Introduction
Psychopathy refers to a personality type expressed in the form of emotional callousness, lack of empathy, a grandiose estimation of self, impulsivity, and persistent antisocial behavior, among other traits (Hare, Reference Hare2003). This profile notably overlaps with features defining the antisocial personality disorder (American Psychiatric Association, 2013). However, such terms are not synonymous. To meet criteria for psychopathy, an individual must exhibit emotional dysfunction (Blair, Reference Blair2012). Emotional features such as callousness, lack of empathy and inflated self-appraisal are not necessary to operatively define antisocial personality disorder in the main Diagnostic and Statistical Manual of Mental Disorders (DSM)-V section, which predominantly considers antisocial deviance. However, the alternative trait-based definition section of the DSM-V includes an antisocial personality disorder variant characterized by low anxiety and a bold interpersonal style, which is closer to the traditional view of psychopathy (American Psychiatric Association, 2013; Venables et al., Reference Venables, Hall and Patrick2014). Analogously, the presence of callous-unemotional traits may be used to define a ‘psychopathic’ variant of conduct disorders in children (Patrick, Reference Patrick2014). A historical account of the use of these and related terms (e.g. sociopathy and dissocial disorder) can be found in Kiehl and Hoffman (Reference Kiehl and Hoffman2011).
The most widely used and validated instrument for the assessment of psychopathy is the interview-based Psychopathy Checklist-Revised (PCL-R; Hare, Reference Hare2003), which contains distinct affective–interpersonal and impulsive–antisocial factors. Various self-report instruments also exist for assessing psychopathy, as well as instruments adapted for children and adolescents (Patrick, Reference Patrick2014). Some inventories are inspired by the PCL-R, while others represent new developments, for instance, the Psychopathic Personality Inventory (Lilienfeld and Andrews, Reference Lilienfeld and Andrews1996) and the Triarchic Psychopathy Measure (Patrick et al., Reference Patrick, Fowles and Krueger2009).
Current brain imaging tools offer a unique window to explore the structural and functional bases of normal and deviant behavior. Historically, researchers have been concerned with seeking arguments to support a neurobiological foundation of psychopathy, as one of the most enigmatic personality disorders. In the last few years, increasingly abundant data have been provided. Nevertheless, further effort is necessary to integrate the new information. In this review, we describe the anatomical and functional features that characterize the brain of psychopathic individuals from a synthesis of neuroimaging research and discuss how such brain anomalies may account for psychopathic behavior. The available neuroimaging literature on psychopathy, defined in accordance with Hare's concept (Hare, Reference Hare2003), is analyzed and the review is complemented by a secondary consideration of selected neuroimaging studies assessing young people showing antisocial behavior and callous emotional dysfunction.
Brain anatomy
Global brain assessment
The brain of primary psychopathic individuals (as opposed to patients with psychopathic-like behavior secondary to focal brain damage) does not show any gross anatomical anomalies upon visual inspection. The whole brain volume is similar to the general population in most studies that have provided the measurement (Dolan et al., Reference Dolan, Deakin, Roberts and Anderson2002; Yang et al., Reference Yang, Raine, Lencz, Bihrle, LaCasse and Colletti2005a, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti2005b; Narayan et al., Reference Narayan, Narr, Kumari, Woods, Thompson, Toga and Sharma2007; Tiihonen et al., Reference Tiihonen, Rossi, Laakso, Hodgins, Testa, Perez, Repo-Tiihonen, Vaurio, Soininen, Aronen, Könönen, Thompson and Frisoni2008; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015). Significant brain volume reductions have only rarely been reported (Barkataki et al., Reference Barkataki, Kumari, Das, Taylor and Sharma2006). When volumes have been measured for specific lobes using conventional anatomical limits, significant volume reduction was solely observed in the temporal lobe and only a minimal effect was appreciated in the prefrontal lobe (Dolan et al., Reference Dolan, Deakin, Roberts and Anderson2002).
Within the samples of high socioeconomic risk, an association has been reported between the persistence of the cavum septum pellucidum – a fluid-filled space between the two leaflets of the septum – and higher scores of psychopathy (Raine et al., Reference Raine, Lee, Yang and Colletti2010). However, the cavum is similarly prevalent in disruptive behavior disorders in the absence of psychopathy (Raine et al., Reference Raine, Lee, Yang and Colletti2010; Toivonen et al., Reference Toivonen, Könönen, Niskanen, Vaurio, Repo-Tiihonen, Seppänen, Aronen, Vanninen, Tiihonen and Laakso2013; White et al., Reference White, Brislin, Sinclair, Fowler, Pope and Blair2013), suggesting that it is mainly a non-specific neuroradiological finding (Saba et al., Reference Saba, Anzidei, Raz, Suri, Piga, Grassi and Catalano2013).
Changes in the content of gray matter
A large number of studies have focused on measuring the regional content of gray matter. These studies were based on trace delineation of gray/white matter boundaries, voxel-based morphometry and automated measurements of cortical thickness. To a large extent, there is general agreement regarding psychopaths’ showing regional cortical reductions in relative gray matter content.
Earlier imaging studies predicted gray matter reductions in the prefrontal lobe of psychopaths (Raine et al., Reference Raine, Lencz, Bihrle, LaCasse and Colletti2000; Yang et al., Reference Yang, Raine, Lencz, Bihrle, LaCasse and Colletti2005a, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti2005b). Subsequent research has both largely confirmed such a prediction and demonstrated that tissue alteration extends to other brain locations (Laakso et al., Reference Laakso, Vaurio, Koivisto, Savolainen, Eronen, Aronen, Hakola, Repo, Soininen and Tiihonen2001, Reference Laakso, Gunning-Dixon, Vaurio, Repo-Tiihonen, Soininen and Tiihonen2002; Narayan et al., Reference Narayan, Narr, Kumari, Woods, Thompson, Toga and Sharma2007; de Oliveira-Souza et al., Reference de Oliveira-Souza, Hare, Bramati, Garrido, Azevedo Ignácio, Tovar-Moll and Moll2008; Müller et al., Reference Müller, Gänssbauer, Sommer, Döhnel, Weber, Schmidt-Wilcke and Hajak2008; Tiihonen et al., Reference Tiihonen, Rossi, Laakso, Hodgins, Testa, Perez, Repo-Tiihonen, Vaurio, Soininen, Aronen, Könönen, Thompson and Frisoni2008; Yang et al., Reference Yang, Raine, Colletti, Toga and Narr2009a, Reference Yang, Raine, Narr, Colletti and Toga2009b, Reference Yang, Raine, Colletti, Toga and Narr2010; Boccardi et al., Reference Boccardi, Frisoni, Hare, Cavedo, Najt, Pievani, Rasser, Laakso, Aronen, Repo-Tiihonen, Vaurio, Thompson and Tiihonen2011, Reference Boccardi, Bocchetta, Aronen, Repo-Tiihonen, Vaurio, Thompson, Tiihonen and Frisoni2013; Raine et al., Reference Raine, Yang, Narr and Toga2011; Sato et al., Reference Sato, de Oliveira-Souza, Thomaz, Basílio, Bramati, Amaro, Tovar-Moll, Hare and Moll2011; Ermer et al., Reference Ermer, Cope, Nyalakanti, Calhoun and Kiehl2012; Gregory et al., Reference Gregory, ffytche, Simmons, Kumari, Howard, Hodgins and Blackwood2012; Ly et al., Reference Ly, Motzkin, Philippi, Kirk, Newman, Kiehl and Koenigs2012; Bertsch et al., Reference Bertsch, Grothe, Prehn, Vohs, Berger, Hauenstein, Keiper, Domes, Teipel and Herpertz2013; Kolla et al., Reference Kolla, Gregory, Attard, Blackwood and Hodgins2014; Kumari et al., Reference Kumari, Uddin, Premkumar, Young, Gudjonsson, Raghuvanshi, Barkataki, Sumich, Taylor and Das2014; Contreras-Rodríguez et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015; Walters et al., Reference Walters, Ermer, Knight and Kiehl2015; Jiang et al., Reference Jiang, Li, Liu, Shi, Wang, Shen, Lee, Hu, Wang and Shen2016), as summarized in Table 1. Although the number of reported brain areas indicates a tendency for changes to affect the cortex globally, gray matter volume reduction is particularly consistent across studies in (i) the rostral temporal lobe and the rostral and ventral frontal lobe including the ventral-medial and orbitofrontal cortex; (ii) the dorsal aspect of the brain medial wall involving a dorsal/medial frontal area extending to the anterior cingulate cortex and the posterior cingulate cortex/precuneus; (iii) the posterior parahippocampal gyri/medial visual cortex; and (iv) the sensorimotor cortex. Figure 1 provides an overall depiction of this consistent pattern of gray matter volume reduction in psychopaths.

Fig. 1. Schematic representation of brain regions showing the most consistent gray matter volume reduction in primary psychopathy according to the literature.
Table 1. Gray matter reduction in psychopathy
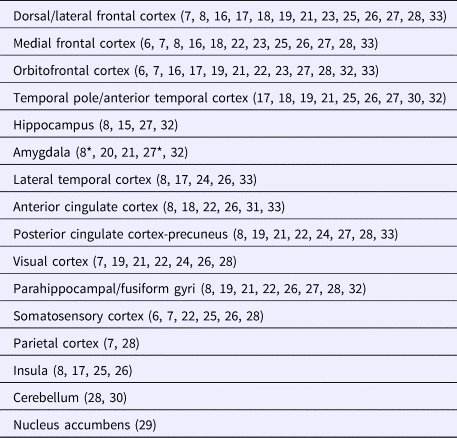
Significant gray matter volume reduction across reported studies distributed in gross brain parcellations. Literature references between parentheses. *marginally involved.
6: Narayan et al., Reference Narayan, Narr, Kumari, Woods, Thompson, Toga and Sharma2007; 7: Tiihonen et al., Reference Tiihonen, Rossi, Laakso, Hodgins, Testa, Perez, Repo-Tiihonen, Vaurio, Soininen, Aronen, Könönen, Thompson and Frisoni2008; 8: Contreras-Rodríguez et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015; 15: Laakso et al., Reference Laakso, Vaurio, Koivisto, Savolainen, Eronen, Aronen, Hakola, Repo, Soininen and Tiihonen2001; 16: Laakso et al., Reference Laakso, Gunning-Dixon, Vaurio, Repo-Tiihonen, Soininen and Tiihonen2002; 17: de Oliveira-Souza et al., Reference de Oliveira-Souza, Hare, Bramati, Garrido, Azevedo Ignácio, Tovar-Moll and Moll2008; 18: Müller et al., Reference Müller, Gänssbauer, Sommer, Döhnel, Weber, Schmidt-Wilcke and Hajak2008; 19: Yang et al., Reference Yang, Raine, Colletti, Toga and Narr2009a; 20: Yang et al., Reference Yang, Raine, Narr, Colletti and Toga2009b; 21: Yang et al., Reference Yang, Raine, Colletti, Toga and Narr2010; 22: Boccardi et al., Reference Boccardi, Frisoni, Hare, Cavedo, Najt, Pievani, Rasser, Laakso, Aronen, Repo-Tiihonen, Vaurio, Thompson and Tiihonen2011; 23: Raine et al., Reference Raine, Yang, Narr and Toga2011; 24: Sato et al., Reference Sato, de Oliveira-Souza, Thomaz, Basílio, Bramati, Amaro, Tovar-Moll, Hare and Moll2011; 25: Gregory et al., Reference Gregory, ffytche, Simmons, Kumari, Howard, Hodgins and Blackwood2012; 26: Ly et al., Reference Ly, Motzkin, Philippi, Kirk, Newman, Kiehl and Koenigs2012; 27: Ermer et al., Reference Ermer, Cope, Nyalakanti, Calhoun and Kiehl2012; 28: Bertsch et al., Reference Bertsch, Grothe, Prehn, Vohs, Berger, Hauenstein, Keiper, Domes, Teipel and Herpertz2013; 29: Boccardi et al., Reference Boccardi, Bocchetta, Aronen, Repo-Tiihonen, Vaurio, Thompson, Tiihonen and Frisoni2013; 30: Kolla et al., Reference Kolla, Gregory, Attard, Blackwood and Hodgins2014; 31: Kumari et al., Reference Kumari, Uddin, Premkumar, Young, Gudjonsson, Raghuvanshi, Barkataki, Sumich, Taylor and Das2014; 32: Walters et al., Reference Walters, Ermer, Knight and Kiehl2015; 33: Jiang et al., Reference Jiang, Li, Liu, Shi, Wang, Shen, Lee, Hu, Wang and Shen2016.
The set of cortical regions shown in Fig. 1 represents a combination of neocortical and paralimbic areas. Although the involvement of other (limbic) elements has frequently been emphasized in psychopathy, only a few studies have actually detected significant volume reduction in structures such as the amygdala, hippocampus, and insula, as indicated in Table 1. Moreover, some analyses specifically targeting the individual limbic structures using manual tracing failed to find the predicted volume reduction in the amygdala (Barkataki et al., Reference Barkataki, Kumari, Das, Taylor and Sharma2006; Boccardi et al., Reference Boccardi, Frisoni, Hare, Cavedo, Najt, Pievani, Rasser, Laakso, Aronen, Repo-Tiihonen, Vaurio, Thompson and Tiihonen2011), hippocampus (Raine et al., Reference Raine, Ishikawa, Arce, Lencz, Knuth, Bihrle, LaCasse and Colletti2004; Barkataki et al., Reference Barkataki, Kumari, Das, Taylor and Sharma2006; Boccardi et al., Reference Boccardi, Ganzola, Rossi, Sabattoli, Laakso, Repo-Tiihonen, Vaurio, Könönen, Aronen, Thompson, Frisoni and Tiihonen2010), and anterior cingulate cortex (Glenn et al., Reference Glenn, Yang, Raine and Colletti2010a). However, the limbic/paralimbic system as a whole (integrated using latent-variable modeling) does appear to be a major contributor to psychopathy (Baskin-Sommers et al., Reference Baskin-Sommers, Neumann, Cope and Kiehl2016).
A number of meta-analyses have been conducted in attempt to summarize common findings across studies of regional brain volumes. However, it is relevant to note that none have focused specifically on psychopathy. Existing meta-analyses have instead broadly pooled finding from studies of antisocial behavior (Yang and Raine, Reference Yang and Raine2009; Aoki et al., Reference Aoki, Inokuchi, Nakao and Yamasue2014), interpersonal violence (Lamsma et al., Reference Lamsma, Mackay and Fazel2017), oppositional defiant disorder (Noordermeer et al., Reference Noordermeer, Luman and Oosterlaan2016), and conduct problems (Rogers and De Brito, Reference Rogers and De Brito2016). Although these analyses have mapped regional volume reductions consistent with the emphasis of our review (Fig. 1), the frequency and extent of such alterations has notably been more discrete. It has been argued that the phenomenological and etiological heterogeneity of antisocial behavior, in particular, as well as methodological differences between studies, may explain the limited results of current meta-analytic studies (Lamsma et al., Reference Lamsma, Mackay and Fazel2017).
In children and adolescents with psychopathic traits, research has demonstrated gray matter changes distributed in regions notably reminiscent of adult psychopathic patterns (De Brito et al., Reference De Brito, Mechelli, Wilke, Laurens, Jones, Barker, Hodgins and Viding2009; Ermer et al., Reference Ermer, Cope, Nyalakanti, Calhoun and Kiehl2013; Fairchild et al., Reference Fairchild, Hagan, Walsh, Passamonti, Calder and Goodyer2013; Cope et al., Reference Cope, Ermer, Nyalakanti, Calhoun and Kiehl2014a, Reference Cope, Vincent, Jobelius, Nyalakanti, Calhoun and Kiehl2014b). However, such studies have produced mixed findings as to the direction of the alteration. Interestingly, young individuals (mean age of 12 years) show a gray matter volume increase (De Brito et al., Reference De Brito, Mechelli, Wilke, Laurens, Jones, Barker, Hodgins and Viding2009) associated with psychopathic traits, whereas in older individuals (mean age of 17 years), psychopathic traits are instead associated with a gray matter volume decrease in both males (Ermer et al., Reference Ermer, Cope, Nyalakanti, Calhoun and Kiehl2013) and females (Cope et al., Reference Cope, Ermer, Nyalakanti, Calhoun and Kiehl2014a, Reference Cope, Vincent, Jobelius, Nyalakanti, Calhoun and Kiehl2014b). One study in female adolescents showed a mixed brain pattern of positive and negative correlations (Fairchild et al., Reference Fairchild, Hagan, Walsh, Passamonti, Calder and Goodyer2013). Taking both young and adult studies together, alterations in gray matter volume in psychopathy may be hypothesized to evolve dynamically throughout life from a relative gray matter increase to a relative decrease.
In support of a developmental dynamic basis of brain tissue alterations in psychopathy, one study reported a significant association of childhood physical abuse with gray matter volume reduction in the temporal pole of adult psychopaths (Kolla et al., Reference Kolla, Gregory, Attard, Blackwood and Hodgins2014). Child abuse was also associated with reduced fronto-temporal cortical thickness predicting antisocial behavior in adolescents (Busso et al., Reference Busso, McLaughlin, Brueck, Peverill, Gold and Sheridan2017). In another study, the combination of relatively lower cortical volumes and higher volumes in subcortical regions was found to partially mediate the relation between adverse life events and antisocial behavior in a sample of 1741 adolescents (Mackey et al., Reference Mackey, Chaarani, Kan, Spechler, Orr, Banaschewski, Barker, Bokde, Bromberg, Büchel, Cattrell, Conrod, Desrivières, Flor, Frouin, Gallinat, Gowland, Heinz, Ittermann, Paillère Martinot, Artiges, Nees, Papadopoulos-Orfanos, Poustka, Smolka, Jurk, Walter, Whelan, Schumann, Althoff and Garavan2017). At the age of 25 years, a voxel-based morphometry analysis demonstrated the association of early life poverty with gray matter volume reduction precisely in the ventral-medial frontal cortex (Holz et al., Reference Holz, Boecker, Hohm, Zohsel, Buchmann, Blomeyer, Barker, Bokde, Bromberg, Büchel, Cattrell, Conrod, Desrivières, Flor, Frouin, Gallinat, Gowland, Heinz, Ittermann, Paillère Martinot, Artiges, Nees, Papadopoulos-Orfanos, Poustka, Smolka, Jurk, Walter, Whelan, Schumann, Althoff and Garavan2015). Symptoms related to early onset cumulative adversity have similarly been associated with reduced gray matter volumes in persistent delinquent youths (Raine et al., Reference Raine, Lencz, Taylor, Hellige, Bihrle, Lacasse, Lee, Ishikawa and Colletti2003).
Changes in the content of white matter
Despite consistency across studies, the extent to which reductions of gray matter volumes in adult psychopaths described above express true gray matter loss (i.e. atrophy) is not obvious. Indeed, results from white matter analyses suggest that white matter increases may contribute to relative reductions of gray matter measurements in the absence of relevant brain volume changes.
When results have been provided, white matter volume changes have generally paralleled the findings of gray matter changes in the opposite direction, with volume increases in global, prefrontal, and corpus callosum white matter correlating with psychopathy scores (Raine et al., Reference Raine, Lencz, Taylor, Hellige, Bihrle, Lacasse, Lee, Ishikawa and Colletti2003; Yang et al., Reference Yang, Raine, Lencz, Bihrle, LaCasse and Colletti2005a, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti2005b). Larger volumes have also been demonstrated for basal ganglia elements with a very high content of white matter (Barkataki et al., Reference Barkataki, Kumari, Das, Taylor and Sharma2006; Glenn et al., Reference Glenn, Raine, Yaralian and Yang2010b; Pujara et al., Reference Pujara, Motzkin, Newman, Kiehl and Koenigs2014).
A similar tendency has also been observed in disorders overlapping with psychopathy. In persistently violent offenders with antisocial personality disorder significantly larger white matter volumes were observed in broadly distributed brain regions grossly coinciding with areas of gray matter volume reduction (Tiihonen et al., Reference Tiihonen, Rossi, Laakso, Hodgins, Testa, Perez, Repo-Tiihonen, Vaurio, Soininen, Aronen, Könönen, Thompson and Frisoni2008). In young males with psychopathic traits, a mixed pattern of changes has been reported combining both increases and decreases in regional white matter volume (De Brito et al., Reference De Brito, McCrory, Mechelli, Wilke, Jones, Hodgins and Viding2011).
It has been argued that the relative increase of white matter in psychopaths may be related to non-optimal brain remodeling (deficient axonal pruning), resulting in excessive anatomical connections (Raine et al., Reference Raine, Lencz, Taylor, Hellige, Bihrle, Lacasse, Lee, Ishikawa and Colletti2003). However, alternatively, we suggest here that larger white matter volumes in psychopathy may also relate to significantly accelerated myelination. It is important to note that white matter segments in conventional (T1-weighted) anatomical magnetic resonance imaging (MRI) scans correspond to myelinated white matter (Paus et al., Reference Paus, Collins, Evans, Leonard, Pike and Zijdenbos2001). Myelination is an active process throughout life (Yakovlev and LeCours, Reference Yakovlev, LeCours and Minkowski1967; Pujol et al., Reference Pujol, Vendrell, Junqué, Martí-Vilalta and Capdevila1993; Narayan et al., Reference Narayan, Narr, Kumari, Woods, Thompson, Toga and Sharma2007), which is physiologically accelerated in early postnatal years and during adolescence (Paus et al., Reference Paus, Collins, Evans, Leonard, Pike and Zijdenbos2001; Pujol et al., Reference Pujol, Soriano-Mas, Ortiz, Sebastián-Gallés, Losilla and Deus2006), and enhanced by repetitive use or skill learning (McKenzie et al., Reference McKenzie, Ohayon, Li, de Faria, Emery, Tohyama and Richardson2014; Pujol et al., Reference Pujol, Fenoll, Forns, Harrison, Martínez-Vilavella, Macià, Alvarez-Pedrerol, Blanco-Hinojo, González-Ortiz, Deus and Sunyer2016a). Pathological acceleration of myelination has been suggested, for example, in melancholic depression (Soriano-Mas et al., Reference Soriano-Mas, Hernández-Ribas, Pujol, Urretavizcaya, Deus, Harrison, Ortiz, López-Solà, Menchón and Cardoner2011), childhood obesity (Ou et al., Reference Ou, Andres, Pivik, Cleves and Badger2015), heavy cannabis use (Matochik et al., Reference Matochik, Eldreth, Cadet and Bolla2005), and pathological lying (Yang et al., Reference Yang, Raine, Lencz, Bihrle, LaCasse and Colletti2005a, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti2005b).
To summarize, measurable changes in gray and white matter regional tissue content can be detected in the brain of psychopaths in the absence of changes of total brain volume. This overall pattern of findings argues against gray matter atrophy as a sole explanation for gray matter volume reductions in psychopaths. We propose that accelerated myelination may be one contributing factor, in that it affects image tissue segmentation by displacing the boundary between white and gray matter.
Changes in the structure of white matter pathways
Potential alterations in the structure of white matter pathways have been investigated using diffusion tensor imaging (DTI). The common measurement across all studies is fractional anisotropy (FA), which quantifies the degree of structural maturation of white matter by estimating water diffusivity along tracts (Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012). DTI studies of psychopathic populations coincide in showing FA reductions mostly involving frontal lobe connections (Craig et al., Reference Craig, Catani, Deeley, Latham, Daly, Kanaan, Picchioni, McGuire, Fahy and Murphy2009; Motzkin et al., Reference Motzkin, Newman, Kiehl and Koenigs2011; Sundram et al., Reference Sundram, Deeley, Sarkar, Daly, Latham, Craig, Raczek, Fahy, Picchioni, Barker and Murphy2012; Hoppenbrouwers et al., Reference Hoppenbrouwers, Nazeri, de Jesus, Stirpe, Felsky, Schutter, Daskalakis and Voineskos2013; Sethi et al., Reference Sethi, Gregory, Dell'Acqua, Periche Thomas, Simmons, Murphy, Hodgins, Blackwood and Craig2015; Wolf et al., Reference Wolf, Pujara, Motzkin, Newman, Kiehl, Decety, Kosson and Koenigs2015; Jiang et al., Reference Jiang, Shi, Liu, Li, Ding, Shen, Lee, Hu, Wang and Shen2017b).
Alterations in the ventral connections between the frontal lobe and the temporal lobe would appear to be a solid finding, which mostly implicate the uncinate fasciculus (Craig et al., Reference Craig, Catani, Deeley, Latham, Daly, Kanaan, Picchioni, McGuire, Fahy and Murphy2009; Motzkin et al., Reference Motzkin, Newman, Kiehl and Koenigs2011; Sundram et al., Reference Sundram, Deeley, Sarkar, Daly, Latham, Craig, Raczek, Fahy, Picchioni, Barker and Murphy2012; Hoppenbrouwers et al., Reference Hoppenbrouwers, Nazeri, de Jesus, Stirpe, Felsky, Schutter, Daskalakis and Voineskos2013; Wolf et al., Reference Wolf, Pujara, Motzkin, Newman, Kiehl, Decety, Kosson and Koenigs2015; Jiang et al., Reference Jiang, Shi, Liu, Li, Ding, Shen, Lee, Hu, Wang and Shen2017b). Significant FA reductions have also been identified in dorsal pathways connecting the frontal lobe with the parietal lobe (Sundram et al., Reference Sundram, Deeley, Sarkar, Daly, Latham, Craig, Raczek, Fahy, Picchioni, Barker and Murphy2012; Sethi et al., Reference Sethi, Gregory, Dell'Acqua, Periche Thomas, Simmons, Murphy, Hodgins, Blackwood and Craig2015; Jiang et al., Reference Jiang, Shi, Liu, Li, Ding, Shen, Lee, Hu, Wang and Shen2017b). Interestingly, the cingulum is altered both ventrally and dorsally, with FA reductions detected in the segment connecting the posterior cingulate cortex with the medial temporal lobe and the segment connecting the posterior cingulate cortex with the frontal lobe (Sethi et al., Reference Sethi, Gregory, Dell'Acqua, Periche Thomas, Simmons, Murphy, Hodgins, Blackwood and Craig2015). Frontal lobe structural connectivity alterations in psychopaths therefore seem to implicate dorsal and ventral bundles that connect brain areas that broadly demonstrate gray matter volume reductions (summarized in Fig. 1). However, frontal lobe connectivity changes are not limited to the anterior–posterior direction, as reduced FA has also been demonstrated in the genu of corpus callosum (transverse connections) (Sundram et al., Reference Sundram, Deeley, Sarkar, Daly, Latham, Craig, Raczek, Fahy, Picchioni, Barker and Murphy2012) and in frontal-basal ganglia connections (Sundram et al., Reference Sundram, Deeley, Sarkar, Daly, Latham, Craig, Raczek, Fahy, Picchioni, Barker and Murphy2012; Hoppenbrouwers et al., Reference Hoppenbrouwers, Nazeri, de Jesus, Stirpe, Felsky, Schutter, Daskalakis and Voineskos2013).
Reductions in FA can be interpreted as expressing a maturation delay in late maturing bundles (Sethi et al., Reference Sethi, Gregory, Dell'Acqua, Periche Thomas, Simmons, Murphy, Hodgins, Blackwood and Craig2015). Nevertheless, accelerated, as opposed to delayed, maturation may better account for the majority of pathway alterations in psychopaths. Indeed, the increase in FA from normal childhood to adulthood reaches a peak between 20 and 40 years of age, and subsequently decreases (Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012). Accordingly, from early adulthood, the normal physiological evolution of FA is one of the progressive reductions, presumably reflecting increased white matter structural complexity accompanying brain maturation (Douaud et al., Reference Douaud, Jbabdi, Behrens, Menke, Gass, Monsch, Rao, Whitcher, Kindlmann, Matthews and Smith2011; Jones e t al., Reference Jones, Knösche and Turner2013). In DTI studies of psychopathy, the mean age of adult samples has broadly coincided with the FA peak, or with the beginning of FA decreases (age range across studies, 30–40 years), with the sole exception of one study (Jiang et al., Reference Jiang, Shi, Liu, Li, Ding, Shen, Lee, Hu, Wang and Shen2017b). Thus, within this age range, reduced FA is better explained by accelerated maturation, or psychopaths reaching FA maturity earlier than what is normally observed. Interestingly, in the DTI study featuring the youngest population (mean age 23 years), a combination of FA decreases (predominant) and increases was reported (Jiang et al., Reference Jiang, Shi, Liu, Li, Ding, Shen, Lee, Hu, Wang and Shen2017b).
DTI analyses in younger populations with callous-unemotional traits have provided mixed results, with different studies reporting higher, lower or no differences in FA v. healthy controls for diverse white-matter tracts (see Waller et al. (Reference Waller, Dotterer, Murray, Maxwell and Hyde2017) for a review). These findings offer further support for the proposal that abnormally accelerated white matter maturation contributes to the nature of anatomical changes in psychopathy and callousness. FA increase will be identified in youths, compared with typically developing controls, at ages prior to the FA age-related peak (the ascending part of the curve) for a given bundle, and FA reduction in older individuals closer to the age at which FA begins to decrease (Waller et al., Reference Waller, Dotterer, Murray, Maxwell and Hyde2017). Here too, early life stress could play a significant role. In one study, adults with life-time exposure to parental abuse showed FA reduction in the hippocampal extension of the cingulum bundle, the fornix, and the arcuate fasciculus (Choi et al., Reference Choi, Jeong, Rohan, Polcari and Teicher2009).
Brain function
Functional connectivity
Functional connectivity is considered a measurement of activity synchrony between brain regions sharing functional properties (Biswal et al., Reference Biswal, Yetkin, Haughton and Hyde1995). In antisocial offenders, the assessment of functional connectivity changes using whole-brain measures has provided evidence of both reduced functional integration and segregation in the organization of large-scale brain networks (Jiang et al., Reference Jiang, Shi, Liao, Liu, Wang, Shen, Hu, Wang and Shen2017a). In global terms, such findings may be interpreted as reflecting deficient brain functional maturation (Fair et al., Reference Fair, Cohen, Power, Dosenbach, Church, Miezin, Schlaggar and Petersen2009; Pujol et al., Reference Pujol, Martínez-Vilavella, Macià, Fenoll, Alvarez-Pedrerol, Rivas, Forns, Blanco-Hinojo, Capellades, Querol, Deus and Sunyer2016b). The integration and segregation of large-scale brain networks is a life-time process that is especially active during adolescence (Sherman et al., Reference Sherman, Rudie, Pfeifer, Masten, McNealy and Dapretto2014). Thus, the hypothetical acceleration of white matter maturation does not seem to imply a more efficient coupling among late maturing networks in the case of psychopaths (i.e. maturation may be faster but deficient).
As in the case of other imaging alterations, global brain changes in functional connectivity have been observed in association with changes that are particularly prominent in specific brain systems. Once again, the frontal cortex is the structure that shows the most relevant connectivity alterations in psychopaths. Some evidence indicates reduced functional connectivity between ventral frontal areas and the anterior temporal lobe and amygdala at rest (Motzkin et al., Reference Motzkin, Newman, Kiehl and Koenigs2011) and during emotional stimulation (Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015; Volman et al., Reference Volman, von Borries, Bulten, Verkes, Toni and Roelofs2016). Other studies have demonstrated reduced functional connectivity of the dorsal frontal cortex (and anterior cingulate cortex) with several limbic–paralimbic structures (Ly et al., Reference Ly, Motzkin, Philippi, Kirk, Newman, Kiehl and Koenigs2012; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015; Philippi et al., Reference Philippi, Pujara, Motzkin, Newman, Kiehl and Koenigs2015) and, particularly, with the posterior cingulate cortex/precuneus complex (Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015; Philippi et al., Reference Philippi, Pujara, Motzkin, Newman, Kiehl and Koenigs2015).
In addition to long-distance connectivity reduction, psychopaths appear to display higher functional connectivity within the dorsal aspect of the frontal lobes at rest (Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015), and between dorsal frontal areas and the striatum during reward expectancy (Geurts et al., Reference Geurts, von Borries, Volman, Bulten, Cools and Verkes2016). Higher frontal cortex connectivity was associated with more severe lifestyle/antisocial traits (Hare factor 2) in the study carried out by Philippi et al. (Reference Philippi, Pujara, Motzkin, Newman, Kiehl and Koenigs2015). In general agreement with such functional connectivity findings, studies of structural connectivity (structural covariance) estimated from cortical thickness measurements, also implicates the superior frontal cortex as a relevant connectivity hub in psychopaths (Yang et al., Reference Yang, Raine, Joshi, Joshi, Chang, Schug, Wheland, Leahy and Narr2012). Reduced functional connectivity also seems to combine with increased connectivity in antisocial personality disorder (Tang et al., Reference Tang, Long, Wang, Liao, Xie, Zhao and Zhang2016).
Brain activation
Brain response to emotional stimuli
A number of studies have examined how psychopaths respond to emotionally provocative stimuli, including emotional faces (Deeley et al., Reference Deeley, Daly, Surguladze, Tunstall, Mezey, Beer, Ambikapathy, Robertson, Giampietro, Brammer, Clarke, Dowsett, Fahy, Phillips and Murphy2006; Decety et al., Reference Decety, Skelly and Kiehl2013b, Reference Decety, Skelly, Yoder and Kiehl2014; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014; Hyde et al., Reference Hyde, Byrd, Votruba-Drzal, Hariri and Manuck2014; Mier et al., Reference Mier, Haddad, Diers, Dressing, Meyer-Lindenberg and Kirsch2014; Pera-Guardiola et al., Reference Pera-Guardiola, Contreras-Rodríguez, Batalla, Kosson, Menchón, Pifarré, Bosque, Cardoner and Soriano-Mas2016; Volman et al., Reference Volman, von Borries, Bulten, Verkes, Toni and Roelofs2016), unpleasant pictures (Müller et al., Reference Müller, Sommer, Wagner, Lange, Taschler, Röder, Schuierer, Klein and Hajak2003; Decety et al., Reference Decety, Skelly and Kiehl2013b; Harenski et al., Reference Harenski, Harenski and Kiehl2014; Sitaram et al., Reference Sitaram, Caria, Veit, Gaber, Ruiz and Birbaumer2014), emotion-laden scenes (Decety et al., Reference Decety, Skelly and Kiehl2013b, Reference Decety, Chen, Harenski and Kiehl2015; Meffert et al., Reference Meffert, Gazzola, den Boer, Bartels and Keysers2013), and emotional words (Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014). Collectively, these studies indicate that the neural processing of emotional stimuli is altered in psychopaths. However, depending on the nature of the experiment, studies have reported either attenuated (Deeley et al., Reference Deeley, Daly, Surguladze, Tunstall, Mezey, Beer, Ambikapathy, Robertson, Giampietro, Brammer, Clarke, Dowsett, Fahy, Phillips and Murphy2006; Harenski et al., Reference Harenski, Harenski and Kiehl2014; Hyde et al., Reference Hyde, Byrd, Votruba-Drzal, Hariri and Manuck2014; Mier et al., Reference Mier, Haddad, Diers, Dressing, Meyer-Lindenberg and Kirsch2014; Volman et al., Reference Volman, von Borries, Bulten, Verkes, Toni and Roelofs2016) or enhanced (Intrator et al., Reference Intrator, Hare, Stritzke, Brichtswein, Dorfman, Harpur, Bernstein, Handelsman, Schaefer, Keilp, Rosen and Machac1997; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014) or indeed a combination of response changes (Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a, Reference Decety, Skelly and Kiehl2013b, Reference Decety, Skelly, Yoder and Kiehl2014, Reference Decety, Chen, Harenski and Kiehl2015) in different elements of the ‘emotion processing’ network. Relevantly, enhanced brain response has been reported for visual sensory areas (Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014), association sensory cortex (Decety et al., Reference Decety, Skelly and Kiehl2013b; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014), insula (Decety et al., Reference Decety, Skelly and Kiehl2013b, Reference Decety, Skelly, Yoder and Kiehl2014), anterior cingulate cortex (Decety et al., Reference Decety, Skelly and Kiehl2013b), basal ganglia (Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a, Reference Decety, Skelly and Kiehl2013b), and frontal (predominantly dorsal) areas (Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014; Decety et al., Reference Decety, Skelly and Kiehl2013b). Thus, the psychopath's brain is not necessarily ‘unemotional’ in terms of its response to emotional stimulation, which may be enhanced at some processing stages. Nevertheless, a disruption of the emotional flow occurs, perhaps in the transition of processing from temporal lobe structures to the ventral prefrontal cortex. Indeed, the amygdala (Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a, Reference Decety, Skelly, Yoder and Kiehl2014; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014; Harenski et al., Reference Harenski, Harenski and Kiehl2014; Hyde et al., Reference Hyde, Byrd, Votruba-Drzal, Hariri and Manuck2014; Mier et al., Reference Mier, Haddad, Diers, Dressing, Meyer-Lindenberg and Kirsch2014) and the ventral prefrontal cortex (Decety et al., Reference Decety, Skelly and Kiehl2013b, Reference Decety, Skelly, Yoder and Kiehl2014) both show only a modest or attenuated response to emotional stimuli in psychopaths, and the normal functional coupling between both elements appear to be reduced during task performance (Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014; Volman et al., Reference Volman, von Borries, Bulten, Verkes, Toni and Roelofs2016).
Brain response to aversively conditioned stimuli (e.g. with pain) has also been tested in psychopaths. Once again, the results have been divergent, with evidence for both attenuated (Veit et al., Reference Veit, Flor, Erb, Hermann, Lotze, Grodd and Birbaumer2002; Larson et al., Reference Larson, Baskin-Sommers, Stout, Balderston, Curtin, Schultz, Kiehl and Newman2013; Decety et al., Reference Decety, Skelly, Yoder and Kiehl2014) and enhanced (Schneider et al., Reference Schneider, Habel, Kessler, Posse, Grodd and Müller-Gärtner2000; Schultz et al., Reference Schultz, Balderston, Baskin-Sommers, Larson and Helmstetter2016) brain activations, which further suggests that the experimental context is critical in determining the magnitude and direction of the response. Attention seems to be a relevant factor that may explain some apparent discrepancies. For example, compared with control subjects, psychopaths showed lower right amygdala and higher left frontal response to a short (200 ms) exposure to a pain-conditioned stimulus when attention was engaged in a neutral task (Larson et al., Reference Larson, Baskin-Sommers, Stout, Balderston, Curtin, Schultz, Kiehl and Newman2013). By contrast, long exposure (8 s) to a pain-conditioned complex image generated a greater response, in this case in the left amygdala and in areas processing visual stimuli features (Schultz et al., Reference Schultz, Balderston, Baskin-Sommers, Larson and Helmstetter2016).
There is also evidence relating psychopathy to altered responses to incentive stimulation. Specifically, psychopathy has been associated with lower basal ganglia activation related to monetary loss (Pujara et al., Reference Pujara, Motzkin, Newman, Kiehl and Koenigs2014), lower medial frontal activation during reward anticipation (Veroude et al., Reference Veroude, von Rhein, Chauvin, van Dongen, Mennes, Franke, Heslenfeld, Oosterlaan, Hartman, Hoekstra, Glennon and Buitelaar2016), and lower anterior cingulate cortex activation in response to reward uncertainty (Prehn et al., Reference Prehn, Schlagenhauf, Schulze, Berger, Vohs, Fleischer, Hauenstein, Keiper, Domes and Herpertz2013), while increased activation has been observed in the posterior cingulate cortex and anterior insula during the task reversal phase (Gregory et al., Reference Gregory, Blair, Ffytche, Simmons, Kumari, Hodgins and Blackwood2015). Also, higher psychopathy scores have been associated with reduced response to drug abuse-related pictures in the basal ganglia and connected brain structures (Cope et al., Reference Cope, Ermer, Nyalakanti, Calhoun and Kiehl2014a, Reference Cope, Vincent, Jobelius, Nyalakanti, Calhoun and Kiehl2014b).
Brain activity during moral challenge
Neuroimaging research has demonstrated the involvement of a well-defined brain network in the mediation of moral judgment in the normal population. This network overlaps with the so-called default mode network connecting the medial frontal cortex, the posterior cingulate cortex, and the angular gyri (Greene et al., Reference Greene, Sommerville, Nystrom, Darley and Cohen2001), but also medial structures of the temporal lobe and upper brainstem (Harrison et al., Reference Harrison, Pujol, López-Solà, Hernández-Ribas, Deus, Ortiz, Soriano-Mas, Yücel, Pantelis and Cardoner2008; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012). In functional MRI studies, psychopaths required to make a judgment in a moral dilemma situation (Fig. 2) have consistently shown deficient activation in the medial frontal and posterior cingulate cortex (Glenn et al., Reference Glenn, Raine and Schug2009; Harenski et al., Reference Harenski, Harenski, Shane and Kiehl2010; Veit et al., Reference Veit, Lotze, Sewing, Missenhardt, Gaber and Birbaumer2010; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015) and, remarkably, in the hippocampus and parahippocampal gyrus extending to the periaqueductal gray (Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012) and amygdala (Glenn et al., Reference Glenn, Raine and Schug2009; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Marsh and Cardinale, Reference Marsh and Cardinale2014; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015). Such experiments may therefore show the inappropriate use of a network that both mediates attention to self as an agent and has access to autobiographical storage (Moll et al., Reference Moll, de Oliveira-Souza, Garrido, Bramati, Caparelli-Daquer, Paiva, Zahn and Grafman2007; Bado et al., Reference Bado, Engel, de Oliveira-Souza, Bramati, Paiva, Basilio, Sato, Tovar-Moll and Moll2014; Leech and Sharp, Reference Leech and Sharp2014). Consistent with this view, criminal psychopaths precisely showed lower activity in the posterior (and anterior) cingulate cortex, parahippocampal gyrus, hippocampus, and amygdala during the performance of an affective memory task (Kiehl et al., Reference Kiehl, Smith, Hare, Mendrek, Forster, Brink and Liddle2001).

Fig. 2. Representation of a typical moral dilemma used in functional MRI experiments in psychopaths. Adapted, with permission, from Pujol et al. (Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012).
A functional ‘breakdown’ in the brain network subserving moral judgment may also extend to neutral situations unrelated to the moral context. Functional MRI signal reduction or deactivation typically observed in the default mode network during conventional cognitive tasks is deficient in psychopaths (Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Juárez et al., Reference Juárez, Kiehl and Calhoun2013; Freeman et al., Reference Freeman, Clewett, Bennett, Kiehl, Gazzaniga and Miller2015). Moreover, the anterior and posterior elements of the default mode network appeared to be significantly uncoupled during the resting state in functional connectivity analyses (Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012). Therefore, the network abnormally responding during moral challenge would also seem to be primarily altered. This idea is consistent with the proposal that attentional deficits in psychopaths are not limited to situations of moral conflict, but extend more generally to other attentional domains (Newman et al., Reference Newman, Curtin, Bertsch and Baskin-Sommers2010; Aharoni et al., Reference Aharoni, Vincent, Harenski, Calhoun, Sinnott-Armstrong, Gazzaniga and Kiehl2013; Newman and Baskin-Sommers, Reference Newman and Baskin-Sommers2016; Rodman et al., Reference Rodman, Kastman, Dorfman, Baskin-Sommers, Kiehl, Newman and Buckholtz2016).
The arousal generated by a moral challenge in psychopaths appears to depend on how the self is taken as a reference. While watching morally laden scenes, psychopaths showed higher frontal activation when identifying the emotional state of the victim and lower frontal activation when identifying the emotional state of the predator (Decety et al., Reference Decety, Chen, Harenski and Kiehl2015). However, other data suggest that if the psychopath is forced to feel like the predator – as opposed to simply having to identify the predator's emotional state – such a response attenuation may be less evident (Sommer et al., Reference Sommer, Sodian, Döhnel, Schwerdtner, Meinhardt and Hajak2010; Decety et al., Reference Decety, Chen, Harenski and Kiehl2013a, Reference Decety, Skelly and Kiehl2013b; Meffert et al., Reference Meffert, Gazzola, den Boer, Bartels and Keysers2013), further suggesting an attentional selection bias (Newman et al., Reference Newman, Curtin, Bertsch and Baskin-Sommers2010; Newman and Baskin-Sommers, Reference Newman and Baskin-Sommers2016).
In brief, while psychopaths are reactive to different forms of emotional stimulation, emotional processing may be incomplete with poor temporal lobe transmission of emotional flow to the ventral frontal system. Also, moral challenge experiments have revealed the inappropriate use of a dorsal network mediating attention to our inner emotional world.
In addition to functional imaging of evoked brain activation, other studies have assessed brain function based on a variety of metabolic parameters. Regional glucose metabolism and cerebral blood flow have generally been reported to be lower in psychopaths, particularly in frontal and temporal areas (Volkow et al., Reference Volkow, Tancredi, Grant, Gillespie, Valentine, Mullani, Wang and Hollister1995; Raine et al., Reference Raine, Buchsbaum and LaCasse1997; Soderstrom et al., Reference Soderstrom, Hultin, Tullberg, Wikkelso, Ekholm and Forsman2002). One study reported lower levels of monoamine oxidase-A, an enzyme that regulates neurotransmitters, in the orbitofrontal cortex and ventral striatum in offenders with high-psychopathic traits (Kolla et al., Reference Kolla, Matthews, Wilson, Houle, Bagby, Links, Simpson, Hussain and Meyer2015). Other authors have reported a positive correlation between striatal serotonin 1B receptor binding and the level of psychopathy (da Cunha-Bang et al., Reference da Cunha-Bang, Hjordt, Perfalk, Beliveau, Bock, Lehel, Thomsen, Sestoft, Svarer and Knudsen2017).
General implications
In this review, we provide a synthesis of available neuroimaging research directly concerning to psychopathy. A variety of imaging methods has been used to date to provide distinct perspectives of brain structure and function. There are notable differences across the findings of existing studies, which is likely to reflect important differences in study methodology, but may also reflect the underlying biological heterogeneity of psychopathy. Nevertheless, most studies coincide in suggesting that the brain of psychopaths differs notably from the typical brain in terms of both anatomy and function. There is a tendency for changes to affect the brain globally, but variations from the normal pattern are particularly evident in a number of functionally related brain structures. One system includes the rostral aspect of both frontal and temporal lobes, which subcortically report to the ventral striatum and anterior hippocampus/amygdala, respectively. Although this system is involved in a vast variety of processes, in the context of psychopathy, we emphasize its role in conveying emotional input to motivated actions (Simpson and Balsam, Reference Simpson and Balsam2016). The second system combines the medial frontal (and anterior cingulate) cortex with the posterior cingulate cortex/precuneus, core elements of the default mode network, which are critical in focusing attention on self and accessing autobiographical storage (Bado et al., Reference Bado, Engel, de Oliveira-Souza, Bramati, Paiva, Basilio, Sato, Tovar-Moll and Moll2014; Leech and Sharp, Reference Leech and Sharp2014). This dorsal system is connected to caudal temporal lobe structures also altered in psychopathy. Relevant changes have been identified in the parahippocampus/posterior hippocampus and visual areas involved in the processing of sensory stimulation in general, but also relevant for the storage of significant autobiographical events (Jeong et al., Reference Jeong, Chung and Kim2015). In addition, alterations have also been reported involving the sensorimotor cortex, which is the primary representation of our bodies.
Functional data suggest that emotionally evocative stimuli are indeed capable of activating the brain and even generating excessive responses in some (mostly dorsal) emotion-relevant brain areas in psychopaths. However, the emotional processing flow may appear to be disrupted in the transition from the temporal lobe to ventral frontal areas, with the consequent failure to integrate emotion into cognition and subsequent decisions, as often proposed (Kiehl et al., Reference Kiehl, Smith, Hare, Mendrek, Forster, Brink and Liddle2001; Raine and Yang, Reference Raine and Yang2006; Blair, Reference Blair2007; Moll et al., Reference Moll, De Oliveira-Souza and Zahn2008; Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Bosque, Ibern-Regàs, Hernández-Ribas, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2014; Blair et al., Reference Blair, Veroude and Buitelaar2018). The alteration in emotional processing resulting from such ventral temporal-frontal blockage may be sufficient to account for psychopathic behavior if taken to the extreme. Indeed, in frontotemporal dementia and after focal damage of rostral fronto-temporal areas, the presence of psychopathic-like behavior is not exceptional (Anderson et al., Reference Anderson, Bechara, Damasio, Tranel and Damasio1999; Brower and Price, Reference Brower and Price2001; Koenigs et al., Reference Koenigs, Young, Adolphs, Tranel, Cushman, Hauser and Damasio2007; Moll et al., Reference Moll, de Oliveira-Souza, Garrido, Bramati, Caparelli-Daquer, Paiva, Zahn and Grafman2007; Diehl-Schmid et al., Reference Diehl-Schmid, Perneczky, Koch, Nedopil and Kurz2013; Birkhoff et al., Reference Birkhoff, Garberi and Re2016; Darby et al., Reference Darby, Horn, Cushman and Fox2018).
Nevertheless, imaging data indicate that emotional processing blockage may also occur as a result of the abnormal functioning of a dorsal brain system. Probably the most consistent finding in functional imaging research in the context of adult psychopathy is the alteration of the network involved in the mediation of moral judgment overlapping with the default mode network. The normal activation of this network serves to focus attention on the self as an agent and thus (as we propose) exposing the individual to be aroused (or ‘touched’) from emotional (positive or negative) memories stored in temporal lobe medial structures. Activation of the default mode network during moral conflict is certainly abnormal in psychopaths (Glenn et al., Reference Glenn, Raine and Schug2009; Harenski et al., Reference Harenski, Harenski, Shane and Kiehl2010; Veit et al., Reference Veit, Lotze, Sewing, Missenhardt, Gaber and Birbaumer2010; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015), which concurs with a similarly poor activation of key memory structures such as the parahippocampal gyrus, hippocampus, and amygdala (Glenn et al., Reference Glenn, Raine and Schug2009; Pujol et al., Reference Pujol, Batalla, Contreras-Rodríguez, Harrison, Pera, Hernández-Ribas, Real, Bosa, Soriano-Mas, Deus, López-Solà, Pifarré, Menchón and Cardoner2012; Marsh and Cardinale, Reference Marsh and Cardinale2014; Yoder et al., Reference Yoder, Harenski, Kiehl and Decety2015).
All in all, a failure to integrate emotion into cognition would appear to result from at least two complementary breakdowns; bottom-up disruption of the emotional flow in the ventral fronto-temporal system and a top-down blockage of emotional self-exposure in the dorsal network mediating moral judgment.
Future directions
In the current review, we have focused on how current neuroimaging research may contribute to a better understanding of pathophysiological mechanisms leading to psychopathic behavior. Nevertheless, another question is whether neuroimaging can provide clues as to its etiology? We would emphasize three potential causal factors deserving future research, in addition to genetic predisposition, which always plays a significant role in complex behavioral disorders.
As discussed earlier, one of the factors that potentially contributes to the development of brain pathology in psychopaths is life-time stress, which may well accelerate brain maturation. Life-time stress could indeed contribute to regional gray matter volume reduction through excessive neural activity leading to subsequent atrophy (a ‘burnout’ effect) or by increasing myelinated white matter with an apparent reduction of the gray matter tissue segment, or both. Future analyses may specifically focus on determining the life-time evolution of cortical white matter content. Additionally, it may be valuable in this context to test the hypothesis of accelerated brain maturation from a functional perspective, by assessing both brain activity and functional network connectivity in children and adolescents with psychopathic traits.
Androgens have a relevant effect on brain shaping. Critical periods include the prenatal androgenization of the brain and androgen activation during adolescence (Arnold and Gorski, Reference Arnold and Gorski1984; Sato et al., Reference Sato, Schulz, Sisk and Wood2008). The male and female brain differs, in part, due to androgen effects (Heany et al., Reference Heany, van Honk, Stein and Brooks2016) and antisocial behavior is substantially more frequent in males (Yildirim and Derksen, Reference Yildirim and Derksen2012). Thus, anomalies in developmental androgenic brain modeling may increase the predisposition to psychopathy (Yildirim and Derksen, Reference Yildirim and Derksen2012). Relevantly, the effects of prolonged high-dose administration of androgenic steroids on brain anatomy are largely reminiscent of gray matter alteration patterns in psychopaths showing reduced cortical thickness in rostral frontotemporal areas, the medial frontal cortex, the posterior cingulate cortex/precuneus, and visual areas (Fig. 3) (Bjørnebekk et al., Reference Bjørnebekk, Walhovd, Jørstad, Due-Tønnessen, Hullstein and Fjell2017).

Fig. 3. Regions showing reduced cortical thickness in users exceeding 10 years of anabolic–androgenic steroid exposure compared with control subjects. Adapted, with permission, from Bjørnebekk et al. (Reference Bjørnebekk, Walhovd, Jørstad, Due-Tønnessen, Hullstein and Fjell2017). Note the notable regional coincidence with the areas showing the most consistent volume reduction in psychopaths illustrated in Fig. 1. The largest coincidences are present in the ventral temporal-frontal system (1) and the dorsal-medial system (2), but cortical reductions are also present in visual (3) and motor cortices (4).
Finally, the results from some analyses suggest that the described brain alterations could show distinct and opposite patterns of association with both affective disturbances and antisocial behavior. Indeed, some brain alterations are reported to be associated with more affective disturbances (Hare factor 1) and less antisocial behavior (Hare factor 2), including reductions in gray matter volume (Contreras et al., Reference Contreras-Rodríguez, Pujol, Batalla, Harrison, Soriano-Mas, Deus, López-Solà, Macià, Pera, Hernández-Ribas, Pifarré, Menchón and Cardoner2015), altered functional connectivity in large-scale cortical networks (Philippi et al., Reference Philippi, Pujara, Motzkin, Newman, Kiehl and Koenigs2015), and amygdala reactivity (Hyde e t al., Reference Hyde, Byrd, Votruba-Drzal, Hariri and Manuck2014). A recent study further indicates that the severity of antisocial behavior is associated with larger prefrontal and striatal subregion volumes and higher functional connectivity between the several areas of the prefrontal cortex (Korponay et al., Reference Korponay, Pujara, Deming, Philippi, Decety, Kosson, Kiehl and Koenigs2017a, Reference Korponay, Pujara, Deming, Philippi, Decety, Kosson, Kiehl and Koenigs2017b). Future research on this issue is therefore of great interest to ascertain whether the described alterations in brain structure and function in psychopaths necessarily predispose them to crime. Interestingly, there are behavioral data indicating that antisocial behavior, as measured in Hare factor 2, is better explained by poorer cognitive functioning (Baskin-Sommers et al., Reference Baskin-Sommers, Brazil, Ryan, Kohlenberg, Neumann and Newman2015).
Conclusions
This review has sought to provide a consolidated perspective on the contribution of brain imaging to understanding of psychopathy. The brain of psychopaths differs from the typical brain in terms of both anatomy and function. Anatomical alterations involve primarily a ventral system connecting the anterior temporal lobe to anterior and ventral frontal areas, and a dorsal system connecting the medial frontal lobe to the posterior cingulate cortex/precuneus complex and, in turn, to medial structures of the temporal lobe. Functional imaging data indicate that a significant disruption or ‘breakdown’ in the flow of emotional information processing may occur in both these two brain systems and suggest specific mechanisms via which emotion is anomalously integrated into cognition in psychopathic individuals during moral challenge. To this end, broader reviews are of interest comparing the results from different fields including, for instance, EEG, genetics, neurochemistry, and neuropsychology.
Acknowledgements
The authors thank Dr Laura Blanco-Hinojo for her valuable comments. Dr Oren Contreras-Rodríguez thanks the Instituto de Salud Carlos III for the Sara Borrell Contract (CD14/00246).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.






