Introduction
Pyrethroids are a major class of synthetic insecticides which are still used to control agricultural pests (Joseph et al., Reference Joseph, Martin, Steinmann and Kosina2017; Parys et al., Reference Parys, Luttrell, Snodgrass and Portilla2018) and disease vectors (Smith et al., Reference Smith, Kasai and Scott2016). Pyrethroids are fast acting insecticides, with high and low insect and mammalian toxicity, respectively (Dong et al., Reference Dong, Du, Rinkevich, Nomura, Xu, Wang, Silver and Zhorov2014). At a cellular level, pyrethroids disrupt nerve function, causing repetitive discharges, membrane depolarization, and synaptic disturbances (Soderlund Reference Soderlund2012; Dong et al., Reference Dong, Du, Rinkevich, Nomura, Xu, Wang, Silver and Zhorov2014). The primary target site of pyrethroids is the voltage-gated sodium channel that underlies the generation of nerve action potential (Soderlund Reference Soderlund2012). The decrease in the sodium channel sensitivity, known as knock-down resistance (kdr) mutation, confers resistance to DDT and pyrethroids. Since its initial report in the house fly (Milani, Reference Milani1956), kdr and kdr-like resistance have been documented globally in almost all agriculturally important arthropod pests and disease vectors (Soderlund, Reference Soderlund, Gilbert, Iatrou and Gill2005, Reference Soderlund2012; Rinkevich et al., Reference Rinkevich, Du and Dong2013). Study of the mechanism of kdr in the past two decades led to the identification of more than 50 sodium channel mutations or combinations of mutations that were associated with pyrethroid resistance in arthropod species (Haddi et al., Reference Haddi, Berger, Bielza, Cifuentes, Field, Gorman, Rapisarda, Williamson and Bass2012, Reference Haddi, Tomé, Du, Valbon, Nomura, Martins, Dong and Oliveira2017; Sierra et al., Reference Sierra, Capriotti, Fronza, Mougabure-Cueto and Ons2016). In the codling moth Cydia pomonella L. (Lepidoptera: Tortricidae), the kdr mutation reported so far is a single nucleotide polymorphism which results in a substitution of leucine for phenylalanine at position 1014 (L1014F) (Franck et al., Reference Franck, Siegwart, Olivares, Toubon and Lavigne2012).
Codling moth is a severe pest of pome fruits worldwide (Grigg-McGuffin et al., Reference Grigg-McGuffin, Scott, Bellerose, Chouinard, Cormier and Scott-Dupree2015) and the fruit damage is caused by the larval stage. Neonates excavate tunnels and feed on the seed. Infested fruit lose their shape and fall prematurely (Danelski et al., Reference Danelski, Kruczyńska, Bielicki and Rozpara2017; Husain et al., Reference Husain, Jagdeesh, Sharma, Raja, Injila Qadri and Waheed Wani2018). Effective C. pomonella management mainly rely on chemical insecticides which has led to the development of insecticide resistance to most classes of insecticides (Sauphanor and Bouvier, Reference Sauphanor and Bouvier1995; Knight et al., Reference Knight, Dunley and Jansson2001; Reyes et al., Reference Reyes, Franck, Olivares, Margaritopoulos, Knight and Sauphanor2009; Rodriguez et al., Reference Rodríguez, Bosch and Avilla2011; Voudouris et al., Reference Voudouris, Sauphanor, Franck, Reyes, Mamuris, Tsitsipis, Vontas and Margoritopoulos2011; Cichón et al., Reference Cichón, Soleño, Anguiano, Garrido and Montagna2013). Insecticide-resistance in codling moth field populations has been related mainly to increased enzymatic metabolization and target-site mutations (Reyes et al., Reference Reyes, Franck, Charmillot, Ioriatti, Olivares, Pasqualini and Sauphanor2007, Reference Reyes, Collange, Rault, Casanelli and Sauphanor2011; Voudouris et al., Reference Voudouris, Sauphanor, Franck, Reyes, Mamuris, Tsitsipis, Vontas and Margoritopoulos2011), including the sodium channel mutation (Reyes et al., Reference Reyes, Franck, Olivares, Margaritopoulos, Knight and Sauphanor2009; Franck et al., Reference Franck, Siegwart, Olivares, Toubon and Lavigne2012; Bosch et al., Reference Bosch, Avilla, Musleh and Rodríguez2018).
Codling moth is widely distributed in Argentina where climate conditions are favorable to apple and pear tree development (commercial and wild varieties). The region of Río Negro and Neuquén valley, located in northern Patagonia (Argentina), contributes with almost 85% of the country pome-fruit production (especially Río Negro province with 78%), followed by the Uco (Mendoza province) and Calingasta (San Juan province) valleys, among others https://inta.gob.ar/sites/default/files/script-tmp-inta_programa_nacional_frutales_cadena_frutales_de_pe.pdf. Pyrethroids have been used to control C. pomonella in the USA (Mota-Sanchez et al., Reference Mota-Sanchez, Wise, Poppen, Gut and Hollingworth2008), France (Bouvier et al., Reference Bouvier, Cuany, Monier, Brosse and Sauphanor1998), Greece (Voudouris et al., Reference Voudouris, Sauphanor, Franck, Reyes, Mamuris, Tsitsipis, Vontas and Margoritopoulos2011), Chile (Fuentes-Contreras et al., Reference Fuentes-Contreras, Espinoza, Lavandero and Ramirez2008), etc. In the Río Negro and Neuquén valley, pyrethroids were introduced in 1982 and have been used for almost 20 years against C. pomonella. Late 90s, many farmers reported control failures to pyrethroid applications, and its use was discontinued (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008). Fruit production area from Mendoza and San Juan provinces has a similar application history but because the pome area is smaller the pesticide pressure is lower. On the other hand, although there are other provinces with pome production, these are of much smaller area and their production is for domestic or family market.
Reyes et al. (Reference Reyes, Franck, Olivares, Margaritopoulos, Knight and Sauphanor2009) reported the kdr mutation in codling moth obtained in 2005 from two orchards in Argentina. However, these orchards were not georeferenced by the authors and no toxicological data were provided.
Recently, the economic crisis of small farmers has led again to the use of lambda- cyhalothrin in C. pomonella control. Therefore, the first objective of the present study was to evaluate the levels of pyrethroid resistance in C. pomonella larvae from three orchards that are currently under pyrethroid treatments. The second objective was to evaluate the frequency of kdr mutation in C. pomonella from the Río Negro and Neuquén valley as well as from other Argentinean areas of pome fruit production.
Material and methods
Study area and insect collection
Field populations of diapausing larvae (FCL) were collected in orchards from 31°27′29″ N to 46°32′60″ S latitudes (1690 km away from each other) (table 1) in 2012. Diapausing larvae were collected from 12 orchards using corrugated cardboards attached to the main branches and trunk trees. Larvae were then transferred to clean corrugated papers and stored at 4° ± 1°C with 12:12 h L:D regime for three months in order to satisfy chilling requirements (Soleño et al., Reference Soleño, Anguiano, Cichón, Garrido and Montagna2012). The laboratory susceptible strain (LSS) was obtained from INTA Alto Valle (Argentina), which has been reared in the laboratory since 1991 without exposure to any insecticide. From the 12 orchards, FCL for bioassays were from Cinco Saltos (CSA), Guerrico (GUE), and Villa Regina (VRG3) orchards, where pyrethroids are currently used. Both LSS and FCL were transferred to post chilling conditions (25°C, 70% relative humidity and 16:8 L:D) for 48 h before bioassays were performed (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008).
Table 1. Orchards names and locations.

Bioassays
Topical concentration–response assays on diapausing larvae were performed by the application of 1 µl of lambda-cyhalothrin (AccuStandard Inc., New Heaven, USA, 98% purity) to the dorsum of each larva using a Hamilton microsyringe. The insecticide was dissolved in acetone and the concentration range assayed was from 1 to 50,000 mg/l (8–10 concentrations). Bioassays were conducted on 3–5 groups of 20 larvae placed in petri dishes according to the available insects. Batches of larvae treated with acetone were used as control. Larvae were subsequently placed under controlled conditions (25°C, 70% RH and 16:8 h L:D photoperiod) for 48 h. Before scoring mortality, larvae were removed and placed in the cap of the corresponding petri dish. Larvae were considered moribund if, after a brush touch, they presented one of the following: they continued lying on their side or in the dorsal position or were unable to move in a coordinated manner. Categories of dead and moribund were combined to assess the percentage of mortality (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008).
Detection of the kdr mutation
Total DNA was individually extracted from half of the diapausing larvae with 150 µl of Chelex 100 (Bio Rad, California, USA). Briefly, tissues were digested 30 min at 56°C and, after boiling for 8 min they were centrifuged at 12,000 rpm during 5 min. The supernatants were used as DNA templates for PCR reaction. DNA concentration was spectrophotometrically determined at 260/280 nm. The genetic variability was analyzed in a 169-bp fragment of the sodium channel gene by polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP) from 351 larvae. This fragment encompasses the molecular target linked to pyrethroid resistance (leucine-to-phenylalanine replacement at position 1014 in trans-membrane segment IIS in the amino acid sequence) (Brun-Barale et al., Reference Brun-Barale, Bouvier, Pauron, Bergé and Sauphanor2005). PCR amplifications were carried out in a 25 µl reaction volume with concentrations according to the manufacturer (Thermo Fisher Scientific, Massachusetts, USA). Specific primers were CpNaF (5′ TAGAGAGCATGTGGGATTGC 3′) and CpNaR (5′ AATTTCGTAGCCCTTGATCG 3′) (Franck et al., Reference Franck, Reyes, Olivares and Sauphanor2007). The sequence of the voltage-gated sodium channel from C. pomonella Rv strain is noted in the GenBank database under the reference AY763097. DNA amplification product was then subjected to overnight treatment with MluCI enzyme (New England BioLabs, Massachusetts, USA). Restriction enzyme MluCI cuts specifically at the AATT site, which is specific for the kdr allele associated with pyrethroid resistance in C. pomonella (Brun-Barale et al., Reference Brun-Barale, Bouvier, Pauron, Bergé and Sauphanor2005). After digestion, DNA fragments of 77 and 112 bp identified kdr and the susceptible alleles, R y S respectively. DNA fragments were visualized in a 4% agarose gel with GelRed™ (Biotium, Fremont, California, USA).
DNA was extracted from 351 larvae from 12 orchards distributed through Argentina along with laboratory susceptible individuals. The assay allowed homozygous resistant (R/R), heterozygous (R/S), and homozygous susceptible individuals (S/S) to be distinguished. Samples with known genotypes were processed in all assays as controls. Codling moth positive controls of kdr mutation were gently donated by Eduardo Fuentes-Contreras of the Molecular Ecology and Evolutionary Applications Center in Agroecosystems of the University of Talca, Chile.
Data analyses
Data from concentration–response bioassays were subjected to PROBIT analysis and the regression lines were compared by Likelihood Ratio χ2 Test. LC50 and LC95 values and their 95% confidence limits (CL) were calculated with Dr Sakuma's PriProbit NM software (USDA, 2019). LC50 and LC95 values for LSS and FCL were significantly different if their 95% CL did not overlap. Resistance ratios (RR) were calculated as the ratio of LC50 FCL/LC50 LSS.
Deviations from Hardy–Weinberg equilibrium (HWE) at each sampling site were analyzed with the χ2 test (P < 0.05) according to the methodology described in Freeland (Reference Freeland2005).
Results
Bioassay
Diapausing larvae were exposed to lambda-cyhalothrin from 1 to 50,000 mg/l for 48 h. The LC50 values obtained from the PROBIT analysis are presented in table 2. The LC50 between the LSS and each FCL was significantly different based on their 95% CL. Diapausing larvae from CSA, GUE, and VRG3 populations showed a high level of resistance to lambda-cyhalothrin, especially GUE (LC50 7.381 µg/larva) and VRG3 (LC50 7.217 µg/larva) compared to susceptible strain (LC50 0.197 µg/larva). The resistance ratios at LC50 level from CSA, GUE, and VRG3 populations ranged from 30 to 37. Moreover, both GUE and VRG3 showed not only the highest LC50 values, but also the lowest slope values. Population genetic heterogeneity is evidenced by low slope values showing an increase of resistant genotypes. Lambda-cyhalothrin concentration-mortality data from the LSS and all three FCL fitted the PROBIT model as indicated by the goodness-of-fit test (P > 0.05).
Table 2. Toxicity of lambda-cyhalothrin in field populations and a laboratory susceptible strain of diapausing larvae of C. pomonella.

RR, resistance ratio = LC50 field population/LC50 laboratory susceptible strain.
a Goodness of fit (P < 0.05)
Detection and frequency of kdr mutation in C. pomonella
The L1014F (kdr) mutation was detected in ten out of the twelve samples analyzed (table 3) as homozygous resistant (R/R), heterozygous (R/S) and homozygous susceptible individuals (S/S). Larvae from SJN and SAR were homozygous wild type. All samples from the Río Negro and Neuquén valley carried the kdr allele with frequencies ranging between 0.24–0.61, although the proportion of homozygotes for kdr alleles within each sample was generally low (fig. 1). The frequency of homozygous kdr genotypes from all samples in this production region was 0.16. The highest frequency of kdr alleles (0.61) was found in VRG1 sample, where almost 30 and 60% of the sampled individuals were homozygotes and heterozygotes for kdr mutation, respectively.
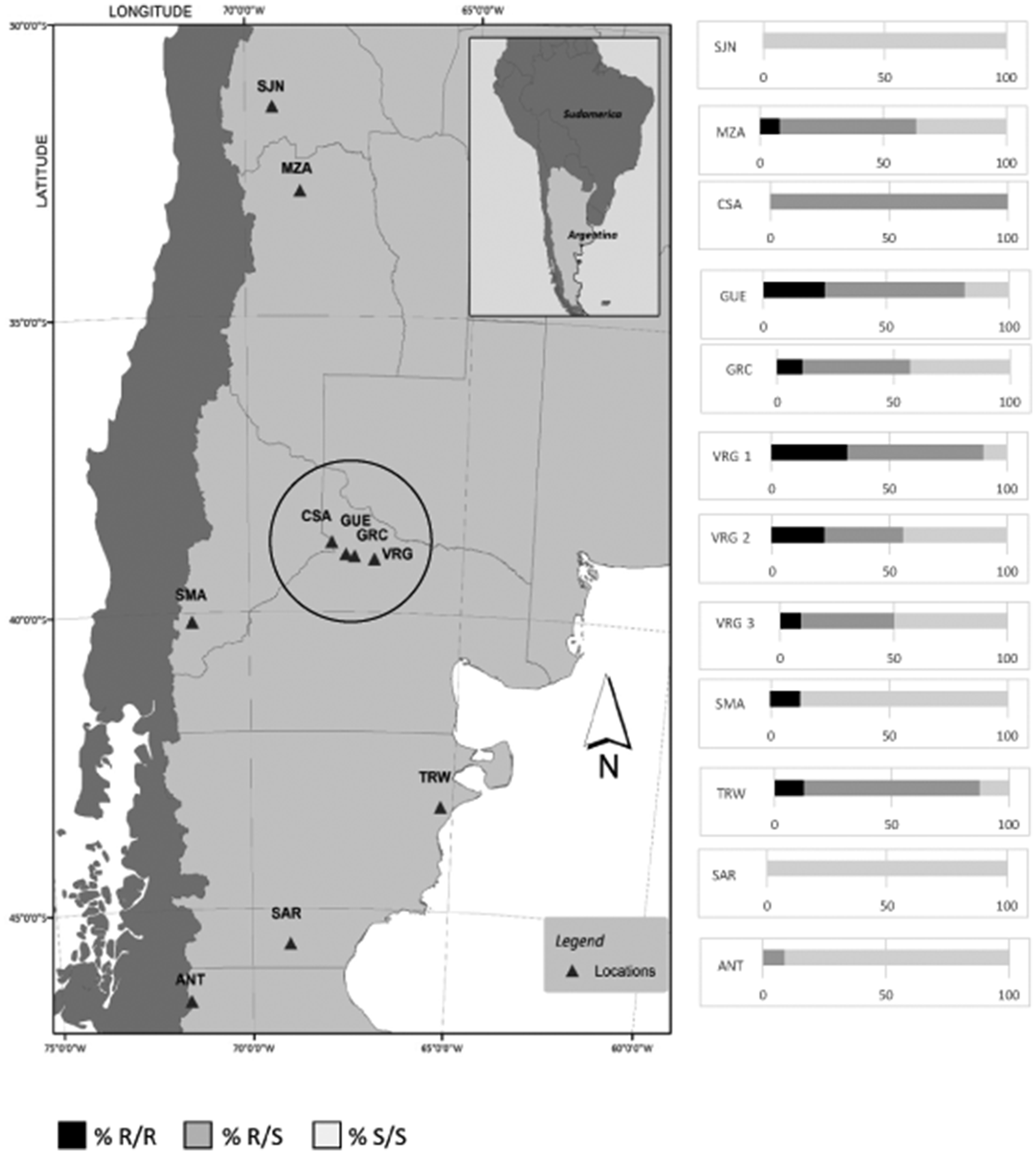
Figure1. Sampling sites of the field populations and kdr percentages throughout Argentina. Samples inside the circle belong to the Río Negro and Neuquén upper valley.
Table 3. Distribution of genotypes and kdr frequency in C. pomonella samples from different orchards throughout Argentina.
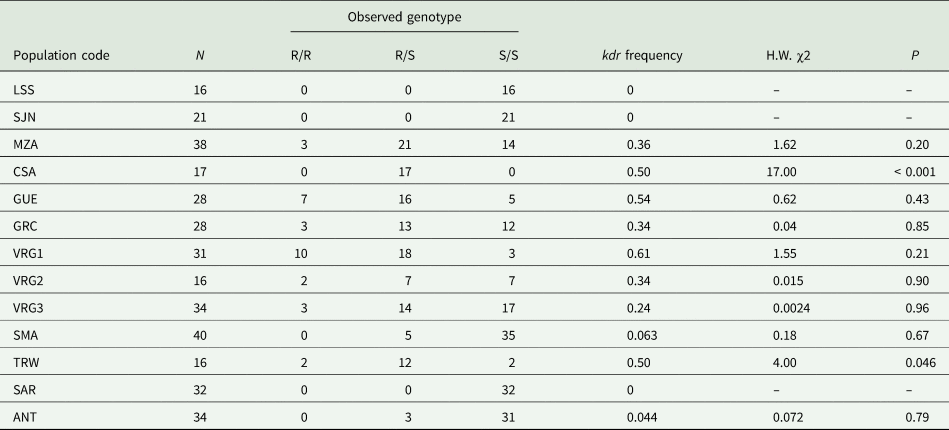
N, diapausing larvae number; R/R, homozygous for kdr; R/S, heterozygous for kdr; S/S, homozygous wild-type; H.W. χ2; P: values for χ2 tests for deviation from Hardy–Weinberg equilibrium (P < 0.05)
Samples from CSA and TRW showed a significant departure from Hardy–Weinberg equilibrium, and the 100 and 75% of the individuals were heterozygotes, respectively.
Discussion
Pyrethroid insecticides were intensively used during the 90s decade against codling moth in apple and pear orchards from the Río Negro and Neuquén valley (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008). The intense use of chemical insecticides produced a severe selection pressure on insects, favoring the increase in pesticide resistance genotypes. In fact, non-target Simulium spp larvae from irrigation channels in this area developed extremely high levels of fenvalerate resistance (400-fold) (Montagna et al., Reference Montagna, Gauna, de D'Angelo and Anguiano2012). Results from the present study showed high levels of resistance to lambda-cyhalothrin in the three populations evaluated (RR > 30). Previously, low levels of resistance to the organophosphate azinphos methyl and the neonicotinoids acetamiprid and thiacloprid have been observed on field populations from the Río Negro province and resistance to these two insecticide families was highly correlated to esterase and ECOD activities, respectively (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008, Reference Soleño, Anguiano, Cichón, Garrido and Montagna2012; Cichón et al., Reference Cichón, Soleño, Anguiano, Garrido and Montagna2013). The increased activities of both enzymes might confer cross-resistance to pyrethroids. Indeed, increased activity of CYP450 and esterase has been related to pyrethroids resistance in codling moth (Sauphanor et al., Reference Sauphanor, Cuany, Bouvier, Brosse, Amichot and Bergé1997) as well as in other several lepidopteran species (Gunning et al., Reference Gunning, Moores and Devonshire1996; Kranthi et al., Reference Kranthi, Jadhav, Wanjari, Kranthi and Russell2001; Chen et al., Reference Chen, Yang and Wu2005; Yang et al., Reference Yang, Chen, Wu, Yue and Wu2006; Sonoda, Reference Sonoda2010).
Besides metabolic resistance, the kdr mutation in the voltage-gated sodium channel has been also linked to pyrethroid resistance in codling moth (Brun-Barale et al., Reference Brun-Barale, Bouvier, Pauron, Bergé and Sauphanor2005; Franck et al., Reference Franck, Siegwart, Olivares, Toubon and Lavigne2012) and in other insect species (Kasai et al., Reference Kasai, Sun and Scott2017; Zibaee et al., Reference Zibaee, Mahmood, Esmaeily, Bandani and Kristensen2018).
The single point substitution L1014F in the voltage-gated sodium channels was analyzed in 12 samples of C. pomonella from apple and pear orchards throughout Argentina. All samples from the Río Negro and Neuquén valley (CSA, GUE, GRC, VRG1, VRG2, and VRG3) and the one from MZA showed the kdr allele with frequencies varying between 0.29–0.61. The frequencies of the homozygous kdr genotypes from larvae collected in Río Negro and Neuquén valley were generally low, with the exception of the sample from VRG1 (0.61). The discontinuation for many years of pyrethroid applications, and the immigration of susceptible individuals from surrounding abandoned and organic orchards could explain the low of kdr frequency in this region. Since the kdr-type resistance allele is partially or completely recessive (Bouvier et al., Reference Bouvier, Bues, Boivin, Boudinhon, Beslay and Sauphanor2001; Gomes et al., Reference Gomes, Purkait, Deb, Rama, Singh, Foster, Coleman, Kumar, Paine, Das and Weetman2017), results from this study suggest that kdr mutation in populations from Río Negro and Neuquén valley may not explain by itself the lambda-cyhalothrin resistance levels. The present and previous studies (Soleño et al., Reference Soleño, Anguiano, de D'Angelo, Cichon, Fernandez and Montagna2008; Cichón et al., Reference Cichón, Soleño, Anguiano, Garrido and Montagna2013) would indicate that lambda-cyhalothrin resistance in C. pomonella from CSA, GUE, and VRG3 is conferred by multiple-resistance mechanisms including kdr mutation in the sodium channel and increased activities of CYP450 and esterases. In French C. pomonella populations, a negative correlation between CYP450 activity and the proportion of homozygous kdr genotypes was found (Franck et al., Reference Franck, Siegwart, Olivares, Toubon and Lavigne2012). The authors hypothesized that metabolic resistance should be sufficient for the codling moth to resist pyrethroid treatments, limiting the selection of sodium channel target mutations in the absence of strong pyrethroid selection.
CSA population showed a significant deviation from HWE, evidencing violation for at least one of the HWE assumptions. We consider that because CSA is the population that showed one of the highest R/S frequencies and no S/S genotype is under mutation and has an effect on allele frequencies.
The kdr mutation was not identified in samples from SJN and SAR and its frequency was very low in ANT (0.044) and SMA (0.063). Neither ANT nor SMA showed the homozygous kdr genotype. Fruit production, including apples and pears, from the irrigated valleys in Chubut (TRW and SAR) and Santa Cruz (ANT) provinces is characterized by family orchards, and the control of C. pomonella usually involve pyrethroids and low toxic pesticides such diatomaceous earth. The sample of C. pomonella from SMA was collected from wild apple trees non-treated with pesticides. According to the low or null pyrethroid selection pressure at these sites (ANT and SMA), it was not expected to found the kdr mutation. Since this pest has low dispersal range (150–300 m) (Basoalto et al., Reference Basoalto, Miranda, Knight and Fuentes-Contreras2010), it is possible that resistant alleles from codling moths from the Río Negro and Neuquén valley had spread to distant sites by the transport of fruits in wooden packages infested with diapausing larvae.
Conclusions
The kdr mutation in C. pomonella is detected in a geographical wide spectrum in Argentina. Moreover, kdr frequency mutations in the Rio Negro valley area are higher than in other tested areas. The kdr frequency is possibly related to the use of pyrethroids against this pest. Target-site insensitivity is, at least, one of the mechanisms involved in resistance to lambda-cyhalothrin in codling moth from the Río Negro and Neuquén valley.
Acknowledgments
We thank MSc Olga Liliana Anguiano for technical assistance in DNA quantification. N. Guiñazu is a member of the research career of CONICET. L. Parra-Morales thanks CONICET for the fellowship granted.
Funding
This study was supported by grants from the Universidad Nacional del Comahue (04/U020) and the Instituto Nacional de Tecnología Agropecuaria (Convenio Fundación ArgenINTA – Dupont no 1131 y PNFRU 1105074).







