labeling atlas (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002). The Craddock-90 (Craddock et al. Reference Craddock, James, Holtzheimer, Hu and Mayberg2012) parcelation atlas was used in supplementary analyses (online Fig. S1 in Supplement 1) to evaluate robustness to the choice of nodes and variation in node size (Zalesky et al. Reference Zalesky, Fornito, Harding, Cocchi, Yücel, Pantelis and Bullmore2010b ). Each individual's network was represented with a symmetric N × N connectivity matrix, where N denoted the number of nodes (Fig. 1 a). Each element of the connectivity matrix was populated with the number of streamlines between the corresponding pair of regions (streamline count), which served as a measure of inter-regional connection strength. In supplementary analyses, connectivity matrices were populated based on FA averaged over all the voxels traversed by the streamlines interconnecting a pair of regions. Streamlines with one or both of their endpoints terminating in white matter were discarded, as were streamlines with both endpoints terminating in the same node. Streamlines that were <20 mm in length were considered spurious and also discarded. For streamlines that interconnected more than two nodes, the first node encountered upon leaving white matter was used. In addition, all connectivity matrices were subject to a group threshold, applied across all subjects, to eliminate spurious connections (de Reus & Van Den Heuvel, Reference de Reus and Van Den Heuvel2013). This involved eliminating any pair of regions that was not interconnected by one or more streamlines in at least 25% of all individuals. This resulted in a connection density of approximately 30% (see online Fig. S2 in Supplement 1 for analyses using connection densities of 20 and 40%).
Statistical identification of disrupted connections
We tested for streamline count differences in the recent-onset and chronic groups, relative to separate age-matched control groups. Two comparisons were performed using two-sample t tests: recent-onset patients v. younger controls and chronic patients v. older controls. First, the total number of streamlines comprising the connectivity matrices was compared to test for whole-brain differences in the streamline count. Second, the network-based statistic (NBS; Zalesky et al. Reference Zalesky, Fornito and Bullmore2010a ) was used to localize differences in the connection strengths (streamline count and FA) to specific networks, while controlling the family-wise error (FWE) rate. The NBS is a widely used, non-parametric method for performing inference on brain networks and has been shown to outperform procedures such as false discovery rate correction (Zalesky et al. Reference Zalesky, Fornito and Bullmore2010a ). Foremost, a test statistic is computed independently for each connection and any connection with a test statistic exceeding a predefined threshold is admitted to a set of suprathreshold connections. Connected components (subnetworks) are then identified in the set of suprathreshold connections. For each connected component, a FWE-corrected p value is computed by generating an empirical null distribution of maximal component size with permutation testing. In this way, the null hypothesis can be rejected at the level of each connected component. Here, a primary t-statistic threshold of 3 was used and 5000 permutations were generated to construct the null distribution of maximal component size (see online Fig. S3 in Supplement 1 for analyses using t-statistic thresholds 2.5 and 3.5). For each permutation, group labels were randomized such that some controls were labeled patients and vice versa. Gender was included as a nuisance covariate.
Statistical testing of overlap in connectivity disruptions between illness stages
Overlap model
Permutation testing was used to test whether any connectivity reductions significantly overlapped between the recent-onset and chronic groups (overlap model; Fig. 1 b). Specifically, we tested whether connections with significantly altered streamline counts in recent-onset patients formed a circumscribed subset of a more widespread network of connectivity disruptions found in chronic patients. This was achieved by quantifying the proportion of disrupted connections in recent-onset patients that were also disrupted in chronic patients. A permutation test was performed to determine whether the proportion of overlap was greater than attributable to chance. For each of 5000 permutations, the observed set of disrupted connections in recent-onset patients was replaced with a randomly chosen set of connections and the proportion of overlap was then computed for this random sample to construct an empirical null distribution.
Linear decline model
Linear regression was used to test for a linear decrease in connectivity strength as a function of illness stage (linear decline model; Fig. 1 c). Specifically, it was tested whether connectivity was strongest in controls (younger and older combined), weakest in chronic patients, and midway between these two extremities in recent-onset patients. This model was encoded as a regression with an explanatory variable in which chronic patients were coded with a 1, recent-onset patients with a 2 and controls with a 3. Age was included as a nuisance covariate. The regression was performed independently for each connection and the NBS (Zalesky et al. Reference Zalesky, Fornito and Bullmore2010a ) was used to control for multiple comparisons.
Statistical analysis of demographic and clinical measures
Two-tailed t tests were used to assess differences in age, premorbid and current IQ, and number of years educated between patient groups and their respective healthy control groups and for differences in clinical variables between the two patient groups. Partial correlations, with gender as a covariate, were used to test relationships between clinical variables (general psychopathology, positive and negative symptoms) and mean streamline counts. All p values < 0.05 were considered statistically significant.
Results
Demographics
There were no significant differences in age between patient groups and their respective healthy control groups (Table 1). Chronic patients displayed significantly lower premorbid [t(93) = 5.54, p < 0.01] and current IQ [t(93) = 6.74, p < 0.01] and were educated for fewer years [t(93) = 4.09, p < 0.01] compared with aged-matched healthy controls. In addition, recent-onset patients displayed lower current IQ compared with aged-matched healthy controls [t(31) = 4.05, p < 0.01]. Compared with recent-onset patients, chronic patients exhibited significantly greater positive symptom (BPRS subscale) scores [t(55) = 2.90, p < 0.01] and negative symptom (SANS total) scores [t(55) = 3.87, p < 0.01]. Although IQ and years of education were significantly different between control and patient groups, disentangling these effects from schizophrenia is generally difficult, given the disorder is characterized by pervasive cognitive impairments (Kahn & Keefe, Reference Kahn and Keefe2013) and in turn, controlling for IQ and education in statistical analyses may be inappropriate (Miller & Chapman, Reference Miller and Chapman2001; Klauser et al. Reference Klauser, Baker, Cropley, Bousman, Fornito, Cocchi, Fullerton, Rasser, Schall, Henskens, Michie, Loughland, Catts, Mowry, Weickert, Shannon Weickert, Carr, Lenroot, Pantelis and Zalesky2016).
Table 1. Demographic and clinical characteristics of study participants
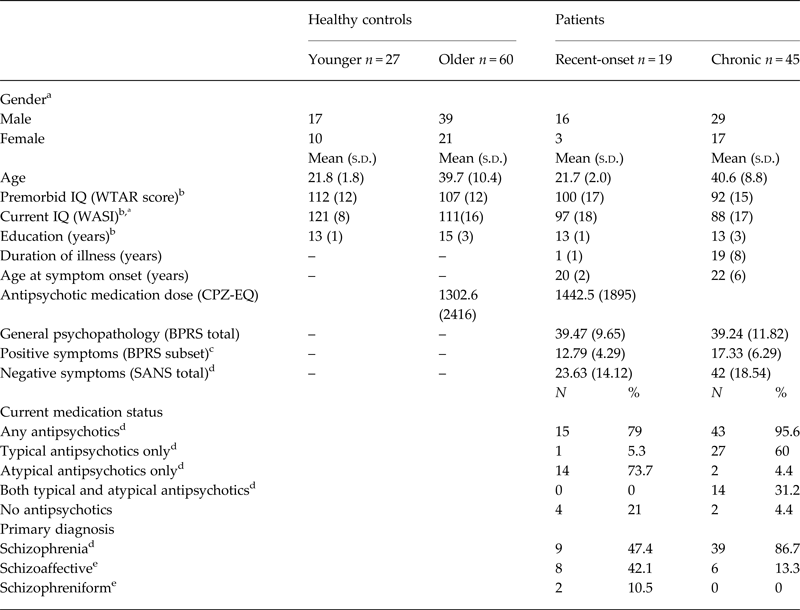
a Significant difference between younger healthy controls and recent-onset patients p < 0.01.
b Significant difference between older healthy controls and chronic patients p < 0.01.
c Significant difference between recent-onset and chronic patients p < 0.01.
d Significant difference between recent-onset and chronic patients p < 0.001.
e Significant difference between recent-onset and chronic patients p < 0.05.
Disrupted connectivity
Compared with the group of younger controls, a relatively localized network of connections with significantly reduced streamline counts was identified in recent-onset patients. This disrupted network was confined to commissural fibers of the anterior corpus callosum, connecting superior frontal and parietal regions (p < 0.01, FWE; Fig. 2 a). The total number of streamlines per individual did not differ between recent-onset patients and younger healthy controls (online Fig. S4 in Supplement 1).

Fig. 2. White matter connectivity disruptions in early stages of schizophrenia overlap with a more extensive network of disruptions found in chronic stages of illness. Connections with a reduced streamline count in recent-onset (a) and chronic (b) patients. Disrupted connections are represented spatially (axial and sagittal brain views) and topologically (circular display). L, left; R, right; C, central; Mid, middle; Supp, supplementary; Ant, anterior. Connection color reflects t-statistic magnitude, ranging from 3 (red) to 5.5 (yellow). Boxplots represent network-averaged streamline counts for recent-onset (RO) patients, younger controls (Young HC), chronic (CH) patients and older controls (Older HC). Boxes represent 25th and 75th percentiles and whiskers represent 10th and 90th percentiles. Surface (beside boxplots) show the proportion of disrupted connections associated with a given region. (c) Disrupted connections in recent-onset patients significantly overlapped with a more extensive network of disrupted connections found in chronic patients. (d) Streamline trajectories representing white matter fiber bundles with a significantly reduced streamline count in both patient groups.
Comparing chronic patients to the older control group revealed a widespread network comprising multiple connections with significantly reduced streamline count in chronic patients (p < 0.01, FWE). Significant connectivity reductions affected the majority of brain lobes and included prominent association fibers such as the uncinate and inferior longitudinal fasciculus, cingulum, and corona radiata (Fig. 2 b). Furthermore, the total number of streamlines per individual was significantly reduced in chronic patients relative to older controls [t(103) = 3.4, p < 0.01; online Fig. S4 in Supplement 1]. Together, these results indicate widespread reductions in white matter connectivity in chronic illness, which were evident at the scale of the entire cortex, whereas early illness was associated with focal disruption to the anterior fibers of the corpus callosum. This finding was not due to the increased sample size of chronic patient group. The widespread reduction in streamline counts identified in the full sample of chronic patients (n = 45) was replicated for five randomly chosen subsamples of 20 chronic patients and 20 older controls. In particular, significantly more connections were disrupted across the five subnetworks derived from random samples (mean = 62, s.d. = 9.73) compared with the disrupted network observed in recent-onset patients (7, p = 0.01).
Overlap in connectivity disruptions between early and chronic illness
Having qualitatively observed more extensive connectivity reductions in chronic illness compared with early illness stages, we next sought to quantify this observation. Overlap model: A significant proportion of disrupted connections in recent-onset patients (86%) overlapped with disrupted connections in chronic patients than attributable to chance (8%, p < 0.01; Fig. 2 c and d). In other words, connectivity reductions in recently diagnosed patients coincided with disrupted connections in chronic patients. Linear decline model: Compared with controls, chronic patients displayed a 14% reduction, while recent-onset patients displayed a 6% reduction in the mean streamline count for all the connections comprising the significant subnetwork (controls > recent-onset patients > chronic patients, p < 0.01, FWE; Fig. 3). This effect was observed in a widespread network of fiber bundles connecting all brain lobes and remained significant after including gender as a covariate. Alternative linear models were not significant (e.g. ‘chronic patients > recent-onset patients > healthy controls’). These results suggest that inter-regional connectivity strength declines from early to chronic stages of illness.

Fig. 3. Widespread network of white matter connections satisfying a linear pattern of decline from early to chronic stages of schizophrenia. (a) Network of white matter connections that satisfied a linear pattern of decline whereby connectivity was strongest in controls, weakest in chronic patients and midway between these extremities in recent-onset patients. Connection color reflects t-statistic magnitude, ranging from red (low) to yellow (high). (b) The proportion of disrupted connections associated with a given region: fibers connecting the orbital–frontal cortex (OFC) and precentral gyrus were particularly affected. (c) Boxplot of network-averaged streamline counts for controls (HC), recent-onset (RO), and chronic patients (CH). (d) Streamline trajectories representing white matter fiber bundles associated with linear decline. Streamlines are colored red for left–right, green for anterior–posterior, and blue for superior–inferior.
FA-weighted connection strength
Reanalysing the data with FA-weighted connectivity instead of the streamline count revealed largely consistent results (see online Figs S5 and S6 in Supplement 1). In particular, a widespread network of reduced FA was identified in chronic patients compared with controls (p < 0.01), whereas significant FA reductions were circumscribed to frontal, parietal, and temporal regions in recent-onset patients. A significant proportion of disrupted connections in recent-onset patients coincided with disrupted connections in chronic patients (p < 0.05). Furthermore, FA-weighted connection strength also demonstrated a linear decline in white matter connectivity from early to chronic illness stages of schizophrenia.
Clinical measures
Age was significantly and negatively correlated with streamline count in both models (linear: r = −0.17, overlap: r = −0.18; p < 0.05) across all subjects. In the network associated with a linear decline (Fig. 3 a), streamline count and positive symptom severity (BPRS-positive subscale) were negatively correlated in recent-onset patients (r = −0.49, p < 0.05; Fig. 4 a). In the same network, a trend was evident toward reduced streamline count and increased general psychopathology (BRPS total) in chronic patients (r = −0.3, p = 0.05; Fig. 4 b). No other relationships were found between streamline count (in the linear and overlap models) and clinical variables, including illness duration, negative symptom scores. No association was detected between antipsychotic chlorpromazine-equivalent dose (Woods, Reference Woods2003) and connection strength defined by streamline count or FA. Further, there was no relationship between IQ and streamline count in patient or control groups.

Fig. 4. Associations between clinical measures and white matter connectivity. Within a network associated with linear decline, reduced streamline count was correlated with increased positive symptom severity in recent-onset patients (a) and increased general psychopathology in chronic patients (b).
Discussion
We mapped brain networks representing white matter connectivity in recent-onset and chronic patients with schizophrenia, and two separate groups of age-matched healthy controls. Inter-regional white matter connectivity was quantified with both the streamline count and tract-averaged FA, as measured from diffusion-weighted imaging and tractography.
We separately tested for reductions in connectivity strength in the recent-onset and chronic patient groups. The recent-onset patients were compared with a group of age-matched (young) healthy controls, while the chronic group was compared with an independent group of older controls. While significant reductions in connectivity strength were identified in both patient groups, chronic patients were associated with a more widespread network of disruptions (153 connections) compared with the recent-onset group (7 connections). Most importantly, the majority of connections found to be disrupted in recent-onset patients also comprised the widespread network of disruptions found in chronic patients. Indeed, the overlap in disrupted connections between the recent-onset and chronic patients was statistically significant (86%, p < 0.01). This is particularly notable given that mutually exclusive control groups were used to identify the disrupted networks associated with both patient groups. In other words, while significant reductions in white matter connectivity strength were circumscribed to frontal and parietal connections in recent-onset patients, these specific connections formed part of the more widespread disrupted network found in chronic patients.
Furthermore, we tested for a trajectory of greater connectivity disruption from recently diagnosed to chronic illness. As expected, we found evidence of a significant linear reduction in connectivity strength whereby chronic patients showed reduced connectivity relative to the control group, while recent-onset patients showed an intermediate reduction in connectivity strength that was approximately halfway in magnitude compared with the chronic patients. This linear model of apparent connectivity decline as a function of illness stage was significant across a widespread network of association and commissural white matter connections that spanned both cerebral hemispheres and all lobes. Therefore, a more widespread network may show reduced connectivity strength in recent-onset patients, although not severely enough to reach statistical significance. Together, our findings point to a disintegrated architecture in white matter connectivity (Collin et al. Reference Collin, Kahn, de Reus, Cahn and Van Den Heuvel2014), which may be progressive from the initial (i.e. within 2 years) to later (>10 years) stages of schizophrenia and frontal and parietal fiber bundles are first affected. Alternatively, it may indicate that such abnormalities represent early markers of poor outcome, or a combination of both. Longitudinal studies will be needed to address this issue.
Thus, one possibility is that our results indicate a worsening, or deterioration, of white matter connectivity from early to chronic illness, subject to the limitations of our cross-sectional design. This notion, supported by previous longitudinal work, indicates progressive reductions in white matter volume following illness onset (Molina et al. Reference Molina, Reig, Sanz, Palomo, Benito, Sanchez, Sarramea, Pascau and Desco2005; Whitford et al. Reference Whitford, Grieve, Farrow, Gomes, Brennan, Harris, Gordon and Williams2007; Walterfang et al. Reference Walterfang, McGuire, Yung, Phillips, Velakoulis, Wood, Suckling, Bullmore, Brewer, Soulsby, Desmond, McGorry and Pantelis2008; Andreasen et al. Reference Andreasen, Nopoulos, Magnotta, Pierson, Ziebell and Ho2011). Of note, a large longitudinal study found white matter volume reductions in patients following psychosis onset, which were more pronounced in early illness (Andreasen et al. Reference Andreasen, Nopoulos, Magnotta, Pierson, Ziebell and Ho2011). This may suggest that white matter deteriorates at specific stages/times, rather than linearly over the course of illness. Although we show greater severity of white matter abnormalities from early to chronic illness, it is unclear at what illness stage deterioration may be more marked. Furthermore, our pattern of results may not reflect a neurodegenerative trajectory, whereby white matter deteriorates following illness onset. Alternatively, it is possible that recently diagnosed patients experienced delayed or arrested white matter maturation, which would also appear as reduced connectivity compared with controls (Kochunov & Hong, Reference Kochunov and Hong2014). Therefore, further research with serial imaging is required to clarify precise trajectories of white matter progression prior to and following the onset of psychosis (Cropley & Pantelis, Reference Cropley and Pantelis2014). Despite this limitation, our findings provide novel evidence of connectivity deficits that are more severe at later stages of schizophrenia.
Our findings suggest that specific fiber bundles are selectively vulnerable to white matter deterioration. Specifically, the anterior portion of the corpus callosum was implicated in connectivity disruption across both connection weights (streamline and FA) and models (overlap and linear decline). Interhemispheric processes, mediated by callosal fibers play a crucial role in lateralizing somatosensory, attention, motor, and language processes (Caeyenberghs et al. Reference Caeyenberghs, Leemans, Coxon, Leunissen, Drijkoningen, Geurts, Gooijers, Michiels, Sunaert and Swinnen2011; Hinkley et al. Reference Hinkley, Marco, Findlay, Honma, Jeremy, Strominger, Bukshpun, Wakahiro, Brown, Paul, Barkovich, Mukherjee, Nagarajan and Sherr2012; Edwards et al. Reference Edwards, Sherr, Barkovich and Richards2014). Due to the wide-ranging functions facilitated by this tract, it is unsurprising that reduced callosal integrity is frequently implicated in schizophrenia pathophysiology and symptomatology (Kubicki et al. Reference Kubicki, McCarley and Shenton2005; Whitford et al. Reference Whitford, Kubicki, Schneiderman, O'Donnell, King, Alvarado, Khan, Markant, Nestor, Niznikiewicz, McCarley, Westin and Shenton2010; Samartzis et al. Reference Samartzis, Dima, Fusar-Poli and Kyriakopoulos2014; Walterfang et al. Reference Walterfang, Wood, Reutens, Wood, Chen, Velakoulis, McGory and Pantelis2008). Furthermore, the regions connecting these fibers, including the superior frontal gyrus and the pre and postcentral gyri are considered hub regions that are integral for global communication across the brain (Van Den Heuvel & Sporns, Reference Van Den Heuvel and Sporns2011) and are disproportionately affected in patients with schizophrenia (Van Den Heuvel et al. Reference Van Den Heuvel, Sporns, Collin, Scheewe, Mandl, Cahn, Goñi, Pol and Kahn2013; Klauser et al. Reference Klauser, Baker, Cropley, Bousman, Fornito, Cocchi, Fullerton, Rasser, Schall, Henskens, Michie, Loughland, Catts, Mowry, Weickert, Shannon Weickert, Carr, Lenroot, Pantelis and Zalesky2016).
The decreased streamline count and FA found in this study may be due to a range of microstructural changes, including demyelination or axon pathology involving degeneration, packing density, number or diameter (Barrio-Arranz et al. Reference Barrio-Arranz, de Luis-García, Tristán-Vega, Martín-Fernández and Aja-Fernández2015). We found a high degree of convergence across our FA and streamline count analyses. Specifically, both measures were reduced in our patient cohorts and across similar white matter fibers. However, more connections were disrupted in the FA-derived chronic network and were concentrated along intrahemispheric fibers, compared with more diffuse reductions observed in the streamline-derived network. Therefore, streamline count, a measure sensitive to focal white matter pathology, may provide increased sensitivity to pathology that is circumscribed to a limited segment of a long-distance interhemispheric fiber. In contrast, averaging FA over the entire fiber length can obscure circumscribed differences because these differences can be diluted in the average.
To assess the clinical relevance of our findings, we examined relationships between connectivity and symptoms. In white matter connections associated with linear decline (i.e. greater severity at chronic stage compared with early illness stages), reduced streamline count correlated to increased positive symptom severity. Positive symptoms have previously revealed associations with both increased and decreased FA in first-episode patients, depending on the regions/tracts investigated and the method (voxel/tract-based) applied to examine FA (Canu et al. Reference Canu, Agosta and Filippi2015). However, to our knowledge, no previous studies have tested the relationship between streamline count, a measure sensitive to focal white matter pathology, and positive symptoms, which limits direct comparison to previous FA reports. In chronic patients, reduced streamline count related to greater general psychopathology or symptom severity, which is consistent with previous studies reporting associations of FA with positive, negative, and total symptom severity in established schizophrenia (Canu et al. Reference Canu, Agosta and Filippi2015). However, this relationship only trended toward significance, and thus requires verification.
Connectivity reductions were not associated with illness duration, which may minimize the contribution of illness chronicity on the connectivity profiles. However, it is possible that illness duration affects specific tracts, or that deterioration in axonal connectivity occurs at specific time windows (illness onset). Furthermore, connectivity disruptions may not decline linearly with illness, and hence precludes detection with a correlation analysis. Although reduced connectivity may relate to symptom severity or disease-related processes, other factors such as age, medication, and cohort effects may contribute. Connection strength was significantly, albeit weakly, associated with age, and elevated age-related FA decline has been observed in schizophrenia (Kochunov et al. Reference Kochunov, Glahn, Rowland, Olvera, Winkler, Yang, Sampath, Carpenter, Duggirala, Curran, Blangero and Hong2013; Douaud et al. Reference Douaud, Groves, Tamnes, Westlye, Duff, Engvig, Walhovd, James, Gass and Monsch2014; Wright et al. Reference Wright, Kochunov, Chiappelli, McMahon, Muellerklein, Wijtenburg, White, Rowland and Hong2014; Cropley et al. Reference Cropley, Klauser, Lenroot, Bruggemann, Sundram, Bousman, Pereira, Di Biase, Weickert, Weickert, Pantelis and Zalesky2017); however, age-related effects unlikely contributed to our findings given patients were age-matched to separate control groups. Likewise, our results did not find a relationship between antipsychotic treatment and connectivity disruptions; however, cumulative treatment may have played a role, and could not be accounted for in this study. Further research is required to differentiate such effects. Cohort effects, including those related to generational or illness-related factors, may provide an alternative explanation for the observed group differences in connectivity. For instance, given recent-onset patients represent a heterogeneous population with many not developing chronic illnesses, the observed differences between patient groups may be due to a subgroup of recent-onset patients who do not develop chronic illness and in turn, display dissimilar neuropathological profiles to chronic patients. However, previous research suggests only 20% of patients remit following a first episode (De Hert et al. Reference De Hert, van Winkel, Wampers, Kane, van Os and Peuskens2007; Emsley et al. Reference Emsley, Rabinowitz and Medori2007). Further, our study only included individuals with a schizophrenia-spectrum disorder (i.e. schizophreniform, schizophrenia, or schizoaffective), and therefore represented a more homogeneous patient population. Conversely, a subgroup of chronic patients may have driven the differences between the patient groups. As such, the connectivity deficits found in this study may represent early markers of later illness chronicity or poor outcome. However, given the connectivity changes occurred in overlapping fiber bundles between the patient groups, it is likely that our findings support the notion of white matter progression in schizophrenia.
Some limitations are worth consideration. The primary limitation of this study is the cross-sectional design, which cannot predict clinical outcomes based on connectivity profiles or elucidate specific trajectories of white matter progression. In addition, while our sample size was sufficient to demonstrate impaired and declining connectivity, a larger sample size would enable examination of subgroups defined by symptom profiles, predictors of clinical outcome (e.g. duration of untreated illness; Whitty et al. Reference Whitty, Clarke, McTigue, Browne, Kamali, Kinsella, Larkin and O'Callaghan2008; Díaz-Caneja et al. Reference Díaz-Caneja, Pina-Camacho, Rodríguez-Quiroga, Fraguas, Parellada and Arango2015), or additional illness stages to better characterize connectivity disturbances over the course of illness. Third, we were unable to comprehensively investigate the impact of medication class (typical or atypical), given that the majority of chronic patients were treated with both typical and atypical medications, whereas the recent-onset patients were treated only with atypical antipsychotics, and thus medication class predicted group membership. It is currently unclear whether typical and atypical drugs differentially affect connectivity, and thus requires clarification in future studies. Finally, FA and streamline count are associated with several caveats when used as measures of connectivity. Specifically, diffusion tensor measures, such as FA, cannot resolve fiber populations with complex architecture – limiting interpretation of FA-derived connectivity under such conditions (Tuch et al. Reference Tuch, Reese, Wiegell and Van2003). Although tractography provides a more direct estimate of underlying connectivity, this method is still susceptible to noise (Jones et al. Reference Jones, Knösche and Turner2013) and suffers from various spatial biases that preclude detection of all white matter connections (Thomas et al. Reference Thomas, Frank, Irfanoglu, Modi, Saleem, Leopold and Pierpaoli2014; Zalesky et al. Reference Zalesky, Fornito, Cocchi, Gollo, Van Den Heuvel and Breakspear2016). Furthermore, the potential influence of morphometric differences on our fiber-tracking results cannot be entirely excluded, although our use of non-linear normalization is suggested to reasonably control for such variation (Andersson et al. Reference Andersson, Jenkinson and Smith2007).
In summary, we found evidence for a trajectory of more severe white matter connectivity deficits from early to chronic stages of schizophrenia. Connectivity disruptions in recently diagnosed patients were confined to frontal and parietal regions, and were contained within the more widespread network of connectivity disruptions found in chronic patients. Future studies should examine connectivity profiles in a longitudinal design to confirm progressive deterioration of connectivity disturbances over the course of illness and to understand the mechanisms involved. In doing so, therapeutic targets aimed at halting white matter progression may be identified.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001313.
Acknowledgments
The authors wish to gratefully acknowledge all participants for taking part in this study. In addition, the authors kindly thank Steven Tahtalian, Annabel Burnside, Sarah Gale, and Hannah Cross for assisting with data collection. Lastly, the authors are also grateful to Melbourne Health and Austin Health for providing access to participant information.
This study was supported by the National Health and Medical Research Council (1065742; C.P. 628386, 1105825; V.C. 628880); the Cooperative Research Centre for Mental Health, funded by the Commonwealth of Australia, Department of Innovation, Industry, Science and Research (20100104); Australian Rotary Health (M.A.D. Ian Scott Ph.D. Scholarship in Mental Health), the Brain and Behaviour Research Foundation (C.P. 18722; V.C. 21660; C.B. 20526) and the University of Melbourne. A.Z. was supported by a NHMRC Career Development Fellowship (GNT1047648).
Declaration of Interest
None.







