INTRODUCTION
For the most part, the burden of parasitic helminth control in animals and humans falls on anthelmintics. Although the currently available portfolio of anthelmintics acts on a broad variety of protein targets, most commonly these drugs serve to disrupt normal motor function in the parasites they are used to treat, often inducing spastic (e.g. levamisole) or flaccid (e.g. piperazine) paralysis. Since the anthelmintics that are in use today were discovered by empirical screening, this largely independent process selected motor function as a favoured target for helminth control. Motor function encompasses many aspects of parasite biology including the ability to move, feed and reproduce and the successful coordination of these activities is essential to the survival of all helminth parasites.
Although nematodes, flatworms and insects appear to be relatively simple metazoans, they all have surprisingly complex neuromuscular systems that enable the coordination of sophisticated behaviours that have contributed to the success of these three phyla (Stretton et al. 1992; Geary et al. 1999; Nässel, 2002; Halton & Maule, 2004). A critical factor in the development of novel anti-parasite drugs is the selection of an appropriate target system. In this respect, the validity of the neuromuscular system as a good drug target is not in question. Parasitic helminths and ectoparasites are characterized by effective attachment organs (flatworms) and sophisticated reproductive systems (flatworms, nematodes and arthropods). In addition, the alimentary tract in both animals is specialized and they have a multitude of sensory receptors that modulate motor activity. A key component of neuromuscular function is the intercellular signalling molecules that act between nerves and between nerves and muscles to enable coordinated behaviours. Classical transmitter molecules and neuropeptides appear to be the main players in the intercellular signalling circuits of helminth and arthropod neuromuscular systems. Interestingly, all of the anthelmintics that are known to directly compromise normal motor function in helminth parasites do so by acting at ion channels or the receptors of classical neurotransmitters (also, commonly ion channels), e.g. levamisole acts at nicotinic acetylcholine receptors, the avermectins act at glutamate-gated Cl− channels, piperazine acts at GABA-gated Cl− channels and praziquantel acts at Ca2+ channels.
As a backdrop to this review, it is worth examining the features of classical signalling pathways in helminths which have made them so receptive to drug intervention: (1) Is it because classical transmitters play the predominant role in neuromuscular coordination? In all studies that have examined the distribution of classical transmitters and neuropeptides, neuropeptides appear to be at least as abundant and widespread and are widely associated with the innervations of muscular organs or tissues. (2) Is it because classical transmitters often dramatically alter neuromuscular activity? Classical transmitters often induce gross changes in muscle activity or tone, either via direct actions on muscle or via indirect actions on associated nerves. Although many neuropeptides are known to modulate the actions of classical transmitters, some neuropeptides can also directly and dramatically alter motor activity in helminths and arthropods. (3) Is it because many classical transmitters act to directly gate ion channels? The anthelmintics that interfere with motor function act at ion channels. Although the majority of neuropeptides act via G-protein-coupled receptors to trigger cytosolic signalling cascades, some neuropeptides also act on ion channels to rapidly alter muscle activity such that their potential as drug targets would appear to be just as great (Purcell et al. 2002a,b). So it appears that many of the facets of classical transmitters that may be responsible for their utility as drug targets are also fulfilled by neuropeptides.
One other attractive feature of neuropeptide signalling as a target for novel parasiticides is the fact that similar or related neuropeptides play important neuromodulatory roles in nematodes, arthropods and flatworms (see Tables 1 and 2; Maule et al. 2002; Mousley, Marks & Maule, 2004; Mousley et al. 2004). What are the implications of this fact? If structurally related peptides can modulate muscle activity in parasites and pests from distinct phyla, then we can hypothesise that the receptors at which these peptides act could offer potential as the targets for drugs that act across a broad spectrum of species. This ability to transcend the phyla boundaries of endoparasites and ectoparasites is a highly sought after commodity as it has the potential to provide a drug that treats multiple parasites and pests simultaneously, thereby enhancing drug utility. The control of helminths and arthropods is of importance due to their massive influence on the profitability of the livestock industry and health status of humans and domestic animals (Londershausen, 1996). Currently, only the macrocyclic lactones (milbemycin/avermectin) act as endectocides and they represent the most successful (in terms of efficacy and spectrum of activity) anthelmintics available today, and they continue to dictate the standards for future novel compounds (Geary, Conder & Bishop, 2004). This review will examine neuropeptide signalling systems across the target phyla in an attempt to evaluate the potential of neuropeptide receptors as targets for the next generation of endectocides. The importance of work in this area is based on the fact that drug resistance is widespread (Kaplan, 2004; Wolstenholme et al. 2004) and, for the foreseeable future, the treatment of parasites and pests will continue to rely on chemotherapy.
Table 1. Native, intra- and inter-phyla activities of nematode FMRFamide-like peptides (FLPs) in helminths and arthropods Data are restricted to peptides for which inter-phyla activity is known. Nematode peptide effects on second messengers are not shown, e.g. KHEYLRFamide is known to stimulate cAMP levels in A. suum (Reinitz et al. 2000; Thompson et al. 2003). Note that KHEYLRFamide has also been shown to potentiate ACh-induced depolarization of A. suum muscle membranes by a mechanism that is thought to involve muscarinic receptors (Trailovic et al. 2005). Also, PF1 has been shown to inhibit ACh-induced contractions in dorsal muscle strips of the chicken nematode, Ascaridia galli (Franks, Walker & Holden-Dye, 2004). The physiology traces are inserted to show the qualitative effects of peptides on an individual muscle preparation and are simply illustrative of response types – scale bars are omitted for clarity. In all cases, the presence of peptide is indicated by the solid bar above the trace. References: [1] Maule et al. 1995a; [2] Bowman et al. 1995; [3] Holden-Dye et al. 1995; [4] Franks et al. 1994; [5] Marks & Maule, personal communication; [6] Bowman et al. 2002; [7] Cowden & Stretton, 1993; [8] Thompson et al. 2003; [9] Maule et al. 1994b; [10] Davis & Stretton, 1996; [11] Maule et al. 1995b; [12] Holden-Dye, Brownlee & Walker, 1997; [13] Kubiak et al. 1996; [14] Moffett et al. 2001; [15] Fellowes et al. 2000; [16] Fellowes et al. 1998; [17] Brownlee & Walker, 1999; [18] Brownlee et al. 1995; [19] Marks et al. 1999b; [20] Rogers et al. 2001; [21] Reinitz et al. 2000; [22] Davis & Stretton, 2001; [23] Nelson, Rosoff & Li, 1998; [24] Li, Kim & Nelson, 1999; [25] Waggoner et al. 2000; [26] Rogers et al. 2003; [27] Totten, Marks & Maule, personal communication; [28] Marks et al. 1997b; [29] Graham, Fairweather & McGeown, 1997; [30] Mousley, Halton, Geary, Thompson, Marks & Maule, unpublished. Genus names: P, Procerodes; F, Fasciola; S, Schistocerca. a, amide.

Table 2. Native, intra- and inter-phyla activities of arthropod type-A allatostatins and FMRFamide-like peptides (FLPs) in helminths and arthropods Data are restricted to peptides for which inter-phyla activity is known. Only the most salient effects of arthropod FLPs and type-A allatostatins are listed. The physiology traces are inserted to show the qualitative effects of peptides on an individual muscle preparation and are simply illustrative of response types – scale bars are omitted for clarity. In all cases, the presence of peptide is indicated by the solid bar above the trace. References: [1] Lange, Bendena & Tobe, 1995; [2] Aguilar et al. 2004; [3] Rankin et al. 1998; [4] Dircksen et al. 1999; [5] Duve, East & Thorpe, 1999; [6] Duve et al. 2000; [7] Vilaplana et al. 1999; [8] Holman, Cook & Nachman, 1986; [9] Cook, Wagner & Pryor, 1993; [10] Predel, Rapus & Manfred, 2001; [11] Cuthbert & Evans, 1989; [12] Robb, Packman & Evans, 1989; [13] Wood et al. 1992; [14] Peeff, Orchard & Lange, 1993; [15] Lange & Orchard, 1998; [16] Fuse & Orchard, 1998; [17] Fuse et al. 1999; [18] Vilaplana, Castresana & Bellés, 2004; [19] Orchard & Te Brugger, 2002; [20] Duve et al. 1993a; [21] Schoofs et al. 1993; [22] Robb & Evans, 1994; [23] Clark & Lange, 2002; [24] Lange, Orchard & Te Brugger, 1991; [25] Lange, Peeff & Orchard, 1994; [26] Peeff, Orchard & Lange, 1994; [27] Facciponte, Miksys & Lange, 1995; [28] Elia & Orchard, 1995; [29] Nachman et al. 1993; [30] Kingan et al. 1996; [31] Mousley et al. 2005; [32] Mousley et al. 2004; [33] Maule et al. 1996. Genus names: P, Procerodes; A, Ascaris. a, amide; *Y, sulphated tyrosyl.

NEUROPEPTIDES IN HELMINTHS AND ARTHROPODS
A wide variety of biochemical, immunochemical and molecular methods have resulted in enormous progress in the identification of neuropeptides and their receptors from species of the phyla Arthropoda, Nematoda, and Platyhelminthes. Indeed, recent years have witnessed an explosion in the number of neuropeptides identified in a broad range of invertebrate species, in particular the model organisms Caenorhabditis elegans and Drosophila melanogaster (Li et al. 1999; Nässel, 2002; Taghert & Veenstra, 2003; McVeigh et al. 2005).
Unfortunately, there is no consistent universal naming scheme for either arthropod or helminth neuropeptides. In arthropods the isolation of novel regulatory peptides including FMRFamide-like peptides (FLPs) and allatostatins has generally been based on the biological activity of peptide fractions, identified from a particular species using a bioassay system, and structural characterization. Therefore, in many cases, peptides have been functionally characterized long before their tissue localisation or structure has been determined. Consequently, arthropod peptides are commonly named after their source and/or description of their activity. The contrary is true for helminths as molecular characterization nearly always precedes functional characterization and therefore helminth peptides are typically named chronologically upon discovery.
In general, commonality of amino acid structure between the numerous identified invertebrate sequences has warranted their delegation into distinct peptide families. It is estimated that some 38 arthropod neuropeptide families exist including the FLPs and allatostatins (Nässel, 2002). In contrast, only 4 neuropeptide groupings are recognized in helminths FLPs, neuropeptide-like proteins [NLPs], insulin-related peptides and the neuropeptide F family) (see Maule et al. 2002); it seems likely that others await discovery. Of the neuropeptides identified in invertebrates, the FLPs are the best known, the most diverse and commonly modulate motor activity in arthropods and helminths, a feature that has elevated their status to components of a signalling system that has potential chemotherapeutic value. Although in this context the FLPs are the most conspicuous candidates, allatostatin-like peptides have recently emerged as potential multi-phyla neuropeptides that could have chemotherapeutic potential.
FMRFAMIDE-LIKE PEPTIDES
The molluscan cardioexcitatory peptide FMRFamide, first isolated from the venus clam Macrocallista nimbosa (Price & Greenberg, 1977), is now considered to be the prototype of a pervasive family of structurally-related peptides, the FLPs. These peptides are classically characterized by a C-terminal motif that most commonly comprises an aromatic residue, a hydrophobic residue, and an Arg-Phe-amide (see Maule et al. 2002). However, with increasing member diversity, small peptides with RFamide at the C-terminus and one of the other two features, are widely viewed as FLPs.
FMRFamide-like peptides in the phylum Nematoda
FLP distribution in nematodes
The traditional application of immunocytochemistry (ICC) interfaced with confocal scanning laser microscopy (CSLM) has not only improved our understanding of the complexity of the nematode nervous system but also fuelled the discovery of many structurally distinct FLPs. A large number of immunocytochemical screens of both parasitic and free-living nematodes have apportioned up to 75% of the neurons as FLPergic making them the most abundant neuropeptide family in nematodes (Schinkmann & Li, 1992; Cowden et al. 1993; Brownlee, Fairweather & Johnston, 1994; Li et al. 1999). These studies have revealed that FLPs occur in all known neuronal sub-types including motor-, sensory- and inter-neurons and it is clear that there is strong conservation of the number and position of FLPergic neurons between diverse nematode species. Of key importance here is the fact that FLP-containing nerves innervate the pharynx, ovijector and somatic muscle of nematodes, all potential target tissues.
Commonly, the C-terminally directed antisera employed in immunocytochemical studies have a broad specificity and cannot reliably discriminate between so many structurally related peptides. It is unfortunate that the multiple antigenic peptide approach to the generation of N-terminally-directed antibodies that has been successfully employed to examine expression patterns of individual FLPs in D. melanogaster (see Nichols, McCormick & Lim, 1997, 1999; Nichols, Lim & McCormick, 1999; Nichols, 2003) has not, to date, been exploited in nematodes. However, the deployment of reporter-flp gene constructs and, more recently, in situ hybridisation (ISH) methods to reveal flp expression has added significant validity to localisation studies in nematodes.
C. elegans flp gene expression data have been collected by inserting green fluorescent protein (GFP) or lacZ reporter constructs into promoter regions for individual flp genes; cell specific expression patterns for most of the C. elegans flp genes have now been reported (Li, Kim & Nelson, 1999; Li et al. 1999; Kim & Li, 2004). Also, ISH has been used to uncover the expression patterns of 5 flp genes (homologous to C. elegans flp-1, flp-6, flp-12, flp-14 and flp-18) in the potato cyst nematode, Globodera pallida (Kimber et al. 2002). This technique is highly specific and can help avoid the specificity issues associated with immunocytochemical and even reporter gene expression studies. These studies have revealed that individual flp genes have restricted and often distinct distribution patterns such that each individual neuron expresses a small subset of flp genes. It was shown that the expression patterns for selected flp gene homologues were not conserved between C. elegans and G. rostochiensis (Kimber et al. 2002). We have now performed ISH methods for selected flp genes in a range of parasitic nematodes and there appear to be both similarities and differences in the expression profiles of individual flp genes in the different nematode species. The implications of this are currently unclear, but identical or highly similar peptides could potentially have distinct functions in different nematode species.
FLP identification in nematodes
A large number of unique FLPs have been characterized by conventional methods involving the collection and extraction of neuron-rich nematode tissue followed by step-wise chromatographic purification and immunometric monitoring of reactive fractions. To date some 42 FLPs have been biochemically characterized from both free-living [C. elegans (14), Panagrellus redivivus (5)] and parasitic [Ascaris suum (20) Haemonchus contortus (3)] nematodes (Cowden, Stretton & Davis, 1989; Geary et al. 1992; Rosoff, Burglin & Li, 1992; Cowden & Stretton, 1993, 1995; Rosoff et al. 1993; Maule et al. 1994a,b, 1995b; Keating et al. 1995; Davis & Stretton, 1996; Marks et al. 1996b, 1997a, 1998, 1999a,b; Edison, Messinger & Stretton, 1997). The molecular approach to nematode FLP characterization, pioneered by Rosoff et al. (1992) and significantly aided by the completion of the C. elegans genome sequencing project, has proved highly fruitful. Indeed, the majority of structural information on nematode FLPs has been gleaned from predictions of flp gene products in C. elegans. Until recently, 23 C. elegans flp genes were recognized to encode some 60 different putative FLPs (Rosoff et al. 1992; Nelson et al. 1998; Li, Kim & Nelson, 1999; Li et al. 1999; Kim & Li, 2004). Published works report six flp genes from A. suum (Edison et al. 1997; McVeigh, Marks & Maule, unpublished) and five from the potato cyst nematode, G. pallida (Kimber et al. 2001). Recently, we undertook examination of the Genbank nematode expressed sequence tag (EST) database and uncovered a substantial number of flp gene candidates from parasitic nematodes (McVeigh et al. 2005). These studies uncovered eight novel flp-encoding gene homologues in multiple species including representatives of four of the five nematode clades; some of these recently uncovered flp-encoding genes have been confirmed through PCR-based cDNA analyses (McVeigh, Mair, Leech, Miskelly, Marks & Maule, unpublished data). These studies have uncovered unprecedented diversity in nematode neuropeptides with approximately 290 distinct FLPs represented amongst the parasitic nematode ESTs. However, even with the rapid identification of putative FLPs through EST-based studies, structural characterization still remains the only way to unequivocally confirm the peptide products of these genes and their associated post-translational modifications.
If we peruse available data (biochemically isolated sequences, characterized flp-encoding genes, and putative flp-encoding ESTs) on nematode FLPs it is clear that while some are unique to a particular nematode species, inter-species conservation of FLP sequences is commonplace. Interestingly, neuropeptide sequence similarities not only occur between the FLPs of free-living nematodes (C. elegans, P. redivivus), but also between those of free-living and parasitic forms (A. suum and H. contortus). It is tempting to speculate that those FLPs that are most highly conserved across nematode species (for example, KHEYLRFamide [AF2], and KSAYMRFamide [PF3]) include those peptides that have important associated physiology and, therefore, may represent key players in targeted drug discovery programmes. However, comparative analysis of the FLP complement of nematode parasites will ultimately require the structural identification of the expressed peptides in representatives of all the target species.
FLP function in nematodes
The ability to exploit the FLPergic system in nematodes is constrained not only by a limited understanding of the structure of endogenous targets (FLP receptors) but also by an ignorance of the functional significance of individual FLPs to worm biology. Indeed the recent surge in FLP identification in nematodes has led to a large disparity between the available structural and associated functional information.
A. suum is commonly used in experiments investigating neuromuscular function in nematode parasites and, although not proven, it is broadly perceived that physiology data determined using this swine parasite could provide baseline information applicable across the nematode phylum; this seems especially so as there is such a high level of conservation in FLP signatures in nematodes. Functional investigations in A. suum have centred around four tissue types: somatic body wall musculature, neurons, the ovijector, and the pharynx (see Maule et al. 2002 for review). In addition, several studies have involved injecting FLPs into the A. suum pseudocoelomic cavity and observing effects on whole worm locomotion (Reinitz et al. 2000; Davis & Stretton, 2001). It should be noted that reports of FLP activities in other parasitic nematodes are restricted to the domestic fowl parasite, Ascaridia galli (Trim et al. 1997, 1998; Franks, Walker & Holden-Dye, 2004), and the sheep parasite H. contortus (Marks et al. 1999b).
With respect to free-living nematodes, functional investigations have been largely constrained by worm size, limiting physiology studies on neuropeptide involvement in feeding and reproduction. With respect to the former, Rogers et al. (2001) have used electrophysiology to reveal FLP effects on pharyngeal tissue in C. elegans. Several studies have looked towards reverse genetics as a tool to unravelling FLP function in C. elegans (see Nelson, Rosoff & Li, 1998; Li et al. 1999) through over-expression or inactivation of target genes. Although a series of aberrant phenotypes were observed in flp-1 knockout C. elegans (Nelson, Rosoff & Li, 1998), profound phenotypes have not been seen with many other flp gene knockouts (Chris Li, personal communication). Recently the involvement of FLPs in other biological processes in free-living nematodes, including the control of social feeding, egg-laying, and male copulatory development and behaviour have been reported (Waggoner et al. 2000; Rogers et al. 2003; Lints et al. 2004; Geary & Kubiak, 2005).
Pooled data indicates that potent and diverse myomodulation is a common outcome of FLP action in nematodes and implicates roles for FLPs in control/modulation of locomotory behaviour, feeding, and reproduction. The most significant findings of the collated physiology data from A. suum are briefly summarised below.
Somatic muscle physiology: Most of the accumulated data on FLP activities in nematodes have been obtained using A. suum somatic body wall muscle strips. Numerous parasitic and free-living nematode-derived FLPs with structurally distinct C-terminal motifs have been shown to induce a diverse array of both pre- and post-synaptic inhibitory (slow and prolonged or fast and transient), excitatory (sustained contraction), and biphasic (transient relaxation/sustained contractility) activities such that four body wall response types (RTs) have been described and designated bwRT1-bwRT4. The ease in preparation of dorsal, ventral and denervated muscle strips has enabled further delineation of FLP activities on somatic muscle; for example KSAYMRFamide (PF3) displays a unique differential activity comprising nerve-cord dependent excitatory effects on ventral and inhibitory effects on dorsal muscle strip preparations (see Maule et al. 2002). It should also be noted that there is compelling physiological evidence that some nematode FLPs activate ligand-gated ion channels (Purcell et al. 2002a,b).
Neuronal physiology: The effects of 18 Ascaris FLPs have been examined on A. suum motorneuron activity (dorsal excitatory type 2 [DE2] and dorsal inhibitor [DI]); approximately five major response types are associated with endogenous FLPs on these motorneurons (Davis & Stretton, 2001).
Ovijector physiology: Preliminary work initiated by Fellowes et al. (1998, 2000) and augmented by Marks et al. (1999a) and Moffett et al. (2001) demonstrated that, in vitro, the A. suum ovijector displays a spontaneous rhythmical activity that can be significantly modulated by some 31 nematode FLPs. The effects displayed by the FLPs were diverse and could be subdivided into five distinct response-types (ovRT1-ovRT5), indicating FLP-receptor/signalling pathway diversity in the ovijector.
Pharyngeal physiology: The influence of FLPs on pharyngeal pumping behaviour has been monitored using a pressure transducer system; serotonin-induced pumping was found to be significantly modulated by two nematode FLPs (Brownlee et al. 1995), however several nematode FLPs had no effect on serotonin-induced pumping (Brownlee & Walker, 1999).
Behaviour: Comprehensive analyses have been carried out on the effects of endogenous Ascaris FLPs and C. elegans flp gene-encoded peptides on locomotory behaviour of intact adult A. suum (Reinitz et al. 2000; Davis & Stretton, 2001). In these studies, FLPs were directly injected into the pseudocoelomic cavity of large female worms. FLP effects were categorized according to their modulation of general locomotion (increased, decreased and abolished), body posture, and head searching activities. A wide variety of behavioural responses were noted.
Nematode FLP receptors
Nematode neuropeptide activated G-protein-coupled receptors (GPCRs) are reviewed elsewhere in this volume (see review by Greenwood, Williams & Geary in this supplement). Until recently, the involvement of seven-pass GPCRs in FLP signalling in helminths has been based on an expanding portfolio of indirect data gleaned from worm physiology, heterologous expression and reverse genetics (Nelson, Rosoff & Li, 1998; Reinitz et al. 2000; Kubiak et al. 2003a,b,c; Thompson et al. 2003). Although the first FLP receptor was characterized in 1995 (a directly ligand-gated sodium channel from the mollusc, Helix aspersa), it was another 8 years before the first nematode FLP receptor was discovered. This breakthrough was aided by the completion of the C. elegans genome sequencing project and preliminary identification of ~54 candidate neuropeptide GPCRs (Bargmann, 1998).
The functional expression of some of these C. elegans GPCRs was first reported in a patent release in 2003 (see Lowery et al. 2003; Greenwood, Williams & Geary in this supplement). Follow-up publications reported the deorphanization of two C. elegans GPCRs as FLP receptors; flp-21 (GLGPRPLRFamide) and flp-18 peptides (possessing C-terminal PGVLRFamide signatures) were matched as ligands for the NPR-1 receptor (Wormbase accession number C39E6.6; Kubiak et al. 2003b; Potter & Luo, 2003; Rogers et al. 2003), previously implicated in the control of the social feeding phenotype by de Bono & Bargmann (1998), and the ‘wormpep’ appointed GPCR, C10C6.2, was found to be activated by flp-15 peptides (possess a GPLRFamide C-terminal signature) and was renamed FLP-15R (Kubiak et al. 2003c). A third C. elegans FLP receptor (C26F1.6), designated the VRFamide receptor 1, has since been cloned and functionally characterised; flp-7 and flp-11 peptides have emerged as the most potent ligands (Mertens et al. 2004) with SMVRFamide being identified as the most active binding motif. The fourth receptor to be ligand-matched had two splice variants (T19F4.1a and T19F4.1b) that were activated preferentially by the FLP-2 peptides (SPREPIRFamide and LRGEPIRFamide) with the active motif being EPIRFamide (Mertens et al. 2005). Unlike the apparent promiscuity of tissue responses to diverse FLPs, these cloned receptors appear relatively selective towards their activating ligand. These expression studies serve to provide very useful preliminary data on potential ligand-receptor pairs but there are several caveats to the process of deorphanization. As all of the possible activating ligands have not been tested in any of these studies, it is premature to assign receptor names that are based on the most potent ligand tested. Also, it is not known if the most potent ligands at each of the receptors act as the in vivo ligands – receptor/ligand expression studies could help support the physiology data.
FMRFamide-like peptides in the phylum Platyhelminthes
Despite the wealth of data available on FLP structure in nematodes and arthropods, complementary data in flatworms has not been so forthcoming; indeed only 4 flatworm FLPs have been structurally characterized (see Maule et al. 2002). Although it is apparent that flatworms do not possess the multitude of FLPs common to nematodes and arthropods, the problems associated with FLP identification in flatworms, including difficulties amassing sufficient nerve-rich tissue and an absence of genomic sequence data, have been significant impediments to this work. As we move away from the classical methods of peptide identification through extraction and purification and towards an era of bioinformatics, it is only a matter of time before more flatworm FLPs are uncovered.
Flatworms do not lend themselves well to physiological manipulation, but they have greatly contributed to our current understanding of FLP function in helminths. Although there are few bioassays available for flatworm parasites, flatworm FLP effects on neuromuscular function have been examined using muscle strip preparations and dispersed muscle fibre assays.
FLP distribution in flatworms
Immunoreactivities to authentic flatworm FLPs have been observed throughout the central and peripheral nervous systems of all the major flatworm taxa including the turbellarians, monogeneans, trematodes and cestodes where they appear widespread and abundant (see Day & Maule, 1999; Halton, 2004; Halton & Maule, 2004). Most of the FLP-immunoreactivities in these species dominate the central nervous system. In the peripheral nervous system, immunostaining is associated with the nerve plexuses that innervate the somatic musculature, the holdfast organs, and the muscles that constitute the alimentary canal and reproductive systems. Although immunocytochemical screens are not reliable indicators of inherent FLP diversity, and indeed the abundant FLP-immunoreactivity observed in every flatworm examined to date is not reflected in the numbers of characterized peptides, they are regarded as an important starting-point to deciphering FLP function in flatworms.
FLP identification in flatworms
Despite concentrated research efforts to characterize endogenous flatworm FLPs, only 4 native FLPs have been biochemically isolated and sequenced. Turbellarians appear to express a limited set of FLPs sharing a YIRFamide motif [GYIRFamide from the turbellarians Bdelloura candida, Girardia tigrina and Procerodes littoralis, RYIRFamide from the land planarian, Arthurdendyus triangulatus, and YIRFamide from B. candida] (Maule et al. 1994c; Johnston et al. 1995, 1996). In contrast, the only known FLP from parasitic flatworms bears an unusual C-terminal FFRFamide motif (GNFFRFamide from the sheep tapeworm Moniezia expansa) (Maule et al. 1993). No flatworm FLP-encoding gene has been reported, although the near-completion of the schistosome genome project and rise in flatworm EST projects will likely uncover putative FLP genes in the near future.
FLP function in flatworms
Whilst experimental investigations of FLP function in parasitic nematodes have been largely restricted to A. suum, several flatworm species (free-living [P. littoralis, B. candida] and parasitic [Schistosoma mansoni, Diclidophora merlangi, Grillotia erinaceus, Fasciola hepatica, Echinostoma caproni, M. expansa and Mesocestoides corti]) have been successfully exploited for elucidation of FLP function (Day et al. 1994; Marks et al. 1996a, 1997b; Graham, Fairweather & McGeown, 1997; Moneypenny et al. 1997, 2001; Day & Maule, 1999; Humphries et al. 2000; Hrčkova et al. 2002). All of the flatworm FLPs isolated to date induce myoexcitation when applied exogenously to muscle strips and dispersed muscle fibres from free-living and parasitic flatworms (see Day & Maule, 1999; Maule et al. 2002; McVeigh et al. in this supplement). However, the flatworm FLPs display potency variations in flatworm bioassays – the turbellarian FLPs were more potent than the cestode FLP in all the turbellarian and trematode bioassays where their activity has been compared.
FLP receptors in flatworms
No flatworm FLP receptors have been characterized to date. Available evidence indicates that flatworm FLP receptors operate via GPCRs to influence intracellular effector proteins through heterotrimeric G-proteins (see McVeigh et al. in this supplement). Indeed several studies have endeavoured to characterize FLP-signalling pathways; Graham, Fairweather & McGeown (2000) have implicated a GPCR and a signalling pathway involving phospholipase C and protein kinase C (PKC) in FLP-induced excitation of F. hepatica muscle strips. In addition, we found that PKC and adenylate cyclase are involved in contractions associated with FLP-induced myoexcitation of P. littoralis dispersed muscle fibres (Totten, Marks, Maule & Day, unpublished).
FMRFamide-like peptides in the phylum Arthropoda
Like the nematode FLPs, arthropod FLPs are structurally diverse (>85 FLPs have been identified in over 23 species of arthropod) and broadly expressed. However, there appear to be no sequence identities between known arthropod and helminth FLPs. Unlike the nematode-derived FLPs, the arthropod FLPs have been divided into distinct sub-groups and, although there are some inconsistencies between authors over the number of FLP sub-groups and the rationale for these divisions, the existence of three FLP sub-groups is the most common train of thought. Indeed, arthropod FLPs are generally delineated into three groups on the basis of their differing C-terminal motifs (RFamide is invariable) and their presence on three different Drosophila precursor genes; (1) the myosuppressins and extended FLRFamides, (2) the extended FMRFamides, and (3) the sulfakinins. Data on FLP complements of arthropods have been enhanced by the application of modern mass spectroscopy methods to elucidate the peptidomes of selected species and tissues (Clynen et al. 2001; Verhaert et al. 2001; Baggerman et al. 2002, 2003, 2005; Schoofs & Baggerman, 2003; Huybrechts, De Loof & Schoofs, 2004; Predel et al. 2004; Verleyen et al. 2004a,b; Reinhard & Gade, 2005). It should be noted that some D. melanogaster genes encode RFamide peptides that have also been identified in additional insect species – the neuropeptide F (NPF)-like peptides exist in short forms (also called the head peptides) and long forms, and are encoded on separate genes (see Vanden Broeck, 2001). These have also been identified in the cockroach, Periplaneta americana (Veenstra & Lambrou, 1995), the horseshoe crab, Limulus polyphemus (Gaus et al. 1993) and the Colorado potato beetle, Leptinotarsus decemlineata (Spittaels et al. 1996; Cerstiaens et al. 1999) and will not be considered in this review. Also noteworthy is the presence of additional RFamides whose structures exclude their assignment into one of the three sub-groups, e.g. the Aedes aegypti head peptides (Matsumoto et al. 1989; Veenstra, 1999).
Elucidation of FLP functions in arthropods are commonly based on visceral muscle bioassay systems including those designed for recording gut (foregut, midgut, and hindgut), oviduct and heart myoactivities in numerous insect species and contrasts markedly to the situation in nematodes where the bulk of available physiology data has been generated using a single parasitic nematode species.
Although, like in the nematodes, in situ hybridisation has been exploited to delineate the expression of FLP-containing neurons in insects, the multiple antigenic peptide approach to characterizing the spatial and temporal distribution of structurally similar FLPs has been more widely utilized. Advantages over the in situ hybridisation technique include the facility to map nerve processes as well as cell bodies facilitating the construction of a complete FLP-specific neuronal atlas.
FLP distribution, identification and function in arthropods
The three sub-groups of FLPs in arthropods are diverse in terms of their structures, precursor organisations, distributions and activities. Current understanding of arthropod FLPs has recently been comprehensively reviewed (Orchard, Lange & Bendena, 2001; Nässel, 2002) and therefore only the most salient features of the three sub-groups are outlined below.
The myosuppressins and the extended FLRFamides: In 1986 Holman, Cook & Nachman isolated a peptide from the cockroach Leucophaea maderae and named it leucomyosuppressin on the basis of its ability to decrease hindgut contractions. Since then four additional myosuppressins have been characterized from five insect species (Schistocerca gregaria [Robb, Packman & Evans, 1989], Locusta migratoria [Schoofs et al. 1993; Peeff, Orchard & Lange, 1994], Neobelliera bullata [Fonaghy et al. 1992a] D. melanogaster [Nichols, 1992a] and Manduca sexta [Kingan et al. 1990]) that conform to the structural definition of this FLP sub-group; all myosuppressins are decapeptides characterized by XDVXHXFLRFamide where X is a variable residue. To date, myosuppressin genes containing a single predicted peptide sequence have been identified in Diploptera punctata, Psuedaletia unipuncta and D. melanogaster (see Nässel, 2002).
Although all of the myosuppressins have been shown to inhibit spontaneous contractions of several muscle systems including foregut, midgut, hindgut, oviduct, heart and skeletal muscles (see Orchard et al. 2001 for review), deviations from the name-giving function have been noted; indeed, the M. sexta myosuppressin stimulates ileum contractions in the sphingid moth, Agrius convolvuli (Fujisawa et al. 1993).
Additional extended FLRFamides have been identified in a number of insects and crustaceans although they do not share the structural or physiological features that would warrant their ‘myosuppressin’ designation (Trimmer, Kobierski & Kravitz, 1987; Krajniak, 1991; Mercier et al. 1993; Lange, Peeff & Orchard, 1994; Kingan et al. 1996, 1997; Sithigorngul et al. 1998, 2001).
The extended FMRFamides: To date, extended FMRFamides are restricted to dipterans. Evidence for their existence arose from immunocytochemical screens of the blowfly Calliphora vomitoria with the subsequent biochemical characterization of 13 FMRFamides (and one MIRFamide), designated calliFMRFamides (Lunquist & Nässel, 1990; Duve et al. 1992). Genes encoding mulitple FMRFamides have since been identified in Drosophila. melanogaster, D. virilus, C. vomitoria and Lucilia cuprina (see Nässel, 2002). The blowfly genes encode 18 putative FLPs, eight of which have been biochemically isolated, and only five of which are homologous between the two blowfly species (Thorpe et al. 1995). The prohormone of the D. melanogaster FMRFamide gene encodes five peptides bearing the C-terminal FMRFamide motif, four of which have been isolated and sequenced or confirmed by tandem mass spectrometry (Nambu et al. 1988; Schneider & Taghert, 1988; Baggerman et al. 2002).
In Drosophila the expression patterns of three FMRFamide-containing peptides have been mapped using the multiple antigenic peptide approach; the use of double and triple staining procedures showed the staining patterns to be unique within the subset of universal FMRFamide staining (see Nichols, Bendena & Tobe, 2002; Nichols, 2003).
Even though extended FMRFamides have not been identified in non-dipteran insects they have been shown to affect several physiological processes including heart rate, gut motility and synaptic activity in a wide range of insect species (see Orchard, et al. 2001).
The sulfakinins: The first sulphated invertebrate peptides were isolated from the cockroach, L. maderae and named sulfakinins after their myostimulatory actions (Nachman et al. 1986a,b). Subsequently, related sulfakinins were identified either via biochemical isolation or molecular characterization of the encoding gene in several insect species including P. americana (Veenstra, 1989), Locusta migratoria (Schoofs et al. 1990), C. vomitoria, L. cuprina (Duve et al. 1995b), N. bullata (Fonaghy et al. 1992b), D. melanogaster (Nichols et al. 1988; Nichols, 1992b; Baggerman et al. 2002) and Anopheles gambiae (Duttlinger, Mispelon & Nichols, 2003).
Sulfakinins are characterized by the common C-terminal structure, X(E,D)DYGHMRFamide, where Y is most commonly sulphated, and all are potent stimulators of cockroach hindgut contractions. Sulfakinins have also been characterized from the black tiger shrimp, Penaeus monodon and the white shrimp, Litopenaeus vannamei (Johnsen et al. 2000; Torfs et al. 2002).
FLP receptors in arthropods
Approximately 44 neuropeptide receptors (GPCRs) have been identified in the D. melanogaster genome (Hewes & Taghert, 2001; Vanden Broeck, 2001) of which more than 18 have been fully functionally characterized (Meeusen et al. 2003; see below); four of these have been identified as FLP receptors.
The first FLP receptor, Drm-FMRFa-R (AC010561), was characterized in 2002 (Cazzamali & Grimmelikhuijzen, 2002; Meeusen et al. 2002) and a homologous gene has since been identified in A. gambiae (Duttlinger et al. 2003) following the completion of its genome sequence. A high degree of structural conservation exists between these two GPCRs, and comparable analogies can be applied to their ligand activation profiles; Drosophila FMRFamides are the native ligands of both FMRFamide receptors, however receptor activation is also induced by myosuppressins and short NPFs, showing some receptor promiscuity.
Two myosuppressin receptors, distinct to the characterized FMRFamide receptors, have been cloned and deorphanized from D. melanogaster (AF544244 and AF545042; Egerod et al. 2003); one has been characterized from A. gambiae (Scholler et al. 2005). Both myosuppressin receptors are specific for TDVDHVFLRFamide (a myosuppressin present in both D. melanogaster and A. gambiae) and are not activated by other insect FLPs.
In addition, two putative D. melanogaster sulfakinin receptors have been identified based on their homology to the mammalian CCK/gastrin receptor family (Brody & Cravchik, 2000; Hewes & Taghert, 2001), one of which has been fully cloned and assigned DSK-R1 (AX128640; Kubiak et al. 2002). Similar receptors have been identified in A. gambiae (Duttlinger et al. 2003).
ALLATOSTATINS
Unlike the FLPs, the primary discovery of allatostatins in insects was not based on their myoactivity but on their inhibitory effects on the production of juvenile hormone (a terpenoid important for development and reproduction) by the corpora allata (endocrine organs near the insect brain).
Allatostatins in the phylum Arthropoda
Allatostatins are loosely organized into a large group of structurally diverse arthropod neuropeptides and were recently sub-divided into three groups based on their differing C-terminal structures: the type-A allatostatins initially identified in cockroaches and delineated by the C-terminal pentapeptide sequence, (Y/F)XFG(L/I)amide (where X is variable); the type-B allatostatins first identified in crickets and characterized by the W2W9amide motif (Lorenz, Kellner & Hoffmann, 1995a); and, the type-C allatostatins, represented by the non-amidated M. sexta/D. melanogaster peptide – pEVR(F/Y)RQCYFNPISCF-OH (Kramer et al. 1991; Price et al. 2002).
Type A allatostatin identification in arthropods
The type-A allatostatins [(Y/F)XFG(L/I)amides], generally referred to as members of the allatostatin superfamily, were first identified in the brain of D. punctata (Pratt et al. 1989, 1991; Woodhead et al. 1989, 1994) and have been the subject of several comprehensive reviews (see Bendena, Donly & Tobe, 1999; Nässel, 2002). Since their initial discovery, both biochemical and molecular biology techniques have been employed to identify a large number of homologous peptides in numerous other insect species including: the cockroaches, P. americana (Weaver et al. 1994; Ding et al. 1995), D. punctata (Donly et al. 1993), Blattella germanica (Bellés et al. 1994, 1999), Blatta orientalis, Blaberus craniifer and Supella longipalpa (Bellés et al. 1999); the locust, S. gregaria (Vanden Broeck et al. 1996; Veelaert et al. 1996a,b); the cricket, Gryllus bimaculatus (Lorenz, Kellner & Hoffmann, 1995b, 1999; Meyerling-vos et al. 2001); the stick insect, Carausius morosus (Lorenz et al. 2000); the silkworm, Bombyx mori (Secher et al. 2001); the fall armyworm, Spodoptera frugiperda (Abdel-latief, Meyering-vos & Hoffmann, 2004); the blowflies, C. vomitoria and L. cuprina (Duve et al. 1993b, 1994, 1995a, 1996; East et al. 1996); the fruitfly, D. melanogaster (Lenz, Williamson & Grimmelikhuijzen, 2000a); the mosquito, A. aegypti (Veenstra et al. 1997); the moths, Cydia pomonella, Helicoverpa armigera (Davey et al. 1999; Duve et al. 1997a,c), Lacanobia oleracea (Audsley & Weaver, 2003) and Manduca sexta (Davis et al. 1997); and the honeybee, Apis mellifera (Rachinsky & Feldlaufer, 2000).
Allatostatins are not restricted to insects. Recently, 39 members of the (Y/F)XFG(L/I) family have been isolated from the tiger prawn P. monodon (Duve et al. 2002) and a further 17 different type-A allatostatins have been isolated from the green crab, Carcinus maenas (Duve et al. 1997b). In addition, 3 members were identified in the crayfish, Orconectes limosus (Dircksen et al. 1999). Indeed, over 100 different arthropod (Y/F)XFG(L/I)amides have been structurally characterized and ~60 additional, novel sequences have been predicted from cDNA.
Type A allatostatin distribution in arthropods
Traditional immunocytochemical studies have revealed a widespread distribution of the (Y/F)XFG(L/I)amides; they not only occur in the central nervous system innervating the cells that project to the corpora allata and the brain, but also in the peripheral nervous system leading to visceral muscles in many different insect species (see Hoffmann, Meyering-vos & Lorenz, 1999 for review).
Type A allatostatin function in arthropods
The allatostatin nomenclature was originally based on the ability of the dipstatins (D. punctata allatostatins) to inhibit the production of juvenile hormone. However, this does not represent the primary role of allatostatins in many arthropod species; indeed true allatostatic function appears to be restricted to only cockroaches and crickets (see Hoffmann et al. 1999). What does seem to be a consistent function of allatostatins is their muscle modulatory activity on a variety of visceral organs; type-A allatostatins have been shown to be potent myoinhibitors of foregut, midgut, hindgut, oviduct and heart muscles in numerous insect species and of skeletal muscles in several crustaceans (for reviews see Bendena et al. 1999; Nässel, 2002).
Type A allatostatin receptors in arthropods
Radioligand binding and photoaffinity-labelling assays were initially used to identify putative allatostatin receptors in brain, corpora allata and midgut of the cockroach D. punctata (Cusson et al. 1991; Yu et al. 1995; Bowser & Tobe, 2000). More recently, mining of the Drosophila genome has revealed two GPCRs, with sequence similarity to mammalian galanin, opioid and somatostatin receptors, for which members of the type-A allatostatin family (drostatin 1–4) are cognate ligands; these receptors were designated Drosophila allatostatin receptor-1 (DAR-1; AF163775) and -2 (DAR-2; AF253526) (Birgül et al. 1999; Lenz, Sondergaard & Grimmelikhuijzen, 2000; Lenz, Williamson & Grimmelikhuijzen, 2000b; Larsen et al. 2001; Lenz et al. 2001).
Expression studies reveal distinct patterns in that while DAR-1 is almost exclusively expressed in the head, the expression of DAR-2 is principally restricted to the gut. The significance of these findings is reflected in the typical bifunctional role assigned to the type-A allatostatins; the (Y/F)XFG(L/I)amides have two major functions, (1) inhibition of juvenile hormone from the corpora allata (in cockroaches and crickets) and (2) inhibition of visceral muscle contractions. Similar receptors have been identified in B. mori, P. americana and C. morosus (Auerswald et al. 2001; Secher et al. 2001).
Type A allatostatins in the phyla Nematoda and Platyhelminthes
Type A allatostatin-like immunoreactivity in the nervous system of non-arthropod groups has been documented; initial immunocytochemical screens have indicated the presence of allatostatin-like peptides in the central and peripheral nervous systems of seven helminths representing the phyla Platyhelminthes and Nematoda (Smart et al. 1994, 1995; Mousley et al. 2005). Although related peptides have not been characterized from these phyla, the completion of the C. elegans genome sequencing project has led to the identification of sequences that bear similarity with the (Y/F)XFG(L/I)amide consensus (neuropeptide-like protein genes [nlp-5 and nlp-6] encode six putative peptides terminating in MGL/FG and one terminating in FGFG) (Nathoo et al. 2001).
NEUROPEPTIDE SIGNALLING AND ARTHROPOD CONTROL
Detailed discussion of research on insect peptide signalling as a target for pest control is beyond the remit of this review and is thoroughly investigated elsewhere (see Gade & Goldsworthy, 2003). However, there are several points to note from the insect work that pertain to the potential of neuropeptide signalling as an endectocide target. Firstly, several studies have reported the rational design of agonists or antagonists of neuropeptide signalling pathways in insects. Structure-activity studies on the insect pheromone biosynthesis activating neuropeptide (PBAN; a 33 amino acid peptide with a C-terminal RLamide), employing truncated peptides and analogues that had one of the L-amino acids replaced with D-phenylalanine, resulted in the discovery of the antagonist, RYFdFPRLamide, that inhibited sex pheromone production in the moth, Helothis peltigera at 100 pmol (Zelster et al. 2000). Peptide backbone cyclization has also been used to design modulators of insect neuropeptide signalling pathways and it is believed that this knowledge will help reveal the conformations of active peptides and thus form a basis for the design of non-peptide agonists/antagonists (Altstein et al. 1999; Altstein, 2001, 2004). Secondly, benzethonium chloride (Bztc) has been reported as a non-peptide agonist of myosuppressin signalling in insects with activities reported on insect crop, gut, malpighian tubules, oviduct and skeletal muscle tissues (Lange et al. 1995; Nachman et al. 1996; Coast, 1998; Lange & Cheung, 1999; Richer et al. 2000). These show that the rational design of drugs that interfere with neuropeptide signalling is possible and that non-peptide ligands for neuropeptide receptors in invertebrates have been discovered.
INTER-PHYLA ACTIVITIES OF NEUROPEPTIDES
With the neuropeptidergic system of parasites and pests emerging as an attractive drug target, receptors to both FLPs and allatostatins have potential as targets for anthelmintics and insecticides respectively. Indeed, several authors have discussed how understanding neuropeptide structure and function is fundamental to the discovery of novel, safe and selective compounds to control pest insects (see Gade & Goldsworthy, 2003).
With respect to helminths, researchers have been absorbed in the expression and screening of target neuropeptide receptors (see Greenwood, Williams & Geary in this supplement) with little or no information on their localization or functional relevance. As yet, it is not known if these receptors are expressed in therapeutically sensitive target tissues whose normal functioning is crucial to worm viability. Clearly, this approach is justified because of the dearth of identified FLP receptors in helminths, but their potential as drug targets would be aided by knowledge on their biological function and expression in relevant species.
In order to make rational decisions about the best receptors (in terms of importance to the physiology of the helminth/arthropod species and spectrum of activity) to choose for mechanism-based screening, basic research into the functional relevance of neuropeptide receptors is imperative. Moreover, analysis of the effectiveness of a range of neuropeptide ligands in the different parasite/pest groups will help highlight those receptor subtypes which offer the greatest opportunities for effective screen development.
Inter-phyla neuropeptide activities are deemed indicative of endectocide potential (Maule et al. 2002), and yet only recently has the subject of inter-phyla activities between helminth and arthropod neuropeptides been addressed. The bulk of FLP and allatostatin functional data known today describes the effects of these peptides in their native species (or related species within the same phylum) (see Maule et al. 2002; Nässel, 2002 for reviews; Tables 1 and 2). Indeed, only three preliminary studies have examined such inter-phyla activities. In 1997, Marks et al. (1997b) and Graham et al. reported the effects of nematode FLPs (KNEFIRFamide, KHEYLRFamide, SDPNFLRFamide, SADPNFLRFamide, KSAYMRFamide and KPNFIRFamide) on the muscle activity of muscle strips from F. hepatica; all peptides examined induced potent excitatory responses. In addition, the effects of three arthropod FLPs were assayed on the nematode somatic body wall muscle where they were found to inhibit contractility in a similar manner to the endogenous FLP, SDPNFLRFamide (bwRT1; Maule et al. 1996). Collectively, these initial observations suggested that a common binding domain on the FLP receptor is conserved across several invertebrate phyla (Thompson, Klein & Geary, 1996) and highlighted a gap in research that could aid the unearthing of prospective endectocides. A summary of the data pertaining to the inter-phyla actions of selected neuropeptides in bioassays from the target phyla (arthropods, nematodes, flatworms) is presented below (see Tables 1 and 2).
Inter-phyla activities of arthropod neuropeptides in helminths
The inter-phyla activities of 10 arthropod FLPs [PDVDHVFLRFamide, pQDVDHVFLRFamide, HVFLRFamide, VFLRFamide, TNRNFLRFamide, SDRNFLRFamide, GNSFLRFamide, DPSFLRFamide, KPNQDFMRFamide and EQFDDY(SO3H)GHMRFamide] have recently been examined in three helminth bioassay systems; A. suum somatic body wall muscle, A. suum ovijector and P. littoralis dispersed muscle fibres (Mousley et al. 2004).
We have found that both native and non-native (P. redivivus, C. elegans) nematode FLPs induce diverse and complex actions on the A. suum body wall and ovijector muscle systems that can be delineated into four (body wall muscle; bwRT1 [slow inhibitory], bwRT2 [fast inhibitory], bwRT3 [excitatory], bwRT4 [biphasic]) or five (ovijector; ovRT1 [inhibitory], ovRT2 [excitatory], ovRT3 [transient contraction], ovRT4 [transient contraction/paralysis], ovRT5 [relaxation/increased activity]) distinct response types (see Maule et al. 2002). Most of the arthropod peptides examined had inhibitory effects on the ovijector that were consistent with ovRT1 (Mousley et al. 2004); two peptides (HVFLRFamide and GNSFLRFamide) induced distinct ovRT4-like responses. With the exception of perisulfakinin, which was inactive, all but one (HVFLRFamide) of the arthropod FLPs tested significantly modulated the activity of the A. suum body wall muscle in a bwRT1-like manner; HVFLRFamide induced a bwRT4-like response.
Despite significant structural deviations from endogenous flatworm FLPs, all of the arthropod FLPs examined induced potent, concentration-dependent contractions of P. littoralis muscle fibres. Previous studies have indicated the presence of a single muscle-based FLP receptor on P. littoralis muscles fibres that favours a ligand with a tyrosine residue in position 4 from the C-terminus. Indeed, Moneypenny et al. (2001) reported the higher potency of the YIRFamide containing FLPs as opposed to the FFRFamide-possessing cestode FLP and hypothesised that the lower potency of GNFFRFamide reflected its non-specific interaction with the endogenous FLP receptor. Mousley et al. (2004) also showed that FLPs deviating from the C-terminal YIRFamide motifs (FLRFamide, FMRFamide and HMRFamide) were less potent on P. littoralis muscle fibres. While it is likely that the range of FLPs bearing diverse C-termini examined in the studies by Moneypenny et al. (2001) and Mousley et al. (2004) interact with a single receptor to induce the observed myoexcitation, the presence of more than one FLP receptor cannot be ruled out.
Allatostatin activities were also recently examined in helminths (Mousley et al. 2005). Indeed, the inter-phyla activities of seven type-A allatostatins [GGSLYSFGLamide, APSGAQRLYGFGLamide, AGPYAFGLamide, AGPYSFGLamide, GDGRLYAFGLamide, DRLYSFGLamide and YSKFNFGLamide] were characterized on P. littoralis dispersed muscle fibres, somatic body wall muscle and ovijector of the parasitic pig nematode A. suum. In this study, all seven members of the allatostatin superfamily induced concentration-dependent contractions of flatworm muscle fibres and pharmacological studies indicated that these peptides interact with a receptor other than that which mediates the FLP-induced contractions. Most of arthropod allatostatins examined did not affect the somatic body wall muscle or the ovijector of A. suum; two allatostatins (GDGRLYAFGLamide and DRLYSFGLamide) exhibited low potency, inhibitory effects on the A. suum ovijector that were ovRT1-like.
The data presented in this study also describe the interrelationships of allatostatin-immunoreactive nerves and muscle systems in a selected flatworm (P. littoralis) and roundworm (P. redivivus) and compares these with native GYIRFamide- and FMRFamide-immunoreactivies, respectively. Comparative analyses of the allatostatin-immunoreactivity and that of known helminth FLPs revealed differences in the distribution of these peptide families; specific differences were noted within the pharyngeal innervation of flatworms and in the cephalic papillary neurons of nematodes. The data indicate that allatostatins and FLPs play distinct roles in helminths.
Inter-phyla activities of helminth neuropeptides in an arthropod
It is clear that both nematode and flatworm FLP receptors are capable of interacting with a wide range of FLP motifs (Mousley et al. 2004), and that flatworms possess allatostatin-responsive muscle based receptors that are distinct from endogenous FLP receptors (Mousley et al. 2005), but are arthropod and helminth FLP receptors similar in terms of their ligand recognition profiles?
Recently the effects of seven nematode FLPs (SDPNFLRFamide [PF1], KSAYMRFamide [PF3], KPNFIRFamide [PF4], KHEYLRFamide [AF2], GLGPRPLRFamide [AF9], AGAKFIRFamide, and DVPGVLRFamide) and one flatworm FLP (GYIRFamide) were examined on spontaneous contractions of the lateral oviduct of the locust, Schistocerca gregaria (Mousley et al. 2005).
The locust (L. migratoria) oviduct has previously been shown to be modulated by native and non-native arthropod FLPs in vitro (Lange, Orchard & Te Brugger, 1991; Fonaghy et al. 1992a; Peeff, Orchard & Lange, 1993; Peeff et al. 1994; Lange et al. 1994). Structure-activity and competitive binding studies of truncated forms of schistoFLRFamide (PDVDHVFLRFamide) using the L. migratoria oviduct bioassay have identified several key features important for biological activity and receptor binding. Whilst HVFLRFamide is the minimum sequence necessary for inhibition of biological activity, VFLRFamide is the minimum sequence required for receptor binding (Wang et al. 1995a). Interestingly, VFLRFamide illustrates activity reversal, possessing minor stimulatory activity (Peeff et al. 1994). Therefore, it was proposed that the His residue, which does not contribute to binding, is a critical amino acid for activation of the response to receptor occupation.
Subsequently, a series of HVFLRFamide analogues were examined to further determine the importance of the His residue to the inhibitory response. Each amino acid in positions 2–6 was substituted with a structurally similar or dissimilar amino acid. It was found that when His remained in position 1 no activity reversal was observed; the analogues were either inhibitory (with reduced potency) or possessed no biological activity (Wang, Orchard & Lange, 1995). However, when His was replaced with Tyr, Leu, Ile or Val, an excitatory response occurred (Wang et al. 1995b). Interestingly, the His residue of position 6 from the C-terminus is common to all members of the myosuppressin subfamily of FLPs.
We have found that a range of nematode FLPs significantly modulate contractile activity of the lateral oviduct, but the platyhelminth-derived GYIRFamide is inactive. The P. redivivus FLPs, PF1 and PF3, the A. suum FLP, AF2 and the putative C. elegans FLPs, AGAKFIRFamide and DVPGVLRFamide induced qualitatively similar inhibitory responses that were comparable to the schistoFLRFamide-induced response. Interestingly only one of the nematode FLPs possesses a His residue in position 6 from the C-terminus, deemed necessary by Peeff et al. (1993, 1994) for an inhibitory response. There are at least two plausible explanations for these findings. Either, contrary to previous suggestions, the His residue of position 6 is not critical for inhibition, or there is at least one other inhibitory receptor on the locust oviduct. Peeff et al. (1993, 1994) have proposed the presence of two FLP receptors on the locust oviduct. One receptor with strict ligand requirements for a C-terminal amide and a specific N-terminal extension containing HVFLRFamide, which leads to inhibition, and a second recognising other extended FLRFamides and leading to excitation. If this is the situation, then the S. gregaria oviduct must possess at least one additional inhibitory receptor capable of recognising PF1, PF3, AF2, AGAKFIRFamide, and DVPGVLRFamide. A qualitatively distinct inhibitory response was observed on addition of AF9 signifying that, if variation in response type is indicative of distinct receptor interaction, then the inhibitory receptor profile on the locust oviduct is more complex than previously thought.
To facilitate further delineation of possible inhibitory receptors endogenous to the S. gregaria oviduct, we examined the effects of three of the inhibitory nematode FLPs (PF1, PF3 and AF9) on proctolin-induced contractions. Proctolin, the first insect neuropeptide to be structurally characterized (Brown & Starrat, 1975), was isolated from the american cockroach, P. americana, and named according to its excitatory properties on the hindgut; proctolin meaning ‘gut factor’. Proctolin is also a potent stimulator of locust oviduct muscle inducing an excitatory effect that can be divided into two distinct components; an initial fast tonic contraction followed by an increase in the frequency and amplitude of phasic contractions. Previous studies have highlighted the antagonistic effect of FLPs on proctolin-induced contractions of the locust oviduct (Lange et al. 1991; Peeff et al. 1993, 1994). Indeed, schistoFLRFamide reduces both the tonic and phasic component of the proctolin-induced contraction (Lange et al. 1991).
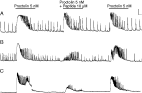
We found that the response of S. gregaria lateral oviducts to proctolin (5 nM) comprised the characteristic initial tonic contraction (tissue shortening) followed by an increase in frequency and amplitude of phasic contractions which could immediately be reversed upon washout (see Fig. 1). Whilst PF3 (10 μM) had no effect on either constituent of the proctolin-induced response, co-application of 10 μM PF1 and proctolin (5 nM) abolished the tonic component, and simultaneous treatment with proctolin (5 nM) and AF9 (10 μM) reduced both the tonic and phasic component of proctolin-induced excitation. In all cases, subsequent addition of proctolin (5 nM) to the same tissue in the absence of peptide induced a characteristic proctolin response (see Fig. 1).

Fig. 1. The effects of [A] KSAYMRFamide (PF3), [B] SDPNFLRFamide (PF1), and (C) GLGPRPLRFamide (AF9) on proctolin (RYLPT)-induced contractions of the Schistocerca gregaria lateral oviduct (see text for details). Presence of peptide is indicated by the horizontal bar above the trace. Scale; horizontal bar represents 2 min, vertical bar represents 2 mg.
The P. redivivus FLP, KPNFIRFamide (PF4), stimulated S. gregaria lateral oviduct contractions. PF4 shares the common C-terminal tetrapeptide motif, FIRFamide, with a native L. migratoria ventral nerve cord FLP, AFIRFamide, which is also excitatory when assayed on locust (L. migratoria) oviduct (Lange et al. 1994). Another FIRFamide, AXXRNFIRFamide (X=unknown), has been isolated biochemically from the locust ventral nerve cord, although as its sequence is incomplete its function remains unknown (Lange et al. 1994). The significance of FIRFamide-possessing FLPs inducing excitatory activities remains to be elucidated, as there are several excitatory peptides that do not conform to the C-terminal FIRFamide consensus. Indeed, the non-native FLPs, FMRFamide, FMRF (non-amidated), YGGFMRFamide, FLRFamide, TNRNFLRFamide, the native locust FLPs, GQERNFLRFamide, PDVDHVFLRF (non-amidated) and the truncated derivative VFLRFamide were all excitatory on the locust (L. migratoria) oviduct (Peeff et al. 1993). In addition, the nematode FIRFamide-containing FLP, AGAKFIRFamide induced an inhibitory response on the S. gregaria oviduct.
Collectively these data indicate the presence of multiple FLP receptors on the locust oviduct, however the prospect that all of these structurally related FLPs are interacting with a single receptor associated with multiple G-proteins mediating diverse downstream effects cannot be ruled out. Indeed such a ligand-receptor reaction system in locust oviduct has been proposed by Wang, Lange & Orchard (1995) and Wang et al. (1995b), whereby all peptides (inhibitory and excitatory) share a single receptor by possessing homologous binding sequences but are able to produce opposite muscle responses due to different activation sites.
A comparison of the effects induced by the nematode-derived FLPs on the S. gregaria oviduct with their endogenous effects on female reproductive function in A. suum reveal some similarities in peptide action and some differences. For example, both PF3 and PF1 inhibit contractility of S. gregaria oviduct and A. suum ovijector; in contrast PF4-induces opposite effects on nematode and arthropod reproductive systems. These nuances between FLP activities on helminth and arthropod muscles systems are interesting from an academic perspective but what is of greater significance is that the same FLPs are capable of modulating homologous organs involved in reproductive function in nematodes and arthropods, demonstrating at least a degree of FLP receptor similarity.
It is interesting to note that the flatworm-derived FLP, GYIRFamide, was inactive on the S. gregaria lateral oviduct. Similarly, we have found that all four flatworm FLPs (GYIRFamide, RYIRFamide, YIRFamide, GNFFRFamide) are inactive on A. suum body wall muscle and ovijector. Although the basis of these findings remains to be determined, it is likely that ligand recognition features for nematode and arthropod FLP receptors have more constraints than those of flatworms. Again, this is reflected in the diverse array of FLPs that have been identified in nematodes and arthropods compared to the handful of structures identified in flatworms. The conservation in FLP receptors between nematodes and arthropods is also reflected in their recent phylogenetic arrangement into an ecdysozoan clade encompassing all animals that shed a cuticle by ecdysis (Aguinaldo et al. 1997).
WHAT HAVE THE INTER-PHYLA STUDIES SHOWN?
These studies have revealed that selected nematode and arthropod FLPs and allatostatins modulate motor function in each of the target phyla (Nematoda, Platyhelminthes and Arthropoda), i.e. individual peptides can activate multiple receptors in multiple phyla (see Tables 1 and 2). Although type-A allatostatins have distinct actions on arthropod and flatworm muscle, their activity in nematodes is restricted. Consistently the most active peptides in a range of assays from each of the three phyla are the FLPs. Selected FLPs have potent effects on sensory and motor function in nematodes, neuromuscular coordination in insects, and muscle activity in flatworms (see Tables 1 and 2). This is significant as it emphasizes the validity of selected FLP receptors as inter-phyla targets for novel, broad-spectrum endectocides. Although FLPergic signalling remains a prime candidate for targeted intervention, other neuropeptide families have not yet been thoroughly investigated for their inter-phyla activities and exploitative potential.
CONCLUSIONS
Currently, there are no drugs marketed that are known to interfere with parasite or pest neuropeptide signalling. Yet, we know that neuromuscular function, a recognized target for parasite and pest control, is largely controlled by the activities of neuropeptides that act on receptors based in their neuromuscular systems. We have gathered evidence to show that FLPs and type-A allatostatins encompass peptides that act across the phylum barriers to modulate muscle activity in roundworms, flatworms and arthropods. Indeed, as structural, physiological and expression data accumulate, FLP receptors are emerging as strong drug target candidates because ligands to these receptors influence such a large number of vital processes across multiple phyla. Significantly, these receptors are now being uncovered in helminths and arthropods and provide handles that facilitate the establishment of screening programmes to identify non-peptide ligands for these receptors (see Greenwood, Williams & Geary in this supplement). We already know from published work on insects that it is possible to design drugs rationally that act at neuropeptide receptors and that non-peptide compounds can act at selected FLP receptors in insects. Taken together these facts suggest there is much merit in attempting to exploit FLP receptors for drug discovery. Time will tell if this is a valid selection and if FLP receptors can be used to uncover a new generation of endectocides.