Introduction
Seed dormancy in environments with high temporal and spatial variability, such as deserts, is often described as a bet-hedging strategy (Eberhart and Tielbörger, Reference Eberhart and Tielbörger2012). Not all mature seeds germinate immediately after dispersal; in many cases, a significant fraction remains dormant for a period that can last for years (Fenner, Reference Fenner1985). These ‘persistent seed banks’ are considered one of the main strategies of desert plants (Figueroa et al., Reference Figueroa, León-Lobos, Cavieres and Pritchard2004; Facelli et al., Reference Facelli, Chesson and Barnes2005; Baskin and Baskin, Reference Baskin and Baskin2014). Larger proportions of dormant species are found in environments with distinctive unfavourable seasons (Jurado and Flores, Reference Jurado and Flores2005); the percentage increase from 40% in tropical rainforests to over 80% in hot deserts (Gutterman, Reference Gutterman2002).
The coastal Atacama Desert of northern Chile and southern Peru has a temperate climate with constant presence of fog, a decisive ecological factor to which the maintenance of formations known as ‘fog oases’ (Ellenberg, Reference Ellenberg1959, cited in Rundel et al., Reference Rundel, Dillon, Palma, Mooney, Gulmon and Ehleringer1991) or ‘lomas vegetation’ (Rundel et al., Reference Rundel, Palma, Dillon, Sharifi and Boonpragob1997) is attributed. These are isolated ecosystems, separated by a hyper-arid habitat (Rundel et al., Reference Rundel, Dillon, Palma, Mooney, Gulmon and Ehleringer1991) located along 3500 km of coastal desert (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009); lomas sustain over 1400 plant species with an endemism of around 40% (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009; Schulz et al., Reference Schulz, Aceituno and Richter2011).
The genus Nolana L. ex L. f. (Solanaceae) currently comprises 90 species, mostly distributed in the costal Atacama Desert, of which 46 are present exclusively in Chile. They are annual or perennial herbaceous plants or small shrubs (Dillon et al., Reference Dillon, Tu, Soejima, Yi, Nie, Tye and Wen2007; Tu et al., Reference Tu, Dillon, Sun and Wen2008) and present foliar succulence and trichomes that capture moisture and restrict transpiration (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009). Nolana is the only genus found in the whole range of lomas, where it stands out as the most conspicuous and diverse floristic element (Tago-Nakazawa and Dillon, Reference Tago-Nakazawa and Dillon1999). Some species are important components of the blooming desert phenomenon, associated with El Niño Southern Oscillation events of heavy rainfall and relatively high temperatures (Dillon, Reference Dillon, Hollowell, Keating, Lewis and Croat2005). Several of these species have high ornamental potential (Freyre et al., Reference Freyre, Douglas and Dillon2005; Riedemann et al., Reference Riedemann, Aldunate and Teillier2006; Fig. 1), and also high conservation value (Tu et al., Reference Tu, Dillon, Sun and Wen2008; Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009) given their endemism to habitats with extreme conditions of aridity and salinity. Dillon et al. (Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009), using molecular markers, identified several strongly supported clades within Nolana, with geographic and morphological fidelity, four of which are confined to Chile and three are mainly Peruvian with some presence in Chile.

Fig. 1. Context, flowers and fruits of four Nolana species included in this study. (A–C) N. divaricata (clade G); (D–F) N. intonsa (clade F); (G–I) N. carnosa (clade C); (J–L) N. jaffuelii (clade B).
Physiological non-deep dormancy, apparently induced by endosperm and testa, has been detected in Solanaceae (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Freyre et al. (Reference Freyre, Douglas and Dillon2005) and Douglas and Freyre (Reference Douglas and Freyre2006), studying Nolana paradoxa and N. aplocaryoides among others, found that germination occurs when opening a ‘funicular plug’ in mericarps and increases when adding gibberellic acid. Douglas (Reference Douglas2007) also reported an increased germination effect on nine Nolana species with gibberellic acid and dry storage for 2 years, suggesting physiological dormancy (PD). Cabrera et al. (Reference Cabrera, Hepp, Gómez and Contreras2015) obtained a higher percentage of germination in N. jaffuelii with scarification in the funicular area and application of gibberellic acid, reporting physical and PD. No reference was found regarding dormancy for any of the other 11 Nolana species included in this research.
Several studies report a decline in lomas vegetation of the coastal desert (Muñoz-Schick et al., Reference Muñoz-Schick, Pinto, Mesa and Moreira-Muñoz2001; Pinto et al., Reference Pinto, Larraín, Cereceda, Lázaro, Osses and Schemenauer2001; Egaña et al., Reference Egaña, Cereceda, Pinto, Larraín, Osses and Farías2004; Pinto and Luebert, Reference Pinto and Luebert2009). Since projections indicate that rainfall will continue to decrease in arid and semi-arid areas, while average temperatures could rise (Santibañez et al., Reference Santibañez, Santibañez, Caroca and González2017), lomas vegetation is expected to continue deteriorating (Schulz et al., Reference Schulz, Aceituno and Richter2011). It is crucial to establish the germination requirements and dormancy mechanisms of these species, so that their storage in seed banks and subsequent propagation for in situ reintroduction or ex situ cultivation can be successful (León-Lobos et al., Reference León-Lobos, Way, Aranda and Lima-Junior2012; León-Lobos et al., Reference León-Lobos, Bustamante-Sánchez, Nelson, Alarcon, Hasbún, Way, Pritchard and Armesto2020).
Seed germination and dormancy of phylogenetically closely related species have not been extensively studied, but there are reports of similar germination requirements and local adaptations mediated by species relatedness (Carta et al., Reference Carta, Hanson and Müller2016) and of the major role of phylogeny in determining seed dormancy occurrence (Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016). Also, a relationship between dormancy level and habitat for certain species (Meyer et al., Reference Meyer, Kitchen and Carlson1995) has been found. However, for Nolana, such relationships have not been established. Therefore, the objective of this study was to characterise dormancy of 12 Nolana species from the coastal desert of Chile, to determine their germination requirements, and to evaluate if there is any relationship between dormancy level and geographical distribution or phylogeny.
Material and methods
Plant material
Between October 2014 and April 2016, mericarps of 12 Nolana species (Fig. 1) were collected at different locations (Table 1) from individual plants at maturity and kept in labelled paper bags. Whenever possible, mericarps were collected randomly within the population from at least 50 different individuals, to obtain a representative sample for each species (León-Lobos et al., Reference León-Lobos, Way, Pritchad, Moreira-Muñoz, León and Casado2003). Fruits were then kept in paper bags, partially dried (they were put inside jars with equal weight of silica gel, for 2–3 d) and stored at 20and 40% RH until used for analysis. Selection of species is the result of availability of suitable fruits (i.e. mature mericarps, black/brown in colour and with dry or senescent calyx) in the field, which explains the overrepresentation of certain clades.
Table 1. Species of the genus Nolana used in the study, latitudinal distribution in Chile, collection site and clade (Flora del Conosur, 2014 and Enciclopedia de la Flora Chilena, 2014)
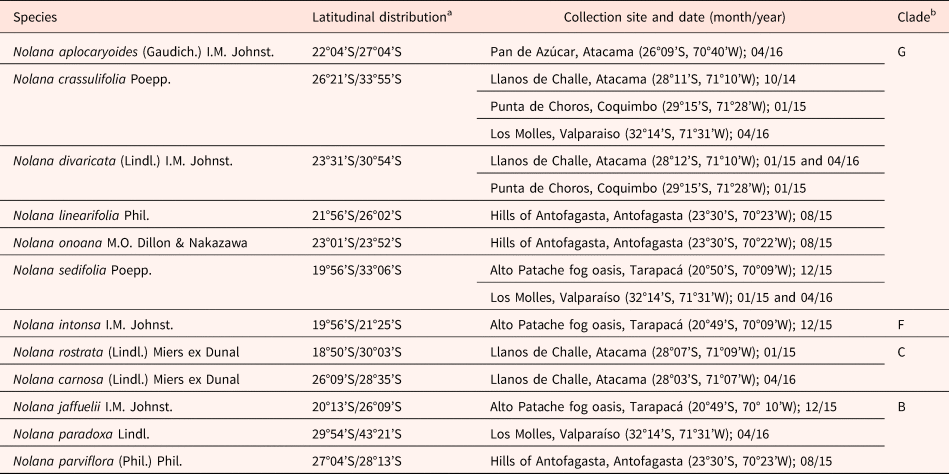
a Latitudinal distribution for all species was obtained from an extensive study of all Herbaria specimens (SGO and CONC), see Hepp (Reference Hepp2019) for a detailed description.
b Clades (and letters) used in this study are those determined by Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009.
In Nolana, dispersal units are fruits called mericarps, which can vary in number between species from 2 to 30 per schizocarp and from laterally united and multi-seeded to completely free and single-seeded (Tago-Nakazawa and Dillon, Reference Tago-Nakazawa and Dillon1999; Knapp, Reference Knapp2002). Seeds within the mericarp remain in independent chambers (Saunders, Reference Saunders1936) and are firmly embedded within the fruit. To facilitate analysis, in this study, we consider only one seed per mericarp, so ‘seed’ will be the unit in which results are expressed.
Seed viability
For evaluation of viability, a tetrazolium test with 2,3,5-triphenyl-2H-tetrazolium chloride (Tz) was performed on a sample of at least 25 mericarps for each seed lot (according to disponibility). This was performed in a separate sample of mericarps, and not in the same fruits after finishing the germination experiments, given the extension of the experiments and the possibility of fungal damage. Mericarps were scarified (one funicular plug per mericarp was removed) and stained with 1% Tz solution for approximately 24 h at 30°C. After that time, mericarps were cut in halves and surfaces were observed using a magnifier. Only seeds that were completely stained red (embryo and endosperm) were considered viable; one seed per mericarp was evaluated.
Seed imbibition
Imbibition in methylene blue (1 g 100 ml−1) was used to evaluate if water was able to penetrate and reach the embryo in intact mericarps. Twenty mericarps of each species were left at room temperature in metal containers with enough dye to cover them. Five mericarps were evaluated after 3, 24, 48 and 196 h. They were washed with distilled water and dried with paper towel, then allowed to air dry for 20 min at room temperature. Finally, stained seed were sectioned using a scalpel and observed under a stereomicroscope system (SZX7, Olympus Corporation, Tokyo, Japan), making sure that the cut allowed to see the plug and half the embryo (longitudinal cut).
Evaluation of germination and characterisation of dormancy
Evaluation of germination for the 12 species was performed in three experiments. Mericarps were placed in 9 cm diameter Petri dishes, over three layers of filter paper, saturated in distilled water or a solution of gibberellic acid (GA3). Plates were sealed with Parafilm to prevent drying and, during evaluation, water or GA3 solution was added when needed in order to keep moisture. Seed was considered germinated if radicle emergence was over 2 mm. Results were reported as total germination percentage.
Experiment 1. Role of funicular plug on germination
Germination tests were carried out using mericarps from ten Nolana species within 1–3 months from their collection. In the case of N. crassulifolia, N. divaricata and N. sedifolia, mericarps from different collection dates and/or sites were evaluated separately. Mericarps were randomly assigned to one of the following treatments: (1) control (Ct), intact dry mericarps imbibed in distilled water; (2) scarification (Sc), removal of funicular plug and imbibition in distilled water; (3) Sc and imbibition in a solution of 500 ppm GA3 (Sc + GA500). A scalpel was used to dissect the pericarp at the funicular scar area avoiding radicle damage, as this proved to be the most effective treatment for the germination of mericarps of N. jaffuelii (Cabrera et al., Reference Cabrera, Hepp, Gómez and Contreras2015). Scarification was performed on one funicular plug, chosen randomly, per mericarp. The presence of an embryo was verified for that selected seed; if there was no embryo, a second funicular plug was removed. Germination was evaluated 3 d a week during 45 d in a chamber at constant 20°C, with constant fluorescent light (10 to 20 μmol m−2 s−1 photosynthetically active radiation), in four replicates of 25 seeds each.
Experiment 2. Alternatives to overcome PD
This experiment was conducted in mericarps of N. linearifolia, which was selected due to the number of available mericarps. The following germination treatments were evaluated: (1) control (Ct), intact mericarps imbibed in distilled water; (2) plug scarification (Sc), removal of funicular plug and imbibition in distilled water; (3) plug scarification with partial removal of endosperm (Sc + en), imbibed in distilled water and (4) Sc + en and imbibition in a solution of 500 ppm GA3 (Sc + en + GA500). Germination was evaluated three times a week in four replicates (25 seeds each) per treatment, during 45 d in a chamber at constant 20°C.
Experiment 3. Role of endosperm on germination
Based on observations after experiments 1 and 2, and to better understand the role of endosperm on germination, a third experiment was performed using mericarps from four species that had high germination percentage in Exp. 1, and two additional species (N. aplocaryoides and N. paradoxa) that were collected and included afterwards. Three of the treatments defined in Exp. 2 were evaluated: Ct, Sc and Sc + en. Germination was evaluated three times a week in four replicates (25 seeds each) per treatment, during 45 d in a chamber at constant 20°C.
A constant temperature of 20°C was chosen for several reasons: (1) it was necessary to have the same set of experiments to apply to all species; (2) the moderate temperatures of the Chilean coast, on average, are around 20°C for the months in which germination usually occurs (Fick and Hijmans, Reference Fick and Hijmans2017); and (3) the constant temperature of 20°C was effective in evaluations conducted by Cabrera et al. (Reference Cabrera, Hepp, Gómez and Contreras2015).
The mean germination time (MGT) was calculated by the following equation (Ellis and Roberts, Reference Ellis, Roberts and Hebblethwaite1980): MGT = ∑ (n × d)/N, where n is the number of seeds germinated in each evaluation day, d is the number of days from sowing, and N is the total number of seeds germinated.
Statistical analysis
Data analysis was performed using R software (version 3.3.3, R core team 2017). The effects of treatments on germination percentages were analysed for each species using generalised linear models (GLM) with binomial distribution and logit link function in the glm function. In cases in which overdispersion for binomial errors occur, quasibinomial distribution and F tests with an empirical scale parameter were used instead of chi-square. When significant differences were detected (P < 0.05), the least significant difference (LSD; α = 0.05) post hoc test was used from glht package to detect significant differences in the comparison between pairs of treatments. Pearson's correlation coefficient was used to evaluate the relationship between germination and latitudinal distribution of species.
Results
Seed imbibition
When mericarps from the 12 Nolana species included in this study were embedded in a solution of methylene blue, we found that water was able to enter until reaching the embryo for all the mericarps and species, usually within 48 h since imbibition started. Images of mericarps from four representative Nolana species after 3 h, 48 h and 8 d of imbibition are presented in Fig. 2. The blue staining allows to see that water entrance to the embryo occurred through the funicular plug.

Fig. 2. Longitudinal sections of intact mericarps (with funicular plug) of N. divaricata, N. jaffuelii, N. linearifolia and N. sedifolia at 3 h, 24 h, 48 h and 8 d after imbibition in methylene blue. Abbreviations: em: embryo; fp: funicular plug; ra, radicle; * indicates regions which have imbibed.
Evaluation of seed germination
Experiment 1
There were significant differences in germination percentages between control and some of the treatments in nine of the ten Nolana species evaluated in Exp. 1 (Table 2). In four species, there was a significant increment of germination when the funicular plug was removed, and in seven species, the imbibition of scarified mericarps in gibberellic acid had a positive effect on germination.
Table 2. Viability and germination percentages of mericarps from 13 accessions of 10 Nolana species in response to three treatments: control, intact mericarps imbibed in distilled water; scarification (Sc), removal of funicular plug and imbibition in distilled water; scarification and imbibition in a solution of 500 ppm GA3 (Sc + GA3)

Percentage of seed viability was determined by tetrazolium test in a separate mericarp sample.
1For each species and collection, germination values with different letters are significantly different according to a LSD test (α = 0.05).
2P value from a generalised linear models (GLM).
Experiment 2
Results from Exp. 2, carried out with N. linearifolia, confirm that some of the treatments had a significant effect in promoting seed germination (P < 0.001). While intact mericarps only reached 2% germination, treatments that combine removal of the funicular plug with partial removal of endosperm reached 54% germination when imbibed in water (Sc + en) and 55% when imbibed in a GA3 solution (Sc + en + GA500) (Fig. 3). When the funicular plug was removed and mericarps were imbibed in water (Sc), germination was 3%, similar to control.

Fig. 3. Cumulative germination of Nolana linearifolia mericarps after different treatments. Control: intact mericarps imbibed in distilled water; Sc: plug scarification, that is, removal of funicular plug, imbibed in distilled water; Sc + en: plug scarification with partial removal of endosperm, imbibed in distilled water; Sc + en + GA500: plug scarification with partial removal of endosperm, imbibed in gibberellic acid (500 ppm GA3). Data presented are an average of four replicates of 25 mericarps each and bars represent the 95% confidence intervals. For each evaluation date, germination values with different letters are significantly different according to an LSD test (α = 0.05).
Experiment 3
Germination and the viability percentages of the six Nolana species evaluated in Exp. 3 are presented in Table 3. In five species, there was a significant promotion of germination when the funicular plug was removed, only in N. linearifolia, this effect was not observed. Additionally, in the six Nolana species, the treatment that combines the removal of the plug with a partial removal of the endosperm had a significantly higher germination than only removing the plug.
Table 3. Viability and germination percentages of mericarps from six Nolana species in response to three treatments: control, intact mericarps imbibed in distilled water; scarification (Sc), removal of funicular plug and imbibition in distilled water; scarification and partial removal of endosperm (Sc + en) with imbibition in distilled water

Percentage of seed viability was determined by tetrazolium test in a separate mericarp sample.
1 For each species and collection, germination values with different letters are significantly different according to an LSD test (α = 0.05).
2 P value from a generalised linear models (GLM).
A summary of germination values from intact mericarps and after the best germination treatment for the 18 accessions evaluated in this study is presented in Table 4. In this case, germination is expressed as a percentage of live seed, which was calculated from the estimations of seed viability that are presented in Tables 2 and 3. The MGT, calculated from the mericarps under the best germination treatment, is also presented in Table 4.
Table 4. Germination percentages of control (intact mericarps imbibed in distilled water) and best germination treatment (BGT) for 18 accessions of 12 species of Nolana, corrected according to viability (% of live seed)
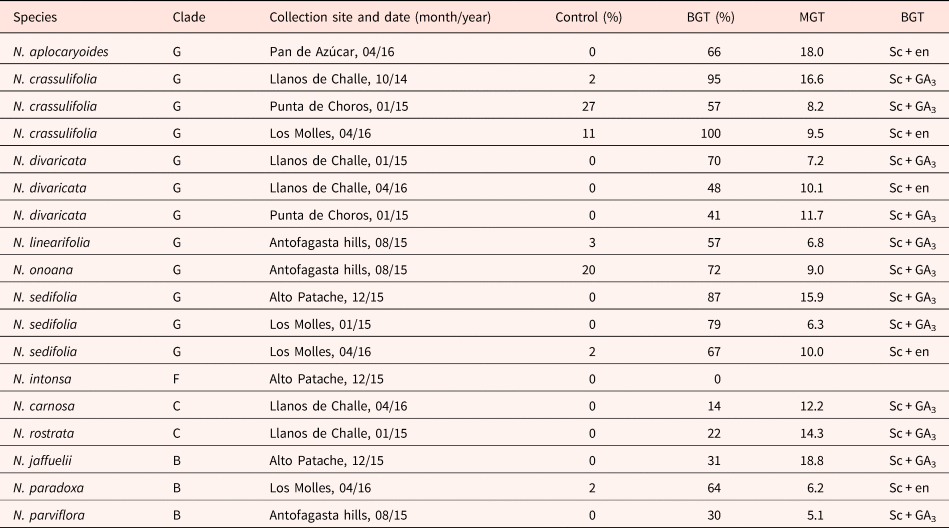
Data from two experiments; in Exp. 1, BGT corresponded to plug scarification and imbibition in gibberellic acid (Sc + GA3), while in Exp. 2, the BGT corresponded to plug scarification and partial endosperm removal (Sc + en). Mean germination time (MGT) calculated from BGT data.
Figure 4 shows the results from the analysis of germination values after the best treatment (Table 4) of Nolana species grouped by clades. In this case, there was a significant difference among the clades, with clade G having higher germination than clades B and C.

Fig. 4. Germination of Nolana species after the best germination treatment (plug scarification and imbibition in gibberellic acid or partial endosperm removal), grouped into clades (B,C,F,G). The number (N) of species included for each clade is noted. Data are expressed as the percentage of live seed and correspond to the average value for species in each clade ± 95% confidence intervals.
When germination results of the studied Nolana species (corrected by the percentage of seed viability) were correlated with the latitudinal distribution of each species (Fig. 5), no evident relationship was obtained (r = 0.45; P-value = 0.06).

Fig. 5. Pearson correlation coefficient (r) and P-value (P) between germination percentage of 19 accessions of 12 species of Nolana for the best treatment (plug scarification and imbibition in gibberellic acid or partial endosperm removal) and latitudinal distribution, expressed as the difference between the northernmost and the southernmost latitudinal distribution point (decimal degrees).
Discussion
The role of the funicular plug on germination
A thick layer of sclereids was identified by Cabrera and colleagues (Reference Cabrera, Hepp, Gómez and Contreras2015) in Nolana jaffuelii mericarps, and authors suggested that this species presented physical dormancy. However, our results show that water was able to enter the mericarps until reaching the embryo in the 12 Nolana species studied and confirm that the entrance route of the water is the funicular plug (Fig. 2). Douglas (Reference Douglas2007) also reports that the imbibition path in Nolana mericarps occurs through tracheid tubes in the funicular plugs. Since physical dormancy is defined as impermeability of the fruit or seed to water (Baskin and Baskin, Reference Baskin and Baskin2004, Reference Baskin and Baskin2014), these results indicate that dormancy in Nolana species would not be physical.
Seeds with PD are permeable to water and possess a physiological inhibiting mechanism that prevents radicle emergence (Baskin and Baskin, Reference Baskin and Baskin2014). In hot semideserts and deserts, PD is the most common type of seed dormancy for shrubs, perennial succulents, herbaceous perennials and annuals (Baskin and Baskin, Reference Baskin and Baskin2014). Taking into account our imbibition data (Fig. 2) and reports by previous studies (Douglas and Freyre, Reference Douglas and Freyre2006; Douglas, Reference Douglas2007), and following the definition by Baskin and Baskin (Reference Baskin and Baskin2014), our results (Tables 2 and 3) indicate that seeds of the studied Nolana species present PD. Morphological or morphophysiological dormancy was discarded as a set of mericarps of all species were dissected prior to imbibition (Hepp, Reference Hepp2019); embryo size was then fully developed and did not change after imbibition.
The role of endosperm on germination
While removal of the funicular plug improved germination in mericarps of six of the 12 Nolana species studied, an additional and significant effect was observed when this scarification was combined with imbibition in gibberellic acid (Table 2) or partial removal of the endosperm (Table 3). The extraction of the funicular plug, therefore, although it is necessary because it is the point from which the radicle emerges, is not as decisive as the removal of the layer immediately below; that is, the endosperm.
PD may be caused by structures that cover the embryo, including endosperm, seed coats and indehiscent fruit walls, among others (Baskin and Baskin, Reference Baskin and Baskin2014). In seeds of Solanaceae species, the embryo is surrounded by two layers: the endosperm and the testa. The micropylar endosperm, covering the tip of the radicle, has been identified as a limiting factor for germination in Solanaceae species and its weakening seems to be a prerequisite for the protrusion of the radicle during germination (Groot and Karssen, Reference Groot and Karssen1987; Sánchez et al., Reference Sánchez, Sunell, Labavitch and Bonner1990). While gibberellins promote weakening, the removal of the endosperm and testa layers opposite the radicle tip may also assist the process to permit radicle protrusion (Groot and Karssen, Reference Groot and Karssen1987; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Both approaches had a positive effect in promoting the germination of scarified mericarps for most of the species included in our study (Tables 2 and 3), which confirms the importance of endosperm in Nolana seed dormancy.
In most of Nolana species included in this study, there was almost no germination when the funicular plug was not removed. This would indicate a very ‘cautious’ germination strategy, as suggested by Gutterman (Reference Gutterman1995), which would be in agreement with the fact that some of these species inhabit places where rainfall occurs every 15 years or more (Orellana et al., Reference Orellana, García, Ramírez and Zanetta2017; Pliscoff et al., Reference Pliscoff, Zanetta, Hepp and Machuca2017). It is possible to ascertain that the funicular plug not only plays an important role in the control of water uptake (Fig. 2) but also represents a physical barrier that the seed must overcome to complete its germination. How does this happen in nature? During the experiments, we noticed that in the case of several species, by keeping the mericarps moistened, it was later easier to remove the plugs. Douglas (Reference Douglas2007) reported that dry storage for 2 years produced significantly more germination than fresh mericarps, so it can be speculated that dormancy is lost over time, and when prolonged rains arrive that ‘loosen’ the plugs, the radicle could be able to expand and eject the plug.
Germination of Nolana mericarps, expressed as a percentage of live seed, ranged from 0 to 27% in non-treated and from 0 to 100% when treated (Table 4), indicating varying levels of depth in their seed dormancy. In our study, it was not possible to ascribe a specific level or type of PD to each species (Baskin and Baskin, Reference Baskin and Baskin2004) since the experiments focused not on determining this classification but on establishing comparisons between treatments. However, the relative levels of PD for each species should be related to the MGT or germination percentage after best treatment. MGT values were very variable within the same clade and species (Table 4), so we considered the germination percentage to be a better indicator. Therefore, we suggest a non-deep level of PD in the case of some species which showed germination after imbibition in distilled water, such as N. onoana and N. crassulifolia; others with a more intermediate level of PD (N. divaricata, N. linearifolia, N. sedifolia, N. jaffuelii, N. parviflora), which showed germination after scarification and the addition of gibberellic acid or endosperm remotion; and finally, some that hardly germinate with any of the treatments, which would correspond to a deeper dormancy (N. carnosa, N. intonsa, N. rostrata). Further experiments, considering varying periods of cold stratification and evaluation of germination at different temperatures, need to be performed in order to establish a more accurate classification of PD levels or types (Baskin and Baskin, Reference Baskin and Baskin2004).
Relationship between dormancy and latitudinal distribution or phylogeny of species
Similar traits and life cycles are expected for species within a genus, and different germination requirements of species may reflect specific adaptations to the occupied habitat and geographic distributions (Van Assche et al., Reference Van Assche, Van Nerum and Darius2002; Barreto et al., Reference Barreto, Santos and Garcia2016). However, our results showed a non-significant correlation (P = 0.06) between germination and latitudinal distribution of the 12 Nolana species studied (Fig. 5). The species that presented higher germination percentages were N. crassulifolia (clade G), a prostrate shrub (Johnston, Reference Johnston1936) with a distribution between 26° and 34°S; and N. sedifolia (clade G), a perennial shrub or sub-shrub (Mesa, Reference Mesa1981) found from 20° to 33°S. Both species share at least one type of habitat (coastal rocks), although one has a very wide distribution (N. sedifolia) and the other, intermediate. N. onoana (clade G), however, which also presented a high germination percentage, is only found at a few locations in Antofagasta region. A particular case is N. paradoxa (clade B), with the most extensive distribution from the centre to the south of coastal Chile (30° to 43°S), where the climate is temperate humid; N. paradoxa had high germination percentages, but not higher than the other species mentioned. While other studies have also found no evidence of a correlation between dormancy and geographic distribution (Giorni et al., Reference Giorni, Bicalho and Garcia2018; Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016), for Nolana, it remains to be studied if species that occupy a greater diversity of ecological niches present a greater plasticity in their dormancy levels.
In a study of several species from campo rupestre grasslands in Brazil, Dayrell et al. (Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016) found no significant correlations between seed dormancy and life-history traits, such as growth habit; in our study, we also did not find such a correlation. Clades B and C, which showed similar germination results, are quite different in terms of growth form, and their mericarps are also highly dissimilar (Hepp, Reference Hepp2019). Clade B includes two subclades: rosette-forming, taprooted plants with large flowers, and erect annuals with slightly smaller flowers (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009). Clade C, on the other hand, are all large-flowered shrubs (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009). A higher level of dormancy would be expected in species with annual habits, since they depend more on seedling survival and/or formation of a seed bank for persistence (Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016; Venable, Reference Venable2007), which was partly the case of N. paradoxa; but as mentioned before, its germination percentage was not higher than those of most clade G species. Clade G, for its part, which was found to have the higher germination percentage (Table 4, Fig. 4), is composed of small to moderate shrubs and annuals, all with highly reduced corollas (Dillon et al., Reference Dillon, Tu, Xie, Quipuscoa Silvestre and Wen2009).
In conclusion, our results indicate that seeds of the studied species present PD with varying levels of depth. The role of the funicular plug and endosperm in dormancy regulation is emphasised. For the Nolana species studied, the results show that similarities in dormancy levels would be more related to phylogenetic proximity than to their latitudinal distribution. Further research should address the mechanism by which the funicular plug is removed under natural conditions, as well as a more accurate classification of PD level for each Nolana species.
Acknowledgements
All species were collected with permission and support of the National Forest Commission (CONAF Pan de Azúcar, CONAF Llanos de Challe), the Agricultural Research Institute (INIA) and owners/administrators of private parks (Punta de Choros, Puquén Los Molles) and research stations (Alto Patache Fog Oasis, managed by Universidad Católica de Chile). CONICYT Scholarship (21130176) to Josefina Hepp made this study possible.












