The most important requirement of milk used in most cheese typologies is its aptitude to coagulate on addition of rennet. Milk with proper rennet coagulation ability gives rise to curds with better rheological properties (syneresis), with positive repercussions on cheese yield and quality. Curds obtained from milk with poor coagulation aptitude are more susceptible to cheesemaking losses during the vat phase and could undergo incomplete and non-homogeneous whey drainage, with possible defects during the early or late phases of ripening (Mariani et al. Reference Mariani, Summer, Formaggioni, Malacarne and Battistotti2001).
The lactodynamography (LDG) is one of the most widespread methods to analyse the rennet coagulation ability of milk (McMahon & Brown, Reference McMahon and Brown1982). This technique detects the change of viscosity in a milk sample induced by rennet-gel formation. The output of LDG analysis is a typical diagram or profile from which it is possible to calculate rennet coagulation parameters (RCP): rennet clotting time, curd firming time and curd firmness. Currently, in the Parmigiano-Reggiano cheese area, RCP are measured every 15 d on herd milk samples and the resulting values are used to reward or penalise milk producers.
Because of the influence of RCP on cheese quality and yield, it is important to investigate reasons for poorly or non-coagulating milk. Naturally occurring variations of RCP are related to genetic (breed and genetic polymorphisms of milk proteins) and environmental factors affecting milk composition (stage of lactation, somatic cell content, feeding, season, etc.) (Okigbo et al. Reference Okigbo, Richardson, Brown and Ernstrom1985; Ostersen et al. Reference Ostersen, Foldager and Hermansen1997; Verdier-Metz et al. Reference Verdier-Metz, Coulon, Pradel, Viallon and Berdague1998; Ikonen et al. Reference Ikonen, Morri, Tyrisevä, Ruottinen and Ojala2004; Malacarne et al. Reference Malacarne, Fieni, Tosi, Franceschi, Formaggioni and Summer2005; Bittante et al. Reference Bittante, Penasa and Cecchinato2012). Most studies underline the key role played by caseins in milk RCP, in terms of their contents, relative proportions, genetic types, and extent of post-translational modifications (Comin et al. Reference Comin, Cassandro, Chessa, Ojala, Dal Zotto, De Marchi, Carnier, Gallo, Pagnacco and Bittante2008; Jõudu et al. Reference Jõudu, Henno, Kaart, Püssa and Kärt2008; Jensen et al. Reference Jensen, Holland, Poulsen and Larsen2012a, Reference Jensen, Poulsen, Andersen, Hammershøj, Poulsen and Larsenb; Vallas et al. Reference Vallas, Kaart, Värv, Pärna, Jõudu, Viinalass and Pärna2012).
Besides caseins, calcium (Ca) and phosphorus (P) are essential constituents of the micelles (Gaucheron, Reference Gaucheron2005). Micellar P can be present as part of colloidal calcium phosphate (CCP) (inorganic-P) or covalently bound to caseins as phosphate groups (casein-P). A fraction of the phosphate groups of calcium-sensitive caseins contribute to micellar casein structure by a Ca2+-mediated secondary interactions with CCP (Horne, Reference Horne1998). The phosphate groups not involved in internal CCP-crosslinks are supposed to play a key role during curd formation (secondary phase of rennet coagulation) by the formation of soluble Ca2+-mediated crosslinks among paracasein micelles (Green & Grandison, Reference Green, Grandison and Fox1993). It is reported that failure in milk coagulation is mostly due to alterations in the secondary phase of rennet coagulation, as the hydrolysis of kappa-casein is also detectable in non-coagulating milk (Frederiksen et al. Reference Frederiksen, Andersen, Hammershøj, Poulsen, Sørensen, Bakman, Qvist and Larsen2011).
The role of micellar Ca and P in milk RCP has not been extensively studied. Higher colloidal contents of Ca, P and Magnesium (Mg) in milk are associated with better rennet coagulation properties in individual milk samples collected from Jersey and Holstein cows (Jensen et al. Reference Jensen, Poulsen, Andersen, Hammershøj, Poulsen and Larsen2012b). These authors do not distinguish between inorganic-P and casein-P. Furthermore, as the content of casein increases from poorly to well coagulating milk, colloidal values are not useful to assess the possible influence of the degree of mineralisation of the casein micelle (expressed as inorganic-P in 100 g of casein) on rennet coagulation of milk. The influence of phosphate groups of casein on rennet coagulation was studied by Pearse et al. (Reference Pearse, Linklater, Hall and Mackinlay1986). These authors observe that dephosphorylation of reconstituted casein micelle has an adverse effect on rennet coagulation and syneresis.
In recent years, an increase of the proportion of herd milk samples with the worst LDG types has been reported in the Parmigiano-Reggiano cheese production area (Tedeschi et al. Reference Tedeschi, Malacarne, Tosi and Sandri2010). Despite the increasing number of studies, the causes of abnormal coagulation of milk are not fully understood. The objective of this study was to contribute to the knowledge of this phenomenon, emphasising relationships between milk characteristics and naturally occurring variations in herd milk LDG profiles, with particular regard to the possible influence of the content of micellar Ca, P and Mg.
Materials and methods
Samples collection
The study was carried out over one year and 118 cattle herds producing milk for Parmigiano-Reggiano cheese were involved. Herds were randomly selected out of 500 cattle herds assisted by the Centro Lattiero-Caseario (Dairy Center) of Parma (Italy). Each herd was sampled once and therefore a total of 118 herd milk samples were collected. About 2 herds (from a minimum of 1 to a maximum of 3) were sampled each week, during the year.
Herds involved milked on average 50 lactating cows, reared in free-stall barns, and parities were equally distributed throughout the year. Feeding of cows was very similar among herds, as this practice was strictly regulated by the Pamigiano-Reggiano Cheese Consortium. (http://www.parmigianoreggiano.com/consortium/rules_regulation_2/default.aspx). Sampling was carried out from the herd tank, at the end of the morning milking, according to the International Dairy Federation standard (IDF, 2008). The milk sample was cooled to 4 °C, transferred to the laboratory within 30 min and analysed immediately.
Milk analyses
The following standard analyses were carried out in duplicate: pH and titratable acidity were measured with a potentiometer (Crison Instruments, Barcelona, E-08328, Spain) and by titration with 0·25 M-NaOH using the Soxhlet-Henkel method (Anonymous, 1963), respectively. Fat and lactose were determined by infrared analysis (Biggs, Reference Biggs1978) with a Milko-Scan 134 A/B (Foss Electric, DK-3400 Hillerød, Denmark). The somatic cell count (SCC) was assessed by the fluoro-opto-electronic method according to Schmidt-Madsen (Reference Schmidt-Madsen1975). Total nitrogen (TN) in milk, non-casein nitrogen (NCN) in pH 4·6 acid whey and non-protein nitrogen (NPN) in milk after treatment with trichloroacetic acid (TCA; 120 g/l), were determined by the Kjeldahl method (Aschaffenburg & Drewry, Reference Aschaffenburg and Drewry1959). Proteose peptone N (PPN) was measured by the Kjeldahl method in acid whey obtained according to van Boeckel & Crijns (Reference van Boeckel and Crijns1994). From these nitrogen fractions, total protein (TN×6·38), casein nitrogen (CN=TN–NCN), casein (CN×6·38), whey proteins N (WN=NCN–NPN), whey proteins (WN ×6·38), proteose peptone (PP=PPN×6·38), casein number (casein nitrogen×100/total nitrogen) were calculated. Total Ca and Mg in milk and soluble Ca and Mg in rennet whey were determined by atomic absorption spectrometry (AAS) (De Man, Reference De Man1962). Total P, soluble P and total acid-soluble P were assessed in milk, in rennet whey and in milk after treatment with TCA (120 g/l), respectively, with the colorimetric method proposed by Allen (Reference Allen1940). Distribution of Ca and P fractions were calculated according to White & Davies (Reference White and Davies1958). Colloidal P was corrected for the quota of P in phospholipids according to Bonaga & Mascolo (Reference Bonaga and Mascolo1977). Dry matter was determined in 10 g milk in a drying oven at a temperature of 102 °C according to Savini (Reference Savini1946). Ash content was determined using the gravimetric method according to Savini (Reference Savini1946) after calcination of the milk sample in a muffle furnace at 530 °C.
A 0·2 ml (1 : 100) rennet solution (1 : 19 000; Chr. Hansen, I-20094 Corsico MI, Italy) was added to milk samples (10 ml). The RCP, milk clotting time (r), curd firming time (k20) and curd firmness (a30), were measured at 35 °C (Malacarne et al. Reference Malacarne, Summer, Fossa, Formaggioni, Franceschi, Pecorari and Mariani2006) using a Formagraph (Foss Electric). Milk clotting time is the time from the addition of rennet to the onset of gelation. Curd firming time is the time from the onset of gelation till the signal attains a width of 20 mm. Curd firmness is the width of the signal 30 min after the addition of rennet. To record k20 values even in milk samples that do not reach a width of 20 mm within 30 min, the analysis was prolonged to 60 min.
Analyses of milk rheological properties resistance to compression and resistance to cut of the coagulum were measured 30 min after the beginning of coagulation, using the Gel Tester apparatus (Marine Colloids Inc. Springfield, NJ 07081, USA) (Annibaldi, Reference Annibaldi and Parmigiano-Reggiano1973).
Classification of milk samples
The milk samples were classified as Optimal, Suboptimal, Poor and Non-coagulating according to their RCP values (Table 1). This classification is in accordance with the original research of Annibaldi et al. (Reference Annibaldi, Ferri and Mora1977) and Pecorari et al. (1984). Briefly, Annibaldi et al. (Reference Annibaldi, Ferri and Mora1977) analysed the RCP of about 15 000 herd milk samples collected in the Parmigiano-Reggiano production area. Originally, the milk samples were classified in eight LDG types identified by capital letters: A, B, C, D, DD, E, F, FF. Later, 5 new types were introduced. Pecorari et al. (1984) grouped LDG types into four classes (Optimal, Suboptimal, Poor and Non-coagulating). This categorisation is made on the basis of data collected by the technicians of the Parmigiano-Reggiano Cheese Consortium during their assistant activity. Each class is constituted including milk LDG types with the same technological behaviour during Parmigiano-Reggiano cheesemaking. These four categories are currently used in the milk quality payment system used for Parmigiano-Reggiano cheese (Pecorari et al., 1984).
Table 1. Method of herd milk samples classification (Annibaldi et al. Reference Annibaldi, Ferri and Mora1977; Pecorari et al. 1984)

Statistical analysis
The average value resulting from the duplicate experiment was submitted to statistical analysis. Data were submitted to ANOVA univariate (IBM SPSS Statics 20, Armonk, New York 10504-1722, USA), using as fixed factor the lactodynamographic classification (4 levels: Optimal, Suboptimal, Poor and Non-coagulating).
Results and discussion
According to their RCP values, the 118 herd milk samples could be classified into 5 (A, B, AE, E or FF) out of 13 potential LDG types and 18 of them (15 %) were grouped within the Non-coagulating class (Table 2). Low proportions of Non-coagulating milk are reported both in Holstein Friesian (8 %, Cassandro et al. Reference Cassandro, Comin, Ojala, Dal Zotto, De Marchi, Gallo, Carnier and Bittante2008) and Finnish Ayrshire (8–13 %, Ikonen et al. Reference Ikonen, Ahlfors, Kempe, Ojala and Ruottinen1999, Reference Ikonen, Morri, Tyrisevä, Ruottinen and Ojala2004) at individual milk level. The high percentage of Non-coagulating observed at herd milk level here may depend on the presence of milk from some infected glands that impair the rennet coagulation properties of the whole mass of milk in the herd tank (Fleminger et al. 2013). Eighty-one milk samples had a somatic cell count below the legal limit of 400 000 cells/ml (Table 2). In this subsample the proportion of Non-coagulation milk was lower than in milk samples with >400 000 cells/ml (6 vs. 35 %). This difference is expected because of the positive relationships existing between the somatic cell count of bulk tank milk and the number of infected glands in the herd (Eberhart et al. Reference Eberhart, Hutchinson and Spencer1982). To reduce the influence of mastitic milk, only data from the subsample with SCC<400 000 cells/ml (n=81) were submitted to ANOVA, whose results are reported and discussed hereafter (Tables 3–7).
Table 2. Classification of herd milk samples above or below the legal limit for somatic cell (400 000 cell/ml). N. of samples

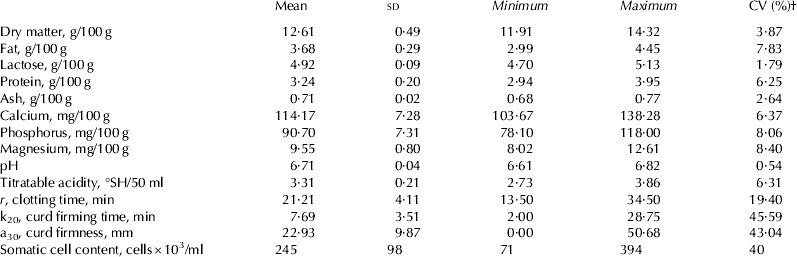
The descriptive statistics of herd milk samples with SCC<400 000 cell/ml are reported in Table 3. Values of pH and lactose were seen to be within the normal range for cow's milk. The contents of fat, protein, lactose and ash are comparable with those reported by Tedeschi et al. (Reference Tedeschi, Malacarne, Tosi and Sandri2010) for herd milk in the Parmigiano-Reggiano cheese production area. About 25 % of samples had a value of titratable acidity ⩽3·20 °SH/ml (hypoacid milk). This confirms the increasing prevalence of hypoacid milk over recent years (Formaggioni et al. Reference Formaggioni, Sandri, Franceschi, Malacarne and Mariani2005). Among RCP, curd firming time and curd firmness showed a higher coefficient of variation (CV) compared with clotting time. Four milk samples showed a clotting time higher than 30 min and 27 samples did not reach a width of 20 mm within 30 min.
Table 3. Descriptive statistics of herd milk samples analysed (N. 81)

† Coefficient of variation.
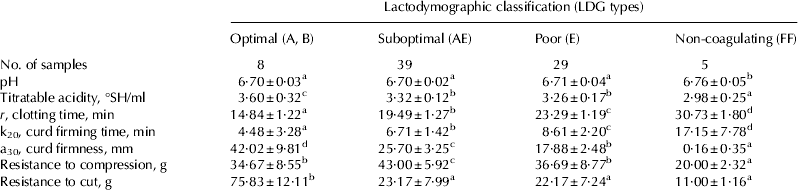
Titratable acidity, pH, RCP and rheological properties are given in Table 4. The highest value of pH was reported in Non-coagulating milk, whereas no significant differences were observed among other classes, although an increasing trend was reported from Optimal to Poor milk. This is in agreement with most studies that report a positive correlation between milk pH and rennet clotting time (Ikonen et al. Reference Ikonen, Morri, Tyrisevä, Ruottinen and Ojala2004; Cassandro et al. Reference Cassandro, Comin, Ojala, Dal Zotto, De Marchi, Gallo, Carnier and Bittante2008). Actually, reducing milk pH increases both the activity of chymosin and the amount of diffusible ionic calcium, with positive repercussions for the first and the second phases of rennet coagulation (Tsioulpas et al. Reference Tsioulpas, Lewis and Grandison2007). Non-coagulating milk showed the lowest titratable acidity value, whereas Optimal milk manifested the highest one. Similarly, Cassandro et al. (Reference Cassandro, Comin, Ojala, Dal Zotto, De Marchi, Gallo, Carnier and Bittante2008) found a favourable genetic correlation between titratable acidity and RCP at individual milk level. As expected, all rennet coagulation parameters were seen to be different among milk classes. Rheological properties of the curd were expressed by the parameters resistance to compression and resistance to cut, both of which were measured 30 min after its formation and are descriptive of the elasticity, contractility, and permeability of the curd and, consequently, of its capacity and rate of syneresis. The higher the values of both parameters, the better the rheological properties of the curd. The value resistance to compression was seen to be markedly higher – and thus more favourable – in Suboptimal milk than in other milks. The highest value of resistance to cut was reported in Optimal milk, while the lowest was observed in Non-coagulating milk. Similarly, Malacarne et al. (Reference Malacarne, Summer, Fossa, Formaggioni, Franceschi, Pecorari and Mariani2006) report the highest values of both parameters in milk with the better rennet coagulation parameters. This confirms the strict relationships between the behaviour of the rennet coagulation and the development physico-mechanical properties of the resulting curd.
Table 4. Values of pH, titratable acidity, rennet coagulation parameters and rheological properties of herd milk samples grouped according to their LDG classification. Mean±sd

Values without a common superscript are different at P<0·05.
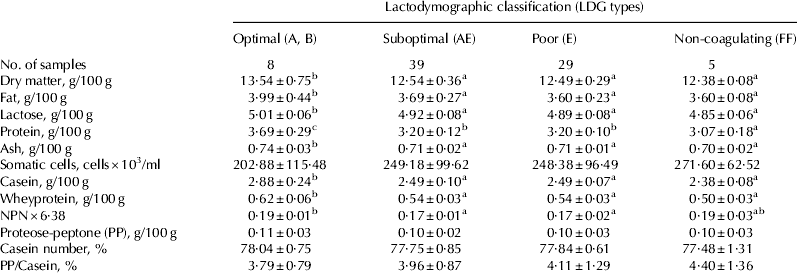
Basic composition, somatic cell content and protein fractions of herd milk samples are shown in Table 5. Optimal milk was characterised by the highest content of dry matter and its constituents (fat, lactose, protein, and ash) than other milk classes. Non-coagulating milk showed the lowest amount of protein. At individual milk level, Auldist et al. (Reference Auldist, Johnston, White, Fitzsimons and Boland2004) report a positive correlation between milk total solids (and its constituents) and curd firmness measured 60 min after rennet addition. Similarly, Martin et al. (Reference Martin, Chamba, Coulon and Perreard1997) report better rennet coagulation parameters in protein-rich bulk milk and Kriščiunaite et al. (Reference Kriščiunaite, Stulova, Taivosalo, Laht and Vilu2012) find a positive correlation between protein content and curd firmness at herd milk level. Furthermore, Optimal milk showed the highest amount of casein and whey protein. Optimal and Non-coagulating milk had the highest content of NPN×6·38. Non-coagulating milk showed the lowest value of lactose, whereas no differences were reported in other classes. As a decrease of lactose is a typical feature of mastitic milk, this observation suggests the presence of a higher quota of milk from infected glands in Non-coagulating milk than in milk from other classes. The presence of milk from infected glands was also confirmed by the increasing trend of proteose peptone (in 100 g of casein) from Optimal to Non-coagulating milk – although differences among classes were not significant – as proteose peptone arose from the breakdown of β-casein by plasmin (Andrews, Reference Andrews1983), which activity increases in mastitic milk (Leitner et al. Reference Leitner, Krifucks, Merin, Lavi and Silanikove2006). Furthermore, some of the breakdown products of β-casein (fragment 1–28) show impairing properties towards milk rennet coagulation, probably by chelating diffusible ionic Ca (Fleminger et al. 2013). However, the somatic cell count of Non-coagulating milk was below the legal limit and not different from other classes. Actually, the predictive value of somatic cell count on milk quality became less effective at herd milk level because of the dilution of mastitic milk with milk from bacterial-free glands (Leitner et al. Reference Leitner, Silanikove, Jacobi, Weisblit, Bernstein and Merin2008).
Table 5. Basic composition, somatic cell content and protein fractions in herd milk samples grouped according to their LDG classification. Mean±sd

Values without a common superscript are different at P<0·05.
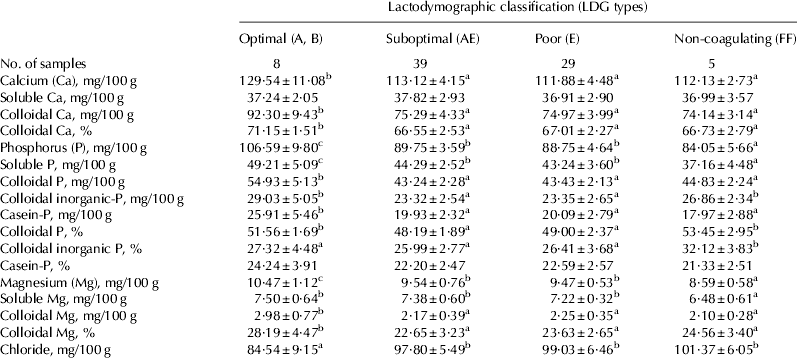
Optimal milk had the highest content of total Ca, total P and total Mg and of their correspondent colloidal fractions (Table 6). No differences were observed among Suboptimal, Poor and Non-coagulating milks for the same parameters. Concerning soluble fractions, no differences, among milk classes, were observed for Ca, whereas soluble P was highest in Optimal milk and lowest in Non-coagulating milk. This latter also had the lowest value of soluble Mg. As colloidal fractions are constituents of the casein micelle, the greater contents of Ca, P and Mg in the colloidal phase were related to the elevated content of casein in Optimal milk. The percentage (relative to corresponding total contents) of colloidal Ca and colloidal Mg were higher in Optimal milk than other milk classes. Interestingly, the values of colloidal inorganic-P (mg/100 g) and colloidal P (%) in Non-coagulating milk were not different from those in Optimal milk. Similarly, Jensen et al. (Reference Jensen, Poulsen, Andersen, Hammershøj, Poulsen and Larsen2012b) underline a higher percentage of colloidal Ca, P and Mg in well than in poor and Non-coagulating milk, at individual level. The lowest value of chloride was reported in Optimal milk, while no differences were observed among milks of other LDG classes. Increase of chloride is associated with a decrease in milk rennetability (Patel & Reuter Reference Patel and Reuter1986) and it is a marker of the presence subclinically infected quarters in the herd.
Table 6. Mineral content and salt equilibria in herd milk samples grouped according to their LDG classification. Mean±sd

Values without a common superscript are different at P<0·05.
Chemical characteristics of casein micelles are shown in Table 7. The casein micelle in Optimal milk showed the highest values of Ca, P, and Mg. As mentioned above, micellar P can be present in two different chemical forms, as inorganic-P (constituents of CCP) or casein-P (as phosphate groups of caseins). Optimal milk showed the highest values of casein-P. No differences for this parameter were observed among other milk classes. The quota of inorganic-P in Optimal milk was seen to be higher than in Suboptimal and Poor milks, and not different when compared with Non-coagulating milk value. According to these results, the higher the content of Ca, P, and Mg in the casein micelle, the better the RCP values of milk. However, even the relationships between the different forms of micellar-P play a role in rennet coagulation of milk. In fact, besides being poorer in colloidal P, the casein micelle of Non-coagulating milk was characterised by the same degree of mineralisation as Optimal milk (expressed by the content of inorganic-P). Consequently, the number of phosphate groups available for curd formation in NC milk is supposed to be low, which could lead to an increase of the time necessary for paracasein to form the curd that, if longer than 30 min from rennet addition, results in the classification of milk as Non-coagulating. The key role play by phosphate groups of casein in micelle aggregation which occur during coagulation was reported by Pearse et al. (Reference Pearse, Linklater, Hall and Mackinlay1986) in artificial micelle. They observe that dephosphorylation of casein (mainly β-casein) negatively influence the aggregation of artificial micelles.
Table 7. Micellar contents of Ca, P and Mg (g/100 g casein) in herd milk samples grouped according to their LDG classification. Mean±sd
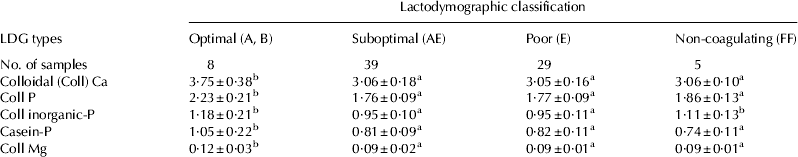
Values without a common superscript are different at P<0·05.
Conclusions
Results observed here emphasised the importance of SCC as a parameter to predict the rennet coagulation aptitude of milk. However, the predictive value of SCC became less effective at herd milk level because of the dilution of mastitic milk with milk from bacterial-free glands. The use of a milking system that separates quarter milk or cow's individual milk on the basis of on-line measurements of its rennet coagulation aptitude would improve the processing quality of milk at herd level. This study suggested that the amount of CCP and the number of phosphate groups inside the casein micelle also seem to influence its behaviour during rennet coagulation. Overall, high content of CCP positively affect micelle rennetability. However, excessive content of CCP could lead to a reduction of the phosphate groups available for curd formation in the secondary phase of rennet coagulation. Given low number of samples analysed in the extreme classes, further observations are necessary to confirm this hypothesis, especially at individual and quarter milk level. Analysis of Ca and P in their different forms will give technicians further parameters to consider when facing the increasing problem of poor or non-coagulating milk recorded in several areas involved in Italian PDO cheeses production. Furthermore, in recent years, particular attention has been paid by Italian cattle breeders associations to improving the content of casein in milk by means of genetic selection, because of its positive effects on cheese yield and quality. Besides casein, the results of this study suggest that the mineral counterpart of the micelle should also be carefully considered, in order to avoid alterations of chemical equilibria inside the micelle. However, to date methods employed are not only time-consuming but also expensive, and therefore can not be used in current milk recording system.