Introduction
Cercariae have a variety of behavioural responses that increase the likelihood of encountering a suitable host and maximising transmission success (Haas, Reference Haas1994). The majority of continuously swimming cercariae have specialized locomotion as well as various taxes and kineses; these features are necessary for reaching the ‘host space’ and the ‘host time’ and successfully infecting the host (Combes et al., Reference Combes, Fournier, Moné and Théron1994; Snyder & Janovy, Reference Snyder and Janovy1996; Haas, Reference Haas2003; Morley, Reference Morley2012; Selbach & Poulin, Reference Selbach and Poulin2018). The nervous system and sensory receptors are essential to these processes.
The chaetotaxy of cercariae frequently attracts attention because of its taxonomic value. Since the advent of the silver nitrate impregnation technique a wealth of data on the topology of sensory receptors has been accumulated. It has become apparent that chaetotaxy is a reproducible and species-specific trait; it also highlights several patterns in the topology of surface receptors among families (Richard, Reference Richard1971; Bayssade-Dufour et al., Reference Bayssade-Dufour, Hugot and Albaret1993; Manafov, Reference Manafov2010). The nomenclature of sensory papillae was developed assuming their colocalization with the orthogonal nerve cords (Richard, Reference Richard1971; Bayssade-Dufour, Reference Bayssade-Dufour1979). This idea was extrapolated to apply to all cercariae. Currently there are not enough data on the structure of the nervous system in xiphidiocercariae. An analysis of their chaetotaxy with a detailed description of the nervous system is presented only in a couple of studies (Grabda-Kazubska & Moczoń, Reference Grabda-Kazubska and Moczoń1981, Reference Grabda-Kazubska and Moczon1990). Reconstruction of the nervous system and chaetotaxy combined with morphological data are presented only for cercariae of Alassogonoporus sp. (Bogéa & Caira, Reference Bogéa and Caira2001).
In comparison with other representatives of Microphalloidea, the cercariae of family Renicolidae have numerous plesiomorphic features such as a simple small stylet, a large number of penetration glands and excretory system organization (Stunkard, Reference Stunkard1950; Odening, Reference Odening1971; Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). In this regard Cercaria parvicaudata Stunkard & Shaw, 1931 (Renicolidae) is an interesting study object due to its phylogenetic position within the evolutionary advanced superfamily (Tkach et al., Reference Tkach, Pawlowski, Mariaux, Swiderski, Littlewood and Bray2001; Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003).
We redescribed the nervous system of C. parvicaudata with the immunostaining of 5-hydroxytryptamine (5-HT) and FMRFamide immunoreactive nerve elements. We analysed the morphology and distribution of sensory receptors with scanning electron microscopy (SEM) and the silver nitrate impregnation technique. All new data were analysed together to describe the colocalization of sensory papillae with nerve cords.
Material and methods
Littorina littorea Linnaeus, 1758 was collected from the intertidal zone in the Chupa Inlet of the Kandalaksha Gulf (White Sea, Russia) in June 2018. Snails were tested for infection in separate dishes filled with seawater. Cercariae that emerged from three infected snails were observed with a Leica DM1000 microscope and were identified as C. parvicaudata (Microphalloidea, Renicolidae). The larvae were collected in small drops of seawater and fixed for further studies.
Distribution of sensory receptors was detected using the standard method of silver nitrate impregnation (Ginetsinskaya & Dobrovolskij, Reference Ginetsinskaya and Dobrovolskij1963). Cercariae were fixed in a 5% solution of silver nitrate for an hour in the dark at 4 °C. Then the cercariae were washed in distilled water and transferred to glycerol. Ten samples were studied with a Leica DM1000 microscope.
To study the tegumental surface and the morphology of sensory receptors, cercariae were fixed in a 2.5% solution of glutaraldehyde in 0.05 m sodium cacodilate buffer (SCB) with post-fixation in a 2% solution of osmium tetroxide in 0.05 m SCB. Then the samples were dehydrated and dried at a critical point using a Leica EM CPD300 station. Images of 22 samples were obtained with a Tescan MIRA3 LMU scanning electron microscope (10 nm platinum sputtering by Jeol JEE-420D).
For immunostaining studies, 52 samples were fixed in a 4% solution of paraformaldehyde in seawater for 6 h at 4 °C, and then washed in 0.1 m phosphate buffered saline (PBS). Next, the samples were kept in 5% Triton X100 solution in PBS for 24 h and in a 1% solution of bovine albumin in PBS for 6 h at 4 °C. All specimens were divided into three groups that were treated with the following antibody solutions in the 0.1% Triton X100 in PBS: rabbit anti-5-HT (S5545 Sigma) (1:1000), rabbit anti-FMRFamide (AB15348 EMD Millipore) (1:1000) and a mixture of mouse anti-acetylated α-tubulin (T6793 Sigma) (1:500) and mouse anti-tyrosinated α-tubulin (T9028 Sigma) (1:500). All cercariae were incubated in primary antibodies for 24 h at 4 °C then washed in 0.1% Triton X100 in PBS. Then they were incubated in the secondary anti-rabbit CF™488 (SAB4600044 Sigma) or anti-mouse CF™647 (SAB4600182 Sigma) antibodies for 8 h at 4 °C. Samples were washed in PBS and transferred to glycerol. The images were obtained with a scanning confocal microscope Leica TCS SP5 MP.
Results
The tegument of C. parvicaudata is creased and contains small spines measuring approximately 1.5 µm (figs 1–3), the tail is devoid of them (fig. 3d, f). The mouth opening and the ventral sucker are both armed with a double row of large spines measuring 2 µm (figs 1b, c and 2b, e).

Fig. 1. Scanning micrographs of the anterior end of C. parvicaudata. (a) The ventral surface (scale 15 µm); (b) surface of the oral sucker (scale 5 µm) with insertions (scale 2 µm); (c) Cmo papillae in the oral cavity (scale 1 µm); (d) spongy papilla in CI group (scale 0.5 µm); (e) sensory receptors in CI and StV groups (scale 1 µm); (f) papillae in CII, CIII groups (scale 1 µm); (g, h) papillae in St2 + St3 groups (scale 1 µm). Arrows, spines; ci, cilia; noCi, non-ciliate papillae; sp, spongy papillae; st, stylet; tco, tegumental collar.

Fig. 2. Scanning micrographs of the ventral surface of C. parvicaudata. (a) General view of the ventral surface (scale 15 µm); (b, e) the surface of acetabulum (scale 5 µm); (c) sensory receptor in the inner S circle (S1) of acetabulum (scale 0.5 µm); (d) papillae in AIV, AIIV, AIIIV groups (scale 5 µm); (f) sensory receptor in the outer S circle (S2) of acetabulum (scale 1 µm); (g) the MV sensory papillae (scale 1 µm); (h) the PI sensory papillae (scale 0.5 µm). Arrows, spines; ci, cilia; noCi, non-ciliate papillae; tco, tegumental collar.

Fig. 3. Scanning micrographs of the dorsal surface of C. parvicaudata. (a) General view of the dorso-lateral surface (scale 10 µm); (b) sensory receptors in StDL group (scale 5 µm); (c) the paired sensory receptors in AIIID group (scale 1 µm); (d) the PIIID paired papillae (scale 1 µm); (e) sensory receptors in AID group and the last StDL papillae (scale 5 µm); (f) single caudal papilla (scale 0.5 µm); (g) sensory receptors in AIID, AIIID groups (scale 5 µm). Arrows, spines; ci, cilia; cp, caudal papilla; ct, caudal tegument; pcs, paired uniciliate sensory receptors; tco, tegumental collar.
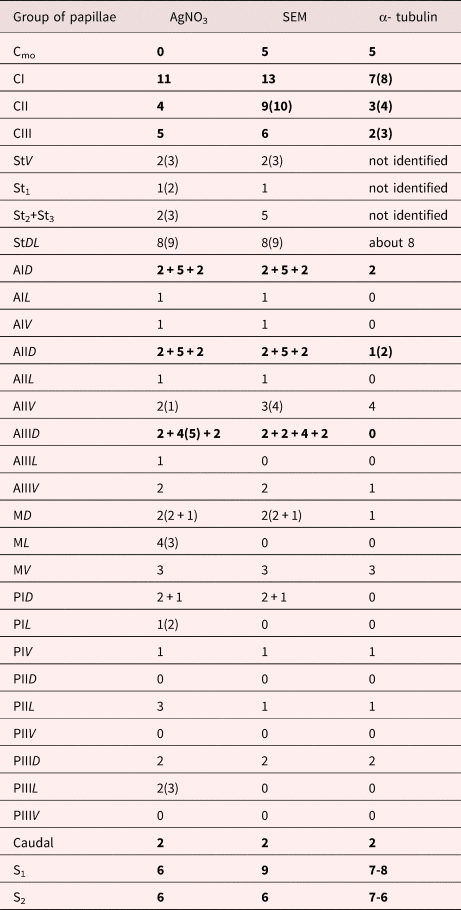
The description of the chaetotaxy (fig. 4, table 1) is based on data obtained using the silver nitrate impregnation technique, SEM and anti-α-tubulin immunostaining. There are three ‘C’ circles at the anterior end of the body (figs 1 and 4c). The first circle CI directly surrounds the oral opening. In the oral opening, we observed small uniciliate papillae (0.5 µm) that we marked as Cmo (figs 1c and 4c). Within the arcs of CI, CII and CIII, similar uniciliate receptors are situated; the cilia length ranges from 0.3 to 1 µm. Each papilla is surrounded by a tegumental collar that ranges from 0.5 to 2 µm in width. Among the 13 receptors of CI there are two that are surrounded by folded tegumental collars (fig. 1b, d). These papillae have a spongy apex that protrudes from a 0.5 µm pore in the tegumental plate. Such sensory structures were found only in one sample; the others had tegumental folds in their place (fig. 1b insertions). There are both uniciliate and non-ciliate receptors of the CII and CIII groups (fig. 1e, f). Among the receptors of the StDL groups (fig. 4a) both single uniciliate (with cilia lengths from 0.3 to 8 µm) and paired uniciliate papillae were found (fig. 3b, e).

Fig. 4. Chaetotaxy of C. parvicaudata. (a) General view of the dorsal surface; (b) general view of the ventral surface; (c) complete picture of the ventral C-region chaetotaxy (silver impregnation combined with SEM).
Table 1. The comparative analyses of chaetotaxy of C. parvicaudata based on different approaches (silver nitrate impregnation, SEM and anti-α-tubulin immunostaining). Total amount of sensory papillae in the entire region is highlighted with bold font, one half from the number of receptors in the region is in regular font. The chaetotaxy described in accordance with the nomenclature proposed by Richard (Reference Richard1971).

Sensory receptors are arranged in a serial pattern on the rest of the body surface, especially on the dorsal side (fig. 4a). Among the dorsal papillae there are three almost identical ‘A’ series (9AID, 9AIID, 8-10AIIID) of similar uniciliate receptors (cilia length from 1 to 10 µm) (fig. 3e, g). The lateral sensory papillae of each dorsal ‘A’ group are paired and united by a common tegumental collar (fig. 3c, e, g). Such paired papillae are also observed in the MD, MV, PIID and PIIID zones, where the ciliae lengths in each pair are 1 µm and 5 µm (figs 3d and 4a). The caudal pair of receptors includes uniciliate papillae; the length of the cilia is 0.7 µm (fig. 3f). The axis between the caudal sensory endings is slightly oblique to the longitudinal axis of the tail (figs 4a and 5b).

Fig. 5. Anti-α-tubulin immunostaining of C. parvicaudata (scale 20 µm). (a) General view of the ventral plane of anti-α-tubulin immunoreactive structures; (b) general view of the dorsal plane of anti-α-tubulin immunoreactive structures; (c) nerve endings of the acetabulum (scale 10 µm). Arrows, sensory receptors (several groups that have been accurately identified are marked); fc, flame cells; gd, penetration glands ducts; cp, caudal papilla (dotted line, the contour of the body).
The sensory receptors on the acetabulum form two ‘S’ circles. The inner circle consists of nine small uniciliate receptors that have 1 µm-long cilia, while the outer circle contains six convex short papillae (figs. 2b, c, e, f and 5c). Receptors of the outer circle are surrounded by a wide convex tegumental collars and the length of their cilia does not exceed 0.5 µm (fig. 2f).
The 5-HT immunoreactive neurons and nerve cords form a metameric orthogonal construction (fig. 6a–c). Fourteen 5-HT neurons were found in the body and two in the tail. The bilobed cerebral ganglion with four pairs of 5-HT neurons is situated behind the oral sucker on the frontal plane of the body (fig. 6b). The other three pairs of neurons are located alongside the ventral nerve cords. Three pairs of longitudinal nerve cords are linked by transverse commissures (fig. 6a, c). In the preacetabular region three ventral commissures are located at equal intervals. The ventral longitudinal nerve cords and the last preacetabular ventral commissure form a plexus in the ventral sucker (fig. 6b). Two ventral and two dorsal nerve cords are located in the tail (fig. 6b). They are a continuation of the body's ventral and the dorsal longitudinal nerve cords.

Fig. 6. 5-HT and FMRFamide immunostaining of C. parvicaudata (scale 20 µm). (a) General view of the ventral 5-HT immunoreactive nerve elements; (b) general view of the 5-HT immunoreactive elements in the plane of the ganglion; (c) general view of the dorsal 5-HT immunoreactive nerve elements; (d) general view of the ventral FMRFamide immunoreactive nerve cords; (e) general view of the FMRFamide immunoreactive elements in the plane of the ganglion. *neuron; clc, caudal longitudinal nerve cord; dlc, dorsal longitudinal nerve cord; G, ganglion; llc, lateral longitudinal nerve cord; p, sucker plexus; tc, transverse commissure; vlc, ventral longitudinal nerve cord (dotted line, the contour of the body of the larva).
The FMRFamide immunoreactive elements (fig. 6d, e) are distributed in a pattern similar to that of the 5-HT ones. The ventral FMRFamide immunoreactive longitudinal nerve cords are the most developed and continue into the tail. There are seven ventral, four dorsal and five lateral transverse commissures.
Discussion
The silver nitrate impregnation technique is a standard for visualization of chaetotaxy in cercariae (Ginetsinskaya & Dobrovolskij, Reference Ginetsinskaya and Dobrovolskij1963; Antonelli et al., Reference Antonelli, Quilichini, Foata and Marchand2014). Some sensory receptors become visible due to silver nitrate but are lost in scanning electron microscope images (Bogéa & Caira, Reference Bogéa and Caira2001a) and vice versa (Denisova & Shchenkov, Reference Denisova and Shchenkov2018) (table 1). Using immunostaining alone also does not give entirely accurate results (fig. 5, table 1). SEM is the most reliable approach for investigating the surface receptors, because this method enables detection of both the position and morphology of papillae. Based on these data it is possible to suggest the role of the individual receptor (Žd’árská & Nebesářová, Reference Žd’árská and Nebesářová2003; Antonelli et al., Reference Antonelli, Quilichini, Foata and Marchand2014). It is assumed that receptors with long cilia respond to low mechanical stimuli, such as water currents, and are localized on the dorsal surface (Bogéa & Caira, Reference Bogéa and Caira2001, Reference Bogéa and Caira2001b), especially in AD groups (fig. 3e, g). Sensory papillae with short cilia and a high tegumental collar are more often located on the ventral surface (figs 1f and 2d, c, f), which is directly in contact with the substrate. The tegumental collar is considered important for tactile mechanoreception (Bogéa & Caira, Reference Bogéa and Caira2001).
Sensory papillae of C. parvicaudata are mainly represented by uniciliate nerve endings surrounded by the tegumental collars (figs 1–3). This type of papillae is common among both cercariae and adults of various digenean families (Bogéa & Caira, Reference Bogéa and Caira2001, Reference Bogéa and Caira2001a, Reference Bogéa and Cairab; Sohn et al., Reference Sohn, Woo and Hong2002; Žd’árská & Nebesářová, Reference Žd’árská and Nebesářová2003; Antonelli et al., Reference Antonelli, Quilichini, Foata and Marchand2014). The sensory endings of the oral sucker of C. parvicaudata are also of the same type (fig. 1e, g, h). Unlike some other stylet cercariae (Denisova & Shchenkov, Reference Denisova and Shchenkov2018), this species does not have complex and polyciliate receptors in the ‘St’ groups. However, in the first circle of ‘C’ groups of C. parvicaudata we observed spongy papillae with powerful extensible tegumental collars (fig. 1d). Such structures are described in cercariae for the first time; however, they may be similar to the papillae of some miracidia (Dunn et al., Reference Dunn, Hanna and Nizami1987; Semyonov, Reference Semyonov1991; Tykhomirov, Reference Tykhomirov2000). A number of other non-ciliate papillae on the ventral surface look like smooth tegumental tubercles and have small apertures (figs 1f and 2d). Non-ciliate receptors have been repeatedly described in other cercariae, where they were suggested to act as mechanoreceptors (Žd’árská & Nebesářová, Reference Žd’árská and Nebesářová2003).
S2 circle receptors are characterized by a large and extensible tegumental collar and short cilia (fig. 2e, f). The similar sensory receptors are located on the acetabulum of the cercariae of Renicola sp. NZ (O'Dwyer et al., Reference O'Dwyer, Blasco-Costa, Poulin and Faltýnková2014). However, they were described as non-ciliate papillae, although the detailed morphology was not presented.
In summary, C. parvicaudata has at least five morphological types of sensory receptors: type 1 – single uniciliate papillae (cilia length from 0.3 to 10 µm) with a small closely fitting tegmental collar (e.g. fig. 2c); type 2 – single non-ciliate papillae (e.g. fig. 1f, noCi); type 3 – paired uniciliate papillae (cilia length 1 µm and 5 µm) grouped by a common tegumental collar (e.g. fig. 3c, d, pcs); type 4 – spongy papillae of the CI group (fig. 1d); type 5 – S2 uniciliate papillae surrounded by a large folded tegumental collars (fig. 2f).
The chaetotaxy of C. parvicaudata is similar to that of other renicolid cercariae (table 2). Distinctive features of C. parvicaudata are the same number and morphology of papillae among its dorsal groups ‘A’ (figs 3e, g and 4a, tables 1 and 2). The serial pattern is also characteristic of the ventral receptor groups (fig. 4b). The presence of the ‘St’ and AID groups in renicolid cercariae are typical for representatives of Microphalloidea (Richard, Reference Richard1971).
Table 2. The number and topology of sensory papillae in some conservative groups in three renicolid cercariae, including new data on C. parvicaudata. The chaetotaxy described in accordance with the nomenclature proposed by Richard (Reference Richard1971).

The process of ‘cephalization’ and the quantitative prevalence of papillae surrounding the stylet and oral opening have been revealed for Pleurogenidae, Lecithodendriidae and Microphallidae families (Bayssade-Dufour et al., Reference Bayssade-Dufour, Hugot and Albaret1993; Manafov, Reference Manafov2010). Compared to the cercariae of these families, C. parvicaudata and other renicolid cercariae have a small number of receptors among ‘C’ and ‘St’ groups. C. parvicaudata has a regular pattern in arrangement of dorsal groups of papillae (AID, AIID, AIIID, MD, PID, PIIID) and a large number of sensory receptors in acetabular groups (9S1, 6S2) (fig. 4a, tables 1 and 2). The caudal pair of papillae in C. parvicaudata is oblique to the longitudinal axis of the tail (figs 4a and 5b, table 2). Thus, the topology of this group is close to that in representatives of Plagiorchioidea (UD group), while in Microphalloidea the caudal pair is located strictly across the longitudinal axis of the tail (UDL group) (Richard, Reference Richard1971; Bayssade-Dufour et al., Reference Bayssade-Dufour, Hugot and Albaret1993).
The distribution of receptors in regions discussed above has never been observed in broad range of cercariae of Microphalloidea. Owing to the wide variety of chaetotaxy in Microphalloidea (Manafov, Reference Manafov2010), it is almost impossible to trace homology of the specific sensory groups without having data on the colocalization of sensory papillae and nerve cords in numerous species of cercariae.
The present immunocytochemical study of C. parvicaudata has revealed clear colocalization of surface sensory receptors with nerve cords and commissures (fig. 7). The StDL, AIV, AIIV, AIIIV, MV, PIV and PID groups of sensory papillae are localized where the longitudinal nerve cords pass (fig. 7 vlc, dlc). The most exact coincidence in the localization is observed between the AID, AIID and AIIID receptors and the dorsal transverse commissures (fig. 7 dlc, dtc2, dtc3, dtc4). The lateral sensory endings of the dorsal groups (morphological type 4) are located at the junctions of each longitudinal dorsal cord with a commissure (fig. 7 dlc, dtc2, dtc3, dtc4, dtc5, dtc6).

Fig. 7. The reconstruction of 5-HT immunoreactive nerve elements and chaetotaxy within the regions of C. parvicaudata body at the dorso-lateral and ventro-lateral planes. ac, acetabulum; dlc, dorsal longitudinal nerve cord; llc, lateral longitudinal nerve cord; n, neuron; os, oral sucker; p, sucker plexus; tc, ventral (v-) or dorsal (d-) transverse commissure with serial number; vlc, ventral longitudinal nerve cord (red arcs, regions of the body, purple dots, surface sensory papillae).
The model of the nervous system of cercariae proposed by Richard (Reference Richard1971) coincides with the orthogon in the broad sense of the term (Richter et al., Reference Richter, Loesel, Purschke, Schmidt-Rhaesa, Scholtz, Stach, Vogt, Wanninger, Brenneis, Döring, Faller, Fritsch, Grobe, Heuer, Kaul, Møller, Müller, Rieger, Rothe, Stegner and Harzsch2010). However, such a pattern has not yet been described in any real nervous system of cercariae. According to our data, the location of nerve elements of C. parvicaudata differs from the hypothetical pattern of the nervous system (Richard, Reference Richard1971) (table 3). These differences primarily concern the position of cerebral ganglion and the unequal number of ventral and dorsal commissures (fig. 7, table 3). Nevertheless, C. parvicaudata has an almost ‘regular rare’ orthogonal pattern (Kotikova, Reference Kotikova1991) of the nervous system that resembles the hypothetical pattern. Such interposition of the nerve cords and commissures is just one of the orthogonal modifications that differ in the stylet larvae studied so far (Grabda-Kazubska & Moczoń, Reference Grabda-Kazubska and Moczoń1981; Bogéa & Caira, Reference Bogéa and Caira2001; Tolstenkov et al., Reference Tolstenkov, Prokofiev, Terenina and Gustafsson2011, Reference Tolstenkov, Akimova, Terenina and Gustafsson2012; Terenina & Gustafsson, Reference Terenina and Gustafsson2014). Among the stylet cercariae whose nervous systems have been described, only the Haplometra cylindracea (Plagiorchiidae) had nerve cords and commissures that had been distributed closely to the intended scheme (Grabda-Kazubska & Moczoń, Reference Grabda-Kazubska and Moczoń1981).
Table 3. Differences and similarities in the structure of the nervous system between the hypothetical model (Richard, Reference Richard1971) and data of the present study.

Author ORCIDs
S.A. Denisova, 0000-0002-5602-5894; S.V. Shchenkov, 0000-0002-0579-1660
Acknowledgements
All data were obtained using the equipment of the Research Park of Saint-Petersburg State University (Centre for Molecular and Cell Technologies) for research project No. 109-9140. The samples were collected and primarily processed using the equipment of the SPbSU Educational and research station ‘Belomorskaia’. We are grateful to Natalia N. Shunatova for all her help during the sampling. We express our deep appreciation to Andrei A. Dobrovolskij, Anna G. Gonchar and Olga V. Knyazeva for help in this publication preparation.
Financial support
The research is supported by RFBR project no. 18-34-00632.
Conflict of interest
None.