Introduction
Patients with major depressive disorder (MDD) have higher risk of suicide compared with the general population (Angst et al., Reference Angst, Stassen, Clayton and Angst2002). Although suicidal ideation (SI) may be distinct from suicidal attempt and behavior (Klonsky and May, Reference Klonsky and May2014), it is a strong indicator of suicide attempt within the first year after SI onset (Nock et al., Reference Nock, Borges, Bromet, Alonso, Angermeyer, Beautrais, Bruffaerts, Chiu, de Girolamo, Gluzman, de Graaf, Gureje, Haro, Huang, Karam, Kessler, Lepine, Levinson, Medina-Mora, Ono, Posada-Villa and Williams2008). It is crucial to elucidate the biological underpinnings of SI in MDD patients.
Biologically, suicidal individuals exhibit genetic and serotonergic differences compared with healthy controls (HCs) and major depression (Joiner et al., Reference Joiner, Brown and Wingate2005). Clinically, SI differs from other depression symptoms (such as insomnia, sad mood, fatigue, and concentration problems) in important dimensions with regard to risk factors and impact on impairments (for instance, SI patients exhibits high pessimism for future, whereas fatigue most impacts on home management) (O'Connor and Nock, Reference O'Connor and Nock2014; Fried and Nesse, Reference Fried and Nesse2015). Moreover, MDD patients with SI are harder to treat and more likely to relapse than those without SI during continuation treatment (Szanto et al., Reference Szanto, Mulsant, Houck, Dew and Reynolds2003). However, few studies have specially concentrated on the different intrinsic brain activity (iBA) or/and connectivity patterns between MDD patients with and without SI (Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016; Chase et al., Reference Chase, Segreti, Keller, Cherkassky, Just, Pan and Brent2017; Du et al., Reference Du, Zeng, Liu, Tang, Meng, Li and Fu2017; Kim et al., Reference Kim, Kim, Myung, Han, Fava, Mischoulon, Papakostas, Seo, Cho, Seong and Jeon2017b).
Previous studies in depressed patients linked SI and suicidal attempt to impulsive behavior and dysfunctional executive and emotional processing (Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek, Eichler, Maier and Wagner2008; O'Connor and Nock, Reference O'Connor and Nock2014; Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016; Johnston et al., Reference Johnston, Wang, Liu, Blond, Wallace, Liu, Spencer, Cox Lippard, Purves, Landeros-Weisenberger, Hermes, Pittman, Zhang, King, Martin, Oquendo and Blumberg2017). Notably, executive function and emotional processing involve brain areas such as the orbitofrontal cortex, anterior cingulate cortex, dorsolateral prefrontal cortex, and temporal pole gyrus (Rogers et al., Reference Rogers, Kasai, Koji, Fukuda, Iwanami, Nakagome, Fukuda and Kato2004; Olson et al., Reference Olson, Plotzker and Ezzyat2007). Compared with MDD patients without SI, SI patients showed reduced fronto-limbic (Du et al., Reference Du, Zeng, Liu, Tang, Meng, Li and Fu2017) and orbitofrontal-thalamic functional connectivity (Kim et al., Reference Kim, Kim, Myung, Han, Fava, Mischoulon, Papakostas, Seo, Cho, Seong and Jeon2017b) and fronto-subcortical white matter connectivity (Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016). Convergent findings suggest the presence of structural and functional magnetic resonance imaging (fMRI) abnormalities in MDD patients with SI (Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016; Du et al., Reference Du, Zeng, Liu, Tang, Meng, Li and Fu2017; Kim et al., Reference Kim, Kim, Myung, Han, Fava, Mischoulon, Papakostas, Seo, Cho, Seong and Jeon2017b). However, these studies relied on the assumption of the ‘static’ of the brain and did not investigate the dynamic brain alterations over time in SI patients. More recently, studies have focused on investigating dynamic functional connectivity or networks, which can provide information on the variability in the strength or spatial dynamic organization of the brain (Bassett and Sporns, Reference Bassett and Sporns2017). Despite the dynamics of inter-regional functional connectivity in resting state are successfully applied in many psychiatric and neurological diseases, such as major depression (Kaiser et al., Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer, Minkel, Smoski, Dichter and Pizzagalli2016), schizophrenia (Damaraju et al., Reference Damaraju, Allen, Belger, Ford, McEwen, Mathalon, Mueller, Pearlson, Potkin, Preda, Turner, Vaidya, van Erp and Calhoun2014), epilepsy (Liao et al., Reference Liao, Zhang, Mantini, Xu, Ji, Zhang, Wang, Wang, Chen, Tian, Jiao, Zang and Lu2014b; Li et al., Reference Li, Liao, Yu, Chen, Guo, Tang and Chen2018), and Parkinson's disease (Kim et al., Reference Kim, Criaud, Cho, Diez-Cirarda, Mihaescu, Coakeley, Ghadery, Valli, Jacobs, Houle and Strafella2017a), knowledge about the dynamics of local brain activity is still limited. This local iBA is supposed to be a reflection of mental activity (Raichle and Snyder, Reference Raichle and Snyder2007), which may cause high time-varying iBA (Fu et al., Reference Fu, Tu, Di, Du, Pearlson, Turner, Biswal, Zhang and Calhoun2017). In this study, we regard time-varying iBA as a potential way to deepen our understanding of SI in depressed patients.
One approach to measure time-varying iBA is to quantify the temporal variability of the amplitude of iBA (Tagliazucchi et al., Reference Tagliazucchi, Carhart-Harris, Leech, Nutt and Chialvo2014; Tomasi et al., Reference Tomasi, Shokri-Kojori and Volkow2016; Fu et al., Reference Fu, Tu, Di, Du, Pearlson, Turner, Biswal, Zhang and Calhoun2017; Yan et al., Reference Yan, Yang, Colcombe, Zuo and Milham2017). The iBA amplitude provides strong temporal information (Zang et al., Reference Zang, He, Zhu, Cao, Sui, Liang, Tian, Jiang and Wang2007). Conventional studies on iBA amplitude assume that brain activity is stationarity over a whole resting-state fMRI scan, while recent investigations of brain activity have taken fluctuation over time into account (Allen et al., Reference Allen, Damaraju, Plis, Erhardt, Eichele and Calhoun2014), which can be quantified by measuring the temporal variability in the iBA amplitude among voxels. One study on temporal dynamics of iBA investigated the relationship between functional connectivity density and temporal variability of iBA (Tomasi et al., Reference Tomasi, Shokri-Kojori and Volkow2016). By investigating the temporal variability of the iBA amplitude in MDD patients with SI, we expect to delineate the brain regions or neural systems related to SI, which can be used as targets in subsequent treatments, and to gain a more thorough understanding of the brain's biological details.
In the current work, to characterize the temporal variability of iBA in MDD patients with SI, we employ a dynamic amplitude of low-frequency fluctuation (dALFF) on resting-state fMRI. We sought to determine (i) whether MDD patients with SI show different patterns in temporal variability compared with MDD patients without SI; and (ii) whether these altered temporal variability of dALFF would provide a neuromarker to predict the severity of SI (Woo et al., Reference Woo, Chang, Lindquist and Wager2017).
Materials and methods
Subjects
This study was approved by the Local Medical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from all subjects. A total of 51 drug-naïve MDD patients including MDD with SI (SI group, n = 30) and without SI (NSI group, n = 21) with single depressive episode were recruited. MDD was diagnosed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID-I/P, Chinese version), with a cut-off score ⩾16 on the 17-item Hamilton Depression Rating Scale (HAMD). Patients were excluded if they had neurological or other psychiatric disorders, history of substance dependence, alcohol, cocaine or other drugs abuse, neurological MRI abnormalities, or any metal or electronic implants.
In addition, the age-, educational level-, gender-matched HCs (n = 30) with no mood disorder or neurologic disorders were recruited. Additionally, HCs were interviewed to confirm that there was no history of psychiatric illness among their first-degree relatives. The exclusion criteria also required that subjects have no history of substance, drug, or alcohol dependence. Three MDD patients (two SI patients, one NSI patient) were excluded due to excessive head movements during the scan. Consequently, 48 patients (SI group, n = 28; NSI group, n = 20) and 30 HCs were included in the final analyses.
Assessment of depression and SI
At enrollment of this study, all MDD patients were assessed for depression severity and SI severity. Depression severity was evaluated using the 17-item HAMD scale. SI severity was measured by the Scale for Suicide Ideation – Chinese Version (SSI-CV) (Li et al., Reference Li, Phillips, Tong, Li, Zhang and Xu2010), which was subsequently verified for satisfactory reliability and validity in evaluating the SI of depressed patients (Wang et al., Reference Wang, Shen, Liang, Luo and Zhang2012). SSI-CV is a 19-item clinical research instrument designed to quantify the intensity of current conscious suicidal intent. The scoring range of each item was 0–2 points (total range 0–38). Items 4 and 5 were used to estimate the current suicidal thoughts (Marzuk et al., Reference Marzuk, Hartwell, Leon and Portera2005). A score of 0 on either item indicates subjects without SI, while a score above 0 indicates current suicidal thoughts (Marzuk et al., Reference Marzuk, Hartwell, Leon and Portera2005). The SI group subsequently completed the remaining 14 items on current suicidal thoughts, which was not required for the NSI and HC groups. There was no strict cut-off point for SSI score (Cochrane-Brink et al., Reference Cochrane-Brink, Lofchy and Sakinofsky2000). Higher scores indicate more severe SI.
Data acquisition
Imaging data were acquired using an echo-planar imaging sequence on 3.0 Tesla GE Medical systems at the First Affiliated Hospital of Chongqing Medical University. During the MRI scan, all subjects were instructed to keep their head still and their eyes closed without falling asleep and do not think of anything in particular. Resting-state fMRI was obtained using the following parameters: repetition time (TR), 2000 ms; echo time, 30 ms; flip angle, 90 degrees; field of view, 240 mm × 240 mm; matrix, 64 × 64; voxel size, 3.75 mm × 3.75 mm × 5 mm; 33 axial slices without slice gap. A total of 240 TRs were collected for each subject.
Data preprocessing
Data preprocessing was performed using the Data Processing & Analysis for Brain Imaging (DPABI, v2.3, www.rfmri.org/dpabi) and SPM12 toolkits (www.fil.ion.ucl.ac.uk/spm/software/spm12). The first 10 volumes were excluded, and the remaining functional images were corrected for slice timing and realignment. Head motion exceeded 2.5 mm translation or 2.5° rotation were excluded from subsequent analyses. The mean frame displacement (FD) was calculated for each subject according to a previously published formula (Power et al., Reference Power, Barnes, Snyder, Schlaggar and Petersen2012). The functional images were further normalized to a standard template (Montreal Neurological Institute) and re-sampled to 3 mm × 3 mm × 3 mm3. After normalization, several spurious variances, including 24 head motion parameters (Friston 24-parameter model) (Friston et al., Reference Friston, Williams, Howard, Frackowiak and Turner1996), cerebrospinal fluid signals, and white matter signals, were regressed out by multiple linear regression analysis. For a precise head motion correction, the parameters from scrubbing data were also regressed out. The bad points were identified by a threshold of FD (>0.5 mm) as well as one-forward and two-back neighbors (Power et al., Reference Power, Barnes, Snyder, Schlaggar and Petersen2012). Then each bad point was modeled as a separate regressor in the regression models. Functional images were spatially smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel. Subsequently, linear trends were removed from time courses. Temporal band-pass filtering was performed between 0.01 and 0.10 Hz. Because of the necessary contiguous time points in ALFF analysis, we did not carry out scrubbing, which altered the temporal structure of the data (Yan et al., Reference Yan, Cheung, Kelly, Colcombe, Craddock, Di Martino, Li, Zuo, Castellanos and Milham2013). While we used the mean FD as a covariate in group-level analysis to reduce motion-related artifact in the fMRI signal.
Dynamic ALFF computation
The dynamic ALFF was computed using a sliding window approach via DynamicBC (v1.1, www.restfmri.net/forum/DynamicBC) (Liao et al., Reference Liao, Wu, Xu, Ji, Zhang, Zang and Lu2014a). Window length is an important parameter in resting-state dynamics computation. According to previous studies, the minimum window length should be no less than 1/f min, because shorter window lengths may increase the risk of introducing spurious fluctuations in the observed dynamic ALFF (Leonardi and Van De Ville, Reference Leonardi and Van De Ville2015). f min was defined as the minimum frequency of time series. Based on this, the optimal window length of 50 TRs (100 s) was selected to compute the temporal variability of ALFF, because a longer window length may hinder the description of the temporal variability dynamics of ALFF. The time series was comprised of 230 TRs (460 s), and the window was shifted by five TRs (10 s). The full-length time series was then divided into 37 windows for each participant. For each sliding window, the ALFF map was obtained. The ALFF of each voxel was divided by the global mean ALFF value to normalize the global effects. To study the temporal variability of the amplitude of iBA, we computed the variance of dALFF maps across sliding-window dynamics. See Fig. 1a for analysis steps.

Fig. 1. Illustration of analysis steps and temporal variability of dALFF pattern. (a) The preprocessed full-length BOLD fMRI time series was segmented into several sliding windows (50 TRs). For each sliding window, the FFT-based ALFF measure was computed for each voxel for the whole brain. The ALFF was defined as the average square root of the activity in the low-frequency band (0.01–0.10 Hz). The ALFF value of each voxel was standardized by dividing the full-brain mean ALFF value. The sliding window was systematically shifted by five TRs and the corresponding ALFF was computed. This process was performed until the entire data length was covered. The temporal variability of the dALFF was defined as the variance of dALFF maps across the sliding windows. The pattern of temporal variability of the dALFF of the SI (b) and NSI group (c). The temporal variability of dALFF was averaged at each voxel across all subjects in each group. Low and high variances of dALFF are shown in red and yellow colors, respectively.
Statistical analysis
Demographic and clinical characteristics were evaluated among three groups. Differences in age and education were analyzed with one-way analysis of variance (ANOVA); χ2 test was used for gender. Illness duration and HAMD score were compared between the SI and NSI groups by Mann–Whitney U test and two-sample t test, respectively.
To determine group-level temporal variability of ALFF, we used DPABI toolkit (v2.3, www.rfmri.org/dpabi) (Yan et al., Reference Yan, Wang, Zuo and Zang2016) to perform one-sample t test for temporal variability of ALFF within-group comparisons (within the gray matter mask) for each group. Two-sample t test analysis was performed to investigate the group differences of temporal variability of ALFF between the SI and NSI groups. Age, gender, educational level, mean FD, and HAMD score were used as covariates. However, liberalizing the statistical threshold can dramatically increase the family-wise error rate (FWER), as recently demonstrated systematically for widely used statistical methods (Eklund et al., Reference Eklund, Nichols and Knutsson2016). Considering the trade-off between ALFF reproducibility and FWER (Chen et al., Reference Chen, Lu and Yan2018), we set the statistical significance level at P FWER < 0.05 under permutation test (PT, 5000 times permutations) using in Permutation Analysis of Linear Models (Winkler et al., Reference Winkler, Ridgway, Douaud, Nichols and Smith2016) implemented in DPABI. Combination of PT-based cluster size inference and height threshold with p < 0.01 as the cluster-forming threshold and the cluster extent threshold at k > 25 voxels. Post hoc comparisons (SI v. HCs and NSI v. HCs) were then performed with a two-sample t test. Bonferroni-corrected for two planned comparisons was used for multiple comparisons.
SSI symptom prediction
To investigate the relationship between altered temporal variability of ALFF and symptom severity measured by the SSI, we predicted the SSI score for each patient in the SI group using a general linear model according to the previous work (Finn et al., Reference Finn, Shen, Scheinost, Rosenberg, Huang, Chun, Papademetris and Constable2015; Shen et al., Reference Shen, Finn, Scheinost, Rosenberg, Chun, Papademetris and Constable2017). We used altered temporal variability of ALFF values in the SI group (compared with the NSI group) as features. We applied a leave-one-out cross-validation (LOOCV) to produce a robust and reliable model. This method is the most popular choice and unbiased strategy (Finn et al., Reference Finn, Shen, Scheinost, Rosenberg, Huang, Chun, Papademetris and Constable2015; Shen et al., Reference Shen, Finn, Scheinost, Rosenberg, Chun, Papademetris and Constable2017). In each LOOCV, we selected one subject's data as a test set, and the remaining subjects’ data were used as a train set so that this subject's SSI score was predicted based on the building prediction model. Finally, we used the Pearson's correlation to determine whether predicted SSI score is correlated with the observed SSI score in patients with SI. To improve the standards correlation analysis, we identified outliers by bootstrapping the Mahalanobis distance D s for each observation from the bivariate mean and excluded all points with an average D s of 6 or greater (Schwarzkopf et al., Reference Schwarzkopf, De Haas and Rees2012). If the statistical significance level of p < 0.05, we then considered that the altered temporal variability of ALFF could predict SSI and vice versa.
In addition, to determine whether dALFF values would provide a neuromarker to predict the severity of SI than static ALFF, we also employed a LOOCV procedure in SSI symptom prediction by static ALFF values.
Validation analysis
To validate our findings of temporal variability of dALFF, we carried out auxiliary analyses. In addition to the window length of 50 TRs (100 s), two additional window lengths [30 TRs (60 s) and 80 TRs (160 s)] were considered to validate the main results.
In addition, the head motion would potentially affect the brain dynamics (Hutchison et al., Reference Hutchison, Womelsdorf, Allen, Bandettini, Calhoun, Corbetta, Della Penna, Duyn, Glover, Gonzalez-Castillo, Handwerker, Keilholz, Kiviniemi, Leopold, de Pasquale, Sporns, Walter and Chang2013). We did not perform the produce for scrubbing bad time points identified as image frames because of the necessary contiguous time points in ALFF analysis. However, the mean FD did not differ between two groups [T (46) = 0.80, p = 0.43]. In addition, the mean FD was considered as a covariate in group-level analysis for correcting motion-related artifact. After we obtained the main results, we additionally performed correlation analysis between head motion parameters (mean FD) and dALFF variance values from group difference regions across subject to further preclude the impact of motion in our results.
Results
Clinical and demographic characteristics
No differences in age (one-way ANOVA, p = 0.27), education (one-way ANOVA, p = 0.83), gender (χ2 test, p = 0.26), and head motion (one-way ANOVA, p = 0.54) were found among the three groups. A significant difference was found in HAMD score (two-sample t test, p = 0.002) (Table 1). The range of SSI score in the SI group was 9–24.
Table 1. Participant demographic and clinical information

SI, major depression disorder patients with suicidal ideation; NSI, major depression disorder patients without suicidal ideation; HCs, healthy controls; HAMD, 17-item Hamilton Depression Scale; SSI, 19-item Scale for Suicide Ideation. FD, framewise-displacement; p, between-group or among-group test p value; t (df), between-group t statistic and degrees of freedom; F (dfn, dfd), one-way ANOVA and degrees of freedom numerator and degrees of freedom denominator; N.A., not available.
Values are mean ± s.d.
a T-value was calculated between the SI and NSI groups.
Temporal variability of ALFF differences between the SI and NSI groups
Temporal variability of ALFF was quantified at each voxel for the SI and NSI groups (Fig. 1b, c). The variance of the dALFF displayed a non-uniform spatial distribution across the brain. The largest temporal variability of dALFF was located in the heteromodal association cortex, including the bilateral prefrontal lobes, the temporal–parietal junction, and the posteromedial cortex. The lowest variability was located in limbic cortices. Brain regions showing a moderate level of variability were anchored to the primary sensory and visual cortices, as well as upstream and downstream of the unimodal cortices.
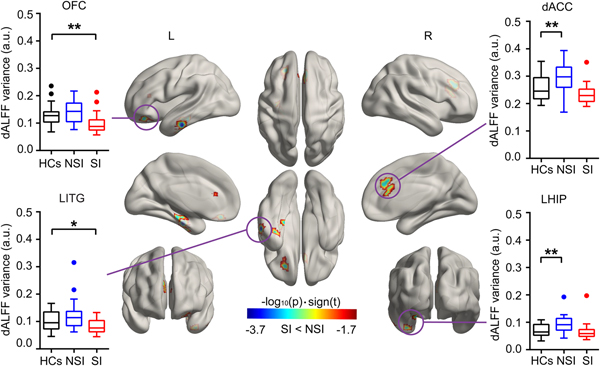
According to two-sample t tests, we found that the temporal ALFF variability in the right dorsal anterior cingulate cortex (dACC), the left inferior temporal gyrus (ITG), the hippocampus/parahippocampus gyrus (HIP/ParaHIP), and the orbital frontal cortex (OFC) was significantly different between the SI and NSI groups. Post hoc tests revealed that patients with SI showed decreased temporal ALFF variability in the OFC, left ITG compared with HCs, and patients with NSI showed increased temporal ALFF variability in the dACC and left HIP/ParaHIP compared with HCs (Fig. 2 and Table 2). These results were presented on inflated surface maps by BrainNet Viewer (v1.8, www.nitrc.org/projects/bnv) (Xia et al., Reference Xia, Wang and He2013).

Fig. 2. Group differences of temporal variability of the dALFF. Temporal variability of the dALFF between the SI and NSI groups was identified using two-sample t tests. The statistical significance level was set P FWER < 0.05 under permutation test-based corrections. The inset box-and-whisker plot indicates the planned post-hoc analysis between SI and HCs, and between NSI and HCs using two-sample t tests. *Denotes p < 0.05, uncorrected. **Denotes p < 0.05 Bonferroni correction with two times planned comparisons, respectively. HCs, healthy controls; NSI, major depressive disorder without suicidal ideation; SI, major depressive disorder with suicidal ideation.
Table 2. Decreased dALFF regions in the SI group compared with the NSI group

MNI, Montreal Neurological Institute.
Statistical value was computed by the equation: statistical value = −log10(p)·sign(t).
To determine whether dynamic ALFF and static ALFF provide overlapping or complementary information, we also computed the static ALFF patterns using full-length time series (Zang et al., Reference Zang, He, Zhu, Cao, Sui, Liang, Tian, Jiang and Wang2007). The group differences between the SI and NSI groups of static ALFF patterns are shown in online Supplementary Fig. S1.
SSI score prediction from temporal variability of ALFF
We found that dALFF values could predict the severity of SI (r = 0.43, p = 0.03), while static ALFF values could not (r = 0.20, p = 0.31) (Fig. 3).

Fig. 3. Temporal variability of the dALFF predicts the severity of SI. (a) The results of dynamic ALFF as features to predict the severity of SI (r = 0.43, p = 0.03). (b) The results of static ALFF as features to predict the severity of SI (r = 0.20, p = 0.31). Filled circles were included in this correlation analysis, while open circles were excluded. Solid lines and dashed lines represented the best-fitted line and 95% confidence interval of the Pearson's correlation analysis, respectively. ALFF, amplitude of low-frequency fluctuation; SSI, Scale for Suicide Ideation.
Validation results
The analysis of the data using different sliding-window lengths supported our main results (online Supplementary Fig. S2). There are no significant correlations between motion parameters and variance of dALFF in abnormal brain regions (all p > 0.05) (online Supplementary Fig. S3).
Discussion
We demonstrated a novel way, temporal variability of amplitude of low-frequency fluctuations, to explore brain dynamics on MDD with and without SI. Specifically, the SI group exhibited decreased brain dynamics (less temporal variability of dALFF) in the dACC, left OFC, ITG and HIP/ParaHIP compared with the NSI group. More broadly, the altered temporal ALFF variability values in these regions could predict the severity of SI.
Brain dynamics would reflect the aspects of neural system functional capacity (Kucyi et al., Reference Kucyi, Hove, Esterman, Hutchison and Valera2017) and serve as a novel physiological neuromarker of various neurological and psychiatric diseases (Damaraju et al., Reference Damaraju, Allen, Belger, Ford, McEwen, Mathalon, Mueller, Pearlson, Potkin, Preda, Turner, Vaidya, van Erp and Calhoun2014; Liao et al., Reference Liao, Zhang, Mantini, Xu, Ji, Zhang, Wang, Wang, Chen, Tian, Jiao, Zang and Lu2014b; Kaiser et al., Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer, Minkel, Smoski, Dichter and Pizzagalli2016; Kim et al., Reference Kim, Criaud, Cho, Diez-Cirarda, Mihaescu, Coakeley, Ghadery, Valli, Jacobs, Houle and Strafella2017a; Li et al., Reference Li, Liao, Yu, Chen, Guo, Tang and Chen2018). Although the presence and severity of MDD is associated with abnormal dynamics of inter-regional functional connectivity (Kaiser et al., Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer, Minkel, Smoski, Dichter and Pizzagalli2016), the dynamics of local brain activity itself remains unknown. In contrast to the inter-regional functional connectivity, fluctuations of local brain activity can also be captured using first-order statistics, that is, with time-resolved analysis of instantaneous activity patterns (Fu et al., Reference Fu, Tu, Di, Du, Pearlson, Turner, Biswal, Zhang and Calhoun2017). The current work expands the dynamics of brain connectivity in depressed patients, and deconstruct the time-varying patterns of iBA in depressed patients with and without SI.
Both OFC and dACC are involved in executive function and emotional processing (Rogers et al., Reference Rogers, Kasai, Koji, Fukuda, Iwanami, Nakagome, Fukuda and Kato2004; Schoenbaum et al., Reference Schoenbaum, Roesch and Stalnaker2006; Frodl et al., Reference Frodl, Bokde, Scheuerecker, Lisiecka, Schoepf, Hampel, Moller, Bruckmann, Wiesmann and Meisenzahl2010), which are implicated in MDD with SI or suicidal attempt (Marzuk et al., Reference Marzuk, Hartwell, Leon and Portera2005; Westheide et al., Reference Westheide, Quednow, Kuhn, Hoppe, Cooper-Mahkorn, Hawellek, Eichler, Maier and Wagner2008; Pan et al., Reference Pan, Hassel, Segreti, Nau, Brent and Phillips2013; Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016). One important aspect of the OFC in executive function is to incorporate emotional salience (e.g. reward and punishment) into decision-making (Mesulam, Reference Mesulam, Stuss and Knight2002; Rogers et al., Reference Rogers, Kasai, Koji, Fukuda, Iwanami, Nakagome, Fukuda and Kato2004). The current finding of the OFC is consistent with the previous studies, suggesting that the OFC abnormalities are related to suicidal behavior in MDD (Monkul et al., Reference Monkul, Hatch, Nicoletti, Spence, Brambilla, Lacerda, Sassi, Mallinger, Keshavan and Soares2007; Jia et al., Reference Jia, Wang, Huang, Kuang, Wu, Lui, Sweeney and Gong2014). The OFC also has a critical role in modulating impulsivity (Matsuo et al., Reference Matsuo, Nicoletti, Nemoto, Hatch, Peluso, Nery and Soares2009). Impulsivity, one of factors related to personality and individual differences affecting cognitive and emotion, is associated with SI, suicidal attempt, and suicide deaths (O'Connor and Nock, Reference O'Connor and Nock2014). Therefore, we speculate that decreased dALFF in the OFC may lead to abnormal executive function and emotional processing related to MDD with SI.
We found another considerable decreased dALFF in the dACC. The dACC plays an important role in executive function (Bush et al., Reference Bush, Luu and Posner2000; Rogers et al., Reference Rogers, Kasai, Koji, Fukuda, Iwanami, Nakagome, Fukuda and Kato2004; Frodl et al., Reference Frodl, Bokde, Scheuerecker, Lisiecka, Schoepf, Hampel, Moller, Bruckmann, Wiesmann and Meisenzahl2010; Lieberman and Eisenberger, Reference Lieberman and Eisenberger2015) in MDD with SI. The dACC is interconnected with prefrontal cortex, parietal cortex, and motor system, playing a central role in processing top-down activation (Posner and DiGirolamo, Reference Posner, DiGirolamo and Parasuraman1998; Zhou et al., Reference Zhou, Hu, Lu, Zhang, Chen, Gong and Huang2017). Top-down mental processes are needed in executive functions (Diamond, Reference Diamond2013). Aberrant brain distinct connectivity and local activity in the dACC may disturb the balance of the default-mode network, resulting in abnormal emotional regulation (Pannekoek et al., Reference Pannekoek, van der Werff, Meens, van den Bulk, Jolles, Veer, van Lang, Rombouts, van der Wee and Vermeiren2014; Zhou et al., Reference Zhou, Hu, Lu, Zhang, Chen, Gong and Huang2017). Furthermore, abnormal brain structure and function in the dACC were related to parasuicidal behavior and SI (Whittle et al., Reference Whittle, Chanen, Fornito, McGorry, Pantelis and Yucel2009; Marchand et al., Reference Marchand, Lee, Johnson, Thatcher, Gale, Wood and Jeong2012; Chase et al., Reference Chase, Segreti, Keller, Cherkassky, Just, Pan and Brent2017). We thus suggest that decreased dALFF activity in the dACC may underlie the phenomenon of abnormal executive function and emotional processing in MDD with SI.
For MDD without SI, previous studies have found altered brain local activity in both dorsal and ventral ACC (Davidson et al., Reference Davidson, Pizzagalli, Nitschke and Putnam2002; Mayberg, Reference Mayberg2003; Liu et al., Reference Liu, Ren, Womer, Wang, Fan, Jiang, Blumberg, Tang, Xu and Wang2014; Zhou et al., Reference Zhou, Hu, Lu, Zhang, Chen, Gong and Huang2017). In line with previous works, we found increased dALFF in the dACC in MDD without SI compared with HCs. The dACC contributes to online performance monitoring by detecting errors and modifies attention bias based on conflict paradigms (Carter et al., Reference Carter, Braver, Barch, Botvinick, Noll and Cohen1998; Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004; Liu et al., Reference Liu, Ren, Womer, Wang, Fan, Jiang, Blumberg, Tang, Xu and Wang2014). Therefore, we indicate that increased ALFF in the dACC may disturb error detection and cognitive control in MDD without SI.
The HIP participants in autographical memory and emotional regulation (Bremner et al., Reference Bremner, Vythilingam, Vermetten, Vaccarino and Charney2004; Viard et al., Reference Viard, Piolino, Desgranges, Chetelat, Lebreton, Landeau, Young, De La Sayette and Eustache2007), which are associated with MDD with/without SI and suicidal attempt (Bremner et al., Reference Bremner, Vythilingam, Vermetten, Vaccarino and Charney2004; O'Connor and Nock, Reference O'Connor and Nock2014; Wang et al., Reference Wang, Xia, Li, Zeng, Su, Dai, Zhang, Jin, Mitchell, Yu, He and Si2015; Johnston et al., Reference Johnston, Wang, Liu, Blond, Wallace, Liu, Spencer, Cox Lippard, Purves, Landeros-Weisenberger, Hermes, Pittman, Zhang, King, Martin, Oquendo and Blumberg2017). We observed decreased dALFF in the left HIP in MDD with SI relative to the NSI group. The dALFF is a mean of capturing brain instantaneous activity patterns based on high time-resolved (Fu et al., Reference Fu, Tu, Di, Du, Pearlson, Turner, Biswal, Zhang and Calhoun2017). The brain instantaneous activity, excessive variability (increased temporal variance), or excessive stability (decreased temporal variance) (Christoff et al., Reference Christoff, Irving, Fox, Spreng and Andrews-Hanna2016) may occur at different times standing as causes of altered cognitive functions and particular pathological state (Preti et al., Reference Preti, Bolton and Van De Ville2017). Therefore, we speculate that decreased dALFF in the left HIP may underlie the phenomenon of disable to recall specific memories and failure to solve emotional problem in the SI group compared with the NSI group. In addition, we found increased dALFF in the left HIP in the NSI group compared with HCs. This finding is consistent with the previous studies demonstrating that MDD exhibits hypoactivation in the HIP in response to positive social stimuli compared with HCs (Fu et al., Reference Fu, Williams, Brammer, Suckling, Kim, Cleare, Walsh, Mitterschiffthaler, Andrew, Pich and Bullmore2007; Sheline et al., Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder, Mintun, Wang, Coalson and Raichle2009).
We observed that the SI groups showed increased dALFF in the left ITG compared with the NSI group. The ITG is involved in a putative output system, which regulates visceral functions connected with emotions (van Tol et al., Reference van Tol, Li, Metzger, Hailla, Horn, Li, Heinze, Bogerts, Steiner, He and Walter2014). Abnormalities in the ITG may cause emotional disturbances, which not only relate to MDD but also influence SI in MDD patients. Moreover, the ITG showed decreased regional cerebral blood flow and it was considered as one of the top 10 regions to predict suicide in depressed suicidal patients compared with depressed non-suicidal patients (Willeumier et al., Reference Willeumier, Taylor and Amen2011). In this respect, impairment in the temporal cortex was not only related to MDD but also associated with the risk of SI in MDD patients. Our study suggests that the ITG may play an important role in detecting NSI development and the risk of SI in MDD patients.
More importantly, the temporal variability of dALFF would predict the severity of SI using. Although several previous studies have found a correlation between neuroimaging features and clinical variables about SI in MDD (Ballard et al., Reference Ballard, Lally, Nugent, Furey, Luckenbaugh and Zarate2015; Pu et al., Reference Pu, Nakagome, Yamada, Yokoyama, Matsumura, Yamada, Sugie, Miura, Mitani, Iwata, Nagata and Kaneko2015; Myung et al., Reference Myung, Han, Fava, Mischoulon, Papakostas, Heo, Kim, Kim, Kim, Kim, Seo, Seong and Jeon2016), we are not aware of any study that has reported employing dynamic values to predict the severity of SI. Interestingly, we found that dALFF values could successfully predict the severity of SI in the SI group while static ALFF values could not, suggesting that dALFF values may be a more powerful predicted neuromarker in the current sample. However, we did not underestimate the key role of static ALFF in disease prediction modal. In the future work, combining the dynamic ALFF and static ALFF (Fu et al., Reference Fu, Tu, Di, Du, Pearlson, Turner, Biswal, Zhang and Calhoun2017), even dynamic local brain activity and remote inter-regional connectivity (Rashid et al., Reference Rashid, Arbabshirani, Damaraju, Cetin, Miller, Pearlson and Calhoun2016) would build better models of brain function and dysfunction.
Limitations and further considerations
Several issues need to be considered. First, although the group size is relatively small, the power analysis showed a large effect size, suggesting the generalizability of our findings to a large sample size. Second, we selected the window size according to the filter bandwidth (0.01–0.10 Hz) utilized in a previous study, which recommended that the minimum window length should be no less than 1/f min (1/0.01 = 100 s) (Liao et al., Reference Liao, Zhang, Mantini, Xu, Ji, Zhang, Wang, Wang, Chen, Tian, Jiao, Zang and Lu2014b). Similarly, albeit less reliable, results from the utilization of different sliding window lengths suggest that the findings of the present study are less influenced by this factor. Third, generalizability of brain predictive models is important (Woo et al., Reference Woo, Chang, Lindquist and Wager2017). The current model using the dynamics of brain activity should be generalized to new individuals and across different centers in future studies. In addition, LOOCV is unbiased but typically has more variance in prediction error than K-fold (i.e. 10-fold) cross-validation (Kohavi, Reference Kohavi1995). However, the K-fold cross-validation can be critical and depends on sample size and effect size in the prediction model (Shen et al., Reference Shen, Finn, Scheinost, Rosenberg, Chun, Papademetris and Constable2017). Considering the small sample size here, we choose the LOOCV for the prediction model. Finally, although we did not perform the produce for scrubbing bad time points, the mean FD was considered as a covariate in statistical analysis and did not correlate with dALFF variance and preclude the impact of motion in our results.
In summary, compared with MDD without SI, the SI group showed decreased brain dynamics (less temporal variability) in the dACC, the OFC, the left ITG, and the left HIP. Our findings suggest that abnormal executive and emotional processing related to MDD with SI. More broadly, these dALFF abnormalities could predict the severity of SI while static ALFF abnormalities could not, indicating the first evidence of changes of brain dynamics in SI. Our findings suggest that this novel predictive model using iBA dynamics could be useful to develop neuromarkers for clinical applications.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001502.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (61533006, 81471653, 81771919, and 61673089), the China Postdoctoral Science Foundation (2013M532229), and the ‘111’ project (B12027).
Conflict of interest
None.
Article information
From the University of Electronic Science and Technology of China (JL, XD, QC, WL, and HC).