Introduction
Diethylstilbestrol (DES), a potent synthetic estrogen, was extensively prescribed worldwide to pregnant women from the 1940s to the 1970s, with the mistaken belief that it could prevent miscarriages and other pregnancy complications.Reference Giusti, Iwamoto and Hatch 1 It was initially given to at-risk pregnancies, but ultimately also prescribed for normal pregnancies to make them ‘healthier’. Estimates ranging upward to 10 million people (mothers, daughters and sons) were exposed to DES in the United States. Subsequently, DES was found therapeutically ineffective in reducing miscarriages, and it was linked to a rare form of cancer termed ‘vaginal adenocarcinoma’ in a small number (<0.1%) of adolescent daughters of women who took the drug while pregnant.Reference Herbst, Ulfelder and Poskanzer 2 Although DES was originally identified as a carcinogen based on its effects in the vagina, the extent of its toxicity was later found to be more extensive as it was associated with numerous other medical consequences. DES was later linked with frequent noncancerous (benign) reproductive problems in approximately 90–95% of DES-exposed daughters: reproductive tract malformations and dysfunction, poor pregnancy outcome, ectopic pregnancies and premature labor and births were reported (for review see Giusti et al. Reference Giusti, Iwamoto and Hatch 1 ). Furthermore, as DES-exposed daughters aged, they were at a higher risk for developing breast cancer than their unexposed, age-matched counterparts. DES-exposed daughters > 40 years exhibited a statistically significant increase (2–2.5×) in risk for developing breast cancer;Reference Hatch, Palmer, Titus-Ernstoff, Noller, Kaufman and Mittendorf 3 – Reference Troisi, Potischman and Hoover 6 this increased risk was more pronounced (3×) in DES-exposed women over 50 years of age, although the sample size was small.Reference Palmer 5 DES-exposed mothers were also found to be at increased risk for breast cancer.Reference Giusti, Iwamoto and Hatch 1 Prenatally DES-exposed sons experienced a range of reproductive tract problems including malformations (hypospadias, microphallus and retained testes) and increased genital/urinary inflammation. 7 – 9
Although reports in the experimental animal literature dating back four decades described the carcinogenic effects of DES, it was not until its effects in humans were reported that its adverse consequences were considered seriously. On the basis of a wealth of accumulated scientific information from humans and experimental animals, DES is now a well-documented ‘transplacental carcinogen’: it crosses the placenta, reaches the fetus, adversely affects developing tissues/organs, and causes a myriad of problems including breast and reproductive tract cancer. 7 – Reference Diamanti-Kandarakis 10 DES caused a major medical catastrophe that continues unfolding today.
Although DES was banned years ago for use during pregnancy, experimental studies continue to explore mechanism(s) through which DES causes its adverse effects. Questions such as how does DES cause abnormalities (ranging from structural malformations to cellular and molecular defects) in breast and reproductive tissues have led to the development of experimental animal models to study the impact of estrogens and other endocrine-disrupting chemicals (EDCs) on differentiating tissues. As the murine model using prenatally DES-exposed outbred mice has successfully duplicated and predicted abnormalities reported in similarly exposed humans (for review see NewboldReference Newbold 11 , Reference Newbold 12 ), this review compares critical windows in breast and reproductive tract differentiation in mice and humans that can be perturbed by DES. For brevity, the focus is on females (Table 1), although DES-induced abnormalities in males are equally important. Investigating DES toxicities has great potential for advancing our scientific knowledge about this chemical and other less-potent environmental estrogens, as well as providing caution in using any drug during pregnancy. Further, studying the vast array of DES health effects will contribute to a better understanding of the role of estrogens in normal and abnormal developmental processes, hormonal imprinting and carcinogenesis.
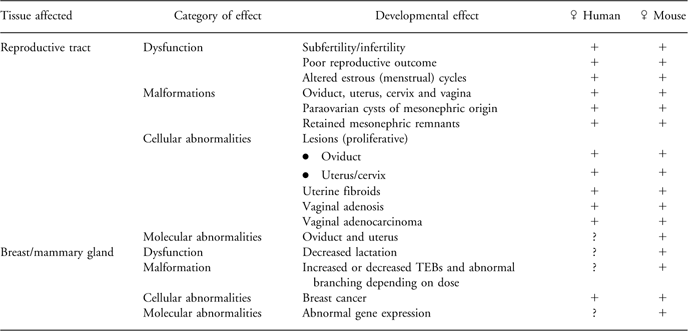
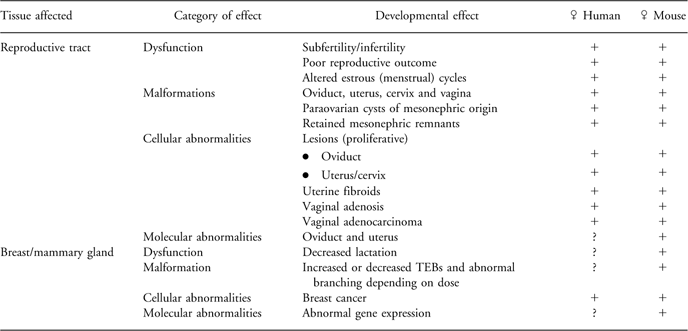
Table 1 Comparative developmental effects of exposure to DES in humans and mice demonstrating the DOHaD phenomena.
DES, diethylstilbestrol; DOHaD, developmental origins of health and disease; TEBs, terminal end buds.
DES exposure provides solid evidence, and indeed proof of principle, that prenatal chemical exposure can have latent effects and lead to diseases later in life in animals and humans. The developmental origins of health and disease (DOHaD) field of scientific investigation points out the vulnerability of the developing fetus to perturbation by DES and other chemicals with estrogenic activity.
The DOHaD concept for environmental chemicals
Although the placenta was once believed to provide complete protection to the unborn child, it is now well established that it only provides partial protection from the external environment, as indicated by the large literature based on the DOHaD paradigm focusing on diet and nutrition, as well as environmental chemicals. Many drugs and chemicals freely pass through the once called ‘placental barrier’. As fetal and neonatal developments are characterized by rapid cell division and differentiation, coupled with complex patterns of cell signaling, exposure to chemicals during these active periods can readily disrupt differentiation and result in abnormal cell proliferation and/or differentiation at inappropriate times. These abnormal cells can then give rise to additional generations of altered cells. Often, damage that occurs before birth to actively dividing cells is irreversible (permanent) and likely due to epigenetic programming, whereas similar adult exposures can be reversible, disappearing when the chemical insult is removed. As development is controlled by a myriad of endocrine signals, chemicals with hormonal activity can easily interrupt or alter these signals resulting in changes that are not apparent until much later in life.
The developing organism lacks many of the protective mechanisms that are available to the adult (detoxifying enzymes, a fully competent immune system, DNA repair mechanisms, mature liver metabolism and blood/brain barrier).Reference Bern 13 In addition, the fetus and neonate have higher metabolic rates as compared with adults, which can cause increased sensitivity to chemicals. Furthermore, prenatal development is an important time of chemical susceptibility as this is when epigenetic marks (programming) are set. Epigenetic events occurring during embryogenesis and development involve heritable changes in gene function and regulation that happen independently from the DNA sequence. Thus, altered epigenetic programming caused by exposure to DES or other EDCs can persist throughout the life of the organism and manifest as latent disease including cancer.Reference Newbold and Kinyamu 14
In the fields of nutritional and endocrine disruption, developmental exposure to environmental stressors, drugs and chemicals has been extensively studied and well recognized to interfere with complex differentiating endocrine signaling pathways resulting in adverse consequences later in life.Reference Gluckman, Hanson and Pinal 15 – Reference Colborn, Dumanoski and Myers 17 Prenatal DES exposure is the best example of DOHaD involving detrimental effects of chemical exposure during critical windows of differentiation and latent occurrence of disease.
An overriding concept in DOHaD suggests a lag time between time of exposure to environmental chemicals and disease manifestation, but there are other key aspects:
-
(1) Time-specific, tissue-specific and dose-specific effects can occur. Chemical exposure may have an entirely different effect on developing organisms as compared with adults. Further, time-specific effects are due to the stage of differentiation of the target tissues when exposure occurred. For example, Hatch et al.Reference Hatch, Herbst, Hoover, Noller, Adam and Kaufman 18 reported that DES-exposed women are at greater risk of developing high-grade squamous cancer in the reproductive tract if exposure occurs early in differentiation within 7 weeks of gestation rather than later. Regarding tissue-specific effects, DES mainly affects estrogen target tissues such as breast and the reproductive tract. Finally, dose may determine the effect with low doses of DES causing functional changes and tumorigenesis, whereas high doses are teratogenic.
-
(2) An environmental chemical such as DES can act alone or with other environmental stressors. Exposure to other environmental stressors could trigger or intensify disease.
-
(3) The abnormality can manifest in various ways as the occurrence of a disease that otherwise would not have happened; an increase in risk for a disease that would normally be of lower prevalence; or perhaps an earlier onset of a disease that would normally have occurred; or an exacerbation of the disease. For example, prenatal DES exposure causes vaginal cancer, a disease that otherwise would not have occurred in a young women; increased breast cancer is an example of disease that would normally be of lower prevalence.
-
(4) The abnormality can have variable latency from onset in development to early childhood, puberty or adulthood depending on the time of exposure and stage of differentiation of the affected tissue or organ.
-
(5) Prenatal exposure to an environmental insult can lead to permanently altered developmental programming and aberrant function of a cell, organ or system, resulting in an individual who is more susceptible to certain diseases later in life. This altered imprinting likely involves epigenetic mechanisms, which are active during differentiation and development, and are known to be influenced by estrogens. With epigenetic alterations, genes are not mutated but changes occur in how the gene is regulated. The effects of developmental exposures persist because altered epigenetic signaling persists as cells divide throughout life. Thus, early exposure to chemicals can alter epigenetic marks that lead to functional changes in genes, which in turn lead to abnormal tissues that lead to diseases including cancer later in life.
-
(6) Prenatal chemical exposure can cause long-lasting adverse effects that may include not only functional, cellular and molecular abnormalities but also structural (malformation) abnormalities (see Table 1 for an example). These different types of effects are probably because of the differences in actual timing and dose of DES exposures with lower doses causing functional changes and higher doses causing structural malformations. These abnormalities may persist and lead to increased diseases such as cancer later in life. Development of new sensitive molecular markers of developmental exposures will contribute useful predictive and preventive information for adult human health.Reference Newbold and Kinyamu 14 , Reference Li 19 – Reference Tang 22
-
(7) Extrapolation of risk from environmental exposures can be difficult because abnormalities do not always follow a monotonic dose–response relationship; low-dose effects may be different from those occurring at higher doses because different mechanisms are involved.Reference Newbold 23 For example, low doses of DES cause increased weight gain, whereas higher doses do not, and may actually cause weight loss. Low-dose effects are of concern because exposure of the general public occurs in this dose range, whereas occupational and pharmaceutical exposures usually occur at higher doses.Reference Diamanti-Kandarakis 10 , Reference Melnick, Lucier, Wolfe, Hall, Stancel and Prins 24 , Reference vom Saal, Akingbemi, Belcher, Birnbaum, Crain and Eriksen 25
-
(8) Individual organisms may have different effects to chemicals because of differences in genetic backgrounds. However, identical genotypical twins may have different effects because of different epigenetic programming.
Together, these are important components of DOHaD and apply to DES and other environmental chemicals. These aspects have recently been reviewed by Heindel and Newbold.Reference Heindel and Newbold 26 To further examine the DOHaD concept and to understand the unique sensitivity of developing tissues to perturbation by chemicals, sensitive windows of breast and reproductive development in humans and mice are described.
Sensitive windows in perinatal development
Mammalian development involves a complex and well-organized series of events to grow from a single cell to a fully developed organism at birth. Processes including cell division, proliferation, differentiation and migration are involved and are closely regulated by hormones that communicate information between specialized cells, tissues and organs. For over 50 years, embryonic and fetal development was assumed to occur by ‘the unfolding of a rigid genetic program’ where the environmental factors played no significant function (for review see Soto and SonnenscheinReference Soto and Sonnenschein 27 ). However, this narrow interpretation of developmental events has evolved because experimental and epidemiological studies reveal the developmental plasticity of the fetus and neonate. In fact, it has become increasingly apparent that environmental factors and stressors including nutrition and toxic chemicals can dramatically alter developmental signals.
Many biological processes involved in mammalian growth and differentiation are conserved across species including differentiation of breast and reproductive tissues. Although developmental processes are similar in humans and mice, exact timing of events varies with many occurring entirely prenatally in humans, but prenatally and neonatally in mice. The website http://www.endocrinedisruption.com/prenatal.criticalwindows.overview.php compare the timing of developmental events in humans and mice. Extrapolations from experimental animals to humans are most successful when similar periods of differentiation are compared rather than same chronological age,Reference Hogan, Newbold and McLachlan 28 and when human exposures are well documented including dose and timing of exposure.
DES and breast/mammary gland development
Critical events in breast development start early in fetal life, continue into postnatal life and are then followed by exponential epithelial outgrowth during puberty; the breast becomes completely competent later in life when it transitions into its lactational role in pregnancy. These well-defined developmental stages occur in humans and mice and are regulated by endogenous hormones, mainly estrogen and progesterone. These hormones mediate their effects by interacting with their receptors (for estrogens, the interaction is with its estrogen receptors (ERs) alpha and beta (α and β) in breast tissue). Excessive estrogen exposure during fetal life is a known risk factor for breast cancer; for example, a positive correlation exists between twin female births (which have increased intrauterine levels of estrogens) and breast cancer later in life of the daughters from these pregnancies.Reference Ekbom, Trichopoulos, Adami, Hsieh and Lan 29 ERs can also interact with exogenous estrogens produced outside the body such as DES, which mimic naturally occurring estrogens, bind to their ERs and interact with developing breast tissue.
Precise mechanisms by which prenatal DES exposure causes breast cancer are unclear; however, the carcinogenic process is complex and multistep, and it likely originates in the terminal duct lobular units (TDLUs) in humans and the counterpart terminal end buds (TEBs) in mice. Support for these as carcinogenic sites comes from the 7,12-dimethylben[a]athracene (DMBA)-induced rodent mammary carcinoma model that is well established and appropriate for studying human breast cancer.Reference Russo 30 TEBs have very high proliferation rates and remove DMBA adducts less efficiently than the more-differentiated epithelial cells in the mammary gland. Further, the presence of TEBs at the time of carcinogen exposure is positively associated with tumor multiplicity and severity.Reference Russo, Gusterson, Rogers, Russo, Wellings and van Zwieten 31
Humans
In the first trimester, as early as 4–5 weeks of prenatal life, breast development appears as a thickening of the milk lines on the ventral surface of the fetus. Epithelial budding and branching starts around 6–20 weeks of gestation; breast buds extend, producing cords of epithelium that grow through the underlying mesenchyme (undifferentiated stroma). The mesenchyme develops supporting stroma between weeks 20 and 32. Epithelial cords become hollow and develop a lumen during the last 2 months of gestation; at this time, ductal and lobuloalveolar branching occurs yielding a primitive gland at birth composed of ducts ending in TDLUs. Near birth, the nipple forms by invagination of the breast surface.Reference Rosso, Hu, Yang and Russo 32
As DES use during pregnancy often started before 9 weeks of gestation and continued throughout pregnancy, it is clear that the primitive breast structure was present at the time of DES exposure. Furthermore, differentiating breast tissue has the necessary cellular machinery including ERs to respond to DES; ERs were localized in normal mammary epithelial cells as early as gestational week 30 and progesterone receptors by week 41.Reference Friedrichs, Steiner, Buettner and Knoepfle 33 Epidemiological studies following prenatally DES-exposed women verify that DES acts as a latent breast carcinogen,Reference Palmer 5 thus providing proof of the DOHaD phenomena.
Mice
Developmental events in the rodent mammary gland are similar to the human breast. Mammary gland development begins approximately on gestational day (d.p.c.) 10.5; by 11.5 d.p.c., five placodes appear along each presumptive mammary line as lens-shaped ectodermal structures that invaginate into the dermis by 13.5 d.p.c. The mesenchyme adjacent to the mammary epithelium becomes denser than the surrounding mesenchyme and develops several concentric layers of fibroblasts, which align around the epithelial compartment.Reference Robinson, Karpf and Kratochwil 34 At 15.5 d.p.c., epithelial buds start to elongate into cords, and on 16 d.p.c. the primary cord undergoes an increase in proliferation as it pushes through the surrounding mammary mesenchyme and grows through the fetal fat pad. By 18 d.p.c., branching of the structure is readily apparent and a lumen starts to form, which differentiates into a mammary duct.Reference Vandenberg, Maffini, Wadia, Sonnenschein, Rubin and Soto 35
ER is first expressed in murine mammary tissue around 12.5 d.p.c. in the mesenchyme surrounding the bud.Reference Lemmen, Broekhof, Kuiper, Gustafsson, van der Saag and van der Burg 36 Autoradiographic studies show specific binding of labeled DES in the mesenchyme surrounding the epithelial anlagen at 16 d.p.c., suggesting functional receptors at this age and the ability of DES to bind to fetal mammary gland tissue.Reference Narbaitz, Stumpf and Sar 37 By 18 d.p.c., just before birth, ERs are found predominately in the stroma and some mammary epithelial cells.Reference Saji, Jensen, Nilsson, Rylander, Warner and Gustafsson 38 In neonatal life, ER-α is mainly expressed in mammary epithelium. After birth, the mammary gland grows with body growth until puberty, approximately 4 weeks when estrogen levels significantly rise. In response to these elevated estrogen levels at puberty, as in the human, extensive mammary gland growth occurs. At the end of the branched mammary ducts, bulbous epithelial TEBs develop, which have high mitotic (proliferative) rates and apoptotic (programmed cell death) activity. The cap cells of the TEBs rapidly divide, permitting ductal elongation and the ability of the duct to change direction in the fat pad, whereas apoptosis is responsible for formation of the ductal lumen and extension of the growing duct.Reference Richert, Schwertfeger, Ryder and Anderson 39 The branching mammary gland structure invades the stroma until it reaches the edge of the fat pad, establishing a network of ducts and a few alveolar buds.Reference Richert, Schwertfeger, Ryder and Anderson 39 This morphology remains quiescent until pregnancy, although fluctuations do occur during different stages of the estrous cycle for mice (v. menstrual cycle for humans). In response to pregnancy, the mammary gland undergoes dramatic proliferation resulting in a massive number of alveolar buds to prepare for lactation. Once lactation is completed, the mammary gland undergoes rapid involution associated with widespread apoptosis and stromal remodeling so that it can return to its pre-pregnancy state. Similar events occur in human breast tissue in response to pregnancy.
Experimental studies with rodents support the carcinogenic effects of DES in the human breast. Fetal DES exposure increases mammary tumorigenesis and tumor multiplicity.Reference Rustia and Shubik 40 – Reference Nagasawa, Mori and Nakajima 48 In addition, neonatal DES exposure of rodents (a time that corresponds to prenatal development in humans) also increases the incidence of cancer in experimental mammary tumor modelsReference Bern, Jones, Mori and Young 49 – Reference Ninomiya, Kawaguchi, Souda, Taguchi, Funato and Umekita 54 (for review see Fenton and NewboldReference Fenton, Reed and Newbold 72 ). Recently, altered gene expression was demonstrated in rat TEBs exposed neonatally to DES, thus showing that differentiation- and development-related genes are involved in altered TEB structural and cellular abnormalities in DES-exposed mammary tissue and its increased propensity to develop cancer.Reference Umekita, Souda, Hatanaka, Hamada, Yoshioka and Kawaguchi 55
Similarities/differences
Developmental milestones in human breast and mouse mammary gland development are similar, although timing of events differs slightly. Ductal branching originates in prenatal life for both species, but minor differences in the extent of ductal branching of the mammary gland occur at birth between species, and alveolar buds are prominent in human infants but not mice. The growing mammary ducts end in TEBs in mice v. TDLUs in humans; these are morphologically analogous structures, which contain actively proliferating cells, and potentially stem cells, accounting for their sensitivity to carcinogenic agents. Most importantly, breast and mammary tissue at all stages of differentiation and development have the ability to respond to endogenous estrogens produced in the body, as well as exogenous estrogens produced outside the body (such as DES).
DES and reproductive tract development
Critical events in mammalian reproductive tract differentiation start in prenatal life similar to mammary gland differentiation. Early in embryonic development, there is an undifferentiated stage (sometimes referred to as the indifferent period) in which the sex of the embryo cannot be determined. At this stage, the gonads have not developed into either testis or ovary and all embryos have a double set of genital ducts, Mullerian (paramesonephric) and Wolffian (mesonephric) ducts. In the female, as sex differentiation proceeds, the Mullerian duct differentiates into oviduct, uterus, cervix and upper vagina, whereas the mesonephric duct regresses and largely disappears. In the male, under the influence testicular secretions, the mesonephric duct forms the epididymis, vas deferens, seminal vesicles and ejaculatory ducts, whereas the Mullerian duct regresses.Reference Tuchmann-Duplessis and Haegel 56 DES exposure during this critical window of sex differentiation results in alterations in female and male gonads, and reproductive tracts including retention of the opposite duct system in both sexes. Permanent alterations were observed in both sexes, but only those seen in the female will be further discussed.
Humans
Primordial germ cells migrate to the genital ridge to populate the primitive gonad around gestation week 5. During weeks 5–6 of embryonic development, the undifferentiated genital tract is comprised of two set of ducts (Wolffian and Mullerian), which are located in the urogenital ridge. By week 7, as the indifferent gonad starts to differentiate into either the testis or ovary, the duct systems responding to gonadal hormones start their differentiation processes. In the female, at approximately 8 weeks, the mesonephric ducts begin to regress cranially to caudally, leaving only vestiges such as in the rete ovarii, broad ligament and cervical area (Gartner's duct), which may give rise to cysts later in life. In contrast, the Mullerian duct differentiates and grows caudally, lateral to the mesonephric duct, fusing caudally as it grows and ending blindly in the urogenital sinus (UGS). The upper portion of the Mullerian ducts, which remains separated and unfused, differentiates into oviducts, whereas the caudally fused Mullerian tube starts differentiation into the uterus by approximately week 9 and into the upper vagina by week 10. ERs have been localized in the primitive uterus as early as gestation week 13. Although major reproductive tract organogenesis is complete by the start of the second trimester, tissue and cell differentiation continue into the second trimester. Birth occurs at approximately38 weeks in gestation.
Mice
Similar differentiation events occur in the mouse reproductive tract; however, analogous to developmental events in the mammary gland, variations occur in timing between humans and mice. Primordial germ cells migrate to the genital ridge during prenatal life starting on approximately gestational day 9. By 12 d.p.c, the undifferentiated genital tract is formed and is composed of the mesonephric and Mullerian duct systems. Regression of the mesonephric ducts and differentiation of the Mullerian ducts start on approximately late 12 d.p.c. and is complete by 16 d.p.c. Tissue and cell differentiation continue into neonatal life.
Similarities/differences
Although developmental milestones are similar, cell differentiation in various regions of the reproductive tract differ between mice and humans. An ovarian bursa is characteristic of mice that aids in guiding fertilized eggs into the oviduct in a mature female, but this structure is not present in women. Oviductal coiling is seen in mice but not in humans, and the utero-tubal junction varies between the species. Further, the cranial Mullerian ducts fuse in humans to form uterus, cervix and upper vagina; however, in mice, fusion occurs further caudally in the cervical region resulting in two separated uterine horns. Although controversy exists over the origin of the vaginal epithelium, it is generally considered that UGS epithelium grows cranially replacing Mullerian epithelium, but the replacement is more complete in humans than in mice. Vaginal adenosis and adenocarcinoma are thought to result from DES interfering with the UGS replacement of Mullerian epithelium, resulting in pockets of Mullerian-derived columnar epithelial cells rather than squamous-derived epithelium from UGS origin. At the molecular level, DES interferes with developmental genes that regulate cellular and tissue patterning.Reference Taylor, Vanden Heuvel and Igarashi 57
Considering the differences in the reproductive tract development between humans and mice, it is apparent that the mouse is well suited for multiple pups per pregnancy with an ovarian bursa and two uterine horns, whereas the human is ideally suited for a single embryo with a unilateral uterine fundus. However, similar to breast tissues, the reproductive tract in humans and mice, at all stages of differentiation and development, has the ability to respond to estrogens such as DES.
DES and latent effects: adult disease/dysfunction following developmental exposure
Exposure to DES has been reported to cause numerous structural malformations in the oviduct, uterus and vagina of both humans and mice. Further, retained mesonephric remnants and paraovarian cysts of mesonephric origin have also been described in both species. Structural malformations in the mammary gland of mice characterized by altered TEBs and abnormal branching have been reported. In addition to malformations, which are teratogenic changes, dysfunction of the reproductive tract resulting in subfertility/infertility, poor reproductive outcome, altered menstrual (estrus) cycles and dysfunction of the mammary gland including altered lactation have all been described (see Table 1 for a summary). Some of these alterations such as oviductal malformations and altered mammary gland structure are apparent early in life, whereas many other alterations such as cancer require a much longer time to manifest.
Breast cancer
Administration of DES causes an increased incidence of breast cancer in prenatally DES-exposed daughtersReference Palmer, Hatch, Rosenberg, Hartge, Kaufman and Titus-Ernstoff 4 , Reference Palmer 5 , Reference Troisi, Hatch, Titus-Ernstoff, Hyer, Palmer and Robboy 58 and their mothers. 7 , Reference Titus-Ernstoff, Troisi, Hatch, Palmer, Wise and Ricker 59 Women > 40 years of age and exposed in utero to DES have an estimated 1.9 times increased risk for developing breast cancer compared with unexposed women at the same age. In addition, the highest risks of breast cancer were correlated with the highest cumulative doses of DES during gestation.Reference Palmer 5
Many studies in rodents show alterations in mammary gland development following perinatal DES exposure. Pregnant rats were treated with DES and their offspring were examined after birth. Although excessive nipple development was observed in male and female offspring, prenatally DES-exposed females were subsequently unable to nurse their offspring after they became pregnant and gave birth; this shows that prenatal DES exposure interferes with the later function of the mammary gland. This was likely because of the absence of the nipple sheath indicating failure to form a connection between the mammary ducts and the nipple, rendering the female unable to nurse.Reference Boylan 60 ACI rats demonstrated elongated nipples, extensive lobuloalveolar proliferation, decreased tumor latency and greater multiplicity of tumors following gestational or lactational exposure to DES.Reference Rothschild, Boylan, Calhoon and Vonderhaar 44 Prenatal or neonatal DES exposure has also been shown to increase mammary tumorigenesis in Syrian golden hamsters and rats treated with DMBA. DES increased the number of mammary tumors, tumor multiplicity and the grade of tumor malignancy, suggesting that in addition to being carcinogenic, it also increased the sensitivity of the mammary gland to other carcinogens (reviewed in NIH 7 and Fenton and NewboldReference Fenton, Reed and Newbold 72 ).
Similar effects were seen in mice dosed just after birth with DES. Increased ductal outgrowth was seen in the mammary gland around the time of puberty, dilated mammary ducts were evident at 12 weeks and morphological changes in mice treated with low doses were accompanied by precocious lactation, suggesting that DES altered structure and function of the mammary gland. The association of increased incidence of mammary cancer in mice exposed either prenatally or neonatally to DES, similar to the increased incidence of cancer reported in humans, has been discussed earlier and is reviewed (see, Fenton and NewboldReference Fenton, Reed and Newbold 72 ).
Altered reproduction and menstrual cycle irregularities
DES also causes alterations in the menstrual cycle in prenatally DES-exposed women such as shortened or prolonged length of cycles, abnormal bleeding between cycles, lack of ovulation, absence of menstruation, subfertility and/or infertility. 7 Similar effects have been seen including abnormal estrus cycles and subfertility/infertility in DES-exposed mice.Reference McLachlan, Newbold, Shah, Hogan and Dixon 61 As menstrual cycle irregularities are not apparent until reproductive maturity, yet are caused by developmental exposure, this abnormality provides another example of DOHaD. Further, DES is also associated with multiple other health problems as it disrupts the endocrine system, which is responsible for communicating cellular signals throughout the body.
Fibroids (Leiomyomas)
Uterine leiomyoma, commonly called fibroids, are tumors of smooth muscle origin, which cause pain, bleeding, infertility and pregnancy complications; they are the leading indication of hysterectomy. Fibroids are the most common type of tumor in women over 30 years of age, and incidence rates have been estimated as high as 77% of women of reproductive age who are afflicted with the disease. In mice, early life exposure to DES causes fibroid development in adult animalsReference McLachlan, Newbold and Bullock 62 and is another example of DOHaD. Data from the DES mouse model showed that fibroids occurred in approximately 9% of the DES-exposed mice as compared with <1% of unexposed animals. Interestingly, the association of fibroids with prenatal DES treatment in humans has also been shownReference Baird and Newbold 63 . Among white women, 76% who reported prenatal DES exposure had fibroids compared with 52% of unexposed women. Further, DES-exposed women tended to have larger tumors. Thus, in this study, the prenatal DES mouse model predicted fibroid occurrence in DES-exposed women. The data with both DES-exposed mice and humans indicate a role for prenatal estrogen exposure in the etiology of uterine fibroids.
Another animal model is relevant to developmental estrogen exposure and fibroid development. Studies using the Eker rat that contains a defective tumor suppressor gene have shown increased susceptibility to uterine fibroids following early life exposure to DES.Reference Cook, Davis, Goewey, Berry and Walker 64 The defective gene resulted in reprogramming of the myometrium leading to an increase in the expression of estrogen-responsive genes. Later in life, these neonatal DES-exposed rats have increased tumor suppressor gene penetrance that correlates with increased tumor size and multiplicity. This effect of DES in the Eker rat is an example of gene–environment interactions during development and provides yet another example of DOHaD.
Together, these data suggest that uterine fibroids observed in women of reproductive age may originate during development and early life, and that altered epigenetic programming in the uterus, due to exposure to environmental estrogens, may play a role in fibroid etiology.
Mechanisms involved in the adverse effects of developmental exposure
ER
Estrogens are linked to the development of breast cancer based on clinical and experimental data; risk factors for breast cancer reflect cumulative exposure of breast epithelium to estrogen.Reference Henderson and Feigelson 65 Two hypotheses involving ER have been proposed to explain this relationship: (1) estrogen binding to ER stimulates proliferation of mammary cells, increasing the target cell number within the tissue, and the increase in cell division and DNA synthesis elevates the risk for replication errors, which may result in the accumulation of detrimental mutations that disrupt normal cellular processes such as cell proliferation, apoptosis or DNA repair; (2) estrogen metabolism leads to the production of genotoxic by-products that can directly damage DNA, resulting in mutations. Evidence has existed for years to suggest that both mechanisms play a role in breast cancer; however, with DES exposure, genetic mutation and/or damage seem to play less of a role than originally expected.Reference Larson, Ungarelli, de lasMorenas, Cupples, Rowlings and Palmer 66 DES has been shown in some studies to be a non-mutagenic carcinogen, therefore epigenetic mechanisms provide an explanation for the ability of DES to cause cancer in a non-mutagenic manner.Reference Szyf 67
Epigenetics
Starting in the 1990s, this mechanism was progressed to explain developmentally induced carcinogenesis and it has since been vigorously studied. Altered epigenetic programming explains how exposure to exogenous estrogen during prenatal life can result in breast cancer some 40+ years later. The explanation lies at the center of the DOHaD paradigm. Although the link of epigenetics and cancer has received current increasing interest, the field of epigenetics is not new; it was first described in 1939 by Conrad Waddington. Epigenetics, meaning in addition to or above the genome, is defined as the study of heritable changes in gene function that occur without a change in the DNA sequence (there is no gene mutation involved, only a change in how the gene is regulated or expressed). Epigenetic programming is essential to regulate normal development and the maintenance of cell/tissue differentiation by dictating cell-fate decisions via regulation of specific genes. Among these developmental genes are the Wnt and Hox family members. Thus, disruption in the balance of epigenetic networks can result in severe pathologies including altered structural development of the breast and reproductive tract, altered protein expression and even cancer later in life.
Environmental chemicals, especially those with estrogenic activity, have been documented in experimental animals to alter epigenetic programming that normally occurs during embryogenesis and development, and result in dysfunction/disease later in life.Reference Newbold and Kinyamu 14 , Reference Li 19 , Reference Li 20 , Reference Tang 22 , Reference Ho and Tang 68 – Reference Prins, Tang, Belmonte and Ho 71 The epigenome is very vulnerable to dysregulation by environmental factors (such as estrogenic chemicals) during critical windows of differentiation because of the high degree of developmental plasticity (especially after fertilization, again at implantation and during organ development) and the high rate of DNA synthesis that is occurring. Most importantly, elaborate epigenetic programming such as DNA methylation patterning required for normal tissue development is established during prenatal life. Alterations in epigenetic events, including changes in DNA methylation, posttranslational modifications of histones involved in chromatin structure and noncoding RNA/DNA, may be permanent and not readily apparent until later in life. Epigenetic reprogramming by early life exposure to estrogenic chemicals is complex and may involve multiple epigenetic and genetic pathways that combine together to initiate disease/dysfunction later in life. DES is a documented epigenetic toxicant and this mechanism likely plays a role in its DOHaD effects.
Summary and conclusions
It has been over 50 years since DES was first prescribed for use during pregnancy, therefore it is very difficult to get solid information on DES-exposed humans because of lost and incomplete medical records. Important data including DES dose and timing are simply not available. Loss of data is a disadvantage for any DOHaD study involving humans because of the lag time between exposure and adverse effects, an thus animal models with their reduced life spans can play significant roles in studying and defining DOHaD events. The importance of animal models to inform human health and vice versa should not be underestimated. Is there a lesson to be learned from the DES scenario? Unfortunately, DOHaD phenomena are not lessons of past exposures but continue to be of concern as estrogens, progesterone and glucocorticoids are still being prescribed during sensitive prenatal and neonatal time periods, thus potentially sensitizing children to a variety of changes later in life.
In summary, ample scientific evidence from experimental animal and humans documents that prenatal DES exposure is an excellent example of DOHaD as it causes an increased risk for breast cancer and reproductive problems including menstrual irregularities and uterine fibroids, in addition to the originally described vaginal adenocarcinoma. Continued follow-up of the DES-exposed population (mothers, daughters and sons) such as the ongoing NCI studies are essential to further determine disease risks as the cohorts age. Regarding mechanisms, current data point to a role for epigenetic mechanisms involving reprogramming of developmental genes during prenatal life, which subsequently leads to altered responses (at the cell, tissue, organ level) to hormones or other environmental factors later in life. Epigenetic reprogramming suggests that DES effects may be passed on to subsequent generations, thus follow-up of DES grandchildren is also crucial. Finally, information gained from research with DES will help predict and prevent future adverse DOHaD events with other environmental chemicals and stressors.
Acknowledgment
The author thanks Dr Jerry Heindel for his critical reading and comments on this paper.