INTRODUCTION
Many African savannas are gaining woody biomass despite differing management practices such as variable fire regimes and stocking rates (Eckhardt et al. Reference ECKHARDT, VAN WILGEN and BIGGS2000, Higgins et al. Reference HIGGINS, SHACKLETON and ROBINSON1999, Reference HIGGINS, BOND, FEBRUARY, BRONN, EUSTON-BROWN, ENSLIN, GOVENDER, RADEMAN, O'REGAN, POTGIETER, SCHEITER, SOWRY, TROLLOPE and TROLLOPE2007, Wigley et al. Reference WIGLEY, BOND and HOFFMAN2010). In fact woody encroachment, also referred to as woody plant invasion or the expansion of woody species into grassland and savanna, has been reported at locations worldwide in the past few decades (see Eldridge et al. Reference ELDRIDGE, BOWKER, MEASTRE, ROGER, REYNOLDS and WHITFORD2011 for a review). The causes of woody encroachment are not clear and could include local drivers such as variable land management or a global driver such as increasing atmospheric CO2 (Bond Reference BOND2008, Bond & Midgley Reference BOND and MIDGLEY2012, Bond et al. Reference BOND, MIDGLEY and WOODWARD2003, Hoffmann et al. Reference HOFFMANN, BAZZAZ, CHATTERTON, HARRISON and JACKSON2000). Regardless of the drivers, the implication of the widespread woody expansion is that large areas of grassland and savanna are in the process of ‘thickening up’ to become woodland or forest. Increasing woody encroachment influences rangeland use, biodiversity and ecosystem functioning, including hydrology and nutrient cycling at both the local and landscape scale (Archer et al. Reference ARCHER, BOUTTON, HIBBARD, Schulze, Harrison, Heimann, Holland, Lloyd, Prentice and Schimel2000, Parr et al. Reference PARR, GRAY and BOND2012). At the regional scale, changes in tree cover could have large feedbacks to the earth–atmosphere system (Archer et al. Reference ARCHER, BOUTTON, HIBBARD, Schulze, Harrison, Heimann, Holland, Lloyd, Prentice and Schimel2000, Asner et al. Reference ASNER, ELMORE, OLANDER, MARTIN and HARRIS2004, Beerling & Osborne Reference BEERLING and OSBORNE2006).
Woody encroachment often leads to increases in ecosystem carbon (C) storage (Geesing et al. Reference GEESING, FELKER and BINGHAM2000, Hibbard et al. Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001, Hudak et al. Reference HUDAK, WESSMAN and SEASTEDT2003). Jackson et al. (Reference JACKSON, CANADELL, EHLERINGER, MOONEY, SALA and SCHULZE1996) found significant variation in SOC storage in ecosystems with different dominant plant functional types (i.e. grass, shrub and tree) and explained the differences as an effect of varying plant production and decomposition rates which ultimately determine SOC storage. Subsequent work has found significant changes in SOC profiles among vegetation types caused by differences in root distribution and above- and below-ground allocation patterns (Jobbágy & Jackson Reference JOBBÁGY and JACKSON2000). Expansion of shrubs in North American rangeland in Texas has led to huge increases in SOC as a result of increased biomass below ground and decreases in turnover and decomposition (Archer et al. Reference ARCHER, BOUTTON, HIBBARD, Schulze, Harrison, Heimann, Holland, Lloyd, Prentice and Schimel2000, Boutton et al. Reference BOUTTON, ARCHER, MIDWOOD, ZITZER and BOL1998). Hibbard et al. (Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001) mention that the contribution of below-ground inputs to observed increases in soil C is not clear but that inputs from fine roots far exceeded inputs from litter in their study which investigated the invasion of Prosopis into Texan savannas.
In certain ecosystems, C and nitrogen (N) storage have been found to decrease as above-ground gains are offset by below-ground soil organic matter losses when grass roots disappear at very high woody densities (Hudak et al. Reference HUDAK, WESSMAN and SEASTEDT2003, Jackson et al. Reference JACKSON, BANNER, JOBBÁGY, POCKMAN and WALL2002). At mesic (but not semi-arid) woody-dominated sites in North America, losses of soil C stocks with encroachment were of such magnitude when compared with grass-dominated sites that the above-ground gains in plant biomass could not compensate for losses of soil C (Jackson et al. Reference JACKSON, BANNER, JOBBÁGY, POCKMAN and WALL2002). This pattern has also been observed in a southern African semi-arid savanna where soil C storage decreased at very high woody densities because the growth of understorey grass was inhibited (Hudak et al. Reference HUDAK, WESSMAN and SEASTEDT2003). The main aim of this study was to test whether soil C storage will increase with woody encroachment. If so, we would expect old established forest and encroaching thicket to contain the most C, and the more grassy savannas and grasslands to contain the least. Our second aim was to investigate which factors are important in driving changes in observed C concentrations and whether these changes are related to the percentage of grass roots (C4) to total roots (C4 and C3, hereafter % C4 roots) in the soil. If soil C concentrations are positively related to % C4 roots, then we expect that C concentrations should decrease in the oldest thicket as only a small percentage of total roots were grass roots (~10%). As previous work has not offered a mechanism to explain the correlation between low % C4 roots and low soil C concentrations, we also tested whether changes in C concentrations were related to changes in fine-root biomass, root N as well as root C : N ratios.
METHODS
Study site
This study was conducted in South Africa, in the Hluhluwe-iMfolozi Park (HiP) complex (28°00′–28°26′S; 31°43′–32°09′E, Figure 1), which consists of the Hluhluwe Game Reserve (225 km2) to the north, the iMfolozi Game Reserve (447 km2) to the south and a corridor (227 km2) joining the two (Whateley & Porter Reference WHATELEY and PORTER1983). The terrain of the park is hilly with altitudes between 60 and 450 m. Rainfall in the park increases with altitude and the higher-altitude Hluhluwe section receives from 700–1000 mm y−1 on the highest hilltops and supports a mesic savanna (Balfour & Howison Reference BALFOUR and HOWISON2001).

Figure 1. Grassland (a), savanna (b), thicket (c) and forest (d) in the Hluhluwe section of the Hluhluwe-iMfolozi Park, South Africa.
The sampling was undertaken in two steps; an extensive approach to compare C stocks and an intensive approach to explore rooting differences and their contributions to C concentrations. During the extensive sampling, we sampled soils along eight thicket-savanna boundaries, the ages of the thicket patches ranging from 30–70-y-old. We also sampled along five forest–grassland boundaries. For the purposes of investigating the effect of changes in root distribution on soil C concentration (i.e. intensive approach), sampling took place at three different sites representing a chronosequence of time since invasion; 10-y-old thicket, 40-y-old thicket and 70-y-old thicket. The soils at the study sites are derived from basalts. They vary from oxidic soils (Fey Reference FEY2010) with black topsoil overlying a red subsoil, to melanic soils (Fey Reference FEY2010) with a black structured topsoil and similar subsoil of varying depth onto weathered rock. Forest and grassland sites were always on oxidic soils. Savanna and thicket sites had more variable soils with both oxidic and melanic soils, with the latter more common on steeper sloping terrain (Table 1). The thicket sites sampled in the intensive sampling approach were situated on oxidic soils.
Table 1. Information on extensively sampled paired boundary sites from Hluhluwe-iMfolozi Park, including position and soils; F–G refers to forest–grassland boundaries and T–S to thicket–savanna boundaries. Classification of soil form follows Fey (Reference FEY2010); that of soil series follows MacVicar et al. (Reference MACVICAR, DE VILLIERS, LOXTON, VERSTER, LAMBRECHTS, MERRYWEATHER, LE ROUX, VAN ROOYEN and VON HARMSE1977). WRB = World reference base.

The vegetation is subtropical and consists predominantly of Northern Zululand Sourveld (Mucina & Rutherford Reference MUCINA and RUTHERFORD2006). The Hluhluwe-iMfolozi Park complex provided an ideal situation to conduct this study as the area is characterized by a mixture of savanna (from open grassland to open woodland) and forest (including thicket which is also referred to as dry seasonal forest in the neotropics (Pennington et al. Reference PENNINGTON, LAVIN and OLIVEIRA-FILHO2009, Ratnam et al. Reference RATNAM, BOND, FENSHAM, HOFFMAN, ARCHIBALD, LEHMANN, ANDERSON, HIGGINS and SANKARAN2011); a single-layer woodland typically with a dense non-grassy understorey). There are sharp boundaries between the grassy and forested biomes (see Figure 1 for examples of different vegetation types), which are often unrelated to the underlying soils.
The forest patches we sampled are classified as Scarp Forest (FOz 5, Mucina & Rutherford Reference MUCINA and RUTHERFORD2006) and although these may burn under unique conditions, their core areas seem to be quite stable. Mucina & Rutherford (Reference MUCINA and RUTHERFORD2006) define these forests as a tall (15–25 m), species-rich and structurally diverse, multi-layered vegetation, with well-developed canopy and understorey tree layers, but with a poorly developed herb layer. The forested sites were dominated by large canopy species such as Protorhus longifolia (Bernh.) Engl., Harpephyllum caffrum Bernh., Combretum kraussii Hochst., Celtis africana Burm.f., and a mid-storey of Englerophytum natalense (Sond.) Heine & J.H. Hemsl. and Maytenus mossambicensis (Klotzsch) Blakelock, and had little or no herbaceous understorey growth. West et al. (Reference WEST, BOND and MIDGLEY2000) used 13C isotopes to establish whether the forests in Hluhluwe are remnants of much larger forests or whether the forest invaded into what was previously grassland. Although they did not date the C in their study, it was shown that the forest used to be grassland or grass-dominated savanna sometime in the distant past (>2000 y, L. Gillson, unpubl. data). This led us to assume that the forest–grassland boundaries we sampled on were temporally stable. Our grassland sites had a low percentage of woody species present (~5% of the total area).
Extensive woody or thicket encroachment has occurred throughout the study area over the past half-century (Balfour & Midgley Reference BALFOUR and MIDGLEY2008, Skowno et al. Reference SKOWNO, MIDGLEY, BOND and BALFOUR1999, Watson Reference WATSON1995, Wigley et al. Reference WIGLEY, BOND and HOFFMAN2010). The vegetation types of these encroached areas are classified by Mucina & Rutherford (Reference MUCINA and RUTHERFORD2006) as Northern Zululand Sourveld. According to Low & Rebelo (Reference LOW and REBELO1996) thicket is a type of vegetation transitional between savanna and forest; almost impenetrable, generally not divided into strata and with variable herbaceous cover. In our study area, thicket consisted of savanna and forest species, typically with a single layer of woody plants which seldom exceeded 6–8 m in height. These thickets are often interspersed with small openings in the canopy where shade-tolerant and fire-intolerant grass species are able to persist (Parr et al. Reference PARR, GRAY and BOND2012). In our chronosequence of thickets the older thicket patches are dominated by trees that are generally shorter and smaller in diameter than the forest species, such as Euclea racemosa subsp. schimperi A.D.C.F. White, Schotia capitata Bolle, Vachellia (Acacia) robusta Burch and Ziziphus mucronata Willd., and thick herbaceous understorey with species of Acanthaceae often prominent. The newly invaded thicket patch is dominated largely by Vachellia (Acacia) karroo Hayne and Dichrostachys cinerea (L.) Wight & Arn. and has a few small individuals of species typically found in thickets present such as Euclea racemosa subsp. schimperi.
Sampling
Sampling took place during the growing season of 2009. Extensive sampling took place along eight thicket–savanna boundaries and five forest–grassland boundaries (see Figure 2 for map). We chose vegetation boundaries which occurred on homogeneous soils and landscape positions (Table 1) to ensure that differences in soil would not impact our results. A 1 × 2-m pit was dug 30 m perpendicular to the boundary at each site (13 boundaries = 26 pits). Samples were taken at set depth intervals of 0–2.5 cm, 2.5–7.5 cm, 7.5–15 cm, 15–30 cm, 30–50 cm, 50–75 cm, 75–100 cm (26 pits × 7 depths = 182 soil samples). In order to determine bulk density a known volume was removed from each layer using a core sampler. Known volumes were dried in an oven at 70 °C until constant weight. They were weighed and sieved to remove all roots and stones, the weight and volume of which were measured. The bulk density of each sample was calculated by dividing the dry mass of soil (minus roots and rocks) by the volume of soil (minus roots and rocks). For the purposes of comparisons between vegetation types, soil layers were combined into intervals 0–15 cm, 15–30 cm and 30–100 cm.

Figure 2. A map of South Africa indicating the position of Hluhluwe-iMfolozi Park with the Hluhluwe section in the inlay, and the 26 extensively sampled sites; (F–G refers to five paired forest and grassland sites and T–S to eight paired thicket and savanna sites).
In order to make rapid assessments of terrestrial carbon stocks it is necessary to develop a quick method of estimating above-ground biomass, and one way of doing this is to find a way to convert stand basal area to biomass, as stand basal area can be determined quickly and easily using a Bitterlich wedge (Šálek & Zahradník Reference ŠÁLEK and ZAHRADNÍK2008). At each site, we sampled basal area at four locations 25 m apart on three parallel transects 25 m apart (12 measurements × 26 sites = 312 basal areas). Basal area is widely used to estimate the biomass of trees, but the only allometric relationships that exist for this region are for individual trees. We followed the approach of Midgley & Seydack (Reference MIDGLEY and SEYDACK2006) in using an equation developed for neotropical forests by Baker et al. (Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, IORE, ERWIN, KILLEEN, LAURANCE, LAURANCE, LEWIS, LLOYD, MONTEAGUDO, NEILL, PATINO, PITMAN, SILVA and MARTINEZ2004) to estimate woody biomass. Baker et al. (Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, IORE, ERWIN, KILLEEN, LAURANCE, LAURANCE, LEWIS, LLOYD, MONTEAGUDO, NEILL, PATINO, PITMAN, SILVA and MARTINEZ2004) estimated above-ground biomass (AGB) of single trees using the equation taken from Chambers et al. (Reference CHAMBERS, DOS SANTOS, RIBEIRO and HIGUCHI2001):
where dbh is diameter at breast height. We then regressed the stand basal area against stand biomasses reported in Tables 1 and 5 in Baker et al. (Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, IORE, ERWIN, KILLEEN, LAURANCE, LAURANCE, LEWIS, LLOYD, MONTEAGUDO, NEILL, PATINO, PITMAN, SILVA and MARTINEZ2004) to get the equation:
 \begin{eqnarray*}
AG\!B &=& \frac{p}{{0.67}} \times (11.2 \times {\it Stand BasalArea})\nonumber\\
&& + 2.12\,{\rm (R}^{\rm 2} {\rm = 0}{\rm .96),}\end{eqnarray*}
\begin{eqnarray*}
AG\!B &=& \frac{p}{{0.67}} \times (11.2 \times {\it Stand BasalArea})\nonumber\\
&& + 2.12\,{\rm (R}^{\rm 2} {\rm = 0}{\rm .96),}\end{eqnarray*}
where ρ is the mean wood density of the stand in our study, and 0.67 is the mean wood density for the neotropical study. This standardization was done in order to remove possible inaccuracy due to differences in mean wood density in our study. However, we determined a mean wood density of 0.672 g cm−3 for our study area (H. Beckett, unpubl. data), so the standardization made little difference. This equation has not been verified for the Hluhluwe area, so it must be used with caution. A carbon : biomass ratio of 0.5 was used to estimate how much C was in the above-ground woody biomass (Martin & Thomas Reference Martin and Thomas2011, Penman et al. Reference PENMAN, GYTARSKY, HIRAISHI, KRUG, KRUGER, PIPATTI, BUENDIA, MIWA, NGARA, TANABE and WAGNER2003).
Grass biomass was estimated using a disc pasture meter (DPM) (Bransby & Tainton Reference BRANSBY and TAINTON1977). The height measurements were converted into biomass measurements using the method developed by Waldram et al. (Reference WALDRAM, BOND and STOCK2008) for the Hluhluwe-iMfolozi area. For the grass, a ratio of 0.37 was used to convert biomass to C (mean of 36.8% for 63 grass samples from Hluhluwe; C. Coetsee, unpubl. data).
Intensive sampling took place at three different sites; 10-y-old thicket, 40-y-old thicket and 70-y-old thicket. The recently invaded thicket patch was part of a burn trial and fire has been excluded from this patch for 10 y. The other thicket patches had not burned since establishment and both were approximately 8 ha in size. Previous studies have measured the distribution of soil C and tree : grass roots through the profile and we combined the methods of Hudak et al. (Reference HUDAK, WESSMAN and SEASTEDT2003), Maclaran & McPherson (Reference MACLAREN and MCPHERSON1995) and Mordelet et al. (Reference MORDELET, MENAUT and MARIOTTI1997). At each site, we sampled at seven locations 10 m apart on three parallel transects, spaced 100 m apart. In order to test whether changes in soil C concentration were related to changes in the overlying vegetation, at each sampling location we noted whether a canopy was present, the height of the lowest and highest canopy and the distance to the nearest woody plant taller than 1 m and taller than 4 m. We also noted the distance to the nearest grass tuft. As the distance to the closest grass tuft or closest tree was respectively very small or very large in the grassland sites, distances to tree and grass tufts were divided into classes. Furthermore, we collected soil samples for soil C measurements, and extracted roots which we used to measure root biomass, root C : N ratios, root N and per cent C4 roots.
Soil samples were extracted with a stainless steel soil auger (7.2 cm diameter) and four depths were sampled at each site; 0–10 cm, 10–20 cm and 20–30 cm (3 sites × 21 sampling points × 3 depths = 189 soil samples). The soil was then sieved using a 2-mm sieve, to remove the bulk of the roots. After soils were sifted, a subsample was taken for the soil C concentration analysis; thereafter soils were submerged in water and floating roots extracted using a 1-mm sieve (after the methods of Aerts et al. Reference AERTS, BAKKER and DECALUWE1992, Hibbard et al. Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001). Fine roots only included roots less than 2 mm in diameter (from both dry sieving and wet sieving) and total root biomass was taken as all the root material collected from each soil core.
Soils for soil C analysis were air-dried and sieved before transportation to the laboratory. Root samples were dried at 60 °C for 3 d and finely ground using a rotary hammer mill (3 sites × 21 sampling points × 3 depths = 189 root samples).
Laboratory analyses
δ13C values can be used to calculate the percentage of C3 (i.e. woody and forb) and C4 (i.e. grass) fine roots in the sample by using the following equation (adapted from Still et al. Reference STILL, BERRY, RIBAS-CARBO and HELLIKER2003):
where % Cgrass is the per cent C4 contribution, δ13Ctree is the carbon isotopic composition of C3 vegetation, δ13Cgrass is the carbon isotopic composition of C4 vegetation, and δ13Cmeasured is the isotopic composition of the measured sample. The root samples prepared as described above were combusted in a Carlo-Erba system (Carlo Erba NCS 2500 Elemental Analyser, Carlo Erba Instruments, Milan, Italy), analysed on a GC-IRMS (Finnigan MAT 252 IRMS, Finnigan, Bremen, Germany) and results were reported relative to the internationally accepted carbonate isotope standard PDB (Chicago Pee Dee Belemnite). Carbon 13C/12C ratios are calculated relative to this standard from the equation:
where Rsample and RStd are the 13C/12C ratios of the sample and the standard, respectively. Replicate samples were reproducible to 0.25‰. Root C concentrations (intensive sampling) and carbon for total ecosystem C (grass, wood and soils – extensive sampling) were measured with the same system as 13C.
Soils sampled during the intensive sampling were analysed for total organic C concentration at the Institute for Plant Production, Elsenburg, Stellenbosch, using the Walkley–Black method (Walkley Reference WALKLEY1947). The coefficient of variation for repeated samples was 0.07. For the extensive sampling, bulk density data (Table 2) were used to convert C concentration to C content.
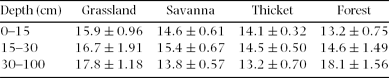
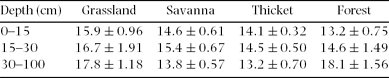
Table 2. Soil bulk density (mean ± SE, g cm−3) for four vegetation types at three depths in the Hluhluwe section of the Hluhluwe-iMfolozi Park.

Statistical analyses
The statistical package R was used throughout for all statistical analyses (R Development Core Team, http://www.R-project.org). To investigate whether there was a trend in how soil organic C (SOC) and above-ground carbon (AGC) responded with an increase in trees, we used a linear regression model to predict differences in soil C and AGC between grassy vegetation and the adjacent woody vegetation at different depths (0–15 cm, 15–30 cm, 30–100 cm). Forest–grassland boundaries were compared separately to thicket–savanna boundaries. All boundaries were on homogeneous soils so differences in C amounts could be assumed to be largely due to differences in vegetation type. To investigate how root quality and quantity changed with woody encroachment, we used a two-way ANOVA to predict root C : N ratios and fine-root mass with site, depth and the pairwise interaction. Values were log-transformed before analyses. We used Tukey HSD to test for differences between levels of each factor. The percentage of C4 grass was compared amongst sites by using the non-parametric, Kruskal–Wallis test.
We used a generalized least squares model in the R package nlme to test which factors were important in estimating the response variable, soil C concentration. We only included values to 30 cm as no root data were collected at deeper depths. The original model included all 12 explanatory variables; site, soil depth, distance to the closest tree over 4 m, distance to the closest shrub over 1 m, presence/absence of a tree canopy, distance to the closest grass tuft, root N, root C, root C : N ratios, per cent C4 roots, total root weight and fine-root mass. A generalized least-squares model was used as the linear model output revealed that assumptions of normality, and non-constant variance were not met. The results of a VIF test (R package MuMIn) revealed no colinearity in the data and the generalized least squares model assumption of non-autocorrelation was met. We used the dredge function in the R package MuMIn to validate output of the generalized least-squares model.
RESULTS
We investigated mean differences between paired woody and grassy sites by using linear regression. Forests contained 12.1 ± 0.73 kg m−2 more AGC than their adjacent grasslands (Figure 3a, t = 16.6, P < 0.0001). Thickets contained significantly more (Figure 3a, t = 6.10, P < 0.0001) AGC (3.33 ± 0.59 kg m−2) than the adjacent savannas, although the difference was not as pronounced as between forests and grasslands.

Figure 3. Differences (mean ± SE, kg m−2) in above-ground and below-ground carbon (SOC) between paired forest–grassland and thicket–savanna plots in the Hluhluwe section of the Hluhluwe-iMfolozi Park.
In the top 15 cm of soil, forests contained 1.32 ± 0.86 kg m−2 more soil C than grasslands, this difference was not significantly different from zero (Figure 3b, t = 1.53, P = 0.15). Thickets contained 1.96 ± 0.68 kg m−2 more soil C than savannas in the top 15 cm of soil, significantly different from zero (Figure 3b, t = 2.87, P = 0.02). Forests contained 0.87 ± 0.21 kg m−2 more soil C than adjacent grasslands in the 15–30 cm layer, which was found to be significantly different from zero (Figure 3c, t = 4.00, P = 0.002). Thickets contained only 0.01 ± 0.17 kg m−2 more soil C than adjacent savannas in the 15–30 cm layer, which was not significantly different (Figure 3c, t = 0.05, P = 0.96). Forests contained 2.35 ± 1.00 kg m−2 more soil C than adjacent grasslands at a depth of 30–100 cm, which was found to be significantly different from zero (Figure 3d, t = 2.34, P = 0.04). On the other hand, thickets contained only 0.52 ± 0.79 kg m−2 more SOC than adjacent savannas at a depth of 30–100 cm, which was not significantly different from zero (Figure 3d, t = 0.66, P = 0.18).
When we investigated differences in the entire soil profile (0–100 cm), we found that forests contained 4.54 ± 1.70 kg m−2 more soil C than grasslands, which was significantly higher than a difference of zero (t = 2.67, P = 0.02). Thickets contained 2.50 ± 1.34 kg m−2 more soil C than savannas in the top 100 cm of soil, which was not significantly different from a difference of zero (t = 1.86, P = 0.09). Soil C: N ratios did not vary in a predictable manner; for instance, forest had the lowest C: N ratios in the surface soil, but the highest C: N ratios at 30–100 cm (Table 3).
Table 3. Soils C : N ratios (means ± SE) for four vegetation types at three depths in the Hluhluwe section of the Hluhluwe-iMfolozi Park.
To determine whether woody encroachment and the associated changes in woody cover have led to changes in the quality and quantity of roots, we measured root C: N ratios, per cent C4 roots and fine-root biomass. The output of the ANOVA revealed that root C: N ratios were similar in the 10-y-old thicket (C: N 33.6) and the 40-y-old thicket (C: N 31.7), but lower in the 70-y-old thicket (C : N 25.7; F2,180 = 19.4, P < 0.0001). In general, root C : N ratios increased with depth, root C : N ratios in the top 10 cm were significantly lower than the C : N ratios found at 20- and 30-cm depths (26.9 vs. 31.1 and 33.0, F2,180 = 10.8, P < 0.0001, Figure 4a). Of the fine-root biomass, 55.2% belonged to C4 grass in the 10-y-old thicket, 22.4% in the 40-y-old thicket and 11.8% in the 70-y-old thicket (Figure 4b). Interactions between site and depth were not significant in the ANOVA for root C : N ratios (P = 0.91) and fine-root biomass (P = 0.32). The older thicket patches had the highest and similar fine-root biomass (10.3 ± 0.39 kg m−2 and 10.9 ± 0.56 kg m−2 for the 40-y-old and 70-y-old thickets), while the recently invaded thicket patch had lower fine-root biomass (3.66 ± 0.12 kg m−2; F2,180 = 64.2, P < 0.0001, Figure 5). An interesting pattern that can be seen in Figure 5 is that fine-root biomass is concentrated in the shallower soil depths in recently invaded thicket patches but increases with depth in the older thicket patches.

Figure 4. Root C : N ratios and % C4 roots (as a percentage of total roots; both grass and trees, mean ± SE) for different levels of woody encroachment in the Hluhluwe section of the Hluhluwe-iMfolozi Park, South Africa; 10-y-old thicket, 40-y-old thicket, and 70-y-old thicket, at different depths (0–10 cm, 10–20 cm, 20–30 cm, as well as averaged over depth). Root C : N ratios (a), C4 grass (%; b). Different lowercase letters indicate significant differences at P < 0.05.

Figure 5. Fine-root biomass (mean ± SE, g m−2, n = 189) for different levels of woody encroachment in the Hluhluwe section of the Hluhluwe-iMfolozi Park, South Africa; 10-y-old thicket, 40-y-old thicket and 70-y-old thicket, at different depths (0–10 cm, 10–20 cm, 20–30 cm). Different lowercase letters indicate significant differences at P < 0.05.
Our analyses indicated that three explanatory variables explained 70% of the variation within soil C concentration (Table 4); these included sites, soil depth and the mass of fine roots. The amount of C4 or grass roots had no effect on soil C concentrations.
Table 4. The output of a generalized least squares model is shown with significance levels. Three explanatory variables (site, depth and fine-root biomass, g m−2) explained 70% of the variation in soil C concentration for thickets in the Hluhluwe section of the Hluhluwe-iMfolozi Park, significance levels indicated by ***P < 0.001, **P < 0.01.

DISCUSSION
Changes in land use have variable effects on soil C stocks; this is reflected in the findings of two meta-analyses on soil C stocks and land-use change. Guo & Gifford (Reference GUO and GIFFORD2002) showed that, across 16 countries, soil C stocks increased when native forest was converted to pasture. A more recent meta-analysis found the opposite, that in the tropics and subtropics, forest conversion to grassland leads to an average loss in soil C stocks of 12% (Don et al. Reference DON, SCHUMACHER and FREIBAUER2011). At the same time, Eldridge et al. (Reference ELDRIDGE, BOWKER, MEASTRE, ROGER, REYNOLDS and WHITFORD2011) have shown in a global synthesis on shrub encroachment that total soil C increased with woody encroachment in the top 15 cm of soil. This is in contrast to our results which suggest that in the top 15 cm of soil the differences in soil C between forests and grasslands, and thickets and savannas, are not great. Total soil C storage in our study increased with woody encroachment into areas previously dominated by grass, but this was due almost entirely to increases in AGC. Besides the top 15 cm of soil, forests contained consistently more C than grasslands, and differences in soil C were significant. In contrast, although on average thickets contained slightly more C than adjacent savannas in all components, these differences were only significant in the AGC.
The second aim of the study was to examine the relationship between C concentrations and measured variables. Previous work has shown that although soil C increases with woody encroachment, decreases may occur at wetter sites and on specific soils when vegetation reaches the closed-canopy stage (Hudak et al. Reference HUDAK, WESSMAN and SEASTEDT2003, Jackson et al. Reference JACKSON, BANNER, JOBBÁGY, POCKMAN and WALL2002). Hudak et al. (Reference HUDAK, WESSMAN and SEASTEDT2003) suggested the loss of grass roots as a possible mechanism of declining soil C concentrations at sites where a closed canopy prohibited the growth of grass. Our results, however, showed that soil C was not affected by the per cent C4 roots across sites and high C concentrations were found with both medium and low per cent C4 roots.
Which factors were then affected by changes in woodiness and how did these relate to soil C concentrations? Woody plant encroachment of grassland often increases the sequestration and cycling of ecosystem C (Geesing et al. Reference GEESING, FELKER and BINGHAM2000, Hibbard et al. Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001, McCulley et al. Reference Mcculley, ARCHER, BOUTTON, HONS and ZUBERER2004). Cebrián & Duarte (Reference CEBRIÁN and DUARTE1995) argue that rates of plant turnover affect decomposition and are important in governing soil C storage. Ecosystems dominated by slow-growing plants accumulate large, slowly decomposing detrital pools which act as C sinks. Previous work has shown that woody AGC inputs and low decomposability in forests may increase C storage (Austin & Vitousek Reference Austin and Vitousek1998, Melillo et al. Reference MELILLO, ABER and MURATORE1982). Similarly, Bird & Pousai (Reference BIRD and POUSAI1997) and Kellman (Reference KELLMAN1979) have shown that higher soil C concentrations in tree-dominated areas may be the result of higher C inputs per area and the longer residence time of woody material derived from trees. We have used root C : N ratios as an indicator of root quality as C : N ratios often govern the rate of litter decomposition and higher C : N ratios often (but not always) mean slower decomposition of litter and as a result higher soil organic C (Enríquez et al. Reference ENRÍQUEZ, DUARTE and SAND-JENSEN1993, Parton et al. Reference PARTON, SILVER, BURKE, GRASSENS, HARMON, CURRIE, KING, ADAIR, BRANDT, HART and FASTH2007). The 70-y-old thicket had the highest root quality (i.e. lowest root C : N ratios) and was also the site with the highest soil C concentrations. This was an unexpected result, as we expected this site with low C : N ratios to have faster decomposition rates and as a result lower C concentrations. Our extensive soil sampling also showed that soil C : N ratios in thickets were generally lower than either grassland or forest (Table 3) and no apparent correlation between soil C : N ratios and C storage.
Apart from altered decomposition rates of above-ground or below-ground inputs, increases of SOC concentrations and storage may be a result of enhanced production below ground (Schlesinger Reference SCHLESINGER1977). Our results showed that fine-root biomass was an important predictor of total soil C concentrations. Increases in fine-root biomass were especially noticeable in the deeper (20–30 cm) soil layers when newly invaded grassland turned to established thicket. Jobbágy & Jackson (Reference JOBBÁGY and JACKSON2000), in a meta-analysis of global root distribution, showed that differing patterns of allocation by vegetation determined vertical distributions of soil C. Other studies have found increased C and N storage with woody encroachment and several mechanisms come into place with higher densities of trees; these include the tree acting as an atmospheric dust trap (Bernhard-Reversat Reference BERNHARD-REVERSAT1988, Escudero et al. Reference ESCUDARO, GARCIA, GOMEZ and LUIS1985), and trees and shrubs affecting nutrient cycling by changing soil structure, microbial biomass, soil moisture, microclimate and by changing N fixation (Hibbard et al. Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001, Hudak et al. Reference HUDAK, WESSMAN and SEASTEDT2003, Jacobs et al. Reference JACOBS, BECHTOLD, BIGGS, GRIMM, LORENTZ, MCCLAIN, NAIMAN, PERAKIS, PINAY and SCHOLES2007, Schlesinger et al. Reference SCHLESINGER, REYNOLDS, CUNNINGHAM, HUNNENNEKE, JARRELL, VIRGINIA and WHITFORD1990). As we did not measure root turnover, it is difficult to conclude whether root biomass alone or together with changes in root productivity affects soil C concentration. Hibbard et al. (Reference HIBBARD, ARCHER, SCHIMEL and VALENTINE2001) found that higher root biomass together with faster root turnover under woody canopies compared with grassy patches in a North American savanna accounted for higher soil C storage in invaded areas. Our results support this and other work that shows that root biomass is important in governing soil C storage (Jackson et al. Reference JACKSON, CANADELL, EHLERINGER, MOONEY, SALA and SCHULZE1996, Reference JACKSON, MOONEY and SCHULZE1997; Jobbágy & Jackson Reference JOBBÁGY and JACKSON2000).
Our findings showed that it takes a very long period of time before C stocks increase over the entire soil profile in thicket-invaded areas. The magnitude of gains in C stored above ground was larger than that gained below ground; e.g. 4.94 kg m−2 and 0.46 kg m−2 contained in thicket and grassland respectively above ground, while below ground thicket and grassland contained similar amounts of C (9.26 kg m−2 and 8.30 kg m−2 in the top 15 cm respectively). We suggest that one possible mechanism that explains the low gains in below-ground C is linked with the fact that fine-root biomass appears to be an important driver of soil C in this savanna. Initially, fine-root biomass increases rapidly with woody encroachment, but as there is little difference between fine-root biomass in the 40-y-old and 70-y-old thicket, so it appears to reach some asymptote. In other words, instead of below-ground C increasing linearly over time since woody encroachment, the increases in soil C seems to slow down within 40 y of the initial woody invasion. However, soil C does increase in mature scarp forest. We suggest that a different mechanism is important in driving soil C in these forests. Fine-root biomass may not be an important predictor of soil C, but high litter inputs of low quality and slow turnover may increase soil C storage.
In conclusion, fire abatement is often advocated in the literature as a way in which to increase C storage in savannas as fire removal leads to increases in C storage (Grace et al. Reference GRACE, SAN JOSÉ, MEIR, MIRANDA and MONTES2006). According to the study by Wigley et al. (Reference WIGLEY, BOND and HOFFMAN2010), 44% of the study area in HiP that was dominated by grassy systems 70 y ago is now dominated by woody vegetation. Thickets support an entirely different and less divergent set of flora and fauna, and nearly half of the plant diversity would be lost with the conversion of savanna to thicket in HiP (Bond & Parr Reference BOND and PARR2010, Parr et al. Reference PARR, GRAY and BOND2012). Furthermore, woody encroachment alters ecosystem functioning by influencing nutrient cycling and hydrology (Asner et al. Reference ASNER, ELMORE, OLANDER, MARTIN and HARRIS2004, Bond Reference BOND2008). Woody plant encroachment may also negatively impact game-viewing experiences in the park, thereby reducing overall visitor numbers in the park (E. Gray, unpubl. data). It is also likely to decrease livestock productivity outside park boundaries (Burrows et al. Reference BURROWS, CARTER, SCANLAN and ANDERSON1990, Mugasi et al. Reference MUGASI, SABIITI and TAYEBWA2000). Gains in C should therefore be weighed up against changes in ecosystem function, losses in biodiversity and economic implications before management decisions regarding fire are made in savannas.
ACKNOWLEDGEMENTS
The authors extend their thanks to: Ezemvelo KZN Wildlife for allowing us to work in HiP; staff and students of the Zululand Tree Project for assistance in the field, staff of the Archaeometry laboratory at the Archaeology Department, UCT. This study was funded by the Andrew Mellon Foundation.