Introduction
Ionising radiation is a type of high-energy electromagnetic wave that releases electrons from atoms and molecules generating highly reactive free radicals which can damage genomic DNA and result in cell death (Ref. Reference Reisz1). Radiotherapy is one of the primary therapeutic strategies for many cancer types, either alone or in combination with surgery, chemotherapy, targeted therapy and/or immunotherapy (Ref. Reference Bahadoer2). For example, the standard treatment of nasopharyngeal carcinoma is radiotherapy, and early-stage laryngeal cancer patients are treated with radiotherapy as a primary therapy, with advanced laryngeal cancers also sensitive to chemoradiation therapy (CRT) (Refs Reference Nishimura3, Reference Anderson4). More recently, radiotherapy has been explored with newer cancer treatment modalities, such as with immunotherapeutic agent pembrolizumab, which significantly increased responses in patients with metastatic non-small-cell lung cancer (NSCLC) (Ref. Reference Theelen5).
Unfortunately, treatment resistance leads to poor outcomes for some patients. A key aspect of tumour biology that affects ionising radiotherapy efficacy is the tumour microenvironment, in particular tumour hypoxia, as the cellular responses to ionising radiation are dependent on how well oxygenated a tissue is (Ref. Reference Gray6). In fact, threefold higher radiation doses are required in hypoxic conditions to achieve the same impact as in normoxic conditions, a factor noted as the oxygen enhancement ratio (OER) (Ref. Reference Goodhead7). Elevated hypoxic content in tumours has therefore been shown to be a factor of poor prognosis and therapy resistance in many tumour types (Refs Reference Brizel8–Reference Nordsmark10). The oxygen levels at which significant radioresistance is observed (<0.13% O2) are also known as radiobiological hypoxia (Ref. Reference Hammond11). Hypoxia is therefore considered a significant challenge to ionising radiotherapy efficiency, so there is an expanding field of study looking at exploring strategies to radiosensitise hypoxic cells. This involves strategies such as increasing oxygen availability, hypoxia-activated prodrugs (HAPs) or targeted therapies for hypoxia-regulated signalling. Interestingly, high-LET (linear energy transfer) radiotherapy modalities have been shown to be less dependent on oxygen levels than low-LET ionising radiation (Refs Reference Goodhead7, Reference Bassler12, Reference Tinganelli13).
The aim of this review is to discuss how hypoxic biology impacts radiotherapy response, how hypoxic radiobiology can be explored therapeutically to avoid radiotherapy resistance and how high-LET modalities might be an alternative approach to hypoxia-induced ionising radiation resistance.
Hypoxia-mediated radiotherapy resistance
An overview of tumour hypoxia
In normal tissue the oxygen supply matches the metabolic requirements of the cells, whereas in tumour tissue oxygen consumption increases significantly and exceeds the supply, resulting in a drop of normal oxygen levels (pO2) from about 20–80 mmHg to hypoxic levels <5 mmHg, or even levels which can cause increased radioresistance (<1–10 mmHg or 0.13–1.3% O2) (Ref. Reference Hill14). In particular, oxygen tensions of lower than 1 mmHg (<0.13% O2) are associated with significant radiotherapy resistance and are therefore called radiobiological hypoxia (Ref. Reference Hammond11).
Chronic hypoxia is caused by the long-term oxygen depletion, which can be derived from increased distance from blood vessels to the tissue, as well as permanent limitations in oxygen diffusion (Ref. Reference Vaupel and Mayer15). Acute hypoxia occurs when a temporary disruption of blood flow to the tumour mass occurs because of the severely abnormal changes in the structure and function of tumour vasculatures, producing oxygen fluctuation in the tumour microenvironment (Ref. Reference Saxena and Jolly16). Because of this, solid tumours contain regions of cycling, or intermittent, hypoxia. The levels of hypoxia and proportion of the tumour that is hypoxic vary significantly due to the disorganised vessels with intermittent blood flow, which generate cyclic changes of oxygen concentrations, resulting in a dynamic microenvironment between hypoxic and reoxygenated states (Ref. Reference Bader, Dewhirst and Hammond17).
Hypoxic adaptation is underpinned by dramatic changes in gene expression patterns, and these are primarily regulated by the hypoxia-inducible factors (HIFs) (Ref. Reference Majmundar, Wong and Simon18). HIFs can transactivate the expression of genes involved in key tumour-promoting hallmarks, such as tumour angiogenesis, energy metabolism adaptation, cell death and autophagy, cell cycle regulation, metastatic spread (including the epithelial-mesenchymal transition), and both chemo- and radio-therapy resistance (Ref. Reference Kabakov and Yakimova19) (Fig. 1). HIF consists of an oxygen-sensitive α subunit (HIF-α, which includes three isoforms: HIF-1α, HIF-2α and HIF-3α), and a constitutively expressed β subunit (HIF1-β). Under normoxic conditions, HIF-α is hydroxylated by both prolyl hydroxylases (PHDs) and factor inhibiting HIF (Ref. Reference Fong and Takeda20). Proline hydroxylation within HIF-α's oxygen-dependent degradation domain by PHDs allows HIF-α to be recognised and bound by the von Hippel-Lindau E3 ligase, resulting poly-ubiquitination and subsequent degradation by the proteasome (Ref. Reference Kubaichuk and Kietzmann21). However, under hypoxic conditions the PHDs are inhibited due to the lack of oxygen as a co-factor, leading to the rapid stabilisation of HIF-α protein levels and increased interaction with its co-activators p300 and CREB binding protein (Ref. Reference Dames22). HIF-α then heterodimerises with HIF1-β, and the heterodimeric transcription factor then binds to hypoxia response elements located in target gene promoters and transactivates these targeted genes (Fig. 1) (Ref. Reference Masoud and Li23).
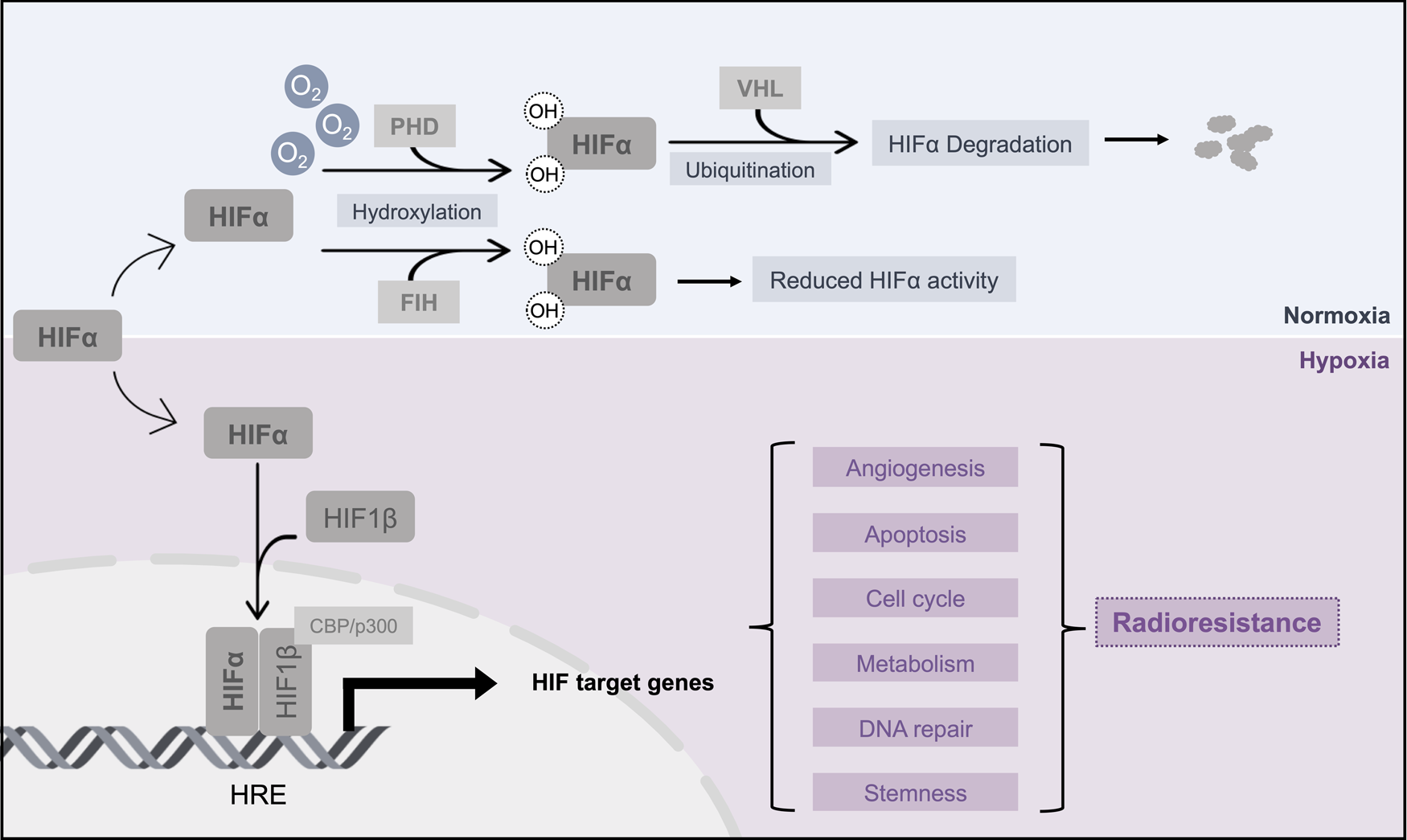
Fig. 1. Mechanisms for HIF-α-mediated radiotherapy resistance. This schematic illustrates the key mechanisms for HIF stabilisation in hypoxic conditions, and highlights key pathways up-regulated by HIF that contribute to hypoxia-mediated radiotherapy resistance. HIF, hypoxia-inducible factor; PHD, prolyl hydroxylases; FIH, factor-inhibiting HIF; VHL, von Hippel–Lindau; OH, hydroxyl groups; CBP, CREB binding protein; HRE, hypoxia response elements.
Hypoxia-mediated radiotherapy resistance
There are primarily two aspects by which hypoxia leads to radiotherapy resistance based on the mechanism of action of ionising radiation. As stated by the oxygen fixation hypothesis, during treatment with ionising radiation DNA radicals are formed either by direct ionisation or indirectly by interaction with free radicals generated by water radiolysis (Ref. Reference Wang24). Molecular oxygen rapidly interacts with these indirect radiation-induced DNA radicals leading to the production of single-strand breaks and oxidised bases, which can be resolved into lethal double-strand breaks (DSBs), leading to cell death (Ref. Reference Lomax, Folkes and O'Neill25). Therefore, in the absence of sufficient oxygen this process is inhibited, and the amount of DNA damage produced by radiation and its impact on cell viability is reduced. Other mechanisms by which hypoxic biology decreases ionising radiation efficacy include changes in reactive oxygen species (ROS) levels, inflammation signalling and HIF-regulated signalling such as induction of angiogenesis and other tumour promoting pathways (Fig. 1) (Ref. Reference Brown26). HIF-1α and HIF-2α expression have been shown to have poor prognostic value for response to radiotherapy or CRT (Refs Reference Aebersold27, Reference Koukourakis28). Counterintuitively, HIF-1α levels have been shown to increase after ionising radiation treatment through a variety of molecular mechanisms (Ref. Reference Dewhirst29). Importantly, hypoxia can also drive increased genomic instability phenotypes through the clonal loss of tumour suppressor p53, repression of the expression of other tumour suppressive factors such as E2F1 as well as key players of DNA repair pathways such as DSB repair (homologous recombination (HR)) and mismatch repair, such as RAD51, BRCA1, MLH1, amongst others (Refs Reference Chan30–Reference Lu32). It is important to note that these latter resistance mechanisms are characteristic of, but not exclusive to, radiobiological hypoxia and are associated with activation of DNA damage response (DDR) signalling and DNA replication downregulation through decreased nucleotide signalling (Refs Reference Hammond11, Reference Pires33–Reference Foskolou35).
Increasing sensitisation to ionising radiation via increased oxygen availability
There are several approaches to target hypoxia-mediated radioresistance, and one of the longest established one is the direct or indirect modulation of oxygen levels in the tumour tissue to reduce hypoxic content and increase radiosensitisation. These utilise three main broad approaches: increasing oxygen diffusion to the tissue, reducing oxygen consumption or using oxygen-mimetic molecules.
Increased oxygen diffusion
Hyperbaric oxygen (HBO) therapy has been used as a treatment for late radiation tissue injury by increasing the availability of oxygen in plasma, which improves oxygen tissue availability (Ref. Reference Bennett36). A meta-analysis of several clinical trials to investigate the effect of HBO as radiosensitisers in patients with squamous cell carcinoma of head and neck showed a significant improvement in overall radiation treatment response, as well as metastasis reduction (Ref. Reference Overgaard37). Radiotherapy after HBO breathing was found to be radiosensitised in a study using experimental models (Ref. Reference Kunugita38). However, this technique is not cost-effective for broad clinical use in later study (Ref. Reference Overgaard37).
A phase II clinical trial investigated the effect of the combination of nicotinamide and carbogen (CON) on radiotherapy outcome for patients with advanced bladder carcinoma (Ref. Reference Hoskin39). Nicotinamide is a vitamin modified to enhance blood flow in the tumours and administered 2 h before radiotherapy while carbogen refers to a gaseous mixture of 2% carbon dioxide and 98% oxygen inhalant (Ref. Reference Tharmalingham and Hoskin40). This study demonstrated improvement in overall response of 50% for those administered with the CON combination therapy, whilst radiotherapy alone only had a 38% overall response (Ref. Reference Hoskin39). A report from a phase III trial for laryngeal cancer also reported positive outcome of accelerated radiotherapy combined with carbon inhalation and nicotinamide compared to radiotherapy alone with a 93% control rate seen in patients with hypoxic tumours treated with the combination therapy (Ref. Reference Janssens41).
Other approaches that enhance oxygen diffusion for reversing tumour hypoxia and improve radiotherapy are also under investigation. Trans sodium crocetinate (TSC) causes physical changes in blood plasma which results in rapid oxygen diffusion from the cell wall to the vascular wall (Ref. Reference Gainer42). TSC was combined with temozolomide and radiotherapy on glioma cells and magnetic resonance imaging (MRI) imaging obtained before and after treatment showed a significant reduction in tumour size when compared with those treated with temozolomide and radiotherapy alone (Ref. Reference Sheehan43). TSC is being developed as a radiosensitiser for improving radiotherapy outcome in glioblastoma multiforme (GBM), pancreatic cancer and brain metastases after a successful phase II clinical trial was completed (Ref. Reference Gainer42).
Oxygen transport agents are also being explored to meet the challenges of hypoxia to radiotherapy. Preclinical investigation of liposome-encapsulated haemoglobin was shown to effectively reverse hypoxia in tumours (Ref. Reference Murayama44). Specifically, the results showed a remarkable reduction of HIF-1α and improved radiation therapy outcome, as tumour growth was significantly inhibited (Ref. Reference Murayama44). OMX is a recent oxygen carrier developed to target hypoxia and improve radiotherapy (Ref. Reference Le Moan45). Preclinical studies showed OMX reduced hypoxia significantly, enhancing T-cell localisation, and increasing CD8 accumulation and other cytotoxic activity previously impaired by tumour hypoxia (Ref. Reference Le Moan45). Fluorocarbon-based agents, through their gas-dissolving and chemically inert properties, can carry and diffuse oxygen at high concentrations (Ref. Reference Johnson, Lapchak and Zhang46). A phase II clinical trial (NCT03862430) in GBM, evaluating the combination of radiotherapy with NVX-108, a dodecafluoropentane-based perfluorocarbon emulsion, is currently recruiting (Ref. Reference NuvOx47).
Decreased oxygen consumption
As well as increased oxygen delivery, suppressors of oxygen consumption have also been explored as radiosensitiser agents.
Nitric oxide (NO) is a free radical that plays a vital role as a vasodilator, as well as inhibitor of tissue oxygen consumption (Ref. Reference Farah, Michel and Balligand48). The mechanism of NO in radiosensitisation is similar to those of oxygen-induced oxidative stress by stabilising radiation-induced DNA damage via the nitrosative stress pathways (Ref. Reference Scicinski49). The radiosensitising effect of NO has been shown both in vitro and in patients, including a phase II study indicating that NO can palliate hypoxia-induced progression in prostate cancer (Ref. Reference Wardman50).
More recently, the anti-microbial agent atovaquone was found to rapidly decrease hypoxic content of tumours, and was identified as a suppressor of oxygen consumption through a high-throughput analysis of FDA-approved drugs (Ref. Reference Ashton51). One clinical study found that atovaquone can increase tumour oxygenation and suppress hypoxic gene expression, therefore improve treatment outcomes for NSCLC patients (Ref. Reference Skwarski52).
Finally, papaverine, another FDA-approved agent, has also been shown as an ideal agent for radiosensitisation of hypoxic tumours as it reduces mitochondrial oxygen consumption (Ref. Reference Benej53). This anti-spasmodic drug was shown to increase oxygenation in tumour and enhanced radiation response directly by inhibiting mitochondrial metabolism with fewer side effects, which makes it a potential clinical radiosensitiser (Refs Reference Benej53, Reference Benej54).
Oxygen mimetics as radiosensitisers
Oxygen mimetics, which are compounds developed with chemical properties of molecular oxygen with a better diffusion ability to low oxygen tissues, have also been explored for their radiosensitising properties (Ref. Reference Coates, Skwarski and Higgins55). These include compounds such as misonidazole and nimorazole, which have been developed to mimic oxygen by promoting fixation of free radical damage during radiation (Ref. Reference Coates, Skwarski and Higgins55). The use of misonidazole was halted at trial in combination with radiotherapy for treatment of inoperable squamous cell carcinoma of lung cancer due to its high toxicity, and a similar effect was observed in an investigation for treatment of advanced uterine carcinoma (Refs Reference Overgaard56, Reference Panduro57). Finally, the NIMRAD phase III trial explored the use of nimorazole in combination with intensity-modulated radiotherapy (IMRT) in head and neck squamous cell carcinoma (HNSCC) (Ref. Reference Thomson58) and has been approved by the Centre for Clinical Practice (Ref. Reference Guidelines59).
Hypoxia-activated prodrugs as radiosensitisers
HAPs are compounds with high specificity for hypoxic tumours, as these are genotoxic compounds which are inactive in the presence of oxygen but are selectively activated under hypoxic conditions, and therefore can accurately target regions of tumour hypoxia (Ref. Reference Coates, Skwarski and Higgins55). These HAPs have been identified and grouped into five main types: nitro compounds, aromatic N-oxides, aliphatic N-oxides, quinones and molecularly targeted HAPs (Ref. Reference Mistry60). Nitro compounds-based HAPs include Metronidazole, PR-104A and TH-302, etc. The most representative N-oxide-based HAPs are Tirapazamine (TPZ), AQ4N and SN30000. Quinone-based HAPs, such as EO9 (Apaziquone), and Mitomycin C (MMC) are the earliest developed HAPs (Ref. Reference Zeng61). Despite promising preclinical data of classical HAPs, limited clinical therapeutic efficacy has been shown in several HAPs, which led to the development of novel molecularly targeted HAPs in recent years, including CH-01 (hypoxia-activated Chk1/Aurora A inhibitor), TH-4000 (hypoxia-activated tyrosine kinase inhibitor) and CH-03 (hypoxia-activated KDAC inhibitor) (Refs Reference Cazares-Korner62–Reference Patterson64). However, none of these have yet been evaluated in combination with radiotherapy. Details of HAPs being investigated in clinical trials as possible radiosensitisers of hypoxic cells are summarised in Table 1, with some examples detailed below.
Table 1. Clinical trials evaluating combination of HAPs with radiotherapy
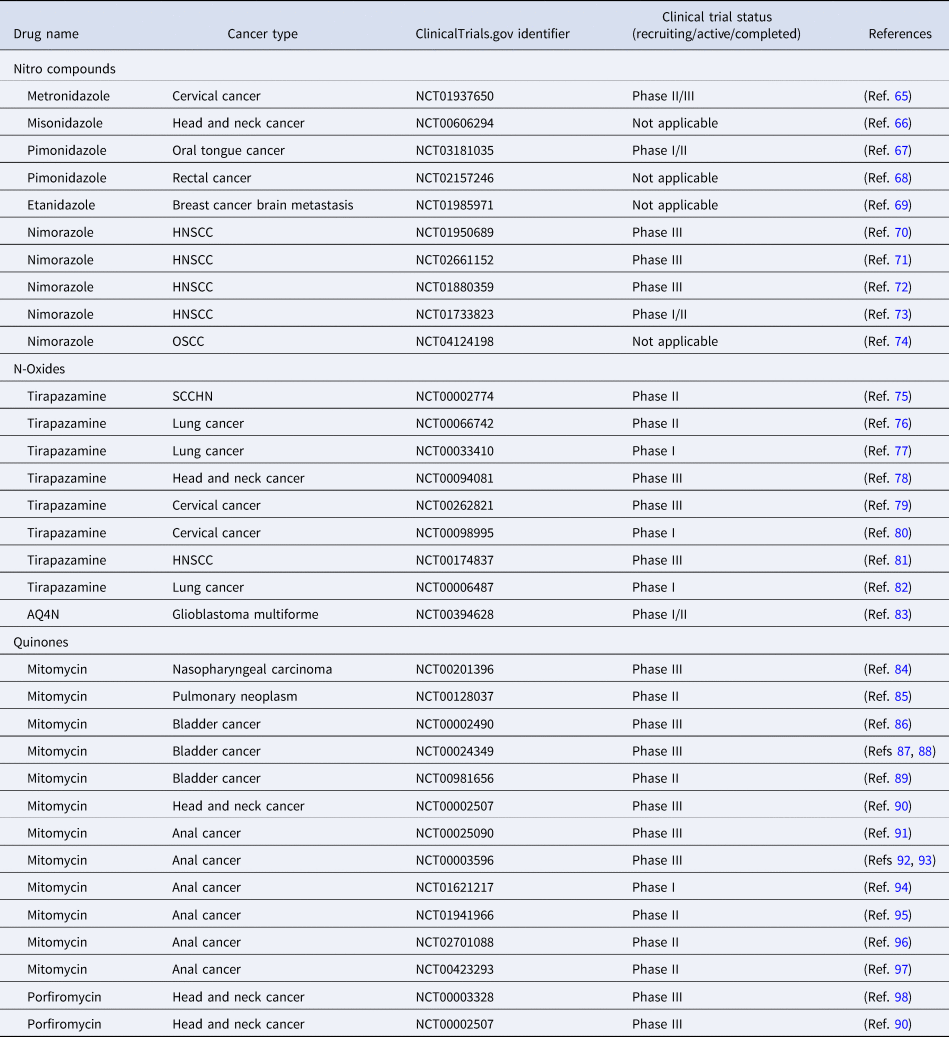
HNSCC, head and neck squamous cell carcinoma; OSCC, oropharyngeal squamous cell carcinoma; SCCHN, squamous neck carcinoma of the head and neck cancer.
Evofosfamide (TH-302)
TH-302 is an inactive compound of bromo-isophosphoramide which is released in hypoxic conditions and leads to alkylation of DNA (Ref. Reference Meng99). Interestingly, it has been shown that TH-302 in combination with radiotherapy enhances therapeutic outcomes (Ref. Reference Peeters100). A further study also found that TH-302 has radiosensitising effects when administered in combination with a VEGF-A inhibitor in preclinical models of sarcoma, increasing DNA damage and apoptosis and decreasing HIF-1α activity (Ref. Reference Yoon101). Further studies combining TH-302 and radiotherapy in vivo and in vitro reported a mild effect of treatment with TH-302 and a significant increase of apoptosis in hypoxic cells (Ref. Reference Takakusagi102). However, a phase III clinical trial of TH-302 reported non-significant benefits and high toxicity, and therefore it has not been adopted clinically (Ref. Reference Cutsem103). There was a phase I clinical trial using TH-302 with chemoradiotherapy in oesophageal cancer (NCT02598687) (Ref. Reference Larue104), however it was withdrawn as phase II/III trials did not meet their primary endpoint, so further development and testing of TH-302 is uncertain.
Tirapazamine
TPZ is an aromatic N-oxide which was first evaluated in 1986 and has been studied for its greater toxicity in anoxia when compared with aerobic conditions in vitro (Ref. Reference Zeman105). TPZ specificity for hypoxic cells initially showed positive results in improving radiotherapy outcomes by using gene-directed enzyme prodrug therapy (GDEPT) in which hypoxia is a trigger for both enzyme expression and drug metabolism (Ref. Reference Cowen106). Preclinical studies in the early 90s had shown great promise. For example, a phase I clinical trial of TPZ in combination with cisplatin and radiotherapy in small-cell lung cancer leading to improved survival rate among patients, and a phase II clinical trial carried out on patients with locally advanced head and neck cancer reporting improved 3-year survival (Refs Reference Le82, Reference Siim107, Reference Rischin108). Unfortunately, a later phase III clinical trial in locally advanced head and neck cancer showed no significant increase of patient survival (Ref. Reference Rischin109).
AQ4N
Banoxantrone (AQ4N) is a bioreductive HAP, which is bioreduced in hypoxic cells by cytochrome P450s to the cytotoxin AQ4 (Ref. Reference McCarthy110). Study found that AQ4N can selectively kill hypoxic cells via an inducible nitric oxide synthase-dependent mechanism when used in combination with radiation (Ref. Reference Mehibel111). Moreover, the use of AQ4N combined with radiotherapy and Temozolomide in glioblastoma entered a phase II clinical trial (NCT00394628), but no results have been published to date (Ref. Reference Clughsey112).
Mitomycin C
MMC is also a HAP that generates DNA-damaging species via DNA cross-linking and has been shown to enhance toxicity against hypoxic compared to normoxic cells (Ref. Reference Mistry60). Preclinical study revealed that MMC could enhance radio response and modulate hypoxic tumour microenvironment in combination with radiotherapy in rectal cancer (Ref. Reference Chen113). Clinical trials that used MMC combination with radiation are listed in Table 1. Combined therapy including 5-fluorouracil, MMC and radiation has become current standard treatments of anal cancers and bladder cancers. RTOG-87-04 study phase III randomised trial suggested that despite greater toxicity of MMC, the use of MMC can be beneficial, especially for those patients with large primary tumours (Ref. Reference Flam114). Long-term update of US GI intergroup RTOG 98-11 phase III trial compares CRT, replacing MMC with cisplatin due to the toxicity of MMC. However, cisplatin-based therapy failed to improve disease-free-survival compared with mitomycin-based therapy, therefore suggested RT + FU5/MMC remains the preferred standard of care of anal cancers (Refs Reference Gunderson92, Reference Ajani93).
Targeting of hypoxia-mediated signalling reprogramming as radiosensitising strategies
Targeting hypoxia-regulated signalling including and beyond direct HIF targeting in cancer has been explored as a therapeutic approach to reduce its tumour-promoting characteristics, and below we explore how targeting various hypoxia-regulated pathways can lead to improvement in radiotherapy responses (Fig. 2).

Fig. 2. Targeting of hypoxia-mediated signalling reprogramming as radiosensitising strategies. This schematic indicates the key hypoxia-regulated or associated signalling pathways targeted in radiosensitising approaches, as detailed in section ‘Targeting of hypoxia-mediated signalling reprogramming as radiosensitising strategies’. RT, radiotherapy; HIF, hypoxia-inducible factor; RTK, receptor tyrosine kinases; DDR, DNA damage response; GLUT-1, glucose transporter 1; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; CHK1, checkpoint kinase 1; PARP1, poly(ADP-ribose) polymerase.
HIF inhibition as a radiosensitiser strategy
As mentioned earlier, HIF is a critical factor in adaptation to the hypoxic microenvironment and is therefore an obvious molecular target to overcome radioresistance of hypoxic tumour cells (Ref. Reference Huang and Zhou115). Several compounds have been studied as inhibitors of HIF-α transcription, translation, and protein stabilisation (Ref. Reference Schönberger, Fandrey and Prost-Fingerle116). Of these, some, such as SN-38 (the active metabolite of irinotecan), alongside its well-established radiosensitiser effect as a topoisomerase I inhibitor, can also lead to increased radiosensitivity through inhibiting radiation-induced HIF-1α in colorectal cancer (Ref. Reference Okuno117). T-type Ca2+ channel blockers, such as Mibefradil, which can block HIF-1 activation by reducing mitochondrial ROS production and increase HIF-1α protein hydroxylation and degradation (Ref. Reference Zhang118), have also been studied in a clinical trial using Mibefradil with hypofractionated irradiation in recurrent GBM (Ref. Reference Lester-Coll119), with results suggesting that Mibefradil can be safely co-administered with RT. STAT3 plays an important role in the response of tumour cells to radiotherapy, and STAT3 inhibitors NSC74859 and Stattic have been found to increase radiosensitivity by downregulating HIF-1α expression in oesophageal cancer (Refs Reference Wang120–Reference Zhang122). YC-1, a NO-independent activator of soluble guanylyl cyclase, was shown to enhance radiosensitivity across different types of cancer cells by inducing HIF-1α protein degradation and hence inhibition of HIF-1α function (Refs Reference Moeller123–Reference Lee125). More recently, other novel small-molecule inhibitors of HIF have been investigated. PX-478 decreases HIF-1α levels by inhibiting HIF-1α translation, as well as inhibiting de-ubiquitination leading to HIF-1α protein degradation (Ref. Reference Schönberger, Fandrey and Prost-Fingerle116). Palayoor et al. have shown a potential role for PX-478 as a clinical radiation enhancer in prostate carcinoma cells (Ref. Reference Palayoor126). HIF-2α inhibitors, including PT2399, PT2977 and PT2385, are also showing promise as single agents in clear cell renal cell carcinoma in phase II clinical trials, but their combination with radiotherapy is not yet explored (Refs Reference Courtney127–Reference Chen129).
Targeting DNA damage response
Hypoxia can drive cancer progression and lead to radioresistance through its impact on genomic integrity by inhibiting DNA repair pathways (Ref. Reference Kaplan and Glazer130). As outlined previously radiation kills cancer cells by damaging their DNA. DNA repair dysregulation provides a promising opportunity to exploit this key vulnerability for overcoming radioresistance, specifically through targeting DSBs repair pathways (Ref. Reference Brown131). This is linked with the concept of ‘synthetic lethality’, which occurs when functional defects of complementary pathways can result in cell death, whereas the perturbation of either pathway does not impact cell survival. Targeting one of the pathways using small molecule inhibitors in cells with a pre-existing defect in the complementary pathway (e.g., use of PARP inhibitors (PARPi) in tumours defective for BRCA1/2) can be very effective, so other such pathway combinations have been explored (Refs Reference O'Neil, Bailey and Hieter132–Reference Lord and Ashworth134). One of these is hypoxia-mediated repression of DNA repair in ‘contextual synthetic lethality’ approaches, for example, through combination with PARPi (Ref. Reference Chan135). Finally, targeting of DDR key factors in combination with radiotherapy has shown a lot of potential for overcoming hypoxic radioresistance (Ref. Reference Begg and Tavassoli136). Details of DDR inhibitors investigated in clinical trials as possible radiosensitisers are summarised in Table 2, and examples of these strategies are detailed below.
Table 2. Clinical trials evaluating the combination of DDR inhibitors and radiotherapy
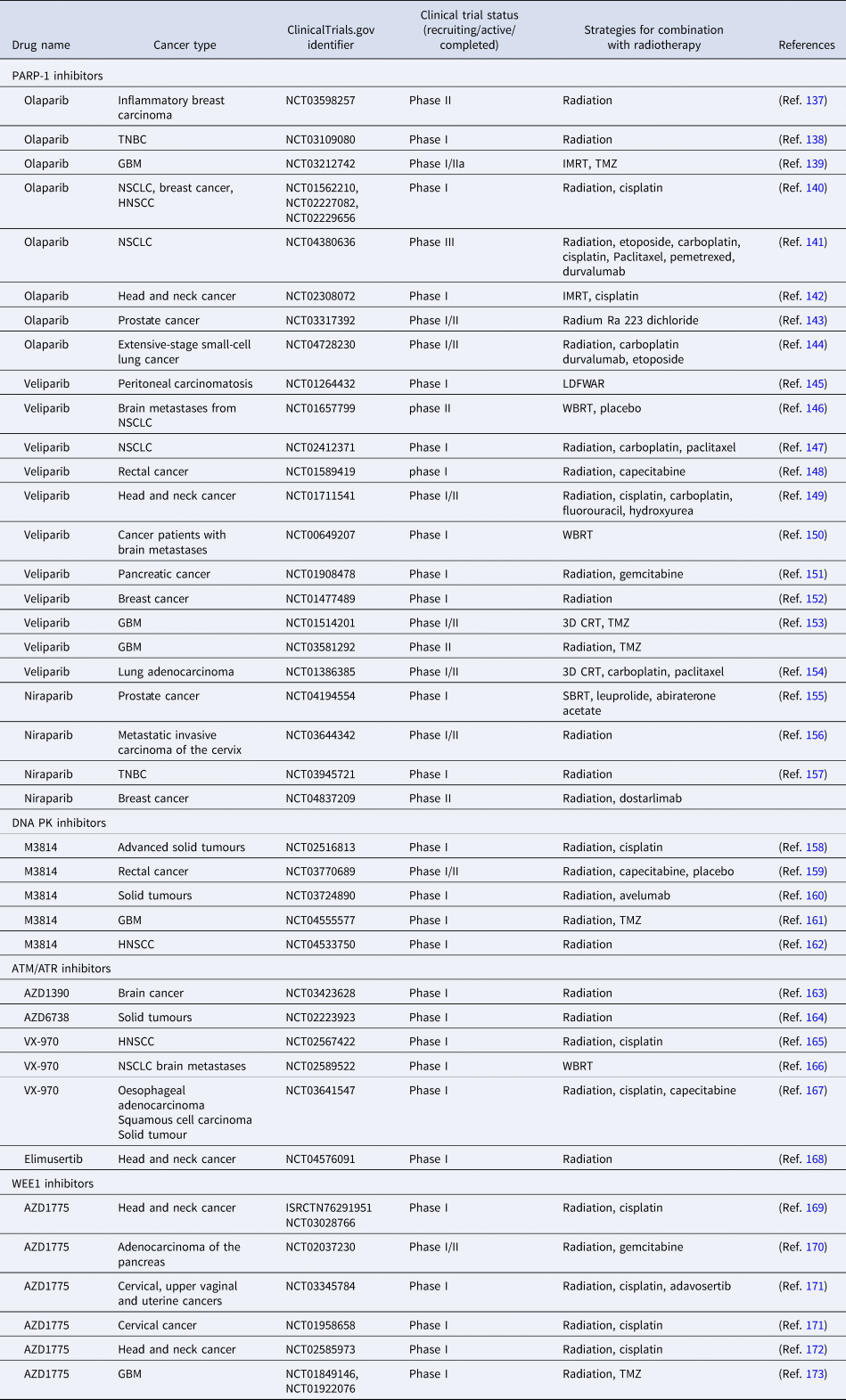
TNBC, triple negative breast cancer; GBM, glioblastoma; NSCLC, non-small-cell lung cancer; HNSCC, head and neck squamous cell carcinoma; IMRT, intensity-modulated radiotherapy; TMZ, temozolomide; LDFWAR, low-dose fractionated whole abdominal radiation; WBRT, whole brain radiation therapy; 3D CRT, 3-dimensional conformal radiation therapy; SBRT, stereotactic body radiotherapy.
PARP1 inhibitors
PARPi, which can effectively prevent the repair of damaged DNA by blocking PARP enzyme activity and PARylation reactions, are the first clinically approved drugs based on the principle of synthetic lethality (Ref. Reference Gogola174). BRCA1/2 are major components of the HR pathway for DSB repair, and deficiency in BRCA1/2 genes leads to high susceptibility for breast and ovarian cancer (Ref. Reference Petrucelli, Daly and Pal175). HR deficiency due to BRCA1/2 mutations leads to an exquisite sensitivity to PARPi through synthetic lethality between these two pathways, a phenomenon also described as BRCAness (Ref. Reference Lord and Ashworth176). Many clinical trials have been carried out in various BRCA-mutated tumours that have evaluated the benefits with the treatments of PARPi both as single agents and in combination with radiotherapy (Ref. Reference Lesueur177). Importantly, a study from 2010 reported that HR-defective hypoxic cells selectively died because of microenvironment-mediated ‘contextual synthetic lethality’, where hypoxia-mediated repression of HR represented a BRCAness-like phenotype, and also enhanced sensitivity to ionising radiation (Ref. Reference Chan135). Other studies have also shown that the combination of PARP1 inhibitor Olaparib with radiotherapy led to radiosensitising effects in hypoxia in NSCLC through this contextual synthetic lethality effect (Ref. Reference Jiang178). Moreover, PARPi also improves the radiotherapy responses, as well as the efficacy of some chemotherapeutic agents, targeted therapy and immunotherapy (Ref. Reference Ramakrishnan Geethakumari179). This has led to a significant number of clinical trials focused on the combination with PARP1i and radiation to improve the response to radiotherapy (Table 2).
DNA-PK inhibitors
DNA DSBs generated by ionising radiation can also be repaired through non-homologous end joining), a more error-prone repair pathway than HR (Ref. Reference Mahaney, Meek and Lees-Miller180). The KU heterodimers (KU70 and KU80) recognise the DNA DSBs, then activate and recruit DNA-PKcs to the DNA break sites. This complex formed at the DSBs consisting of DNA, Ku70/80 and DNA-PKcs is referred to as DNA-PK (Ref. Reference Goodwin and Knudsen181). The expression and activity of DNA-PK in cancers is correlated with the response to anticancer therapy, including radiotherapy (Ref. Reference Hsu, Zhang and Chen182). A study showed that DNA-PKcs inhibition led to increased sensitivity of gastric cancer cells to ionising radiation (Ref. Reference Geng183). Moreover, another study also found that DNA-PK inhibitor NU5455 may preferentially sensitise chronically hypoxic tumour cells to radiotherapy in vivo (Ref. Reference Jiang184). Another study showed that DNA-PKcs inhibition potentially overcome hypoxia-induced radioresistance in NSCLC by the combination of ionising radiation treatment with the DNA-PK inhibitor M3814 (Ref. Reference Klein185). To our knowledge, M3814 is the only DNA-PK inhibitor currently in clinical development (see Table 2).
ATM/ATR inhibitors
Ataxia-telangiectasia mutated (ATM) is one of the central kinases of the DDR and has a critical role in cancer suppression and DNA DSBs repair (Ref. Reference Meschini186). Like ATM, ataxia telangiectasia and Rad3 related (ATR) is also a central kinase involved in the DDR (Ref. Reference Szurman-Zubrzycka187). Inhibition of ATM or ATR has been shown to sensitise the cancer cells to radiation treatments. Moreover, ATR and ATM have a role to play in hypoxia/re-oxygenation (Refs Reference Pires188–Reference Bencokova190), which led to the exploration of ATM/ATR inhibitor treatment in overcoming hypoxia-mediated radioresistance in cancer. Inhibition of ATM or ATR has been shown to be potential radiosensitisers under hypoxic condition in several studies. One study found ATM inhibition can increase the radiosensitising effect under hypoxic conditions in NSCLC (Ref. Reference Klein185). ATR inhibitor VE-821 has reported to increase sensitivity of pancreatic cancer cells to radiation and chemotherapy in pancreatic cancer under both normoxic and hypoxic conditions (Refs Reference Pires188, Reference Prevo191). Another ATR inhibitor from the same chemical series as VE-821, Berzosertib (formerly VE-822, M6620 and VX-970), has also been shown to sensitise response to chemo/radiotherapy, which could improve the treatment efficacy in oesophageal cancer (Ref. Reference Leszczynska192). Clinical trials regarding combination of ATM or ATR inhibitors with radiation are ongoing, such as ATM inhibitors AZD1390 and AZD6738, and ATR inhibitor VX-970 (Table 2). ATM and ATR target kinases CHK1 and CHK2 also represent attractive targets to be combined with established cancer therapies, including radiotherapy, but to date only CHK1 inhibitor Prexasertib/LY2606368 combined with radiation has entered clinical trial and suggest that this combination therapy may increase clinical benefit (Refs Reference Zeng193, Reference Zeng194).
WEE1 kinase inhibitor
WEE1 kinase is a key regulator of the G2/M phase transition that allows DNA repair before mitotic entry (Ref. Reference Li195). Amongst several WEE1 inhibitors evaluated in combination with radiotherapy (Table 2), combination of AZD1775 and ionising radiation has shown significantly increased apoptosis in cervical cancer cells (Ref. Reference Lee196). Another study also highlighted the radiosensitised effect of WEE1 kinase inhibitor AZD1775 through inducing replication stress in hepatocellular carcinoma (Ref. Reference Cuneo197). Furthermore, another study investigated the impact of WEE1 inhibition using the MK-1775 on hypoxic cells in combination with radiation, showing MK-1775 sensitised radiation under normoxia, but not hypoxic conditions (Ref. Reference O'Brien198).
Targeting cell metabolism
There are an increasing number of studies that conclude that metabolic alterations in cancer are one of the major reasons contributing to radioresistance (Ref. Reference McCann, O'Sullivan and Marcone199). The PI3K/AKT/mTOR is a key signalling pathway that can stimulate glucose uptake, therefore controlling cell metabolism in cancer cells. The PI3K/AKT/mTOR pathway is involved in hypoxia-ischemia signalling, and HIF-1α is regulated by PI3K/Akt signalling pathway (Ref. Reference Zhang200). PI3K inhibition by LY294002 radiosensitises human cervical cancer cell lines (Ref. Reference Lee201). Studies have also found that PI3K/Akt/mTOR pathway inhibitors (BEZ235 or PI103) enhance radiosensitivity in radioresistant tumour cells such as prostate cancer cells (Ref. Reference Chang202). A dual PI3K and mTOR inhibition NVP-BEZ235 has been shown to significantly reduce tumour hypoxia by normalising tumour vasculature (Ref. Reference Fokas203). PI3K/mTOR inhibitors BEZ235 and BKM120 were shown to significantly reduce oxygen consumption in cancer cell lines, with associated reduced mitochondrial respiration (Ref. Reference Kelly204). Several clinical studies have now evaluated the efficacy of PI3K/Akt/mTOR inhibitors in combination with radiotherapy, and these are summarised in Table 3. Nelfinavir, which is AKT phosphorylation inhibitor, has entered clinical trial phase III in cervical cancer (Ref. Reference Sastri and Goda205). Another study using Nelfinavir with concurrent CT-RT is associated with acceptable toxicity. Moreover, the results from metabolic response and tumour response suggested the benefit of Nelfinavir is promising in stage IIIA/IIIB NSCLC (Ref. Reference Rengan206).
Table 3. Clinical trials evaluating the combination of PI3K/AKT/mTOR inhibitors and radiotherapy

GBM, glioblastoma; NSCLC, non-small-cell lung cancer; HNSCC, head and neck squamous cell carcinoma; TMZ, temozolomide; WBRT, whole brain radiation therapy; SBRT, stereotactic body radiotherapy; IMRT, intensity-modulated radiotherapy.
Glucose transporter 1 (GLUT1) is an essential factor for glucose metabolism and is also a canonical HIF target gene (Ref. Reference Kido225). Studies found increased GLUT1 levels in radioresistant tumour cells, which indicates that GLUT1 expression may be used as an indicator of the sensitivity to radiation and prognosis of radiotherapy (Refs Reference Boström226–Reference Kunkel228). Targeting GLUT1 and related signalling pathways may therefore represent an effective way to improve radiotherapy efficacy. A small molecule inhibitor of GLUT1, WZB117, can increase the sensitivity of radiation in breast cancer cells (Ref. Reference Zhao229). Another study found that modulating the glucose metabolism sensitised glioblastoma cells to ionising radiation (Ref. Reference Shen230). However, there are no GLUT1 inhibitors combined with radiation entered in clinic trails yet.
Combined immunotherapy
During radiotherapy treatment, radiation not only damages cancer cells directly, but also activates an immune response (Ref. Reference Storozynsky and Hitt231). Meanwhile, hypoxia also plays a pivotal role in the regulation of immunosuppressive molecules and participates in the activation of immunosuppressive cells (Ref. Reference Deng232). For example, IL10 and TGFβ are increased under hypoxia, which induces the differentiation of tumour-associated macrophages into M2 macrophages and therefore activates immune-suppressive activities (Ref. Reference Boutilier and Elsawa233). Hypoxia also regulates the differentiation and activation of dendritic cells (Ref. Reference Winning and Fandrey234). On the other hand, hypoxia activates immunosuppressive cells, such as myeloid-derived suppressor cells, regulatory T cells and decreased infiltration and activation of CTCs, which suggests that targeting HIF in the immune system could be beneficial for anti-tumour immune responses (Ref. Reference Kumar and Gabrilovich235).
Radiotherapy has both pro-immunogenic and immunosuppressive effects on immune response in various levels. This includes the induction of immunogenic cell death, promoting the recruitment and function of T cells within the tumour microenvironment, and improving the recognition and killing of cancer cells by CD8+ CTLs (Ref. Reference Vanpouille-Box, Formenti and Demaria236). This is key to the synergistic effect of radiation with immune checkpoint inhibitors, antibodies targeting inhibitory receptors on T cells, including cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1(PD-1), and has become an optimal partner for immune check point inhibitors (Ref. Reference Pilones, Vanpouille-Box and Demaria237). In fact, several completed clinical trials evaluated the efficacy of combining immunotherapy approaches using immune checkpoint inhibitors with radiotherapy, and the completed clinical trials are summarised in Table 4.
Table 4. Clinical trials evaluating the combination of immunotherapy therapeutics and radiotherapy

GBM, glioblastoma; NSCLC, non-small-cell lung cancer; IMRT, intensity-modulated radiotherapy; TMZ, temozolomide; LDFWAR, low-dose fractionated whole abdominal radiation; WBRT, whole brain radiation therapy; 3D CRT, 3-dimensional conformal radiation therapy; SBRT, stereotactic body radiotherapy, SIRT, selective internal radiation therapy; SABR, stereotactic ablative radiotherapy; SBRT, stereotactic body radiation therapy; HFRT, hypofractionated radiation therapy.
Furthermore, studies also found that immunosuppressive macrophages were recruited by radiation, which induced upregulation of CSF-1. Depletion of these macrophages by using anti-CSF antibody (aCSF) significantly delays tumour regrowth following radiation. Moreover, the addition of an anti-PD-L1 antibody to aCSF resulted in improved tumour suppression and even regression in a highly resistant murine pancreatic cancer model (Ref. Reference Jones265); therefore, macrophage depletion may play a role in immune checkpoint blockade-resistant tumours. Ultimately, as suggested by Eckert et al., as hypoxia mediates radioresistance and immune escape, the combination of immune checkpoint inhibition and radiotherapy might be a promising strategy to improve outcome in tumours with high hypoxic content (Ref. Reference Eckert266).
IMRT combination with radiosensitiser approaches
IMRT is a radiotherapy modality that delivers highly conformal dose distributions (Ref. Reference Cho267). It is designed by inverse optimisation algorithms, with the following inputs: the dose required to the ‘tumour’ to gain control of the disease; and constraints or dose limitations for proximal tissues and ‘organs at risk’. The optimisation process is controlled by cost functions, these essentially compare dose distributions achieved by a set of x-ray beams, to the desired outcome; they then guide modulation of each beam in a systematic manner until a solution close to that originally specified is obtained. In simple terms the described process results in a set of beams, each consisting of a number of segments whose individual dose patterns superpose to create exquisite dose distributions that acknowledge the 3D nature of tumours and the discrete hypoxic and normoxic regions present in tumour masses (Ref. Reference Marta268). Commonly, the degrees of freedom available to the optimiser is increased by using arc-based treatment beams rather than a discrete set of fixed directions. IMRT has had a clear impact on the success of modern radiotherapy strategies. However, given it typically is implemented with high-energy x-rays which are low-LET radiation, further developments considering strategy modification related to hypoxia management may be limited, see section ‘High-LET modalities as alternatives to oxygen-dependent low-LET ionising radiation’.
Combining precise delivery via IMRT with radiosensitiser approaches such as DDR inhibitors (section ‘Targeting DNA damage response’) and immunotherapy (section ‘Combined immunotherapy’) has the potential to improve patient outcomes. Furthermore, nanotechnology has potential to provide a new dimension to this strategy with metallic nanomaterials being developed as possible hypoxic radiosensitisers (Ref. Reference Wang269). Gold nanoparticles (GNPs), for example, are gaining attention due to gold's ability to readily donate electrons and thereby promote the production of ROS, even in low oxygen environments. In a study of colon cancer, CT26 cells were incubated in hypoxia both with and without GNPs prior to radiotherapy application. Significantly improved responses were observed in the GNP group, suggesting dual IMRT-GNP therapeutics could improve the relative biological effectiveness (RBE) and OER of low-LET modalities compared to x-ray application alone (Ref. Reference Kim270).
High-LET modalities as alternatives to oxygen-dependent low-LET ionising radiation
LET is the energy loss of a radioactive particle per unit of distance travelled and in radiotherapy, a measure of the amount of energy transferred from the radiation source to the patient. High-LET radiation sources include alpha particles, with high mass and positive charge, and low-energy neutrons which have no charge and are approximately ¼ mass of an alpha particle (Ref. Reference Huefner271). Low-LET radiation sources, most commonly x-rays or gamma-rays, are photons having no mass or charge and wavelengths below 10−8 m (Ref. Reference Ilicic, Combs and Schmid272). High-LET particles deposit their energy within a short distance from the radiation source, following a discrete pathway and causing significant cellular disruption localised to a smaller area close to the target (Ref. Reference Costes273). Low-LET waves however penetrate tissues more readily and are widely scattered as they transverse through the patient, causing less intense damage to a larger area of tissue (Ref. Reference Costes273).
The radiobiology of high-LET RT modalities
Tumours with oxygen-deficient areas experience increased radioresistance termed the OER, a comparison of the dose of radiation needed to cause the same damage in normoxic verses hypoxic tissue environments. Experimentally, the OER is inversely proportional to LET suggesting a potential clinical advantage of high-LET radiotherapy compared to low-LET irradiation (Ref. Reference Wenzl and Wilkens274). RBE (relative biological effectiveness) is a comparison of biological efficacy of one type of ionising radiation compared to another (such as DNA damage and apoptosis levels), and indicates the dose of different ionisation sources that are needed to produce the same biological effect (Ref. Reference Ilicic, Combs and Schmid272). High LET radiation has an increased biological effectiveness compared to photons of low LET, causing more extensive and clustered DNA damage (Ref. Reference Nikjoo275). Specifically, application of high-LET radiotherapy causes closely interspaced DSBs leading to high local concentrations of repair proteins and perturbed DNA damage owed to its discrete pattern of energy deposition compared to low-LET X-ray irradiation (Fig. 3) (Ref. Reference Roobol276). Contemporary proton particle therapy utilises scanning beam technology which facilitates intensity modulated proton therapy, wherein the benefits afforded by intensity modulation and high-LET delivery are combined (Ref. Reference Cao277).

Fig. 3. Water radiolysis in high vs low LET radiation. Water radiolysis, propagated by ionising radiation, can follow numerable reaction pathways resulting in a snowballing mechanism that produces numerable ROS (oxygen containing radicals) that damage DNA, known as the indirect effect. Additionally, radiation treatment can damage DNA through impact alone and subsequently damage molecular structure, known as the direct effect. Application of high-LET radiotherapy sources induce a larger degree of the direct effect compared to low-LET sources, owed to the particles high mass and charge, while low-LET modalities rely more on the presence of sufficient oxygen to be efficacious. Figure created in ChemDraw 20.1.1 and adapted from (Refs Reference Le Caër278, Reference Desouky, Ding and Zhou279).
FLASH
FLASH radiotherapy is a treatment method that decreases the damage caused to the normal tissue (tissue sparing) whilst maintaining a tumour response compared with conventional low dose rate radiotherapy (Refs Reference Wilson280, Reference Diffenderfer281). The FLASH technique involves application of a single, ultra-high dose of radiation over a short time period. When compared to conventional radiotherapy in vitro, FLASH radiotherapy caused significantly less DNA damage to normal tissue than conventional radiation. The mechanisms underpinning the tissue sparing effect of FLASH are hypothesised to be diverse, including rapid radiochemical depletion of oxygen leading to transient hypoxia in normal tissue, radical interaction or inhibition of activation of genes that drive inflammation and proliferation of tumours (Ref. Reference Moon, Petersson and Olcina282). In the oxygen depletion/transient hypoxia hypothesis, normal tissue with physiological oxygen levels would experience rapid oxygen depletion after FLASH, leading to transient radioresistance which would in turn would lead to decreased damage and ultimately a tissue sparing effect. Further investigation on post irradiation effect showed that FLASH halted repopulation, whilst significantly reducing radio-induced senescence (Ref. Reference Fouillade283). Importantly, FLASH radiotherapy has increased RBE when delivered in high-LET modalities harnessing a proton beam radiation source compared to low-LET x-ray sources (Ref. Reference Hughes and Parsons284). Experiments to validate its efficacy in hypoxia however suggest FLASH radiotherapy has a high OER in vitro, with tissue oxygen concentrations above 4.4% needed for the technique to match the efficacy of conventional RT as hypoxic regions lack the oxygen availability to support the rapid oxygen consumption occurring in local tissues during FLASH therapy (Ref. Reference Adrian285). The mechanism and biological nature of the FLASH effect is complex, but it is expected this will be an area of increased interest in the radiobiology field.
Dose painting
Positron emission tomography (PET) and MRI are functional, non-invasive imaging modalities utilised to identify hypoxic tissue regions in patient tumours (Ref. Reference Busk, Horsman and Overgaard286). Such imaging allows clinicians to define areas likely to be resistant to radiotherapy, such as areas of tumour hypoxia. Therefore, strategic delivery of higher ionising doses to hypoxic areas while reducing the dose delivered to more oxygenated regions thereby limiting dose-related side effects, a process also known as dose painting (Refs Reference van der Heide287, Reference Donche288).
In a study of 12 patients with locally advanced HNSCC, hypoxia-specific tracer 18F-Fluoroazomycin arabinoside was harnessed alongside PET technology to assess the capabilities of hypoxia-guided dose painting. FAZA accumulation successfully identified hypoxic voxels in 80% of the cohort, while hypoxic volume made up to 54% of the patients' total tumour masses. Subsequently, 86 Gy doses were delivered to hypoxic voxels while a 70 Gy mean dose was administered across other regions and results revealed that dose escalation had no impact on adjoining healthy tissues (Ref. Reference Moon, Petersson and Olcina282). Another dose painting study involving 10 HNSCC patients harnessed hypoxic tracer 18F-Fluoromisonidazole in combination with PET to identify and image chronic hypoxic voxels. Post imaging, one sub-group received 35 fraction schedules of 2 Gy irradiation (70 Gy total) homogenously while a second sub-group received an escalated dose of 2.28 Gy to hypoxic regions (79.8 Gy total). Comparison of the two treatment plans demonstrated dose escalation to hypoxic regions can be delivered safely and efficaciously, without any increased delivery to at-risk organs (Ref. Reference Fouillade283). Therefore, the literature suggests that combining dose-painting methodologies with high-LET radiation could therefore increase the benefit of hypoxia mapping as patients could benefit from the improved OER and RBE that high-LET therapies provide, accompanied by increased precision of application, allowing potent radiation doses to be delivered with minimal damage to healthy cells. However, a caveat of this approach is that it is based on a plan prior to treatment. A course of radiotherapy is delivered over a period of 1 and 7 weeks and oxygen level distribution can change in response to the treatment, thus impacting on the efficacy of this approach.
Concluding thoughts and future directions
Radiotherapy remains one of the most effective non-invasive treatments for solid tumours, but the impact of tumour biology on response of tumour cells to radiation remains a fundamental limitation to what radiotherapy can ultimately achieve. Challenges associated with radiotherapy response include inherent radioresistance of cancer cells, lack of discrimination between normal tissue and tumour cells, and, pertinent to this review, tumour hypoxia-mediated radioresistance. State-of-the-art dual treatment modalities for cancer patients have previously relied upon radiotherapy accompanied by surgery, chemotherapy and more recently, immunotherapy. However, these combinations have been unable to abolish treatment-resistant hypoxic regions often resulting in poor survival rates and disease recurrence. Furthermore, radiotherapy technology (instrumentation and software) and delivery has improved significantly over last 15 years, but has potentially encountered an era of diminishing returns, where increased accuracy in radiotherapy delivery may not substantially improve outcomes alone.
We suggest that hypoxia targeting in radiotherapy treatment strategies should encapsulate the mainstream treatment strategy for cancer, especially solid tumours, with experimental and clinical evidence suggesting some of these strategies even carry the benefit of reduced off-target effects. Of particular interest are treatment plans that strategically exploit the hypoxic tumour microenvironment by targeting hypoxia-mediated radioresistance signalling, such as HIF inhibition and targeting DDR, as well as employment of HAPs. However, further studies using accurate evaluation of hypoxic content of tumours are needed to validate their efficacy in combination with radiotherapy and advance such strategies towards the clinic.
Clinical validation of existing hypoxia-targeted radiosensitisers should therefore continue to be a priority area in radiotherapy research, alongside prioritising treatment metrics that include hypoxic indices of tumours, capitalising on the disease-specific, druggable targets in the hypoxic microenvironment. This should include evaluating combination approaches of radiotherapy with relevant hypoxia signalling targeting small molecule inhibitors (such as HIF and DDR inhibitors) as well as immunotherapy. These strategies should be also combined with current radiotherapy delivery modalities, including developing the use of hypoxia content scores in increasing the effectiveness of fractionated radiotherapy strategies using machine-learning in in silico modelling. It will also involve a shift towards high-LET radiotherapeutics over low-LET options to provide relatively immediate benefits to the cancer patient group.
Ultimately, the use of these various strategies targeting hypoxic radiobiology, combined with cutting-edge precise radiotherapy delivery and modelling, should lead to improvement in patient outcomes.
Acknowledgements
The authors would like to acknowledge all the studies were not able to include due to space limitations.
Financial support
CL and IMP are funded by a University of Hull studentship part of the Adaptive Radiotherapy PhD cluster, which is a collaborative project with the NHS Trust, led by IMP (University of Hull) and AWB (Hull University Teaching Hospitals NHS Trust). IMP, LW and RR are funded by a Bowel Research UK PhD studentship (reference 000100009).
Conflict of interest
None.









