Introduction
The globally distributed taeniid cestode Taenia hydatigena Pallas, 1766 (class: Cestoda; subclass: Eucestoda; order: Cyclophyllidea; Family: Taeniidae) infects a wide range of definitive and intermediate hosts (Solusby, Reference Solusby1982). The parasite is usually transmitted in a dog–sheep cycle and can cause high economic losses to the sheep industry (Nourani et al., Reference Nourani, Pirali Kheirabadi, Rajabi and Banitalebi2010; Scala et al., Reference Scala, Pipia, Dore, Sanna, Tamponi, Marrosu, Bandino, Carmona, Boufana and Varcasia2015).
Taenia hydatigena infections were documented in sheep worldwide. In Egypt, T. hydatigena cysticerci were molecularly identified in sheep from the Southern governorates (Omar et al., Reference Omar, Elmajdoub, Al-Aboody, Elsify, Elkhtam and Hussien2016), but not from the Nile Delta, the largest agricultural region in Egypt. Thus, our objective was to determine for the first time the molecular characteristics of T. hydatigena cysticerci from sheep in Dakahlia governorate, the Nile Delta, Egypt. Additionally, we aimed to give a comprehensive overview on different epidemiological, clinical and molecular aspects of T. hydatigena infections in hosts other than pigs and cattle as for these species T. hydatigena infections have been reviewed previously (Nguyen et al., Reference Nguyen, Gabriël, Abatih and Dorny2016).
Materials and methods
Experimental study
Animals and study area
Carcasses and visceral organs of 200 local breed sheep aged 1–2 years old and of both sexes, but mostly males, were examined during a routine veterinary inspection in the main municipal slaughterhouse of Dakahlia governorate, Egypt in the period from January to August 2015. Dakahlia is a large (3500 km2) agricultural governorate located in the Nile Delta (North to Cairo) at approximately 31°50′N and 31°00′E with an annual average temperature of 22–28°C. This governorate has about 6 million inhabitants, many of them working in the agriculture and livestock production. Besides Dakahlia, four other governorates (Gharbia, Sharkia, Menoufiya and Kafr Elsheikh) are located in the Nile Delta, and all share their geographical boundaries with each other. In the Nile Delta, frequent movement of sheep for feeding on crop residues in the agricultural areas is common among different governorates. No official estimates for the sheep population in Dakahlia are available; however, approximately 5.5 million sheep are reared in Egypt (FAO, 2015).
Taenia hydatigena cysticerci in the slaughtered sheep were identified morphologically according to Loos-Frank (Reference Loos-Frank2000) and viable cysticerci with no signs of caseation or calcification were collected. From these, the invaginated protoscolices were harvested, washed several times with PBS and stored in 70% ethanol at −20°C. Samples were then transferred to the Institute for Parasitology, University of Veterinary Medicine Hannover, for further analysis.
Molecular analysis
DNA was extracted from the individual protoscolices using the NucleoSpin® Tissue kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. The primer pair 12SRF (5′-AGGGGATAGGACACAGTGCCAGC-3′) and 12SRR (5′-CGGTGTGTACATGAGCTAAAC-3′) was used to amplify a part of the 12S rRNA gene (Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2015). PCR reactions were carried out in a total volume of 50 μL containing 1 μL (25 μ m) of each primer, 1 μL (10 mm) dNTP mix, 5 μL PCR buffer (10×), 0.5 μL 5 Prime Perfect Taq DNA polymerase (5 Prime GmbH), 4 μL template DNA and 37.5 μL nuclease-free water. Thermocycling comprised the following conditions: 5 min at 94°C as an initial hot start, followed by 35 cycles of 30 s at 94°C, 45 s at 57°C, 35 s at 72°C and final extension for 10 min at 72°C. A negative control (no template DNA) was included in each experiment.
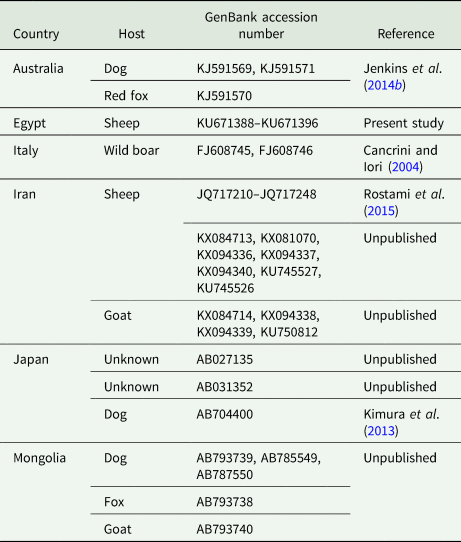
PCR products were visualized on 1% agarose gels stained with Gel Red™ nucleic acid stain (BIOTREND Chemikalien GmbH, Cologne, Germany). Bands of about 500 bp were cut off the gel and centrifuged at 5000 g for 2 min to squeeze out the amplification products for subsequent commercial Sanger-sequencing (Seqlab, Göttingen, Germany). Obtained sequences were confirmed as T. hydatigena using the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi). Alignment of the obtained sequences with sequences deposited in GenBank (Table 1, including available sequences from pigs) was conducted employing the software Bioedit (https://bioedit.software.informer.com/). Different diversity indices (haplotype and nucleotide), Tajima D neutral index (Tajima, Reference Tajima1989) and the Neighbor-Joining phylogenetic analysis were established using the software MEGA version 6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
Table 1. Partial 12S rRNA nucleotide sequences of Taenia hydatigena isolates included in the cluster analysis
Searching strategy
A systematic search was conducted using various databases including PubMed, Science Direct and Google Scholar. The following keywords were used: T. hydatigena, Cysticercus tenuicollis, canids, sheep, goat, ruminants and wild animals. Publications on cattle and pigs were excluded except one study in cattle and three recent studies in pigs not cited by Nguyen et al. (Reference Nguyen, Gabriël, Abatih and Dorny2016). Published papers in local journals from Egypt were also included. Criteria of inclusions were the full text of papers; articles available only as conference or proceeding abstracts were excluded. In total, 251 articles met the criteria to be selected for this review. Of these, 138 reported T. hydatigena infections in intermediate hosts, 101 in definitive hosts and 4 in both kinds of hosts. Information on animals, sampling year, country/region, sample size, number of positive samples and organ distribution was gathered from these studies. No statistical methods were used in this review.
Results
Detection of T. hydatigena cysticerci in Egyptian sheep and molecular analysis
Taenia hydatigena cysticerci were detected in 42 (21%) of the 200 examined sheep. Most of the cysts were found attached to the omenti and mesenteries; however, five animals carried cysts on the visceral liver surface. Cysticerci from nine animals, mostly old-aged, were caseated or calcified. Each cyst of the 33 animals carrying viable cyst was subjected to molecular analysis.
PCR analyses of the collected viable T. hydatigena cysts resulted in single gel bands at the expected size (489 bp). Sequencing of the PCR products yielded high-quality sequences of nine isolates, which were verified as T. hydatigena using BLAST, and deposited in GenBank under accession numbers KU671388–KU671396.
Cluster analysis and diversity indices
Sequence alignment of the nine Egyptian T. hydatigena isolates revealed 14 polymorphic sites. Overall, six haplotypes were determined. Of these, one comprised three isolates, another one comprised two isolates, and the four remaining haplotypes were detected in single isolates. In addition to the nine isolates obtained in this study, available T. hydatigena 12S rRNA nucleotide sequences deposited in GenBank (n = 63) were subjected to cluster analysis. Initial phylogenetic analysis displayed a bizarre clustering of two Iranian isolates from sheep (KX084713 and KX094337) and one from goats (KX094338) in a separate branch apart from T. hydatigena and Taenia saginata. BLAST search with these isolates showed the highest identities with Echinococcus granulosus (KX084713) and Echinococcus ortleppi (KX094337 and KX094338), respectively. Consequently, these isolates were supposed to be misidentified as T. hydatigena, and were excluded from subsequent analyses.
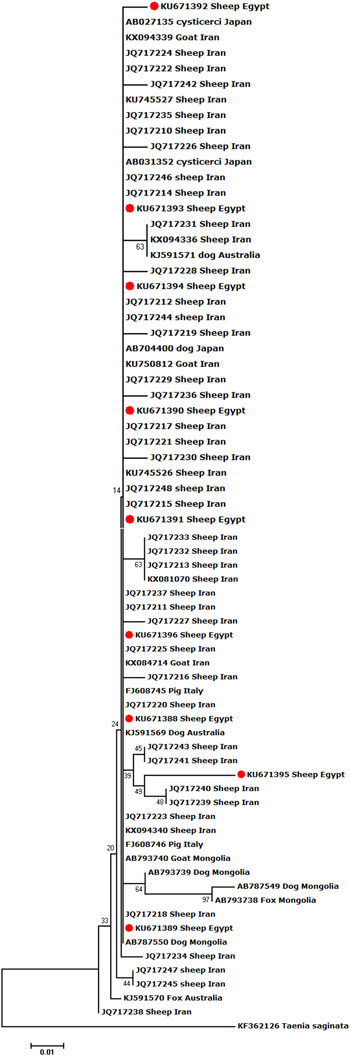
In total, 88 polymorphic sites and 55 haplotypes were detected among the remaining 69 isolates, indicating high haplotype diversity (0.797). Low nucleotide diversity (0.00339) and a negative value (−2.702) for the Tajima D neutrality index were also noted. Phylogenetically, our isolates clustered with those of T. hydatigena from different definitive and intermediate hosts in different geographical regions; however, the isolate KU671395 displayed marked genetic variation (Fig. 1).

Fig. 1. Neighbour-Joining phylogenetic tree of T. hydatigena isolates from different hosts and geographical regions worldwide. Taenia saginata was used as an outgroup. The tree was constructed by sequence sections (394 bp) of the mitochondrial 12S rRNA. Scale bar indicates the proportion of sites changing along each branch.
Discussion
Taenia hydatigena can cause serious implications to the livestock economy. In the following sections, we gathered the most important findings of the published papers on T. hydatigena infections worldwide, and our results were discussed within the respective sections.
Taenia hydatigena morphology
Morphological characters of the different taeniids overlap considerably (Edwards and Herbert, Reference Edwards and Herbert1981). Taenia hydatigena was first described by Verster (Reference Verster1979), then redescribed by Edwards and Herbert (Reference Edwards and Herbert1981). Loos-Frank (.Reference Loos-Frank2000) and Hoberg et al. (Reference Hoberg, Jones, Rausch, Eom and Gardner2000) tabulated the results of the earlier studies. Adult T. hydatigena from dogs measures up to 1 m in length. The scolex measures 601–682 μm in diameter and bears four suckers (228–273 μm) and a rostellum (373–382 μm) with two rows of 22–44 alternatively arranged large (175–228 μm) and small (118–157 μm) hooks. The mature proglottids have two ovarian lobes of unequal size; the poral lobe is smaller than the aporal one. The vagina has a characteristic dilatation proximal to the genital pore but devoid of the vaginal sphincter. Testis are 400–1000 in number, arranged in one layer and are not confluent posterior to the vitellarium. The cirrus pouch extends to the excretory vessels. The gravid proglottid is wider (4–8 mm) than long and the uterine branches (n = 6–10) have terminal bifurcations. The larval stage (C. tenuicollis) from the intermediate hosts can be easily identified. The cysticerci measure up to 7–10 mm in size and consist of a yellowish-white bladder completely filling a transparent capsule of walnut to apple size with a scolex suspended with a long neck (Loos-Frank, Reference Loos-Frank2000).
Taenia hydatigena infections in definitive hosts
Dogs are the main definitive hosts for T. hydatigena; however, sylvatic cycles for transmission are also considered, in which the parasite utilizes wild canids such as foxes, wolves and jackals (Jenkins et al., Reference Jenkins, Urwin, Williams, Mitchell, Lievaart and Armua-Fernandez2014b). Furthermore, occasionally cats may serve as definitive hosts (Karamon et al., Reference Karamon, Sroka, Dąbrowska, Bilska-Zając, Zdybel, Kochanowski, Różycki and Cencek2019).
Definitive hosts become infected after ingesting the larval stage (C. tenuicollis) in tissues of intermediate hosts. Protoscolices evert in the intestine, attach to the intestinal wall and start to grow. The number of the resultant worms is proportional to the number of the cysticerci fed; however, individual weight, length and number of proglottids of adult worms decrease with increasing infection dose (Parmeter et al., Reference Parmeter, Heath and Twaalfhoven1981). After growth, the ripened proglottids and/or eggs are shed in the feces. Taenia hydatigena eggs are environmentally resistant; they can survive for 250–400 days on pastures (Duthy and van Someren, Reference Duthy and van Someren1948; Cabrera et al., Reference Cabrera, Haran, Benavidez, Valledor, Perrera, Lioyd, Gemmell, Baraibar, Morana, Maissonave and Carballo1995), and in different relative humidity conditions; however, eggs are very sensitive to dry conditions (Thevenet et al., Reference Thevenet, Alvarez and Basualdo2017). Eggs lose their viability after heat treatment at 60°C for 5 min (Buttar et al., Reference Buttar, Nelson, Busboom, Hancock, Walsh and Jasmer2013a).
Worldwide reports on the prevalence of T. hydatigena in dogs are listed in Table 2. However, reports are not always comparable because of the highly varying number of dogs sampled, and utilization of different diagnostic tools. Diagnosis of cestode infections in alive dogs requires detection of the proglottids in feces (often after purgative treatment) or in the perianal region by the adhesive tape method. Shape and morphometric characteristics of the proglottid and the gravid uterus offer a sensitive tool of species identification, while detection of eggs in feces by coproscopy does not allow species identification because of identical morphological characteristics of taeniid eggs (Edwards and Herbert, Reference Edwards and Herbert1981). Indirect detection of T. hydatigena coproantigen in feces or antibody detection in serum using different types of antigens was tested, but hindered by cross-reactions with other taeniids (Jenkins and Rickard, Reference Jenkins and Rickard1985; Deplazes et al., Reference Deplazes, Gottstein, Stingelin and Eckert1990). In addition, coprophagia of feces of infected definitive hosts may lead to absorption of the antigens through the gut, resulting in false-positive serological reactions (Jenkins et al., Reference Jenkins, Gasser, Romig and Zeyhle1991), as well as false-positive coproscopic results. Molecular methods using e.g. PCR and employing different genetic markers provide a precise species diagnosis (Cabrera et al., Reference Cabrera, Canova, Rosenzvit and Guarnera2002).
Table 2. Worldwide reports on Taenia hydatigena prevalence in dogs
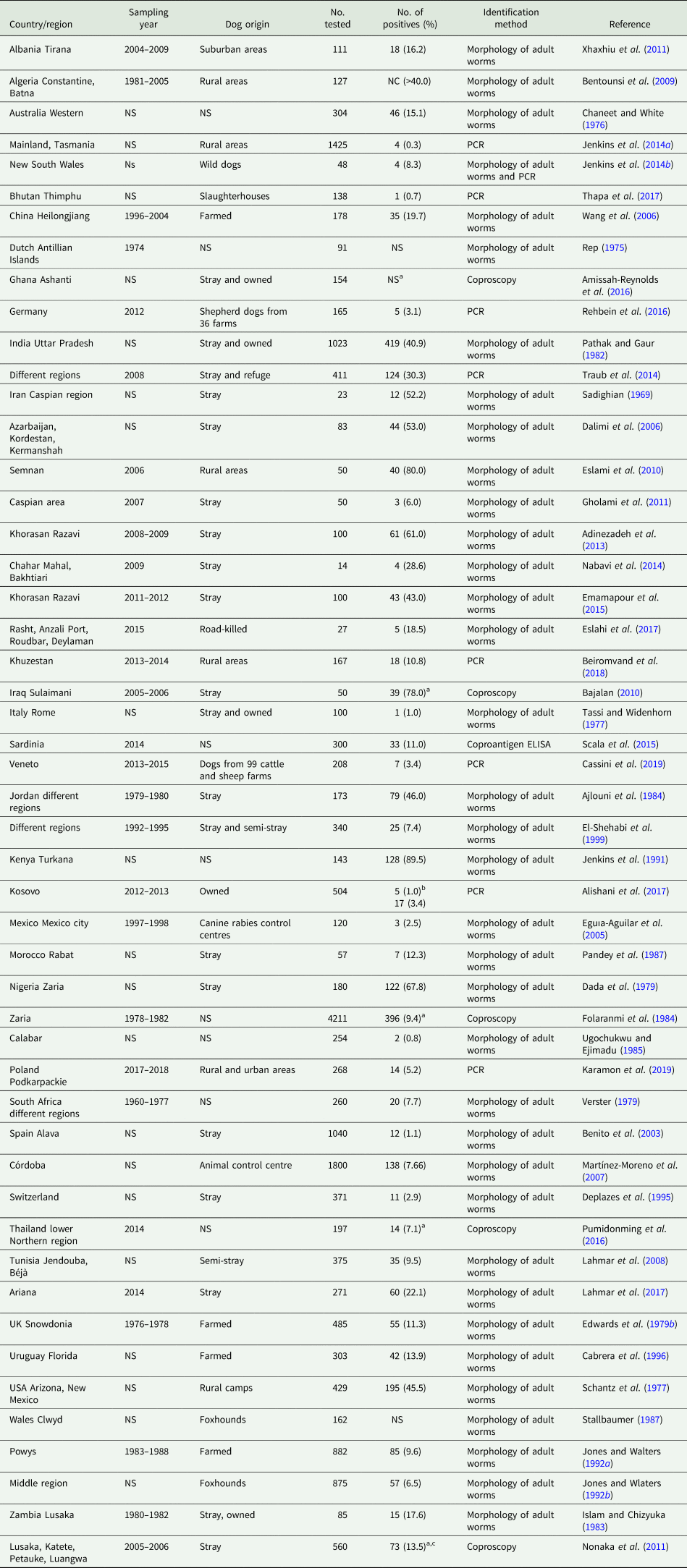
NS, not stated; NC, not clear.
a Unidentified Taenia spp.
b Dogs were inspected before and after feeding on sheep offal during a religious ceremony (Eid Al-Adha).
c Taenia hydatigena in 35 out of 38 PCR teaniid-positive samples.
Among the worldwide reports, the prevalence is very high in dogs from African countries and the Middle East, where sheep and goats are also highly infected with the larval stage (C. tenuicollis). Various reasons contribute to this high prevalence in both definitive and intermediate hosts: (1) most dogs are stray and widely spread T. hydatigena eggs in the environment, (2) shepherd dogs within sheep flocks are widely used, (3) most dogs do not receive anthelmintic treatment, (4) a nomadic pastoralism system for sheep rearing is used in these countries with sheep feeding on residues of agricultural crops and moving between lands, which increases the infection possibility with T. hydatigena eggs, (5) uncontrolled home slaughter of sheep and goats with raw offal usually fed to dogs, and (6) incorrect disposal of infected carcasses in slaughterhouses (Varcasia et al., Reference Varcasia, Tanda, Giobbe, Solinas, Pipia, Malgor, Carmona, Garippa and Scala2011).
Although stray dogs are usually exposed to parasitic infections more than farmed/owned ones, the effect of dog lifestyle on the prevalence of T. hydatigena is not entirely clear due to scarce reports comparing the prevalence in stray and owned or farmed dogs from the same geographical area. Moreover, T. hydatigena infections are more common in adult than in young dogs (Lahmar et al., Reference Lahmar, Arfa, Othmen, Jguirim, Saïd, Dhibi and Boufana2017), while no significant difference between males and females was reported (Ajlouni et al., Reference Ajlouni, Saliba and Disi1984). The prevalence is increased after certain ceremonial events such as Eid Al-Adha in Muslim countries, in which many sheep are slaughtered mostly outside slaughterhouses (Alishani et al., Reference Alishani, Sherifi, Rexhepi, Hamidi, Armua-Fernandez, Grimm, Hegglin and Deplazes2017).
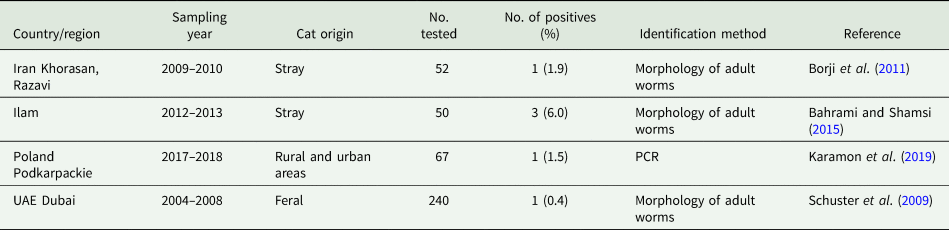
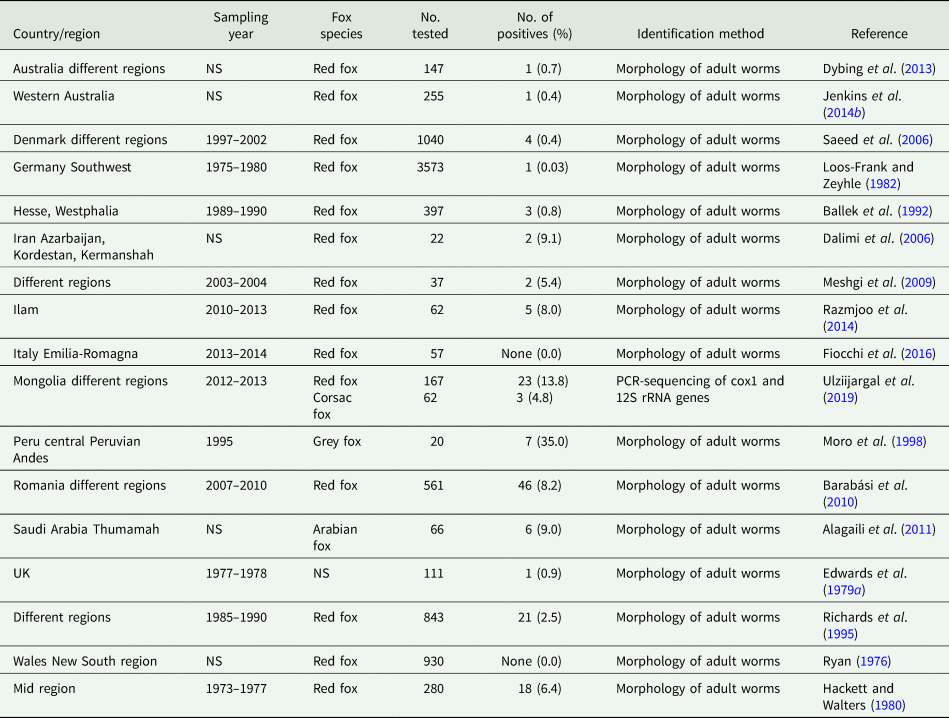
Besides domestic cycles including dogs and, more rarely, cats (Table 3), sylvatic T. hydatigena transmission cycles have been investigated in different, mostly European, countries. Data on the prevalence of T. hydatigena infections in foxes, jackals as well as wolves are given in Tables 4–6, respectively. Taenia hydatigena was also found in the intestines of 3.3% (3/91) free-ranging black bears (Ursus americanus) in Alberta, Canada (Dies, Reference Dies1979). Additionally, T. hydatigena was detected in wild felids. Of Eurasian Lynx (Lynx lynx), 3% (1/37) were reported to be infected in Estonia (Valdmann et al., Reference Valdmann, Moks and Talvik2004), and in the feces of two adult lions from the University of Ibadan Zoological Garden, Nigeria, proglottids were noted. Both lions were donated by the Zoo Leipzig in former East Germany and fed daily on raw goat meat (Ogungbade and Ogunrinade, Reference Ogungbade and Ogunrinade1984).
Table 3. Worldwide reports on Taenia hydatigena prevalence in cats

Table 4. Worldwide reports on Taenia hydatigena prevalence in foxes

NS, not stated.
Table 5. Worldwide reports on Taenia hydatigena prevalence in golden jackals

NS, not stated.
Table 6. Worldwide reports on Taenia hydatigena prevalence in grey wolves

a 13.3% (2/15) in wild wolves and 35.9% (14/39) in captive wolves.
Taenia hydatigena infections in the intermediate hosts
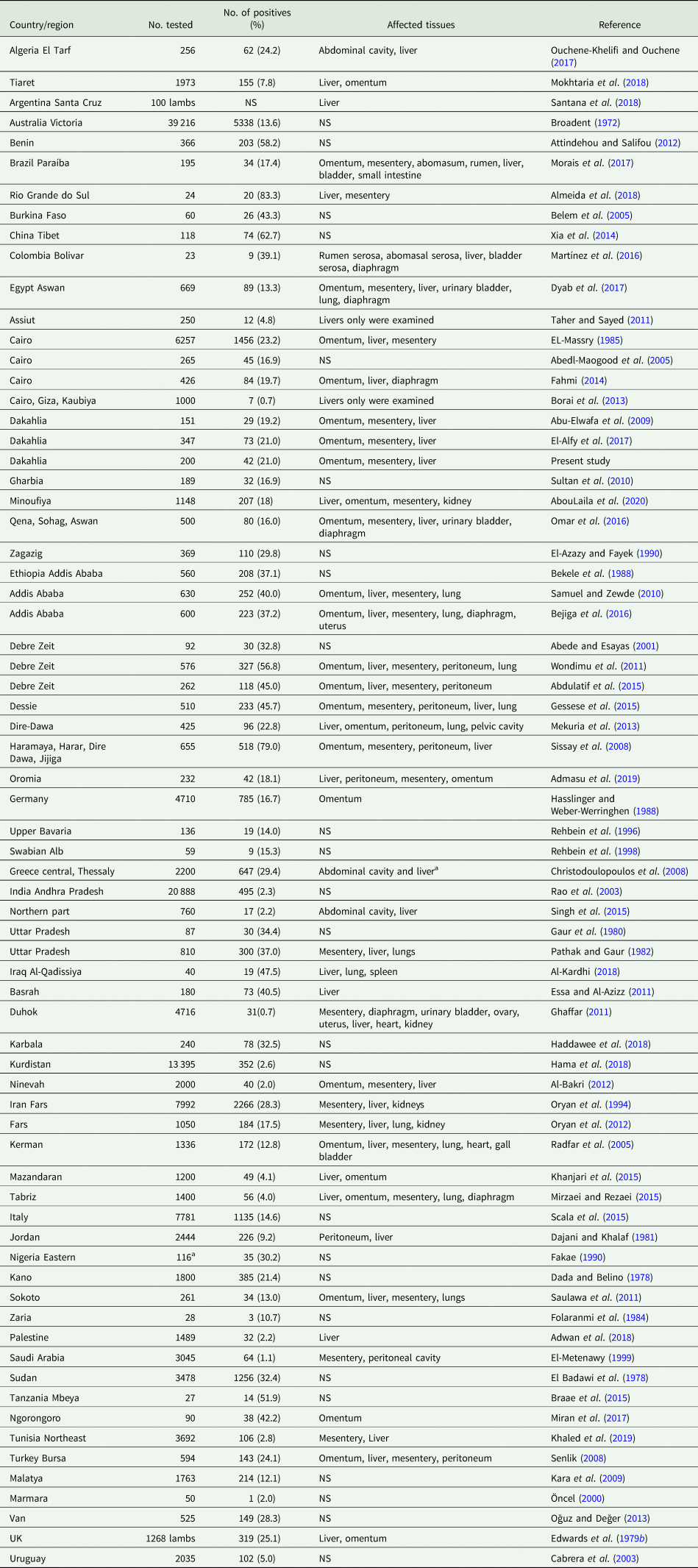
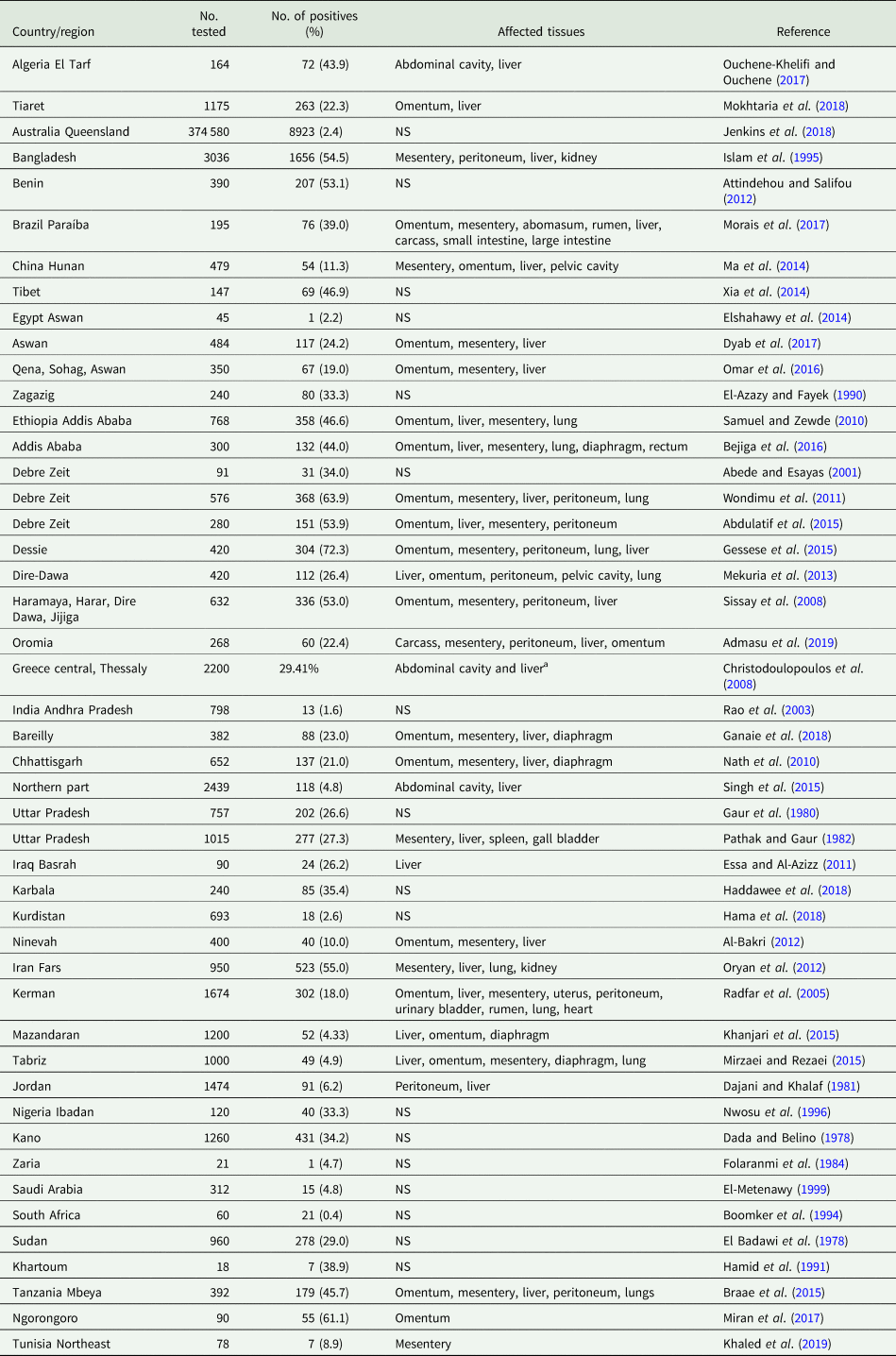
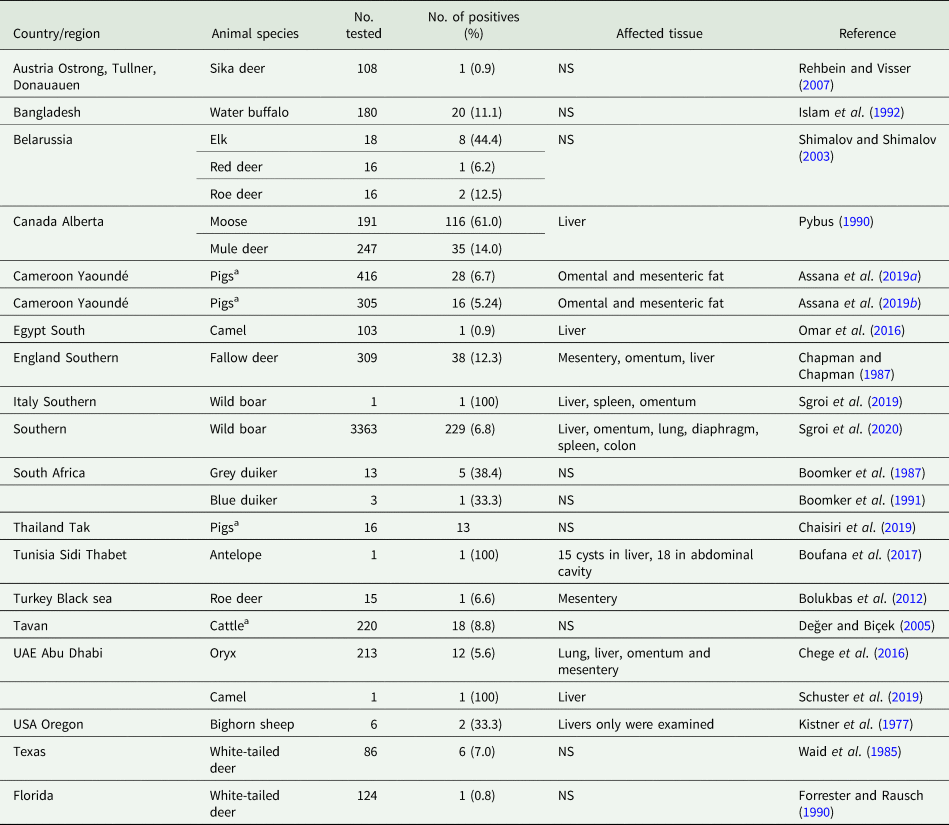
During its life cycle, T. hydatigena utilizes various intermediate hosts. Besides goats, sheep are the principal intermediate host for this parasite; and often sheep and goats co-graze on the same pastures. Reports on the prevalence of T. hydatigena cysticerci in sheep and goats worldwide supplemented with the results from the present study in Egyptian sheep are listed in Tables 7 (sheep) and 8 (goats). Noteworthy, the prevalence in sheep remained similar (19–21%) throughout three reports from Dakahlia governorate, covering the last 10 years. Overall, small ruminants from African countries and the Middle East showed to be infected at quite high percentages (up to 79%) for reasons given in the above section. Other domestic ruminants such as cattle, buffaloes and camels are also infected (Table 9). Wild boars are considered good hosts (Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Pacifico, Buono, Neola, Fusco, Santoro, Toscano, Fioretti and Veneziano2019, Reference Sgroi, Varcasia, D'Alessio, Varuzza, Buono, Amoroso, Boufana, Otranto, Fioretti and Veneziano2020), and wild ruminants including different deer species could play a role in T. hydatigena transmission (Table 9). Taenia hydatigena cysticerci were also observed attached to the omentum of Cynomolgus and Rhesus macque monkeys (Hobbs et al., Reference Hobbs, Colgin, Maginnis and Lewis2003; Tsubota et al., Reference Tsubota, Nakatsuji, Matsumoto, Fujihira, Yoshizawa, Okazaki, Murakami, Anagawa, Oku and Oishi2009).
Table 7. Worldwide reports on the prevalence of Taenia hydatigena cysticerci in sheep (tissues were ordered according to affection rates)
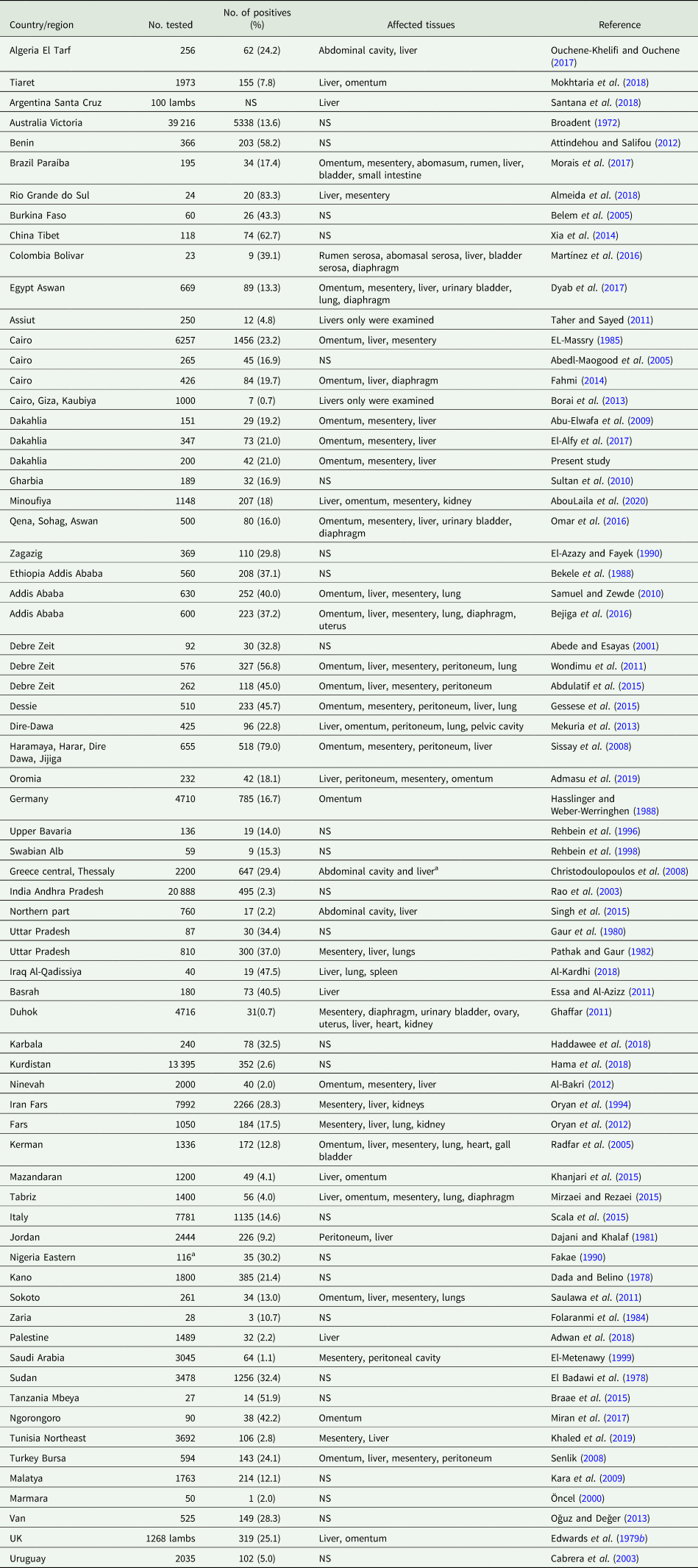
NS, not stated.
a 116 sheep and goats were examined; authors did not specify the number of animals examined in both species.
Table 8. Worldwide reports on the prevalence of Taenia hydatigena cysticerci in goats (tissues were ordered according to affection rates)
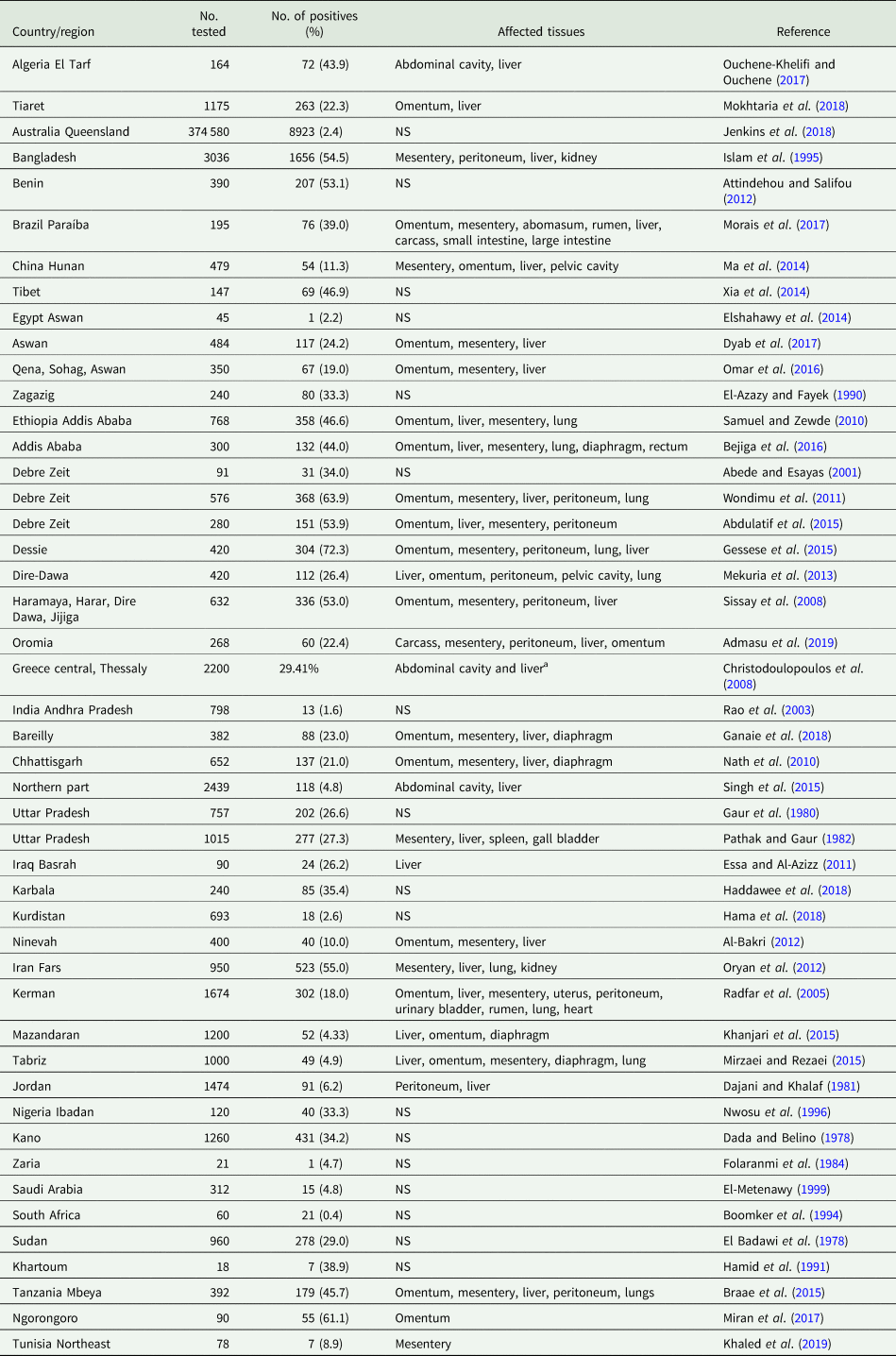
NS, not stated.
a 116 sheep and goats were examined; authors did not specify the number of animals examined in both species.
Table 9. Worldwide reports on the prevalence of Taenia hydatigena cysticerci in miscellaneous intermediate hosts

a Those studies have been included as it has not been mentioned in the review of Nguyen et al. (Reference Nguyen, Gabriël, Abatih and Dorny2016).
Epidemiology with focus on sheep
The large number of possible definitive and intermediate hosts for T. hydatigena is one key factor for the wide distribution of this parasite. Intermediate hosts acquire infection accidentally via ingestion of food or water contaminated with T. hydatigena proglottids and/or eggs from infected definitive hosts. The presence of infected dogs on the pastures increases the rate and intensity of infection. Gemmell and Macnamara (Reference Gemmell and Macnamara1976) compared these parameters in different groups of lambs before (group A) and after (group B) the removal of infected dogs from the pasture. At slaughter 6 months later, significantly higher T. hydatigena infection rates (65%) and cysts count (4–104) were found in group A compared to group B (6.5% infection rate and 0–3 cysts count).
In addition, mechanical transmission of T. hydatigena eggs has been documented in dung beetles (Vargas-Calla et al., Reference Vargas-Calla, Gomez-Puerta, Pajuelo, Garcia and Gonzalez2018), blow flies (Lawson and Gemmell, Reference Lawson and Gemmell1985), potatoes (Buttar et al., Reference Buttar, Nelson, Busboom, Hancock, Walsh and Jasmer2013b), and vegetable and fruits (Federer et al., Reference Federer, Armua-Fernandez, Gori, Hoby, Wenker and Deplazes2016). Upon ingestion, eggs hatch in the small intestine. Oncospheres are liberated, penetrate the wall of the gut, and usually reach the liver through the portal blood stream. Sometimes, oncospheres reach other organs such as lungs and kidneys (Scala and Marrosu, Reference Scala and Marrosu1997). In sheep, oncospheres reach the liver 7 days after ingestion and the immature cysticerci migrate to the liver surface 18 days later (Sweatman and Plummer, Reference Sweatman and Plummer1957). Cysticerci may remain in the liver or drop in the abdominal cavity and attach to the omentum or mesentery. Additionally, cysticerci may develop in the lungs or the serosal surface of the peritoneal cavity. Within 8 weeks, the cysticerci become mature and remain infective to dogs for several months. Cysticerci that died during liver migration may remain surrounded by granulomatous lesions, and calcium salts are deposited around them (Gemmell and Lawson, Reference Gemmell and Lawson1985).
Cysticerci are more common in lambs than in old-aged sheep as well as in fattening lambs than slower growing ones. Due to immunity (see below), cysticerci are rather uncommon in ewes, in which they may be of considerable volume, sometimes dead and organized, whereas in fatting lambs the cysticerci are usually small (Edwards and Herbert, Reference Edwards and Herbert1980).
Predilection sites of the cysticerci
Cysticerci of T. hydatigena are frequently found attached to the omentum, mesentery, liver and peritoneum (cf. Tables 7 and 8). Lungs, kidneys and brain are less common attachment sites. Sometimes, the cysticerci attach to ovaries, uterine tubes, cervix and the outer surface of the uterus (Smith et al., Reference Smith, Parkinson and Long1999), and were recently noticed inside the chorio-allantoic membranes of goat fetus, indicating materno-fetal transmission of T. hydatigena onchospheres (Payan-Carreira et al., Reference Payan-Carreira, Silva, Rodrigues and Dos Anjos Pires2008; Al Salihi et al., Reference Al Salihi, Rahee and Ali2016).
Clinical signs and pathology in intermediate hosts and resulting economic loss
Taenia hydatigena infections in intermediate hosts are usually asymptomatic; however, hepatitis cysticercosa caused by migrating cysticerci is common sequelae of infection (Blazek et al., Reference Blazek, Schramlova and Hulinska1985; Nourani et al., Reference Nourani, Pirali Kheirabadi, Rajabi and Banitalebi2010), and may be fatal in heavy infections producing massive destruction to the liver parenchyma (Scala et al., Reference Scala, Urrai, Varcasia, Nicolussi, Mulas, Goddi, Pipia, Sanna, Genchi and Bandino2016). Acute fatal cysticercosis in sheep and goats has been reported from the UK (Livesey et al., Reference Livesey, Herbert, Willis and Evans1981), Italy (Manfredi et al., Reference Manfredi, Ghirardelli and Zanzani2006), Turkey (Yildirim et al., Reference Yildirim, Iça, Beyaz and Atasaver2006), Greece (Koutsoumpas et al., Reference Koutsoumpas, Psychas, Papadopoulos, Panousis, Karatzias and Giadinis2013) and Israel (Perl et al., Reference Perl, Edery, Bouznach, Abdalla and Markovics2015). Lung involvement is also commonly observed during acute cysticercosis (Darzi et al., Reference Darzi, Pandit, Shahardar and Mir2002). Additionally, severe hepatic alterations due to the involvement of the liver with multiple small (3–6 mm) cysticerci of T. hydatigena were documented in a camel calf from a farm in Dubai, UAE. This calf died shortly after translocation with his mother from Pakistan, and the majority of the cysticerci were in a stage of caseous degeneration; their identity was molecularly confirmed (Schuster et al., Reference Schuster, Kinne, Boufana and Varcasia2015).
The infection dose correlates with the severity of the disease. Edwards and Herbert (Reference Edwards and Herbert1980) fed six lambs with T. hydatigena eggs at different doses; four lambs showed clinical signs, and two of them died. Both dead lambs were infected with 25 000 eggs and exhibited fever, lethargy, anorexia and jaundice. Livers were grossly enlarged with numerous haemorrhagic tracts. The liver from one lamb harboured 5318 juvenile cysticerci (8 mm), which were also recovered from washings of the abdominal cavity and lungs. The two recovered lambs showed clinical signs 3–4 days after infection, which continued for 18–20 days; one of them was infected with the same dose, whereas the other lambs, as well as lambs with no clinical signs, were given trickle doses each of 5000 eggs in alternate days. Elevated levels of liver enzymes (GOT and GPT) were observed in all infected lambs.
In chronic infections with low parasite burdens, most liver lesions become resolved. Livers may pass for human consumption or may be partially or completely condemned (Trees et al., Reference Trees, Owen, Craig and Purvis1985). Based on liver condemnations of lambs, resulting economic losses were exemplarily estimated to be $65 000 and $370 600 annually in Ethiopia and Italy, respectively (Wondimu et al., Reference Wondimu, Abera and Hailu2011; Scala et al., Reference Scala, Pipia, Dore, Sanna, Tamponi, Marrosu, Bandino, Carmona, Boufana and Varcasia2015).
Diagnosis
Diagnosis of T. hydatigena infections in definitive hosts including the limitations of e.g. coproscopy are described in the respective section above. In intermediate hosts, macroscopic examination for T. hydatigena cysticerci during meat inspection is the diagnostic gold standard. However, routine meat inspection can be of low sensitivity because immature small cysts often remain unnoticed. Antibody detection assays offer diagnosis in sera from live animals. However, as described above, these assays are limited regarding species identification, because antigens of different Taenia species are very similar and, therefore, cross-reactions between Taenia spp. are common in immunodiagnosis (Craig and Rickard, Reference Craig and Rickard1980). Craig and Rickard (Reference Craig and Rickard1981) used the ELISA technique to diagnose T. hydatigena and T. ovis in experimentally infected lambs. They found onchospheral antigens prepared from eggs to be non-species-specific, and the antibodies raised against these antigens were detectable 1 week post-infection, reached their peak after 3–4 weeks and lasted for 8–12 weeks, while antibodies resulting from strobilate antigen extracts lasted for a longer time (18 weeks). El-Massry (Reference El-Massry1999) tested sera from 500 sheep for T. hydatigena and found 151 (30.2%) individuals to be ELISA-positive. During postmortem inspection, 100 sheep were diagnosed to harbour T. hydatigena cysticerci. Of these, 91 tested seropositive, while in the remaining 60 seropositive sheep, no T. hydatigena cysticerci were found. Recently, hepatic ultrasonography was proven to be effective for the diagnosis of acute cysticercosis in lambs (Corda et al., Reference Corda, Dessì, Varcasia, Carta, Tamponi, Sedda, Scala, Marchi, Salis, Scala and Pinna Parpaglia2020).
Immunity
In dogs as definitive hosts, no immunity develops after T. hydatigena infection and re-infection is common (Heath et al., Reference Heath, Parmeter and Osborn1980). For example, Cabrera et al. (Reference Cabrera, Parietti, Haran, Benavidez, Lloyd, Perera, Valledor, Gemmell and Botto1996) noted T. hydatigena re-infection in dogs 2 months after anthelmintic treatment. This lack of immunity in dogs and presumably other definitive hosts sustains the persistence and distribution of the parasite.
In contrast, sheep develop immunity against T. hydatigena already after primary infection. This immunity manifests by (1) absence or reduction in the number of cysticerci, (2) loss of cysticerci viability or (3) both (Gemmell et al., Reference Gemmell, Lawson, Roberts and Griffin1990). Gemmell (Reference Gemmell1964) reported solid immunity in sheep after oral challenge infection with T. hydatigena eggs (n = 2500) following parenteral injection of viable T. hydatigena eggs or active embryos. In addition, the use of culture antigens of hatched T. hydatigena onchospheres provided a good level of protection against reinfection (Onawunmi and Coles, Reference Onawunmi and Coles1980). A study investigating the possible involvement of neutrophils in the immunity against T. hydatigena infection showed that there might be an interplay between these cells and antibodies: in vitro experiments with neutrophils collected from the mammary glands of both T. hydatigena infected and uninfected sheep, attached to and killed oncospheres in the presence of serum from infected sheep (Beardsell and Howell, Reference Beardsell and Howell1984).
Acquired immunity to T. hydatigena is transferred from the dam to her offspring. After grazing on contaminated pastures for 10 days, Gemmell et al. (Reference Gemmell, Lawson, Roberts and Griffin1990) noted a lower proportion of viable T. hydatigena cysticerci in lambs from ewes previously exposed to T. hydatigena than those from previously unexposed ewes. Additionally, short-acting protection of 1–3 weeks was detected in lambs receiving colostrum from infected ewes (Jacobs et al., Reference Jacobs, Moriarty, Charleston and Heath1994).
Treatment and control
Taenia hydatigena infections in dogs or other definitive hosts can easily be treated with the isoquinoline derivates praziquantel and epsiprantel. Additionally, several benzimidazoles, niclosamide and nitroscanate are effective against taeniosis (Saari et al., Reference Saari, Näreaho and Nikander2010). No reports on anthelmintic resistance of T. hydatigena are available. Praziquantel in high doses (15 mg kg−1) was also used successfully in lambs suffering from acute T. hydatigena cysticercosis (Scala et al., Reference Scala, Urrai, Varcasia, Nicolussi, Mulas, Goddi, Pipia, Sanna, Genchi and Bandino2016); however, no product is licensed in this indication. Unlike Taenia solium and Taenia saginata, no vaccination trials to control T. hydatigena infections in both definitive and intermediate hosts are reported.
Improved regional epidemiological data on T. hydatigena occurrence is essential for its control, especially in endemic areas. Careful routine meat inspection procedures in slaughterhouses including tissues other than the cysticerci predilection sites, and proper condemnation of the infected tissues or organs are mandatory. Various strategies were evaluated to control this ubiquitous parasite. Of them, regular simultaneous treatment of both definitive and intermediate hosts proved its efficacy (Harris et al., Reference Harris, Revfeim and Heath1980).
Molecular characterization of T. hydatigena isolates
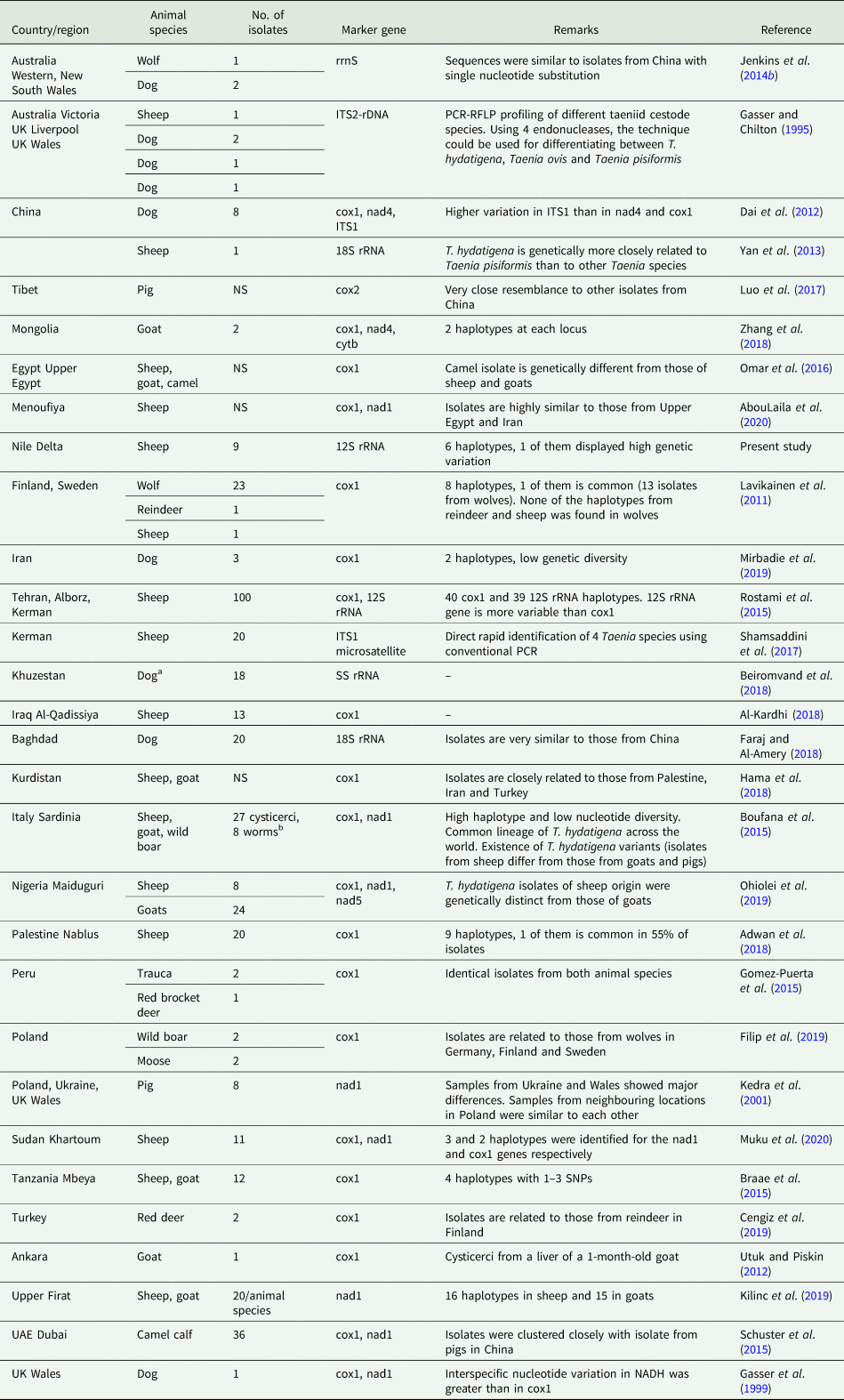
Molecular analysis of T. hydatigena isolates, adult worms and cysticerci, aids in understanding the circulation patterns of this parasite among different definitive and intermediate hosts, and in the implementation of effective control strategies. Based on the mitochondrial 12S rRNA gene, the present study is the first to describe the molecular characteristics of T. hydatigena isolates from sheep in Dakahlia governorate, Egypt. Using this marker, one of our isolates showed to be genetically different from the others. By use of another marker (cox1), Omar et al. (Reference Omar, Elmajdoub, Al-Aboody, Elsify, Elkhtam and Hussien2016) investigated the molecular characteristics of isolates from sheep, goats and camels in Southern Egypt. Earlier molecular analyses using mitochondrial genes reported nad1 displaying greater inter-taxon sequence variations than cox1 (Gasser et al., Reference Gasser, Zhu and McManus1999), and 12S rRNA displaying greater T. hydatigena intra-taxon variation than cox1 (Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2015). Furthermore, variable sections of the nuclear 18S rRNA gene are highly discriminative among members of the genus Taenia including T. hydatigena (Yan et al., Reference Yan, Lou, Li, Ni, Guo, Li, Zheng, Dyachenko and Jia2013).
Many different T. hydatigena haplotypes have been recorded in different geographical regions. Based on our analysis including 69 worldwide T. hydatigena isolates, high haplotype diversity was noted, which could be attributed to (1) different definitive and intermediate hosts, (2) hosts population and dispersal in countries, (3) farming practices, and (4) infection rate (Boufana et al., Reference Boufana, Scala, Lahmar, Pointing, Craig, Dessì, Zidda, Pipia and Varcasia2015; Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2015). However, the existence of a common lineage of T. hydatigena circulating worldwide was suggested (Boufana et al., Reference Boufana, Scala, Lahmar, Pointing, Craig, Dessì, Zidda, Pipia and Varcasia2015; Sgroi et al., Reference Sgroi, Varcasia, D'Alessio, Varuzza, Buono, Amoroso, Boufana, Otranto, Fioretti and Veneziano2020), possibly resulting from the worldwide circulation of the parasite through human and animal transportation (Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2015). Based on the biochemical, morphological and molecular differences in T. hydatigena cysticerci from intermediate hosts, the possibility of strain variations is stated (Abidi et al., Reference Abidi, Nizami, Khan, Ahmad and Irshadullah1989; Radfar et al., Reference Radfar, Jajalli and Jalalzadeh2005; Boufana et al., Reference Boufana, Scala, Lahmar, Pointing, Craig, Dessì, Zidda, Pipia and Varcasia2015; Singh et al., Reference Singh, Sharma, Gill and Sharma2015). Significant results on the genetic variation and population structure of T. hydatigena are summarized in Table 10.
Table 10. Worldwide reports on molecular characterization of Taenia hydatigena isolates

cox, mitochondrial cytochrome c oxidase subunit gene; nad, mitochondrial NADH dehydrogenase subunit gene; ITS, internal transcribed spacer; SS rRNA (rrnS), small-subunit ribosomal RNA gene; cytb, cytochrome b; ND, not done; NS, not stated.
a Eggs from dog feces.
b Taenia hydatigena tapeworm isolates from necropsied dogs in Sicily (n = 1), Tunisia (n = 5) and Wales (n = 2) were included for comparison.
Conclusion
Taenia hydatigena is a ubiquitous parasite circulating worldwide and utilizing various intermediate and definitive hosts in domestic and sylvatic cycles of transmission. In this review, we gathered the available information from the earlier publications about T. hydatigena in both kinds of hosts. In addition, we updated the prevalence of T. hydatigena cysticerci from sheep in Egypt, where sheep are mainly reared in small flocks including one or two dogs for protection. No reports on this parasite in dogs from Egypt are available, thus a study on the dispersal of T. hydatigena in dogs, particularly shepherd ones, from Egypt is needed. For the first time, molecular characteristics of isolates from sheep in the Nile Delta were determined. Analysis of newly generated and published Genbank-retrieved 12S rRNA partial nucleotide sequences of T. hydatigena elucidated genetic variants among the Egyptian isolates and a future large-scale study with a large number of samples from various intermediate and definitive hosts is desirable to confirm the present findings.
Acknowledgements
We thank Mansoura University, Egypt, for supporting Ibrahim Abbas with a 1-month externship at the Institute for Parasitology, Center for Infection Medicine, University of Veterinary Medicine Hannover, Germany.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
All national and international ethical guidelines were followed during the handling of the sampled animals. This study was approved by the ethics committee at the Faculty of Veterinary Medicine, Mansoura University, Egypt.