Significant outcomes
• Selective breeding for low and high levels of positive affective vocalisations in juvenile rats promotes differential responsivity to social but not physical stress, confirming its potential utility for understanding affective temperamental traits.
• Rats selected for high positive affectivity exhibit increased coping with negative affective circumstances, suggesting selection for a stress-resilient phenotype.
• The genetic selection for differential positive and negative affects may provide preclinical models with implications for understanding human psychopathologies, especially depression.
Limitations
• Because only maternal odour was used to evaluate maternal potentiation effects, additional contrasts to the physical presence of the mother would be useful.
• Inclusion of a random selection line would have been desirable, but was unavailable when the research was conducted.
• The genetic characterisation of these selection lines remains to be conducted.
Introduction
In recent years, several genetic models of human-type emotional traits have been developed in rodents in order to facilitate the study of the neural mechanisms of emotionality (Reference Burgdorf, Panksepp, Brudzynski, Kroes and Moskal1–Reference Dichter, Brunelli and Hofer6). Among these efforts, the characterisation of ultrasonic vocalisations (USVs) in laboratory rats has been a powerful tool to investigate negative as well as positive affective states (Reference Knutson, Burgdorf and Panksepp7–Reference Borta, Wöhr and Schwarting11) with important implications for psychiatric medicinal development.
Rats emit USVs at the earliest stages of life and the production of USVs varies greatly as a function of age (Reference Nitschke, Bell, Bell and Zachman12). Infant rats exhibit short periods of high rates of 35–45 kHz USVs (Reference Ehret13) when separated from their mother and littermates (Reference Hofer14). These stress-induced USVs show a developmental curve with a peak at postnatal day (PND) 10–12 and a steep decline after PND 14 (Reference Shair15). Given the aversive circumstances (social isolation, hypothermia, etc.), it has been assumed that these infant USVs are associated with a state of negative affect or distress (hence they are called ‘distress calls’).
Differences in distress call rates have been used as a procedure for selective breeding of emotional dispositions that may model human temperamental traits: Brunelli and Hofer (Reference Brunelli and Hofer16) selectively bred rats based on high and low rates of distress USVs emitted by isolated infants. These two lines exhibited distinct coping styles across the life span, with results suggesting a negative affect phenotype in their high distress USV line. Similarly, Burgdorf et al. (Reference Burgdorf, Panksepp, Brudzynski, Kroes and Moskal1) developed an animal model for positive affective phenotypes. Whereas Brunelli and Hofer selected for infant distress USVs that may index negative-affect, Burgdorf's group selected for positive USVs: Low and High Lines of Long Evans rats were selectively bred based on the number of positive 50 kHz ‘chirping’ USVs emitted during a brief hand-play procedure (i.e., ‘tickling’) conducted in the juvenile period. The number of USVs presumably reflects the intensity of a rat's positive affective social experience during tickling [(Reference Burgdorf, Panksepp, Brudzynski, Kroes and Moskal1,Reference Panksepp17), and for a review, see Panksepp and Burgdorf (Reference Panksepp and Burgdorf18)], and it has been validated that the neural circuitry that mediates these calls (our objective measure of affect), has positive reinforcing properties (Reference Knutson, Burgdorf and Panksepp7,Reference Burgdorf, Wood, Kroes, Moskal and Panksepp19). Burgdorf's two lines have exhibited variations in behavioural phenotypes such as social motivation in response to social isolation in infancy (i.e., isolation distress calls) (Reference Harmon, Cromwell and Burgdorf20), anxiety- and depressive-like responses (Reference Burgdorf, Panksepp and Brudzynski21), and cocaine-induced hedonic-like responses (Reference Mu, Fuchs, Saal, Sorg, Dong and Panksepp22), suggesting differential affective traits.
In order to extend these results, we investigated potential Line differences in USV emission rates in response to social isolation, ambient temperature and relevant social odours within an isolation–reunion–reisolation paradigm first described by Shair (Reference Shair15). This paradigm evinced in rats and mice the so-called ‘maternal potentiation’ phenomenon, which is considered to be one of the strongest forms of evidence for the social-bonding relevance of infant isolation calls (Reference Shair15,Reference Hofer, Masmela, Brunelli and Shair23–Reference Hofer, Brunelli, Masmela and Shair25), with a potential resemblance to human infant emotional behaviour (Reference Hofer, Masmela, Brunelli and Shair23). Previous studies have shown that isolation-induced USVs increased markedly during the second of two closely spaced social-isolation periods, especially if the rat pups were briefly reunited with their mothers between the two sessions (Reference Hofer, Brunelli and Shair26). This response was not evident when pups were reunited with littermates or unfamiliar females [see Dichter et al. (Reference Dichter, Brunelli and Hofer6), for a review] suggesting it reflected a mother-specific form of social bonding.
In our study we used the isolation–reunion–reisolation paradigm to evaluate differences in social motivation, as measured by isolation USVs, between rats selectively bred for low and high levels of tickle-induced 50 kHz USVs – namely for low and high positive affect, respectively (Reference Burgdorf, Panksepp, Brudzynski, Kroes and Moskal1). However, rather than uniting pups with mothers and strangers between isolation periods, they were exposed to either social (familiar or unfamiliar), or neutral nest-odour cues, because it has been previously demonstrated that the USV distress emission of infant rats can be differentially attenuated by home-nest odours (Reference Hofer14,Reference Oswalt and Meier27–Reference Conely and Bell29). We chose this modified paradigm, because rat pups find home nest shavings rewarding and because our target was the pups’ vocal activity with no direct influence of maternal interactions. We predicted that both lines would increase rates of USVs in the second isolation, compared with the first isolation, and would decrease rates of USVs in the presence of social odours (both familiar and unfamiliar, but perhaps differentially) compared with neutral (control) odour, as previously found in both rats and mice (Reference Moles, Kieffer and D'amato3,Reference Hofer, Brunelli and Shair26). The rate of infant USVs is also affected by environmental temperature: a decrease in temperature is generally associated with elevated rates of USVs, which may reflect age-related thermoregulatory deficits, yielding distress from increasing hypothermia (Reference Hofer14,Reference Hofer and Shair30,Reference Kraebel, Brasser, Campbell, Spear and Spear31). Thus, we carried out all sessions at two different ambient temperatures: (1) the nest equivalent temperature of 35°C; and (2) a cool temperature of 23°C, in order to evaluate whether the two lines also vocalised differently in response to physical discomfort: we predicted that Low Line rats, which seem more susceptible to negative affect (Reference Harmon, Cromwell and Burgdorf20), would call more than High Line animals.
Materials and methods
Animals and housing
We used rat pups from the 17th generation of Long Evans rats selectively bred, bidirectionally, for low (Low Line) and high (High Line) rates of 50 kHz USVs emitted in response to a standard repeated (4-day) tickling procedure (Reference Burgdorf, Panksepp, Brudzynski, Kroes and Moskal1). Parenthetically, we did not use animals from a random-breeding line because those animals were exhibiting apparent inbreeding sterility; thus, we no longer had sufficient animals of that genotype available. The colony was maintained at a constant room temperature (20 ± 1°C and 60 ± 10% relative humidity) and on a 12/12 light-dark cycle (light on at 07:00 a.m.). Male and female pairs were housed in opaque plastic cages (73 × 54 × 24 cm) with food and water ad libitum. When the females were visibly pregnant, they were individually housed to avoid postpartum pregnancy, so no male odour was present in the bedding at birth. The day of birth was considered PND 0. The litters (Low Line, n = 16; High Line, n = 16) were left undisturbed until testing at PND 12 or 13. On the testing day, no pups had yet opened their eyes. Animals were tested in counterbalanced order across ages.
Procedure
On the testing day, eight Low Line litters and eight High Line litters were assigned to the ‘nest temperature’ condition, while the remaining litters were assigned to the ‘cool temperature’ condition. For this purpose, we used acoustically isolated temperature-regulated environmental test chambers (Model 352602, Hotpack, Philadelphia, PA, USA), set at either nest or cool temperatures (35 ± 0.5°C or 23 ± 0.5°C, respectively), and illuminated with a dim, red light. This value for the nest temperature condition was determined by monitoring the ambient temperature of a litter immediately after the dam vacated it.
From each litter, three pups were arbitrarily selected and assigned to one of the following odour conditions: familiar bedding (FB), unfamiliar bedding (UB), or clean bedding (CB), reflecting which odour cue they were exposed to between successive isolation sessions. For the FB condition we used 400 g of bedding from their own home cage, while for the UB condition we used the same quantity of bedding from a cage housing a different litter of the same age.
Each pup underwent three test sessions: Isolation 1 (Iso1), Bedding exposure (Bedding), Isolation 2 (Iso2). Fifteen minutes before the test, the dam was removed and the home cage of each litter was placed in a separate acoustically isolated temperature-regulated environmental chamber maintained at 35 ± 0.5°C. After 15 min of habituation to the move, each test subject was placed alone into an empty opaque plastic test box (29 × 19 × 12 cm) in its respective, temperature-regulated isolation chamber for 2 min (Iso1). Afterwards, each animal was placed into another box with identical features, but containing 400 g of FB, UB, or CB and monitored for 2 min (Bedding). The pup was then re-isolated (Iso2) for 2 min in the empty isolation box (Fig. 1). Following testing, body weight and axillary temperature (measured by insertion of a microprobe (Sixth Sense LT300 Infrared Thermometer, Instrumart, Carlsbad, CA, USA)) were recorded and the test box was cleaned with a diluted alcohol solution. All tests for a particular pup occurred in the same isolation chamber.

Fig. 1 Experimental design. CB, clean bedding; FB, familiar bedding; Iso, isolation; UB, unfamiliar bedding.
For the FB and UB conditions, faecal boli were removed from the soiled bedding before use. During all the sessions and for all the conditions, the testing boxes were covered with a Plexiglas lid that maintained a constant concentration of odour and also served to prevent odour dispersal into the isolation chamber. Experiments were carried out between 01:00 p.m. and 04:00 p.m. (i.e., starting 6 h into the light cycle), and all pups were handled with nitrile gloves.
The experiment was conducted in compliance with standard institutional guidelines for the care and use of research animals, and all experimental protocols were approved by the Washington State University Institutional Animal Care and Use Committee.
Data collection
USVs were recorded with a high quality ultrasonic microphone (Condenser ultrasound microphone CM16/CMPA; Avisoft Bioacoustics, Berlin, Germany) placed inside the isolation chamber and suspended ∼5 cm above the centre of the plastic test box. The signal was then amplified and converted to digital format using a digital audio card (Avisoft USG Ultra Sound Gate; Avisoft Bioacoustics). Digital acquisition and analysis was performed on a PC with SeaPro Ultra software developed by Pavan at CIBRA (http://www.unipv.it/cibra). We report the total number of USVs emitted during each session. The numbers of USVs were scored offline by two trained observers blind to the treatments, and exhibiting high inter-observer concordance (>95%).
Statistical analysis
Analyses were performed using a mixed-model analysis of variance (ANOVA) for repeated measures. The model included between factors (Line: Low and High; Temperature: Nest and Cool; Odour condition: FB, UB and CB) and within factors (Sessions: Iso1, Bedding, Iso2). The model included the litter as the blocking factor. Post hoc comparisons were performed using Tukey's honestly significant differences test, which is permissible in the absence of significant ANOVA effects (Reference Wilcox32). Statistical analyses were conducted using Statview II software (Abacus Concepts Inc., Berkeley, CA, USA).
Results
Statistical analysis revealed a main effect of Line [F(1, 28) = 52.8, p < 0.0001, η = 1], with the Low Line vocalising more than the High Line (Low: 238.3 ± 9.5; High 97.7 ± 4.2) over all sessions. Low Line rates of USV emissions did not change over the three sessions. In contrast, post hoc analyses on the Line × Session interaction [F(2, 56) = 4.9, p = 0.01, η = 0.7; Fig. 2] revealed that High Line pups increased USVs during the Bedding (Bedding > Iso1, p < 0.01) and Iso2 sessions (Iso2 > Iso1, p < 0.01).

Fig. 2 Line × Session interaction. Mean (±SE) of USVs emitted by Low and High lines during a three-session test: Iso1, Bedding, and Iso2. Iso, isolation; USVs, ultrasonic vocalisations. **p < 0.01.
In addition, there was a significant main effect for Temperature [F(1, 28) = 5.2, p = 0.03, η = 0.6], with both lines emitting more vocalisations at the cool temperature compared with the nest temperature (Line × Temperature interaction: F(1, 28) = 2.0, p = 0.17, η = 0.2; High: Cool = 106.4 ± 6; Nest = 89.2 ± 5.9; Low: Cool = 274 ± 11.8, Nest = 202.5 ± 13.7). No differences were observed for body weight (High: 22.9 ± 0.4 g; Low: 23.8 ± 0.7 g) and axillary temperature (High: 33.8 ± 0.3°C; Low: 34.3 ± 0.2°C) between lines (p > 0.05). For the odour conditions, the Line × Odour interaction was not significant [F(2, 56) = 0.2, p > 0.05 overall]. However, a post hoc inspection of the data on the interaction Line × Session × Odour [F(4, 112) = 1.6, p = 0.17, η = 0.3; Fig. 3] revealed that whereas Low Line USV rates were clearly not affected by exposure to any of the odours during any session, there were odour-induced changes in the High Line animals, which showed lower overall rates of USVs. In particular, High Line pups showed comparable increases of vocalisations both during as well as after the exposure to the soiled bedding (i.e., both FB and UB conditions), but not during and after the exposure to the CB condition (High-Iso1<High-Bedding in FB, p < 0.01; High-Iso1< Bedding and High-Iso1<High-Iso2 in UB, p < 0.01). The elevation effect seemed to be sustained with the UB condition, but restricted to the odour manipulation in the FB condition. The Line × Session × Odour × Temperature interaction was not significant [F(4, 112) = 0.3, p = 0.87]. Descriptive statistics for the two Lines across Sessions and Odour conditions are reported in Table 1.

Fig. 3 Line interaction × Session × Odour condition. Mean (±SE) of USVs emitted by Low and High Lines during the three-session test and the exposure to the three odour conditions. CB, clean bedding; FB, familiar bedding; UB, unfamiliar bedding; USV, ultrasonic vocalisation. **p < 0.01.
Table 1 Descriptive statistics
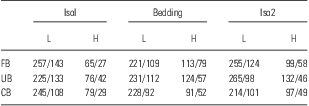
CB, clean bedding; FB, familiar bedding; UB, unfamiliar bedding.
Mean/SD of USVs emitted by Low (L) and High (H) Lines during the three-session test and the exposure to the three odor conditions.
No differences between lines were found for latencies to emit the first vocalisations (p > 0.05 for main effect and interactions).
Discussion
Previous work with these selectively bred lines has found that Low Line pups emit more USVs than High Line pups during a single isolation session (Reference Harmon, Cromwell and Burgdorf20). Our results confirm those data and extend the findings to new testing conditions using a novel nest-cue potentiation paradigm within the isolation–reunion–reisolation test procedure.
The sensitivity threshold to isolation does not differ between the two lines, as evidenced by a lack of difference in initial USV emission latencies following isolation. However, when comparing the total number of USVs, it becomes clear that the two lines differ in their overall response to a stressful condition, i.e., isolation. Low Line pups in all conditions consistently vocalised at higher levels than High Line pups, suggesting an enhanced responsiveness to negative stimuli in the Low Line animals, which were selectively bred for a lower responsiveness to positive stimuli. In addition, at this age and under these experimental conditions, the two lines responded comparably to variations in our thermal stressor, with both lines vocalising at higher rates at cool temperatures, revealing comparable susceptibility to physical distress, which suggests that these animals were differentially sensitive to social–emotional stress in particular.
Moreover, the lines varied in their responses to the presentation of a social odour. Low Line animals did not alter the amount of separation calls in response to any odours, while High Line animals, contrary to our prediction, increased the rate of calling during exposure to both social odours (FB and UB) and, accordingly with our prediction, the subsequent isolation (Iso2), although the increase from Iso1 to Iso2 was not quite significant in the FB condition. High Line animals did not show this ‘potentiation’ effect when exposed to a neutral odour (CB). The increase of USVs in the presence of social odours (but not in presence of the control odour) partially resembles the vocal behaviour of another rodent, the Octodon degus, under similar testing conditions (Reference Fuchs, Iacobucci, Mackinnon and Panksepp33). In that study, given a choice between FB and UB material, 12-day-old degus easily discriminated between the two kinds of bedding and showed a clear preference for FB. Interestingly, young degus emitted higher rates of separation calls when on FB, as compared with UB (Reference Fuchs, Iacobucci, Mackinnon and Panksepp33). Also, when tested in an isolation–reunion–reisolation paradigm, where animals were either exposed to FB or a social partner (mother or unfamiliar female) between isolations, degu pups exposed to bedding continued to vocalise, but those in the social partner conditions did not (T. Fuchs, personal observation). It seems reasonable that the odour cue serves as an indicator of proximity to the nest, stimulating the pups to vocalise, perhaps as an adaptation to request attention from a possibly nearby mother or caregiver. Our results partially corroborate this explanation in that High Line animals increased USVs in the presence of social odour, and this effect also persisted into the second isolation, though the increase was significant only for the UB condition. However, the High Line response to general social odour does not necessarily suggest that they form the maternal bonds that degus form. In fact, their similar response to both familiar and unfamiliar odours may speak against the capacity of infant rats to ‘bond’ to their home environments, even though this may happen at older ages when they are motorically mature enough to become lost (Reference Panksepp and Deeskinazi34). At that age, they may be more comparable to precocial species that show clear social bonding in infancy when ‘proximal separation’ of infants from mothers (e.g., being separated by a barrier) typically elevates separation distress type calls (Reference Wiener, Bayart, Faull and Levine35–Reference Colonnello, Iacobucci and Panksepp37).
The observed lack of USV potentiation in response to odour cues in Low Line animals could be due to a diminished responsiveness to social reward (social odour), as indicated by the low rates of 50 kHz USVs emitted in a variety of other, rewarding paradigms (Reference Knutson, Burgdorf and Panksepp7,Reference Burgdorf, Panksepp and Brudzynski21). Since, in our experiment the Low Line pups already had a high rate of vocalisation in Iso1, it would have been reasonable to expect that the social reward of the smell of mother's bedding might result in a reduction of calls. But this did not happen, suggesting a diminished ability for Low Line animals to experience social reward. Similarly to the High Line, we do not wish to suggest that they also showed diminished social bonding because, to the best of our knowledge, infant rats may not really bond to their mothers or homes as robustly as other mammals, perhaps because of reduced selection pressure under historically long-term laboratory breeding, and/or because their altricial status does not permit them to get lost on their own (Reference Panksepp, Newman and Insel38).
How do our results relate to the one other social-vocalisation breeding programme that has been undertaken? As mentioned earlier, Brunelli and Hofer (Reference Brunelli and Hofer16) developed bidirectional rat lines selected for high and low emissions of social isolation calls. Interestingly, they evidenced that their Lines exhibited differences in play behaviour and 50 kHz USVs during adolescence, with their High Line showing a depression of both pinning and 50 kHz USVs. Although a direct data comparison between the 50 kHz emitted during tickling and the isolation distress calls in response to social isolation is not possible, it is noteworthy that data from Brunelli and Hofer's high-isolation-call rats resemble results from our Low Line animals, especially in terms of sensitivity to first social isolation (i.e., total number of vocalisations emitted during the first isolation). Brunelli's group also found that USV differences detected in early infancy during isolation tests predicted divergent coping styles in later developmental stages (Reference Hofer, Shair, Masmela and Brunelli5,Reference Brunelli, Keating, Hamilton and Hofer39,Reference Brunelli40). Our results are consistent with that observation: the offspring of animals selected in the juvenile period for a positive social affective trait (high 50 kHz USVs) were more resistant to separation distress. Related studies have indicated that the High Line animals are resistant to negative affective challenges (Reference Burgdorf, Panksepp and Brudzynski21). This suggests that both breeding lines have selected for stable affective-temperamental traits, presumably within presently unidentified brain emotional networks, which may be important for understanding human social-affective temperaments.
Notably, low positive affectivity in children is a key factor in increased risk of developing psychological disturbances such as anxiety and depression (Reference Forbes and Dahl41), particularly following adverse life experiences. Nonetheless, little attention has been devoted to the study of positive affectivity as a protective factor against adversities throughout the lifespan. Along these lines, it is noteworthy that the main classic temperamental theories, while focusing on traits associated with negative affectivity as correlates of psychopathology (Reference Stelmack42,Reference Zuckerman43), may have underestimated the protective role that constitutional positive affectivity plays in psychopathology during development. Indeed, in recent human temperamental models and emotional problems, high positive affectivity has been considered a stable trait and a protective factor in youth against the emergence of psychopathological symptoms (Reference Lonigan, Phillips and Hooe44). From this perspective, further elucidation of the role of positive emotionality across the life-span, as modelled by our Low and High 50 kHz USV genetically selected lines, may help shed some light on how constitutional positive and negative emotional processes of the brain may regulate susceptibility to affective disorders and other psychiatric problems in later life.
A major limitation of the present work was our inability to include a randomly selected line of animals, since that line was exhibiting inbreeding sterility. We note that in a few more generations, both the High and Low Line animals exhibited similar breeding problems. We harvested brains and other bodily tissues from all lines for further analysis.
Acknowledgements
This research was supported by a Hope for Depression Research Foundation grant to J.P. V.C. and L.D. were supported by a fellowship from the National University Council of Italy (Sapienza University of Rome, IT). The authors are grateful to Sheri Six for her helpful editing and comments and to Professor Gianni Pavan (University of Pavia, IT) for providing free access to SeaPro software for recording and analysis of audible and ultrasonic vocalisations. The authors report no financial or other conflicts of interest.
Authors contributions: J.P. and P.I. conceived and designed the experiment, P.I. ran the experiment. All the authors analysed and interpreted the data, drafted the manuscript, and critically revised it.




