It is well known that milk protein or milk protein hydrolysate is beneficial to the absorption of calcium (Osborne et al. Reference Osborne, McTyre, Dudek, Roche, Scheuplein, Silverstein, Weinberg and Salkeld1996). With the increased production of casein from milk, more and more whey protein is discarded, the reasonable exploitation of whey protein becomes particularly significant. However, whey protein is sensitive to acid or heat conditions, which cause decreases in solubility. The modification of whey protein has become an immediate area of research focus all over the world. Enzymatic modification can produce biological active peptides.
Calcium deficiency results in hypertension, osteoporosis and intestine cancer (Osborne et al. Reference Osborne, McTyre, Dudek, Roche, Scheuplein, Silverstein, Weinberg and Salkeld1996). The intake of calcium could increase the bone density of children and it is essential among the middle-aged and the aged to prevent osteoporosis (Guénguen & Pointillart, Reference Guénguen and Pointillart2000; Cilla et al. Reference Cilla, Lagarda, Alegría, Ancos, Cano, Sánchez-Moren, Plaza and Barberá2011). With the increase in population of the aged throughout the world, there is a growing interest in developing calcium supplements as medicine to prevent and treat bone disease (Kim & Lim, Reference Kim and Lim2004). The ionised calcium has served as main calcium supplements for human beings in recent years (Lee & Song, Reference Lee and Song2009). However, the disadvantage of ionised calcium is that it is prone to form calcium phosphate deposition in basic intestine environment (Bronner & Pansu, Reference Bronner and Pansu1998). As a result, the bioavailability of dietary calcium is severely lowered. The organic calcium supplement including calcium-binding peptides has been becoming a popular research topics (Narin et al. Reference Narin, Benjamas, Nualpun and Wirote2013).
Studies showed that vitamin D-dependent calcium-binding protein and calmodulin were the main source of calcium supply in the body. As the main component of milk protein, whey protein (WP) has calcium-binding sites, such as β-lactoglobulin, α-lactalbumin and lactoferrin. Moreover, α-LA has especially strong calcium-binding sites (Jeyarajah & Allen, Reference Jeyarajah and Allen1994; Feng et al. Reference Feng, van der Does and Bantjes1995; Bennett et al. Reference Bennett, Desmond, Harrington, McDonagh, FitzGerald, Flynn and Cashman2000; Kim et al. Reference Kim, Shin and Lim2004).
The objective of this study was to purify and characterise a highly specific calcium-binding peptide from whey protein hydrolysates. The research would be of significance in utilising the hydrolysed peptides from whey protein as calcium-binding peptide ingredients in functional food.
Materials and methods
Materials
Whey protein was purchased from Hilmar Corporation (Batch No. 20111107) (USA). The commercial protease, Flavourzyme (EC. 3.4.11.1, 2 × 106 U/g) and Protamex (EC. 3.4.21.62 and EC. 3.4.24.28, 1·5 × 106 U/g) were purchased from Novo (Novozymes, Denmark). TOYOPEARL DEAE-650M and Sephadex G-25 were offered by Amersham Pharmacia (Amersham Pharmacia Co., Uppsala, Sweden). All the other chemicals and solvents were analytical grade reagents and high performance liquid chromatography (HPLC) grade.
Preparation of whey protein hydrolysates
In order to prepare whey protein hydrolysates, 5% (w/v) whey protein solution was denatured at 80 °C for 20 min, and the pH was adjusted to 7·0. The sample was hydrolysed using Flavourzyme and Protamex in a 2 : 1 (w/w) mixture, with substrate to enzyme ratio of 25 : 1 (w/w) at 49 °C for 7 h. Hydrolysate was heated in boiling water for 10 min to inactive the enzymes and cooled to room temperature. The mixture was subsequently centrifuged at 16 000 g for 20 min, then the supernatant named whey protein hydrolysates (WPH) was lyophilised and stored at −20 °C for subsequent purification.
Isolation and purification of calcium binding peptide
DEAE anion exchange chromatography
The slurry of TOYOPEARL DEAE-650M was packed in a column (20 × 2·5 cm), then equilibrated at 5 column volume (CV) 20 mm Tris-HCl buffer (pH 9·0) as equilibrating buffer. Afterwards, 100 mg lyophilised hydrolysates that had been through 0·45 μm filter film were dissolved in 10 ml of the same buffer (pH 9·0) and loaded on the column. Then washed with the equilibrating buffer, the collected peak was labelled as non-absorbed fraction. The bond peptides were eluted by a gradient elution with the same buffer containing 0–0·5 m NaCl. The flow rate was 0·5 ml/min, fraction volume was 5 ml/tube, and elution was monitored at 214 nm, all peaks were collected and calcium-binding capacities of the fractions were determined.
Sephadex G-25 gel-filtration chromatography
The highest calcium-binding capacity fraction was pooled and lyophilised, 200 mg of the sample were dissolved in 5 ml deionised water and loaded onto a Sephadex G-25 column (100 × 2·0 cm) which had previously been equilibrated with deionised water, then eluted with deionised water at flow rate of 0·3 ml/min. The eluate was monitored by measuring the absorbance at 214 nm. After calcium-binding capacity was determined, the fraction with highest activity was pooled and lyophilised.
Reversed phase HPLC
The lyophilised sample collected from G-25 was dissolved in distilled water approximately equivalent to 30 mg/ml and purified by semi-preparation reversed phase high-performance liquid chromatography (RP-HPLC) on a C-18 reversed-silica gel chromatography (Gemini 5 μ C18, 250 × 10 mm, Phenomenex Inc., Torrance, CA, USA). Elution was performed with solution A (0·05% trifluoroacetic acid (TFA) in water) and solution B (0·05% TFA in acetonitrile) with a gradient of 0–30% B at 1·0 ml/min for 50 min. The elution was monitored at 214 nm, the absorption peaks were fractionated for measuring the calcium-binding activity. The injection volume was generally 200 μl.
The active fraction from preparative RP-HPLC was further applied on analytical HPLC for purity analysis. The elution condition was the same as semi-preparation RP-HPLC.
Amino acid sequence of calcium binding peptide
Amino acid sequence of purified calcium-binding peptide was determined using a liquid chromatography/electrospray ionisation (LC/ESI) tandem mass spectrometer (Lee & Song, Reference Lee and Song2009) (Delta Prep 4000, Waters Co., USA) over the m/z range 300–3000.
Calcium binding activity analysis
Lyophilised whey protein hydrolysate was dissolved in deionised water at 1·0 mg/ml, and mixed with 5 mm CaCl2 in 0·2 m sodium phosphate buffer (pH 8·0). The solution was stirred at 37 °C for 2 h and pH was maintained at 8·0 with a pH meter. The reaction mixture was centrifuged at 10 000 g at room temperature for 10 min in order to remove insoluble calcium phosphate salts. The calcium contents in the supernatant were determined using a colorimetric method with ortho-cresolphthalein complexone reagent (Gitelman, Reference Gitelman1967). The absorbance at 570 nm was determined after adding the working solution to the sample. The experiments were performed in triplicate, and values were expressed as mean ± standard deviation (sd).
Structural characterisation of whey protein hydrolysate-calcium complex
Preparation of the peptide-calcium complex
The calcium-binding complex was prepared by adding 5 ml 1% (w/v) CaCl2 into 20 ml of 2·5% (w/v) calcium-binding peptide solution. The reaction was placed in a controlled water bath with constant agitation (100 rpm) at 37 °C for 2 h after the pH of the solution was adjusted to 7·0 by the addition of 0·1 m NaOH. Then the mixture was mixed with absolute ethanol (9 times of solution volume) to remove free calcium and centrifuged at 10 000 g for 10 min, the precipitates were lyophilised for analysis.
Ultraviolet absorption spectroscopy
The ultraviolet spectra of calcium-binding peptide and its calcium complex were recorded over the wavelength range from 190 to 400 nm by a UV-Vis spectrophotometer (UV-2600, UNICO Instrument Co. Ltd., Shanghai, China) as described by Chen et al. (Reference Chen, Liu, Huang, Zhao, Dong and Zeng2013). The calcium-binding peptide of 0·2 mg/ml was prepared. The peptide-calcium complex was prepared by adding 0, 20, 40, 60, 80 and 100 μm CaCl2 to 0·2 mg/ml calcium-binding peptide solution, respectively. The mixed solution reacted at room temperature for 30 min.
FTIR measurement
One milligram of dry powder sample of calcium-binding peptide/peptide-calcium complex was mixed with 100 mg KBr and Fourier Transform Infrared (FTIR) spectrograph was performed. The FTIR spectra were recorded using an infrared spectrophotometer from 4000 to 400 cm−1 (360 Intelligent, Thermo Nicolet Co., USA) (Wang et al. Reference Wang, Zhou, Tong and Mao2011). The peak signals in the spectra were analysed using OMNIC 8·2 software (Thermo Nicolet Co., Madison, WI, USA).
Sequestered calcium percentage determination
The sequestered calcium percentage determination of EG-Ca and CaCl2 was examined according to the method described by Wang et al. (Reference Wang, Zhou, Tong and Mao2011) and Zhou et al. (Reference Zhou, Wang, Ai, Cheng, Guo, Teng and Mao2012). EG-Ca and CaCl2 were separately dissolved in deionised water equivalent to 10 μg/ml. The pH of these solutions was adjusted to 2·0, 3·0, 4·0, 5·0, 6·0, 7·0, 8,0. After incubation in a shaking water bath for 2 h at 37 °C, the solutions were centrifuged in a refrigerated centrifuge at 10 000 g for 10 min. The calcium amount in supernatant and the total calcium in the whole solution were determined by colorimetric method with ortho-cresolphthalein complexone reagent. The sequestered calcium percentage was calculated as follows:
Statistical analysis
All experiments were conducted in triplicate. Data were presented as means ± sds. Analysis of variance and Duncan's multiple range tests were performed to analyse the results using the SPSS software program (SPSS Inc., Chicago, IL, USA). The linear relationship between the peptide concentration and the binding ratio was performed using Origin 8·0 (Origin Lab Co., USA).
Results and discussion
Purification of WPH
Fraction 2 from DEAE was taken forward for further purification by Sephadex-G25 gel-filtration chromatography (Table 1). Fraction 23 showing the highest calcium binding activity was further purified using semi-preparative reversed-phase HPLC and was separated into thirteen fractions (Fig. 1a). Fractions 7 and 13 possessed the highest calcium binding abilities (Fig. 1b) and there was no significant difference between their activities (data not shown). The purified fraction 13 named WPH-1 was loaded onto analytical RP-HPLC column to identify the purification (Fig. 2). The calcium-binding capacity of WPH-1 reached 67·81 μg/mg, and the calcium-binding amount increased by 95% compared with unpurified whey protein hydrolysate complex (33·92 μg/mg). The result was similar to that of peptide derived from porcine blood plasma protein hydrolysate (Lee & Song, Reference Lee and Song2009).

Fig. 1. Semi-preparative RP-HPLC chromatography of fraction F23 derived from G-25. (a) The elution profile monitored at 214 nm. (b) The calcium-binding capacity of fractions 1 to 13 of F23 separated from semi-preparative HPLC. All data were expressed as mean values (mean ± sd, n = 3)

Fig. 2. The analytic RP-HPLC for purification of the peak named WPH-1 (whey protein hydrolysate-1). The elution was monitored at 214 nm.
Table 1. Calcium-binding capacity of fractions from DEAE and G-25

F2 exhibited negative charges in the same buffer condition. The result that calcium-binding capacity of F2 was higher than that of F1 was consistent with the former reports about the strong ion-binding activity of negative charged peptide (Chaud et al. Reference Chaud, Izumi, Nahaal, Shuhama, Lourdes Pires Bianchi and Freitas2002). The difference of ion-binding activity between F2 and F3 are possibly associated with their other properties including molecular weight, hydrophilicity/ hydrophobicity. The ion-binding capacity of peptide is related to its net charge (Chaud et al. Reference Chaud, Izumi, Nahaal, Shuhama, Lourdes Pires Bianchi and Freitas2002). The phosphopeptides from α-casein and β-casein hydrolysates have negative charges which are efficiently bound to divalent cations (Vegarud et al. Reference Vegarud, Langsrud and Svenning2000). The coordination abilities of the peptides due to the donor groups, the polar groups would be deprotonated in high pH value (Kállay et al. Reference Kállay, Várnagy, Micera, Sanna and Sóvág2005; Wu et al. Reference Wu, Liu, Zhao and Zeng2012).
Identification of WPH-1
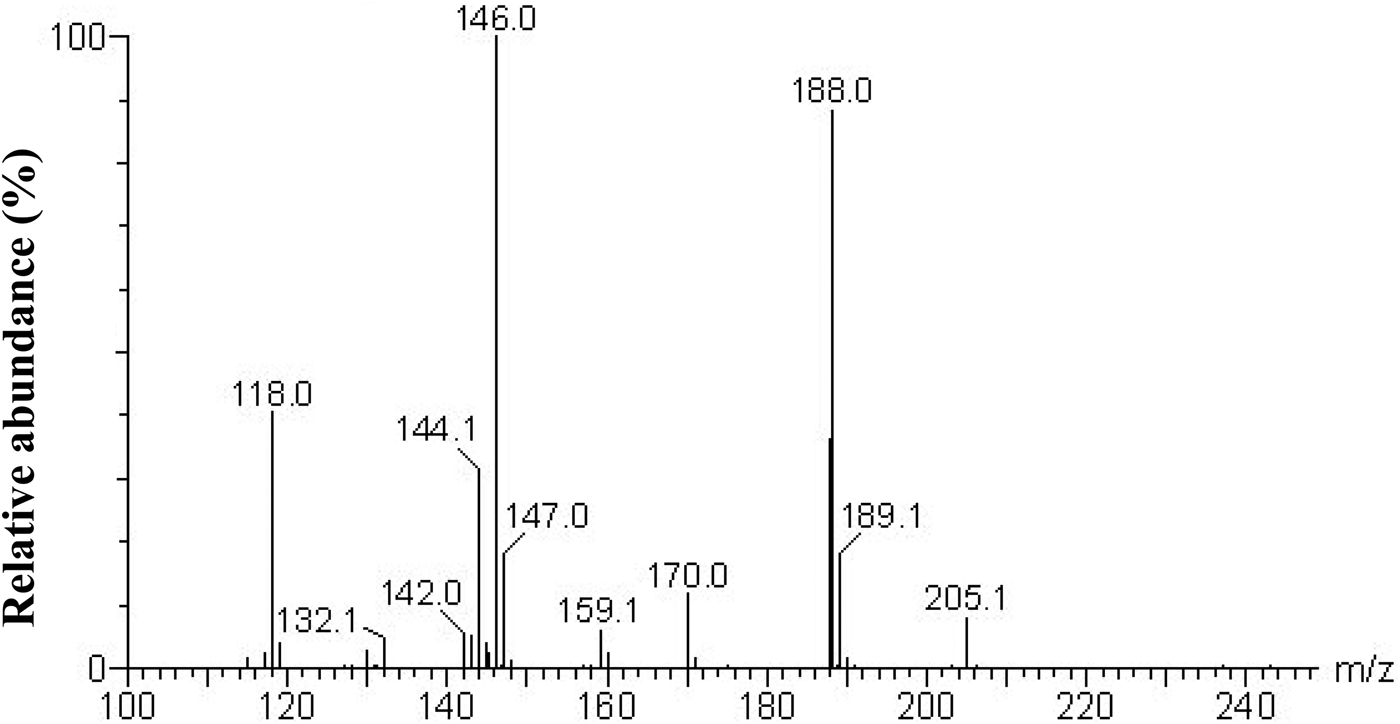
WPH-1 was analysed by LC/ESI tandem mass spectrometer for molecular weight and amino acid sequence determination. The purified calcium-binding peptide from whey protein hydrolysate was identified to be Glu-Gly (EG), which has a molecular weight of 204 Da (Fig. 3). Flavourzyme is known as leucyl aminopeptidase, contains both endo- and exopeptidase activity. The hydrolysis results in release of an N-terminal amino acid and is preferably Leu, but may be other amino acids such as Glu, Gly. And protamex is a Bacillus protease complex (EC 3.4.21.62 and 3.4.24.28), and the cleavage sites is aromatic amino acid, hydrophobic amino acids including Tyr, Phe, Gly and Trp. Therefore, the purified dipeptide EG is possibly obtained through the cleavage sites of the commercial enzymes used in the study. The amino acid sequence was confirmed when compared with the data from the National Center for Biotechnology Information database (NCBI).

Fig. 3. Identification of amino acid sequence of calcium-binding peptide using LC/ESI tandem mass spectrometer.
The result that the calcium-binding peptide was identified to contain Glu residue was similar to the report of Tamura et al. (Reference Tamura, Oku and Hosoya1982) which stated Glu was abundant in milk protein. When cheese whey protein was hydrolysed, the content of Glu increased to 30·08 from 17·8%, which become the highest percentage of amino acid component (Kim & Lim, Reference Kim and Lim2004). The Glu and Ser residues of αs1- and β-casein phosphopeptides were responsible for calcium binding (Sato et al. Reference Sato, Shindo, Gunshin, Noguchi and Naito1991). Generally speaking, peptides with side chains composed of carbonyl and carboxyl functional groups usually have relatively strong activity to interact with ions such as calcium and zinc (Nemirovskiy & Gross, Reference Nemirovskiy and Gross2000; Lee & Song, Reference Lee and Song2009). The major amino acids including Gly, Glu and Asp obtained from the hoki bone peptides had proved to show high affinity to calcium ion (Jung et al. Reference Jung, Park, Byun, Moon and Kim2005). Besides, Wang et al. (Reference Wang, Li, Yang, Wang and Shen2009) characterised the metal complexes of aluminium with reduced glutathione. It is reported that the principal sites are the negatively charged COO− of Glu and Gly. The carbonyl group (CO), amino group (NH2) and peptide amino (NH) probably participate in coordination in the bidentate and tridentate complexes of Al(III).
Therefore, the negative charge of amino acid residue like Glu and Asp play an important role in calcium-binding peptide. It is apparent that Glu residue in Glu-Gly is the important amino acid of the dipeptide for calcium binding in the study, it is speculated that the carboxyl group of Glu, carbonyl group (CO) and peptide amino (NH) in Glu-Gly might participate in coordinating with calcium. The extra carboxyl group makes the Glu negatively charged at high pH. Furthermore, the low molecular weight of the purified dipeptide EG is conductive to the calcium-peptide complex absorption in intestinal epithelial cells, since the whole dipeptide chelation could pass through the intestinal epithelial cells rather than the calcium ion being deposited in the basic intestinal condition. The purified calcium-binding dipeptide has the potential to be used as ion-binding ingredient in dietary supplements.
UV spectra of EG
As the spectra shown in Fig. 4, strong absorption peak observed near 200 nm could be explained the characteristics of peptide bond, no absorption around 280 nm probably means that there is the absence of aromatic amino acid residue in EG. Besides, the absorption intensity of dipeptide-calcium complex in near ultraviolet region is higher than that of the dipeptide alone. As the increased addition of calcium chloride, the spectra had the tendency of redshift.

Fig. 4. UV spectra of EG and EG-Ca complexes over the wavelength range from 190 to 400 nm. Calcium concentration 0–100 μm
The result is consistent with the related reports. Previous studies have indicated the spatial structure with the chirality of the chromospheres (C = O, -COOH) and auxochromes (-OH, -NH2) of peptides changed after binding with calcium (Houser et al. Reference Houser, Fitzsimons and Barton1999; Armas et al. Reference Armas, Sonois, Mothes, Zazarguil and Faller2006) inducing hyperchromicity and redshift in the UV spectra. In addition, a small absorption band appeared at around 192 nm along with the increased additive amount of CaCl2, which perhaps reflected the difference of relevant valence electron transition after more calcium reacted with EG.
Infrared spectrometer analysis
FTIR absorption peak changes of organic ligand groups in EG can reflect the interaction of calcium with EG. As shown in Fig. 5, the absorption at high frequency of 3448·35 or 3432·01 cm−1 is the N-H and O-H bonds referred to water of hydration (Huang et al. Reference Huang, Ren and Jiang2011). As the calcium coordinated with EG, the Ca-N bond replaced N-OH (hydrogen bonds) and was responsible for the shift to low frequency of 3432·01 from 3448·35 cm−1. The absorption band of EG at 1679 cm−1 as an amide I band attributed to the C = O stretching vibrations and bending vibrations of NH, it shifted to lower frequency (1572·76 cm−1) after interacting with calcium. The amide II band at about 1550 cm−1 in peptide assigned to the C-N stretching vibration coupled with N-H bending disappeared in the dipeptide-calcium complex (Fabian et al. Reference Fabian, Schultz, Naumann, Landt, Hahn and Saenger1993). The band at 1221·34 cm−1 of the -COO− carboxyl group in the EG could not be found in the spectrum of dipeptide-calcium complex. Several absorption bands at around 800 cm−1 caused by the vibration of the N-H linked to C-N bonds were not present in the dipeptide-calcium complex. The possible reason why the absorption bands of -COO−, N-H and C-N disappeared is that the calcium binding to EG affects the stretching and bending vibration of these functional groups. The FTIR results demonstrate carboxyl oxygen and amino nitrogen atoms are the interaction sites between calcium and EG.

Fig. 5. Infrared spectra of EG and EG-Ca complexes.
Sequestered calcium percentage of EG-Ca complex and CaCl2
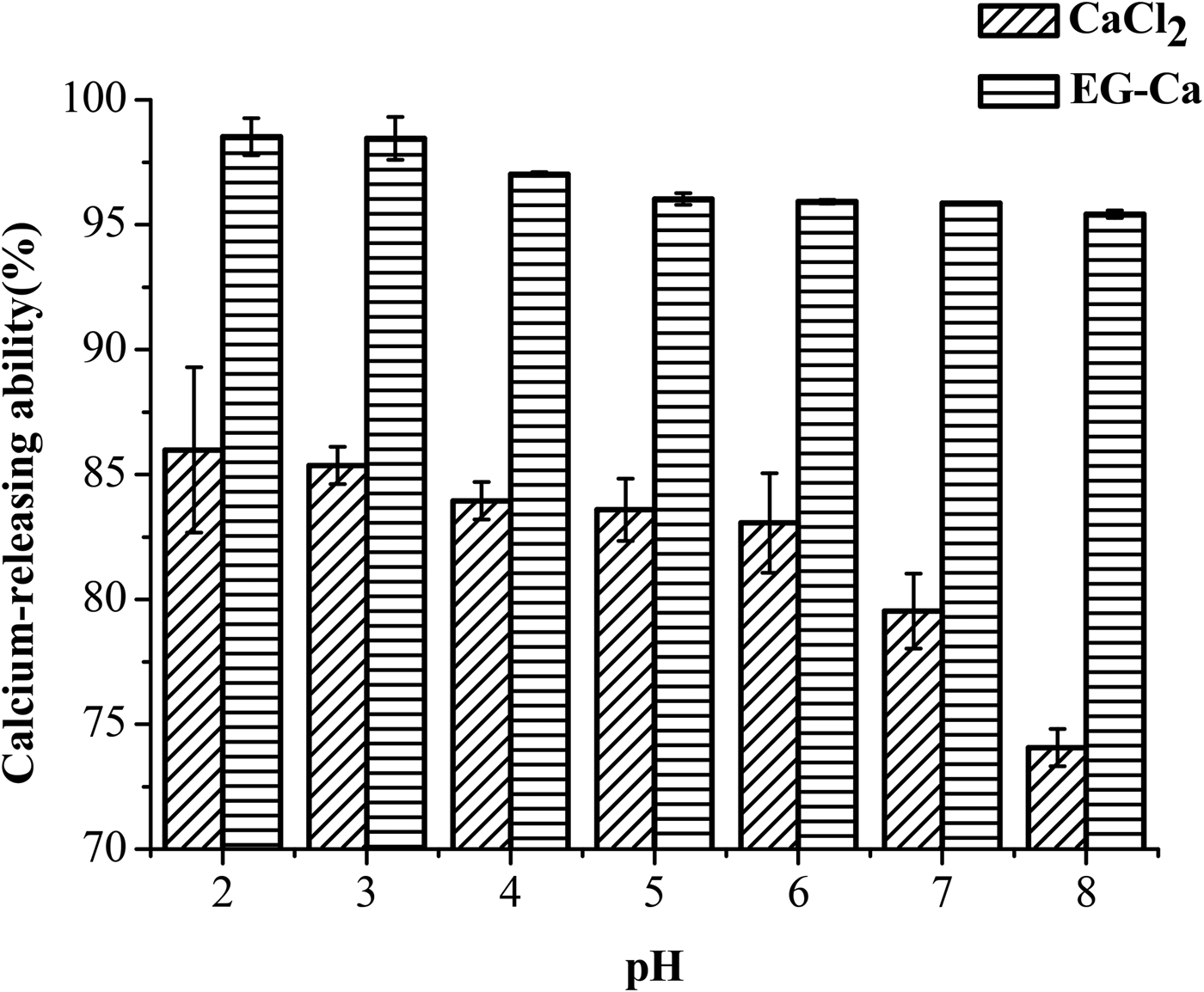
To discuss the calcium sequestered effect in human gastrointestinal tract in vitro, the sequestered calcium percentage of EG-Ca and CaCl2 at different pH values from 2·0 to 8·0 was investigated. The sequestered calcium amount in EG-Ca complexes was relatively stable in the pH range from 2·0 to 8·0, whereas that of CaCl2 decreased significantly from 85·98% at pH 2·0 to 74·06% at pH 8·0 (Fig. 6). Meanwhile, the sequestered calcium percentages of EG-Ca at various pH values were obviously higher than that of CaCl2. The higher sequestered calcium percentage in these pH ranges is important for ion bioavailability improvement in the physiological environment of human gastrointestinal tract. Especially, the high sequestered calcium amount in pH 2·0 equivalent to the pH value of gastric environment is in favour for the transportation of calcium to intestinal environment. The high sequestered calcium percentage in the basic intestinal tract can prevent calcium from producing precipitation so that it can be effectively absorbed by intestinal epithelial cells (Wang et al. Reference Wang, Zhou, Tong and Mao2011). The finding suggests that EG can improve calcium solubility under gastrointestinal tract pH values.

Fig. 6. Calcium sequestered percentage of EG-Ca complexes and CaCl2 at pH values of 2·0, 3·0, 4·0, 5·0, 6·0, 7·0 and 8·0.
Conclusions
In summary, a dipeptide EG with strong calcium-binding capacity was purified from whey protein hydrolysate, and the Glu residue of EG played an important role in binding to calcium ion. EG-Ca chelate demonstrated stability under either acidic or basic conditions.
This work was supported by the National Marine Public Welfare Projects (No. 201305022), Natural Science Foundation of China (No. 31471623, 31201287), Fujian Natural Science Foundation, China (No. 2013J01132), the open funding of Fujian Provincial Key Laboratory of Photocatalysis–State Key Laboratory Breeding Base, China (No. 038018).