1. Introduction
Encrusting tubicolous microconchids are a Late Ordovician – Middle Jurassic group of mostly marine taxa. On the basis of their regularly coiled calcareous tubes, microconchids, including Permian and Triassic forms, were variously classified as either the polychaete tubeworm Spirorbis, spirorbids as a whole, serpulids, tubicolous worms or sometimes microgastropods (e.g. Gall, Reference Gall1971; Peryt, Reference Peryt1974; Kelber, Reference Kelber1987; Adachi et al. Reference Adachi, Ezaki and Liu2004; Vaslet et al. Reference Vaslet, Le Nindre, Vachard, Broutin, Crasquin-Soleau, Berthelin, Gaillot, Halawani and Al-Husseini2005; Kukhtinov, Reference Kukhtinov, Ivanov, Novikov and Yashkov2017). However, in-depth studies of the tube ultrastructure and morphology proved that they were neither polychaetes nor molluscs, but resembled most closely the tentaculitoids, together with which the order Microconchida forms the class Tentaculita, probably an extinct lineage of the lophophorates (Weedon, Reference Weedon1991; Taylor & Vinn, Reference Taylor and Vinn2006; Taylor et al. Reference Taylor, Vinn and Wilson2010; Vinn, Reference Vinn2010). Compared with serpulids, which possess aporose tubes open at both ends and chevron-shaped growth increments in their wall structure, microconchids had a closed bulbous protoconch at the proximal end of the tube and a foliated wall fabric traversed by pseudopunctae and pores similar to brachiopods and bryozoans (Taylor & Vinn, Reference Taylor and Vinn2006; Vinn et al. Reference Vinn, ten Hove and Mutvei2008; Zatoń & Olempska, Reference Zatoń and Olempska2017).
Microconchids appeared in the Late Ordovician seas and began colonizing continental settings probably as early as during the Early Devonian Epoch, populating a wide range of environments in brackish and fresh waters (Taylor & Vinn, Reference Taylor and Vinn2006; Caruso & Tomescu, Reference Caruso and Tomescu2012; Zatoń et al. Reference Zatoń, Vinn and Tomescu2012, Reference Zatoń, Wilson and Vinn2016 b; Zatoń & Peck, Reference Zatoń and Peck2013; Matsunaga & Tomescu, Reference Matsunaga and Tomescu2017). Recently, the autochthonous origin of brackish- and fresh-water microconchids has been challenged and their presence in such settings has been explained by invasions of marine waters that brought into continental lowland aquatic systems detached tubes or short-term surviving larvae during storm surges and tsunamis (Gierlowski-Kordesch & Cassle, Reference Gierlowski-Kordesch and Cassle2015; Gierlowski-Kordesch et al. Reference Gierlowski-Kordesch, Falcon-Lang and Cassle2016).
Here we describe microconchids from lacustrine and fluvial settings in the uppermost Permian strata of the Tunguska and Kuznetsk basins and the Lower Triassic deposits of the southern Cis-Urals (Russia). We further summarize palaeoecological and palaeobiogeographical data on latest Palaeozoic and earliest Mesozoic microconchids to interpret these new occurrences as unique disaster eurytopic survivors of the Permo-Triassic mass extinction (Fig. 1).
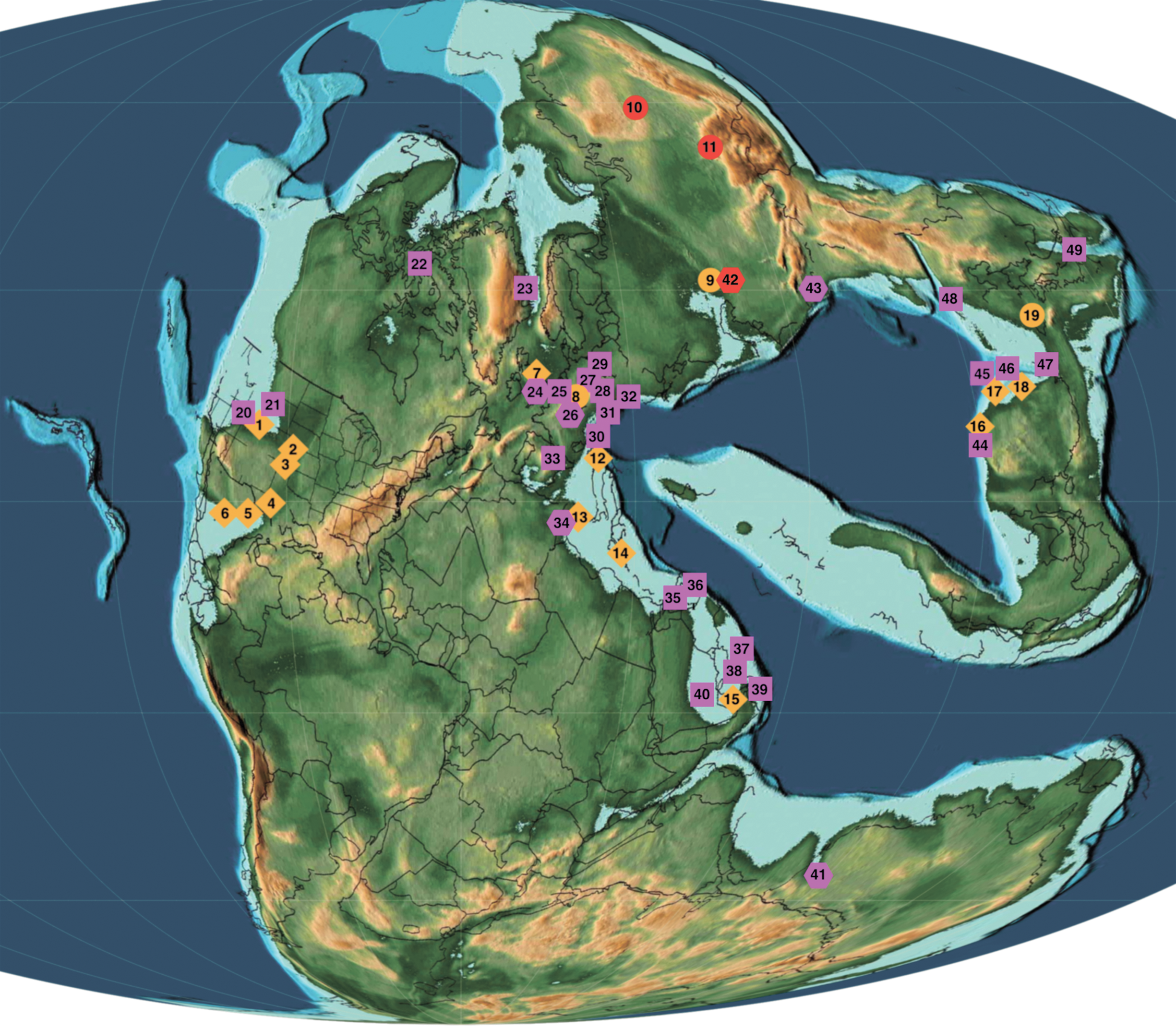
Fig. 1. Early Triassic, Olenekian (250 Ma) palaeogeography (generated from https://www.earthbyte.org/paleomap-paleoatlas-for-gplates/ with GPlates 2.1.0) showing Permian and Lower–Middle Triassic localities with microconchids (locality numbers correspond to those in Table 2). Orange rhombs – Permian marine localities; orange circles – Permian lacustrine localities; violet squares – Triassic marine localities; violet hexagons – Triassic lacustrine localities; red symbols – lacustrine localities under discussion (10 – uppermost Permian Tunguska Basin, 11 – uppermost Permian Kuznetsk Basin, 42 – Lower Triassic southern Cis-Urals; Russia).
2. Geological background
2.a. Uppermost Permian Tunguska Basin
The Tunguska Basin of the Siberian Platform hosts the Permian–Triassic Traps large igneous province (LIP), the eruption of which caused the most severe mass extinction known. Trap formation started at the end of the Permian Period from initial volcanic ejections leading to volcanic ash (tuff) deposition, followed by an increasing volcanic activity and the emplacement of mafic sills and dykes, and finally by the vast lava floods of dominantly basaltic compositions that bracket the Permian–Triassic boundary interval (Fedorenko & Czamanske, Reference Fedorenko and Czamanske1997; Reichow et al. Reference Reichow, Pringle, Al’Mukhamedov, Allen, Andreichev, Buslov, Davies, Fedoseev, Fitton, Inger, Medvedev, Mitchell, Puchkov, Safonova, Scott and Saunders2009; Ivanov et al. Reference Ivanov, He, Yan, Ryabov, Shevko, Palesskii and Nikolaeva2013). The lowermost Triassic volcanoclastics in this sequence contain a number of combusted woody fragments and char particles embedded in the volcaniclastic matrix (Elkins-Tanton et al. Reference Elkins-Tanton, Grasby, Black, Veselovskiy, Ardakani and Goodarzi2020).
The strata bearing microconchids are exposed along the middle reaches of the Nizhnyaya Tunguska River (50–90 km east of the settlement of Tura, Krasnoyarsk region), which crosses the flood basalt plateau in its southern area. These beds are a part of the terrestrial volcanic-siliciclastic Bugarikta Formation, the deposition of which corresponds to the interval immediately preceding the major basalt flooding event (Fig. 2, sites 1–4, Table 1). Going west and downstream along the Nizhnyaya Tunguska River, these exposures are at Degigli (64° 01’ N, 102° 01’ E), Anakit (64° 07’ N, 101° 52’ E), Khungtukun (64° 10’ N, 101° 42’ E) and Nizhnyaya Lyulyuikta (64° 07’ N, 101° 15’ E). The Bugarikta Formation conformably overlies the Upper Permian Uchami Formation here, which consists primarily of massive coarse-grained volcanic tuffs and xenomorphic tuffs and, in places, agglomerated unsorted volcanomictic breccia, and is overlain by the dominantly basaltic Nidym Formation. The Bugarikta Formation is 50–270 m in thickness, consisting of variegated volcanic-sedimentary medium- to coarse-grained volcanic ash-rich tuffite, medium- and coarse-grained tuff, grey and dark-brown thin-bedded fine- to coarse-grained siltstone and fine-grained sandstone, with lenses of siltstones consolidated by a calcareous cement; taxite-type basalt sills are interbed with the other lithologies (Saks et al. Reference Saks, Gol’bert, Dagis, Mesezhnikov and Shatskiy1981; Sadovnikov & Orlova, Reference Sadovnikov and Orlova1995; Sytchevskaya, Reference Sytchevskaya, Arratia and Schultze1999; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009).

Fig. 2. Map of Russia indicating sections discussed here: (1–4) uppermost Permian (Changhsingian) (1) Degigli, (2) Anakit, (3) Khungtukun and (4) Nizhnyaya Lyulyuikta sections of the Tunguska Basin, Krasnoyarsk region; (5) uppermost Permian (Changhsingian) Babiy Kamen’ section of the Kuznetsk Basin, Kemerovo region; (6) Lower Triassic (Olenekian) Petropavlovka III section, southern Cis-Urals, Orenburg region. Base map source: https://en.wikipedia.org/wiki/Wikipedia:WikiProject_Geographical_coordinates.
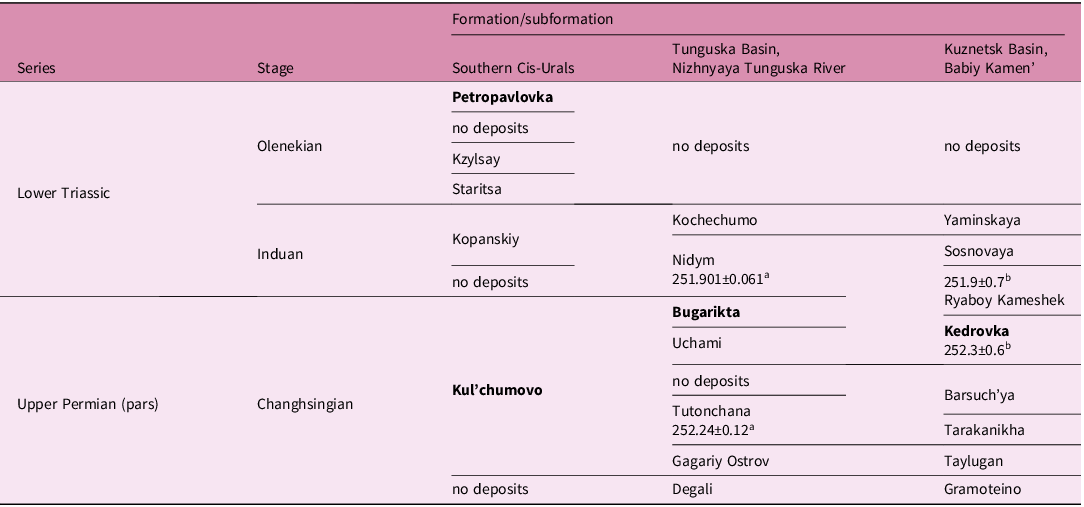
Table 1. Lithostratigraphic chart of the Permian-Triassic boundary strata in the Cis-Urals and the Tunguska and Kuznetsk basins of Central Siberia (Saks et al. Reference Saks, Gol’bert, Dagis, Mesezhnikov and Shatskiy1981; Krasnov et al. Reference Krasnov, Savitsky, Tesakov and Khomentovsky1982; Kazakov et al. Reference Kazakov, Konstantinov, Kurushin, Mogutcheva, Sobolev, Fradkina, Yadryonkin, Devyatov and Smirnov2002; Tverdokhlebov et al. Reference Tverdokhlebov, Tverdokhlebova, Minikh, Surkov and Benton2005; Knyazev et al. Reference Knyazev, Knyazeva, Snachev, Zhdanov, Karimov, Aydarov, Masagutov and Arslanova2013; Kukhtinov et al. Reference Kukhtinov, Yaroshenko, Shishkin, Sennikov, Minikh, Minikh, Tverdokhlebov, Levina, Prokhorova and Voronkova2016); microconchid-bearing units in bold; the Ryaboy Kameshek, Kedrovka, Barsuch’ya and Tarakanikha subformations compose the Mal’tsevo Formation

a Geochronological dates (Ma; Burgess & Bowring, Reference Burgess and Bowring2015).
b Geochronological dates (Ma; Svetlitskaya & Nevolko, Reference Svetlitskaya and Nevolko2016).
The macroflora and miospore assemblages characterizing the entire Bugarikta Formation have a transitional Permian–Triassic appearance: they lack cordaitaleans and yield mostly ferns, peltasperms and conifers with less common sphenophytes (Prinada, Reference Prinada1970; Krugovykh, Reference Krugovykh and Dagis1987; Mogutcheva, Reference Mogutcheva and Dagis1987, Reference Mogutcheva (Mogucheva)2016; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009; Sadovnikov, Reference Sadovnikov2015 a, b). Cordaitina pollen grains, which were earlier attributed to cordaitaleans (Romanovskaya et al. Reference Romanovskaya, Tabachnikova, Dryagina and Boytsova1973), possess a different exin ultrastructure and therefore do not provide evidence for the presence of the cordaitaleans in transitional Permian–Triassic strata of the Tunguska and Kuznetsk basins (Zavialova et al. Reference Zavialova, Gomankov, Yaroshenko and Rovnina2004). In addition to terrestrial vascular plant remains, charophycean gyrogonites are extremely common together with various shelly fossils, within the grainstone–packstone lenses in the Khungtukun and Nizhnyaya Lyulyuikta sections (Fig. 3c). Siltstone and sandstone beds from the localities under discussion contain a rich assemblage of freshwater clam shrimps or spinicaudatans (Anakit and Khungtukun; Sadovnikov & Orlova, Reference Sadovnikov and Orlova1995; Sadovnikov, Reference Sadovnikov2008; Figs 3a, 4a), ostracods (Anakit, Khungtukun and Nizhnyaya Lyulyuikta; Sadovnikov, Reference Sadovnikov2008; Fig. 3c), a rich insect fauna (Anakit, Khungtukun and Nizhnyaya Lyulyuikta; Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013), neopterygian fishes (Berg, Reference Berg1941; Sytchevskaya, Reference Sytchevskaya, Arratia and Schultze1999) and the temnospondyl amphibian Tungussogyrinus bergi (Anakit and Nizhnyaya Lyulyuikta; Efremov, Reference Efremov1939; Shishkin, Reference Shishkin1998).

Fig. 3. Microconchid tubes from the uppermost Permian (Changhsingian), Siberia, Russia; ESEM (BSE). (a) PIN 2716/3, aggregation encrusting spinicaudatan carapace, Bugarikta Formation, Degigli section, Tunguska Basin. (b) Detail of (a), two tubes showing embryonic chambers (arrowed) and microsculpture. (c) PIN 2402/33 packstone consisting of microconchid tubes (mostly) with groove-like attachment scar (bottom left), ostracod carapaces and charophycean gyrogonites, Bugarikta Formation, Nizhnyaya Lyulyuikta section, Tunguska Basin. (d) PIN 4887/822, attachment scars of three microconchids (arrowed) encrusting a bivalve shell, Mal’tsevo Formation, Babiy Kamen’ section, Kuznetsk Basin. Scale bars: (a, c) 1 mm; (b) 0.5 mm; and (d) 0.2 mm.

Fig. 4. Microconchid tubes encrusting spinicaudatan carapaces from the Bugarikta Formation, uppermost Permian (Changhsingian), Anakit section, Tunguska Basin, Krasnoyarsk region, Russia. (a) PIN 2362/27, large aggregation of mature individuals. (b) PIN 3061/27, ESEM (BSE), large individual. (c) PIN 5381/344, ESEM (BSE), showing lower attachment area and microsculpture, a spinicaudatan carapace with striated ornamentation is on the right. (d) PIN 5381/350a, ESEM (BSE), showing attachment area, bulbous embryonic chamber and microsculpture. Scale bars: (a, b) 2 mm; (c) 0.5 mm; and (d) 0.2 mm.
In the regional stratigraphic chart (Saks et al. Reference Saks, Gol’bert, Dagis, Mesezhnikov and Shatskiy1981) and in a number of palaeontological publications dealing with fossil flora and fishes, these strata are ascribed to the Lower Triassic (e.g. Mogutcheva, Reference Mogutcheva and Dagis1987; Sytchevskaya, Reference Sytchevskaya, Arratia and Schultze1999; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009). Despite the fact that these strata occupy a higher stratigraphic position than the presumable interval corresponding to the mass extinction, which eliminated the Permian cordaitalean flora, and also than the base of the coal gap (reviewed by Retallack et al. Reference Retallack, Veevers and Morante1996), they lack any typical Triassic elements. Even their most specific genera, such as the fish Evenkia and the conifer Quadrocladus, are known from the Upper Permian strata of Europe and other regions (Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013; Sadovnikov, Reference Sadovnikov2015 a, b; Bajdek et al. Reference Bajdek, Qvarnström, Owocki, Sulej, Sennikov, Golubev and Niedźwiedzki2016; Bernardi et al. Reference Bernardi, Petti, Kustatscher, Franz, Hartkopf-Fröder, Labandeira, Wappler, Van Konijnenburg-van Cittert, Peecook and Angielczyk2017; Blomenkemper et al. Reference Blomenkemper, Kerp, Hamad, DiMichele and Bomfleur2018; Karasev et al. Reference Karasev, Naumcheva, Arefiev, Golubev and Nurgaliev2018). Additionally, based on the high-precision (uranium/lead (U/Pb) chemical abrasion – thermal ionization mass spectrometry) geochronology of the northern Maymecha–Kotuy area within the Tunguska Basin (Burgess & Bowring, Reference Burgess and Bowring2015) and on its correlation with the Nizhnyaya Tunguska River area (Kazakov et al. Reference Kazakov, Konstantinov, Kurushin, Mogutcheva, Sobolev, Fradkina, Yadryonkin, Devyatov and Smirnov2002; Table 1), the Bugarikta Formation occurs below the geochronologically dated 251.902 ± 0.061 Ma base of the Hindeodus parvus conodont Zone, which defines the Permian–Triassic boundary in marine facies (Kozur & Weems, Reference Kozur, Weems and Lucas2010; Sadovnikov, Reference Sadovnikov2015 a, b, Reference Sadovnikov2016). These biostratigraphic and geochronological constraints restrict the age of the microconchid-bearing strata of the Tunguska Basin to the uppermost Permian Changhsingian Stage (Table 1).
2.b. Uppermost Permian Kuznetsk Basin
The Kuznetsk Basin is a vast late Palaeozoic – Mesozoic depression occupying the NW part of the Altay–Sayan Foldbelt of southern Siberia (Kemerovo region), which was superimposed onto an older terrane that was accreted to the main Siberian (Angara) Craton by the Silurian Period (Sennikov, Reference Sennikov, Albanesi, Beresi and Peralta2003; Cocks & Torsvik, Reference Cocks and Torsvik2007; Fig. 2, site 5). As in the Tunguska Basin, substantial coals were deposited here during Permian time under northern temperate paralic non-marine conditions. This accumulation was interrupted by a basalt trap eruption dated at 252.9 ± 0.4 – 251.9 ± 0.7 Ma (based on plagioclase 40Ar/39Ar). Geochemical and petrological features, palaeomagnetic characteristics and the age coincidence suggest a common LIP genetic source of Kuznetsk Basin lavas with those of the Tunguska Basin (Kazansky et al. Reference Kazansky, Metelkin, Bragin and Kungurtsev2005; Reichow et al. Reference Reichow, Pringle, Al’Mukhamedov, Allen, Andreichev, Buslov, Davies, Fedoseev, Fitton, Inger, Medvedev, Mitchell, Puchkov, Safonova, Scott and Saunders2009; Buslov et al. Reference Buslov, Safonova, Fedoseev, Reichow, Davies and Babin2010; Svetlitskaya & Nevolko, Reference Svetlitskaya and Nevolko2016). In the Kuznetsk Basin, radiometric ages were obtained for basaltic andesite and trachyandesite units and basalt sills occurring within the uppermost Permian (Changhsingian) Mal’tsevo Formation. The formation is subdivided into four subformations (in ascending order: the Tarakanikha, Barsuch’ya, Kedrovka and Ryaboy Kameshek) and composed of a well-expressed cyclic alternation of sandstone (with conglomerate lenses), siltstone, mudstone and argillaceous marlstone formed in lacustrine and fluvial environments and containing a significant proportion of pyroclastics (Neuburg, Reference Neuburg1936; Vasil’eva, Reference Vasil’eva1962; Vladimirovich et al. Reference Vladimirovich, Lebedev, Popov, Radchenko, Shvedov and Greyner1967; Kazakov et al. Reference Kazakov, Konstantinov, Kurushin, Mogutcheva, Sobolev, Fradkina, Yadryonkin, Devyatov and Smirnov2002). The Mal’tsevo Formation is underlain by the Upper Permian continental coal-bearing siliciclastic Taylugan Formation and overlain by the Lower Triassic non-marine volcanic-siliciclastic Sosnovaya Formation (Table 1). The key section, Babiy Kamen’ (54° 23’ N, 87° 32’ E), occurs on the right bank of the Tom’ River (75 km NNE of the town of Novokuznetsk); the microconchids are found in the upper Kedrovka Subformation, which is 75–150 m thick. This unit consists of massive and laminated mudstone, massive, laminated and ripple cross-stratified siltstone and sandy tuff, and is suggested to be deposited in braided river channel systems and low-energy long-lived lakes within a vast fluvial floodplain (Neustrueva & Bogomazov, Reference Neustrueva, Bogomazov, Martinson and Neustrueva1987; Shcherbakov et al. Reference Shcherbakov, Kabanov, Ponomarenko, Esin, Tatarinov and Golubev2002; Davies et al. Reference Davies, Allen, Buslov and Safonova2010).
In addition to microconchids, the Kedrovka Subformation yielded rich faunas of freshwater ostracods (Kukhtinov & Neustrueva, Reference Kukhtinov, Neustrueva, Oleynikov and Zhamoyda1986); spinicaudatans (Davydov et al. Reference Davydov, Zharinova and Silantiev2019); the bivalve Utschamiella (Silantiev et al. Reference Silantiev, Urazaeva, Nurgalieva, Alekseev and Nazarova2020; Fig. 3d); various insects (Shcherbakov, Reference Shcherbakov2008 c; Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013); gastropods; millipedes; scales of juvenile Acropholidae, Elonichthyidae and Palaeoniscidae actinopterygian fishes; tetrapod bones (Neuburg, Reference Neuburg1936; Shcherbakov et al. Reference Shcherbakov, Kabanov, Ponomarenko, Esin, Tatarinov and Golubev2002); and macrofloras consisting of the sphenophyte Neokoretrophyllites, the ferns Cladophlebis, Katasiopteris, Kedroviella and Kchonomakidium, the peltasperm Lepidopteris, the putative ginkgophyte Rhipidopsis and the conifer Quadrocladus (Neuburg, Reference Neuburg1936; Betekhtina et al. Reference Betekhtina, Mogutcheva, Batyaeva, Kushnarev, Yanshin and Dagis1986; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009; Karasev, Reference Karasev2015). The palynoflora demonstrates the dominance of a fern-ginkgophyte vegetation (Romanovskaya et al. Reference Romanovskaya, Tabachnikova, Dryagina and Boytsova1973). Based on the similarity of their faunal and floral fossil assemblages, the Kedrovka and Ryaboy Kameshek subformations are correlated with the Uchami and Bugarikta formations of the Tunguska Basin (Kukhtinov & Neustrueva, Reference Kukhtinov, Neustrueva, Oleynikov and Zhamoyda1986; Kazakov et al. Reference Kazakov, Konstantinov, Kurushin, Mogutcheva, Sobolev, Fradkina, Yadryonkin, Devyatov and Smirnov2002; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009; Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013; Davydov et al. Reference Davydov, Zharinova and Silantiev2019; Table 1). Similarly, these strata are assigned to the Lower Triassic in the regional stratigraphic chart (Saks et al. Reference Saks, Gol’bert, Dagis, Mesezhnikov and Shatskiy1981), although geochronology supports their Changhsingian age (Svetlitskaya & Nevolko, Reference Svetlitskaya and Nevolko2016; Table 1).
2.c. Lower Triassic Petropavlovka Lagerstätte
The fossiliferous Permian–Triassic succession of the Cis-Urals is well known for diverse fossil tetrapods and sections that allow for a detailed study of changes in climate, landscapes, vegetation, and insect and vertebrate communities across the Permian–Triassic boundary (Ochev & Shishkin, Reference Ochev and Shishkin1989; Ochev & Surkov, Reference Ochev, Surkov, Benton, Shishkin, Unwin and Kurochkin2000; Shishkin et al. Reference Shishkin, Ochev, Lozovskii, Novikov, Benton, Shishkin, Unwin and Kurochkin2000; Benton et al. Reference Benton, Tverdokhlebov and Surkov2004; Gomankov, Reference Gomankov2005; Shcherbakov, Reference Shcherbakov2008 a; Benton & Newell, Reference Benton and Newell2014). During the Early Triassic Epoch (Olenekian), orogenic movements were renewed in the Ural Mountains and the Peri-Caspian Depression was inundated by a transgression of the Palaeotethys, leading to increased rates of siliciclastic deposition in the Cis-Urals (Tverdokhlebov, Reference Tverdokhlebov, Martinson and Neustrueva1987). In the Cis-Ural Trough and on the nearby southeastern slope of the Volga-Ural Anteclise, a vast lacustrine-deltaic floodplain was formed, bordering the northern Peri-Caspian marine basin of the Palaeotethys (Fig. 2, site 6).
The Petropavlovka area was a part of this floodplain that accumulated grey and reddish-grey siliciclastics, mostly a rhythmic alternation of cross-laminated coarse-grained polymictic sandstone, parallel-bedded fine-grained sandstone, reddish-yellow, reddish-brown or grey subparallel-layered clay, siltstone and fine-grained clayey sandstone, reaching 400–800 m in total thickness (Tverdokhlebov, Reference Tverdokhlebov, Martinson and Neustrueva1987; Shishkin et al. Reference Shishkin, Ochev, Tverdokhlebov, Vergay, Gomankov, Kalandadze, Leonova, Lopato, Makarova, Minikh, Molostovskiy, Novikov and Sennikov1995). Conglomerate lenses are also common containing igneous and metamorphic pebbles originating in the Urals. Mud cracks and rhizoliths are generally restricted to the finer parallel-bedding lithologies; coarser sediments represent alluvial deposits while finer lithologies constitute shallow-water lacustrine deposits (Tverdokhlebov et al. Reference Tverdokhlebov, Surkov and Tverdokhlebova2007). These facies characterize delta floodplain and delta front complexes of the Petropavlovka Formation. This unit disconformably overlies the lower Olenekian coarse-grained Kzylsay Formation, and is disconformably overlain by the Middle Triassic siliciclastic Donguz Formation (Tverdokhlebov, Reference Tverdokhlebov and Morozov1967, Reference Tverdokhlebov, Martinson and Neustrueva1987; Table 1).
The Petropavlovka Formation itself is ascribed to the upper Olenekian strata based on the Parotosuchus tetrapod fauna, the lungfish Ceratodus multicristatus, miospore assemblages rich in Densoisporites nejburgii associated with the lycophyte Pleuromeia, and magnetostratigraphy (Shishkin et al. Reference Shishkin, Ochev, Tverdokhlebov, Vergay, Gomankov, Kalandadze, Leonova, Lopato, Makarova, Minikh, Molostovskiy, Novikov and Sennikov1995; Tverdokhlebov et al. Reference Tverdokhlebov, Tverdokhlebova, Surkov and Benton2003; Novikov, Reference Novikov2018). One of its key sections (locality Petropavlovka III, bed 43; Tverdokhlebov, Reference Tverdokhlebov and Morozov1967, p. 119) occurs along the Sakmara River near the village of Petropavlovka c. 45 km NE of the town of Orenburg (coordinates 52° 02’ N, 55° 38’ E) and consists of fossiliferous coarse-grained red beds containing a metre-thick lens of grey fine-grained micro-wavy to parallel-laminated polymictic siltstone to sandstone. Plant and animal fossils are not restricted to certain bedding planes but are randomly distributed in the rock, thus preserving some three-dimensionality. Such a sediment probably accumulated in an ephemeral pond during a flood event. The lens contains abundant plant megafossils including sphenophytes (Equisetites sp. and Neocalamites sp.) and gymnosperms – Carpolithus sp. seeds and Voltziopsis sp. conifer ovuliferous scales (Dobruskina, Reference Dobruskina1994; Shishkin et al. Reference Shishkin, Ochev, Tverdokhlebov, Vergay, Gomankov, Kalandadze, Leonova, Lopato, Makarova, Minikh, Molostovskiy, Novikov and Sennikov1995). The fossil vertebrate coenosis represented by the lungfish Ceratodus (Minikh & Minikh, Reference Minikh and Minikh1997) and temnospondyl amphibians (Shishkin et al. Reference Shishkin, Ochev, Tverdokhlebov, Vergay, Gomankov, Kalandadze, Leonova, Lopato, Makarova, Minikh, Molostovskiy, Novikov and Sennikov1995; Novikov, Reference Novikov2018) is typical of the entire formation. Red beds yield spinicaudatans and ostracods as well as crayfish burrows (Tverdokhlebov, Reference Tverdokhlebov and Morozov1967; Sennikov & Novikov, Reference Sennikov and Novikov2018).
During the 2018 and 2019 field seasons, numerous insect wings and fragments including various roaches, beetles and hemipterans, rare dragonflies, grylloblattids and orthopterans, as well as tomiulid millipedes, horseshoe crabs, microconchids and a microdrile oligochaete worm, were excavated along with additional terrestrial plant remains (Sphenopteris sp. pinnules and lycophyte fragments), ostracods, clam shrimps and fish scales (Hannibal & Shcherbakov, Reference Hannibal, Shcherbakov, Dányi, Korsós and Lazányi2019; Shcherbakov et al. Reference Shcherbakov, Bashkuev, Vasilenko, Karasev, Lukashevich, Tarasenkova, Strelnikova, Felker and Alekseev2019, Reference Shcherbakov, Timm, Tzetlin, Vinn and Zhuravlev2020).
3. Methods
Microconchid images were obtained with a Leica M165C stereomicroscope coupled to a Leica DFC425 digital camera. High-resolution images were taken on TESCAN VEGA variable-pressure and environmental scanning electron microscope (ESEM) using backscattered electron (BSE) and secondary electron (SE) detectors at the Borissiak Palaeontological Institute, Russian Academy of Sciences (PIN RAS). Elemental analysis of uncoated and unpolished samples of both fossils and adjacent matrices was performed with a quantitative energy-dispersive spectrometer (EDS) X-ray Inca coupled to the TESCAN VEGA SEM, at an accelerating voltage of 20 keV, in PIN RAS. All samples are housed in PIN RAS, under collections numbers 2362, 2402, 2716, 3061, 4887, 5381 and 5640.
4. Results
4.a. Late Permian microconchids of the Tunguska and Kuznetsk basins
Several hundred complete or partially preserved microconchid tubes were observed, either attached to the outer surface of spinicaudatan carapaces (up to 10 individuals per valve of 4 mm in length) or detached from their original substrate within the Changhsingian Bugarikta Formation. Three individuals were found on a bivalve shell in the Kedrovka Subformation (Fig. 3d).
Various attachment scar morphologies are observed in the form of substrate bioimmuration (Figs 3c, d, 4c, d). Linear depressions are visible at the basal surface of some detached tubes, which may have been formed due to microconchid attachment to firm elongated objects such as plant stems (Fig. 3c). In the Anakit-2 locality, dense detached flattened tube accumulations (over 60 individuals per 10 cm2) are found on the same bedding plane covered with a thin volcanic ash layer. EDS analysis indicates that the tubes have a low magnesium-calcite composition, except for those of Anakit-2 locality where EDS data yield oxygen and silicon with subordinate amounts of iron, aluminium, calcium, potassium and sulphur in proportions broadly similar to those present in the sedimentary matrix. At Anakit-2 the microconchid tubes are crushed and fractured, and elemental analysis reveals their replacement either by silica or by a K,Ca-silicate, most likely a clay mineral (Fig. 3b).
Tubes are generally small (0.1–1.6 mm in diameter) planispiral tightly coiled with rapidly increasing diameters, but some specimens display slight uncoiling in later growth stages (Figs 3c, 5a). Dextrally (clockwise) and sinistrally (anticlockwise) coiled tubes co-occur in the same aggregations of the similarly well-preserved shelly specimens, but dextral forms prevail. The embryonic chamber is bulbous, closed, up to 0.2 mm in diameter (Figs 3b, 4d, 5a). As the successive whorls overlap minimally, coiling is tight evolute showing older whorls in a broad and almost circular umbilicus. The umbilical width ranges from 0.3 to 0.4 mm, increasing slightly in larger individuals where it is not directly correlated with an increase in tube diameter. The umbilical edge is rounded and characterized by a relatively low-angle slope. Tubes reach their maximum height about the midline. The lower attachment surface of the tubes is flattened and their upper free surface is inflated (Fig. 4c, d). The aperture is round to oval, up to 0.6 mm in diameter. Externally, the tubes are regularly ornamented by fine, poorly to moderately developed, transverse growth bands (Figs 3b, 4c, 5b). The bands are 11–12 µm wide and are spaced at regular intervals of the same width accentuated by transverse ribs, which do not cross the entire tube width. The outer surface is covered with evenly spaced tubercles (c. 1 µm in diameter), which are mostly arranged in transverse rows following the ribs, and with a faint wavy transverse striation (Figs 4c, d, 5b). The tube wall is microlamellar, foliated and is regularly traversed by pores 1–3 µm in diameter that are restricted to inter-rib depressions and are somewhat scarcer than the tubercles (Fig. 5b). The inner tube surface is covered with evenly spaced inward inflections of microlamellae-forming tubercles that are penetrated by radial canals (punctae) connecting the outer pores with the tube interior, and have somewhat smaller pits corresponding to the outer surface tubercles (outwardly pointed microlamellar inflections devoid of canals, i.e. pseudopunctae; Fig. 5a, c, d).
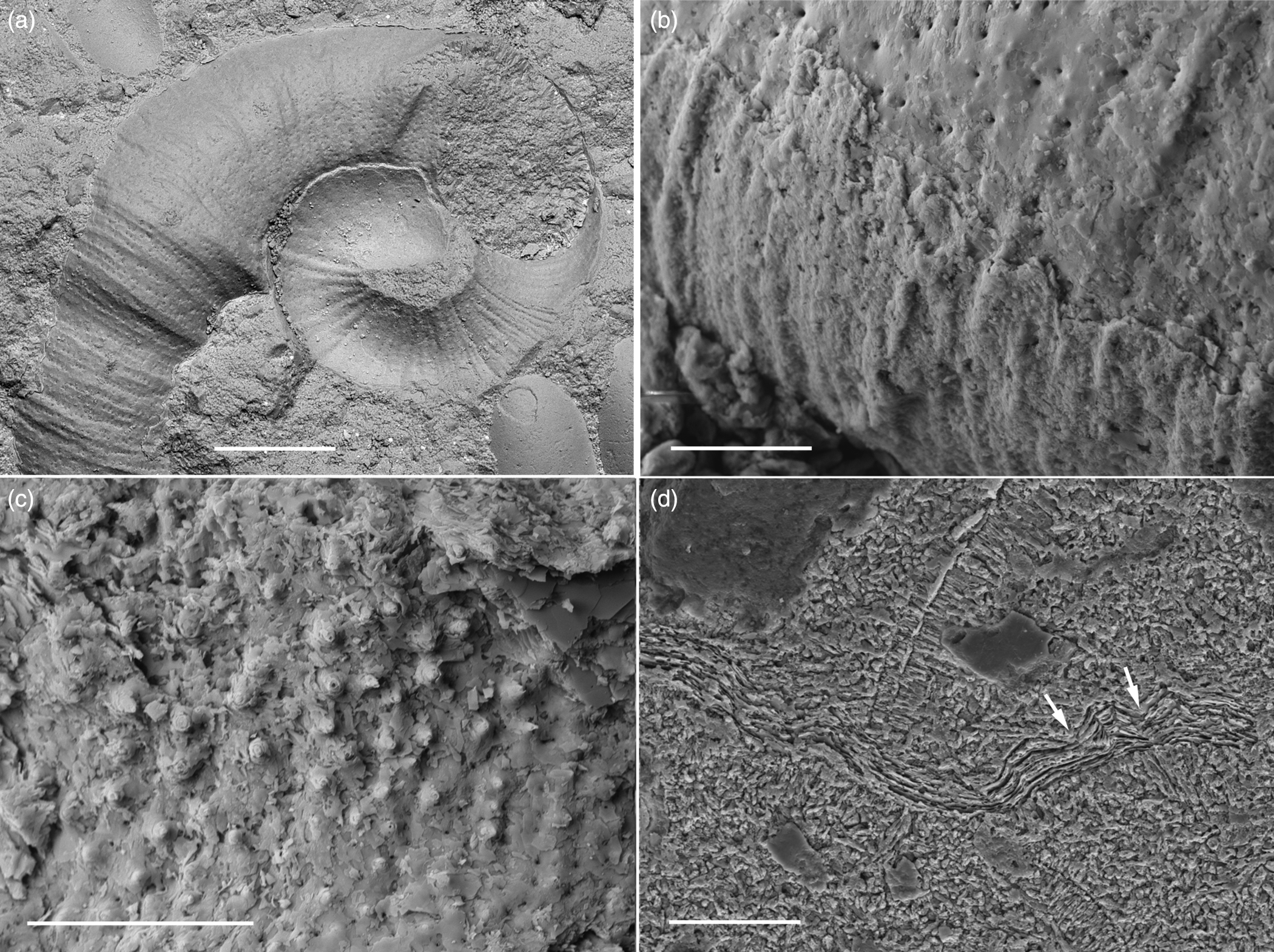
Fig. 5. Microconchid tube microsculpture and microstructure from the Bugarikta Formation, uppermost Permian (Changhsingian), Tunguska Basin, Krasnoyarsk region, Russia; ESEM. (a) PIN 2402/36a, shell showing bulbous embryonic chamber and imprint of inner surface microsculpture; tube is surrounded by several ostracod carapaces, Nizhnyaya Lyulyuikta section. (b) PIN 5381/349, lamellar tube and inner mould showing microsculpture and punctae, Anakit section. (c) PIN 2362/27, BSE, tube inner surface showing tuberculate microsculpture, Anakit section. (d) PIN 2402/36b, SE, section of lamellar tube wall with inflections pierced by canals (arrowed), Nizhnyaya Lyulyuikta section. Scale bars: (a) 0.2 mm and (b–d) 0.05 mm.
In general, these tube characteristics conform to the genus Microconchus Murchison, Reference Murchison1839 (family Microconchidae Zatoń in Zatoń & Olempska, Reference Zatoń and Olempska2017). However, the specimens of the Tunguska and Kuznertsk basins differ in detail from all formally described Late Permian and Early Triassic microconchids, and likely belong to a new species that is unique among known microconchids in having both punctae and pseudopunctae (Fig. 5a, c, d).
4.b. Early Triassic microconchids of Petropavlovka
In the Olenekian Petropavlovka Lagerstätte, complete or partially preserved ferruginous moulds of microconchid tubes are found on a horseshoe crab head shield (2) and aggregated on fragments of terrestrial plant stems (over 30), but not on the leaves. The moulds are accentuated by bright reddish accumulations of unspecified iron (oxyhydr)oxide minerals resulting from the oxidation of pyrite. SEM and EDS investigations show the crystal concentrations to consist of densely packed clusters of dodecahedral pyrite pseudomorphs ranging from 0.5 to 10.0 µm in size (Figs 6d, 7c, d).

Fig. 6. Moulds of microconchid tubes from the Petropavlovka Formation, Lower Triassic (Olenekian), Petropavlovka III section, southern Cis-Urals, Orenburg region, Russia; ESEM (BSE). (a) PIN 5620/217, moulds on horseshoe crab head shield. (b) Detail of (a). (c) PIN 5640/218, moulds on plant stem with veins. (d) PIN 5640/213, SEM, detail of Figure 7a showing stem venation. Scale bars: (a, c) 2 mm; (b) 0.5 mm; and (d) 1 mm.

Fig. 7. Microconchid tubes encrusting plant stems from the Petropavlovka Formation, Lower Triassic (Olenekian), Petropavlovka III section, southern Cis-Urals, Orenburg region, Russia. (a) PIN 5640/213. (b) Detail of (a), diffuse light. (c, d) Details of (a) showing pyrite dodecahedron clusters replaced with iron (oxyhydr)oxides, ESEM (BSE). Scale bars: (a) 5 mm; (b, c) 1 mm; and (d) 0.1 mm.
The tubes are planispiral tightly coiled, small (0.5–2.5 mm in diameter) and with no tendency to uncoil in later growth stages (Figs 6b, 7b). Tube diameter increases continuously, resulting in a moderate overlapping of successive whorls. Tubes reach their maximum height about the umbilical edge, next to the midline. Dextrally and sinistrally coiled tubes co-occur in the same aggregations. The lower attachment tube surface is flattened and its upper free surface is convex. The tube umbilicus is open, circular and moderately wide in all specimens; the umbilical width ranges from 0.35 to 0.70 mm, slightly increasing in larger individuals. The umbilical edge is rounded and characterized by a relatively flattened low-angle slope. The umbilical width is not always correlated with an increase in tube diameter. The tube aperture is rounded to oval, and up to 0.7 mm in diameter.
Based on the planispiral coiling and open umbilicus, the specimens from the Petropavlovka Lagerstätte resemble to some extent the type species Microconchus carbonarius Murchison, Reference Murchison1839, including the specimens from its Pennsylvanian (Carboniferous) population of Nova Scotia, Canada described by Zatoń et al. (Reference Zatoń, Grey and Vinn2014 a), and are tentatively assigned to the genus Microconchus. An almost invariable tube shape independently of dextral or sinistral coiling indicates that all the specimens probably belong to the same species, but unequivocal identification is not possible as neither skeletal carbonate nor surface ornamentation are detected. It is, nevertheless, possible that two approximately equally represented species with differently coiled tubes are present, similar to some other microconchid populations (Brönnimann & Zaninetti, Reference Brönnimann and Zaninetti1972).
5. Discussion
5.a. Lacustrine microconchid associations of the Tunguska and Kuznetsk basins
5.a.1. Latest Permian continental environments and palaeocommunities of Siberia
Despite the relative remoteness of the Tunguska and Kuznetsk basins, these vast Siberian regions occur within the same northeastern Asian area of Pangea that witnessed one of the major LIP eruptions in the Earth’s history, at the end Permian – Triassic transition (Fig. 1). All the Changhsingian sections of both basins were located far inland among volcanic landscapes, which hosted numerous lakes and rivers under dense mesic forests (Neustrueva & Bogomazov, Reference Neustrueva, Bogomazov, Martinson and Neustrueva1987; Davies et al. Reference Davies, Allen, Buslov and Safonova2010; Budnikov et al. Reference Budnikov, Kutygin, Shi, Sivtchikov and Krivenko2020). The forests were dominated by ferns, peltasperms and conifers (Dobruskina, Reference Dobruskina1994; Mogutcheva, Reference Mogutcheva and Dagis1987, Reference Mogutcheva (Mogucheva)2016; Sadovnikov, Reference Sadovnikov2008; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009; Karasev, Reference Karasev2015) and provided ecological niches for abundant and diverse insects. Over 600 fossil insect specimens were collected along the Nizhnyaya Tunguska River, comprising abundant and diverse beetles, grylloblattids, mayflies, scorpionflies, cockroaches, hemipterans, neuropterans and orthopterans; beetles belonging to eight different families and grylloblattids (four families) dominated here (Aristov, Reference Aristov2011; Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013; Bashkuev, Reference Bashkuev2013; Sinitshenkova, Reference Sinitshenkova2013; Yan et al. Reference Yan, Beutel and Lawrence2018). Similarly, a rich insect fauna of beetles, grylloblattids, mayflies, cockroaches, hemipterans, neuropterans, orthopterans, stoneflies and webspinners is discovered in the Kedrovka Subformation of the Kuznetsk Basin at Babiy Kamen’ (Shcherbakov, Reference Shcherbakov2008 c; Aristov et al. Reference Aristov, Bashkuev, Golubev, Gorochov, Karasev, Kopylov, Ponomarenko, Rasnitsyn, Rasnitsyn, Sinitshenkova, Sukatsheva and Vassilenko2013; Ponomarenko & Volkov, Reference Ponomarenko and Volkov2013).
The lakes were populated by abundant and diverse charophyceans, bivalves, gastropods, spinicaudatans and ostracods (Neuburg, Reference Neuburg1936; Kukhtinov & Neustrueva, Reference Kukhtinov, Neustrueva, Oleynikov and Zhamoyda1986; Sadovnikov & Orlova, Reference Sadovnikov and Orlova1995; Sadovnikov, Reference Sadovnikov2008, Reference Sadovnikov2016; Mogutcheva & Krugovykh, Reference Mogutcheva and Krugovykh2009; Davydov et al. Reference Davydov, Zharinova and Silantiev2019; Silantiev et al. Reference Silantiev, Urazaeva, Nurgalieva, Alekseev and Nazarova2020). In the Tunguska Basin, unequivocally aquatic insects were represented by mayfly nymphs Khungtukunia sibirica of the family Vogesonymphidae (Sinitshenkova, Reference Sinitshenkova2013) and by the earliest whirling beetle Tunguskagyrus planus (Gyrinidae) featuring a smooth streamlined drop-shaped body with very specific completely divided compound eyes and paddle-shaped antennal pedicels (Yan et al. Reference Yan, Beutel and Lawrence2018). Aquatic insects of the Kuznetsk Basin are more diverse and include mayflies (of which only adults have been found), various beetles (Schizophoridae, Haliploidea, Hydrophilidae) that typically live in the water in all stages of their life cycle, and presumably semiaquatic chaulioditid grylloblattids (Shcherbakov, Reference Shcherbakov2008 c; Ponomarenko & Volkov, Reference Ponomarenko and Volkov2013). For instance, the extinct family Schizophoridae was characterized by an elytra-thoracic interlock (‘schiza’) considered an amphibiotic adaptation (Shcherbakov, Reference Shcherbakov2008 b).
Increased alkalinity of the lakes is thought to be due to intense volcanic outgassing and evidenced by the development of a calcareous cement that consolidates shellbed lenses, montmorillonite seams and common zeolite pseudomorphs after shells and plant fragments (Neuburg, Reference Neuburg1936; Neustrueva & Bogomazov, Reference Neustrueva, Bogomazov, Martinson and Neustrueva1987). Specifically, the increased alkalinity could be due to the breakdown of volcanic ash glass particles reacting with lake pore water. The result was water supersaturation in silica (Calvert, Reference Calvert, Hsü and Jenkyns1974; Hethke et al. Reference Hethke, Fürsich, Jiang and Klaus2013; Fürsich & Pan, Reference Fürsich and Pan2016), which facilitated dissolution of calcareous shells and their preservation as fine fabric replacive silica, as in the Anakit-2 locality that was especially rich in pyroclastic sediments (Fig. 3b). The same process could inhibit the proliferation of a rich aquatic insect fauna in the Tunguska Basin. Such a fauna, although present, is restricted to the few low-abundance species listed above. However, even this depauperate aquatic insect palaeocommunity required fresh- to slightly brackish water conditions (salt concentrations below 3–8‰) judging by the presence of mayfly larvae and aquatic beetles, which lack physiological mechanisms for proper osmoregulation (Chadwick et al. Reference Chadwick, Hunter, Feminella and Henry2002; Bauerfeind, Reference Bauerfeind and Dathe2003). In turn, the abundance of filter feeders (microconchids, small bivalves) and fine-deposit feeders (ostracods, spinicaudatans and certain mayfly larvae) points to eutrophication of the water bodies.
Fishes of the Tunguska Basin comprised heavily armoured predator neopterygians Tungusichthys acentrophoroides, Arctosomus sibiricus, Evenkia eunotoptera and Eoperleidus bergi. These represent four families from orders affiliated with stem groups of relict freshwater gars in the orders Lepisosteiformes and Amiiformes (Arratia, Reference Arratia, Arratia and Tintori2004), two of which were restricted to a few freshwater basins of northern Asia (Sytchevskaya, Reference Sytchevskaya, Arratia and Schultze1999). The only local amphibian Tungussogyrinus bergi was a small neotenic newt-like temnospondyl that was thought to have maintained external gill breathing during its entire life cycle, and possessed uncommon tricuspid dentition similar to that of anuran tadpoles, adapted to scrape algae; this is a feature typical of the latest representatives of branchiosaurids (Shishkin, Reference Shishkin1998; Werneburg, Reference Werneburg2009). Some elements of this rich fauna (certain spinicaudatan species and the newt-like amphibia) suggest the possibility that lotic conditions were present at least temporarily (Sadovnikov, Reference Sadovnikov2008; Werneburg, Reference Werneburg2009).
The overall abundance and diversity of the floras and faunas present in the Tunguska and Kuznetsk basins indicate that they were neither depauperate nor stressed, despite high levels of local volcanic activity, which resulted in ash fall ‘killing’ beds. In general, areas fertilized by nutrient-rich volcanic ashes were suitable for rapid plant growth during calm episodes, which in turn provided abundant food for rapidly reproducing insects. For instance, tree fern species, which comprised more than half of the diversity in the Tunguska Basin flora, proliferate and demonstrate high growth rates on volcanic substrates in Hawaii and New Zealand (e.g. Nicholls, Reference Nicholls1959; Durand & Goldstein, Reference Durand and Goldstein2001; Shepherd et al. Reference Shepherd, Perrie and Brownsey2007), while abundant bacterio- and phytoplankton blooms occur as a result of fertilization with volcanic ash leachate nutrients (Zhang et al. Reference Zhang, Jiang, Tian, Xie, Zhou, Li and Jiao2017). It is noteworthy that large vertebrates were not detected either in the Tunguska Basin, or in the coeval Kuznetsk Basin lakes, where the lacustrine vertebrate fauna was represented only by 10 to 300-mm-long individuals (Sytchevskaya, Reference Sytchevskaya, Arratia and Schultze1999; Shcherbakov et al. Reference Shcherbakov, Kabanov, Ponomarenko, Esin, Tatarinov and Golubev2002; Werneburg, Reference Werneburg2009). The abundance of endemics of a high taxonomic rank (fishes, amphibians) and progenitors of Mesozoic groups (plants, insects) is typical of rapidly evolving and changing volcanic landscapes, similar to the present Great Lakes of the East African Rift, which provide a wide test site for adaptive radiation and explosive speciation (Salzburger et al. Reference Salzburger, Van Bocxlaer and Cohen2014; Lyons et al. Reference Lyons, Scholz, Cohen, King, Brown, Ivory, Johnson, Deino, Reinthal, McGlue and Blome2015).
5.a.2. Palaeoecology of the latest Permian microconchids of Siberia
Microconchids became part of this freshwater biota but were the only epibenthic encrusting filter feeders. They settled on spinicaudatan carapaces and bivalve shells and commonly formed aggregations of tubes of a wide size range densely covering this substrate (Figs 3a, 4a). Such an ecological strategy resulting in a specific encrusting morphology, which is probably plesiomorphic for microconchids, is commonly found on different hard substrates under many conditions (e.g. Sandberg, Reference Sandberg1963; Kelber, Reference Kelber1987; Zatoń et al. Reference Zatoń, Taylor and Vinn2013; Matsunaga & Tomescu, Reference Matsunaga and Tomescu2017). Dense settlements of microconchids representing the full local size range are present on small areas of the same substrate. There is no evidence of differentiation of growth conditions among microconchids of various sizes randomly distributed along the same substrate, which suggests coexistence of different generations of the same population rather than unequal growth rates among individuals of the same age. Several generations of these diminutive encrusters commonly grew in aggregations (e.g. Zatoń & Krawczyński, Reference Zatoń and Krawczyński2011; Caruso & Tomescu, Reference Caruso and Tomescu2012; Zatoń & Peck, Reference Zatoń and Peck2013). In the Nizhnyaya Tunguska microconchid populations, a settlement of older individuals likely facilitated further larval settlings because parental aggregations even by themselves provided hard substrates for attachment. Similar aggregative behaviour is ubiquitous for many extant encrusting invertebrates, such as the small polychaete tubeworm Spirorbis (Knight-Jones, Reference Knight-Jones1951).
Based on the specific ornamentation consisting of concentric growth lines and fine wavy radial striation, it is possible to ascertain that the majority of spinicaudatan carapaces settled by microconchids are belonged to Bipemphigus gennisi. This species was among the largest spinicaudatans (over 5 mm long) and was probably a benthic deposit-feeding crustacean resting on the lateral surface of one valve at the water–sediment interface, similar to its extant relatives (Vannier et al. Reference Vannier, Thiéry and Racheboeuf2003). Due to a specific spinicaudatan exuviation with preservation of the old carapace outer layers, it cannot be ruled out that these microconchids settled on living animals but eventually sentenced the host to death by locking the valves. Such extremely dense and heavy microconchid aggregations consisting of large individuals were able to develop on empty carapaces accumulating at the bottom of the lakes (Figs 3a, 4a, 8). On a bivalve shell, encrusting microconchids were small and left scars featuring an irregular network formed by a partial dissolution of the host valve surface (Fig. 3d). Because freshwater bivalves are covered with thick periostraca (Harper et al. Reference Harper, Palmer and Alphey1997), scars formed on a calcareous layer are indicative of a post-mortem settlement. Among the local fauna, the small branchiosaurid armed with tricuspid teeth adapted for scraping might have been a threat for these tiny encrusters (Fig. 8).

Fig. 8. Reconstruction of a latest Permian (Changhsingian) lacustrine community in the Tunguska Basin: neopterygian fishes Tungusichthys acentrophoroides and branchiosaurid amphibian Tungussogyrinus bergi in the water, mayfly larvae Khungtukunia sibirica, ostracods Darwinula and clam shrimp Bipemphigus carapaces encrusted by gregarious microconchids at the bottom among charophyceans (artwork: Andrey Atuchin).
In the Nizhnyaya Lyulyuikta section, microconchid tubes formed shellbeds of a grainstone–packstone grade together with calcareous charophycean gyrogonites, small gastropod conchs, spinicaudatan and ostracod carapaces of the same size range. The presence of attachment scars on microconchid tubes indicates that they were encrusters when alive, as noted in other microconchids from the same localities when they are preserved in situ. Such microconchid tubes preserve linear groove-like attachment scars, the shape of which indicate that such individuals, when alive, have been settled on cylindrical plant stems or algal thalli (Fig. 3c, bottom left specimen).
5.b. Lacustrine Petropavlovka microconchid association
5.b.1. Early Triassic continental environments and palaeocommunities of the southern Cis-Urals
Sedimentological and palaeontological data indicate that the Petropavlovka Lagerstätte in the southern Cis-Urals was probably formed within an ephemeral pond on a vast floodplain. This allowed rapid colonization by opportunistic animals until the next dry season. This fauna was represented by ostracods and spinicaudatans with resting egg clutches, which could endure long periods of desiccations (Horne & Martens, Reference Horne, Martens, Brendonck, De Meester and Hairston1988; Vannier et al. Reference Vannier, Thiéry and Racheboeuf2003), lungfish aestivating in burrows (Hasiotis et al. Reference Hasiotis, Mitchel and Dubiel1993), and microdrile clitellates forming cocoons that are very resistant to physical and chemical decay (Manum et al. Reference Manum, Bose and Sawyer1991). These organisms are preserved in the Petropavlovka Formation either as body fossils – ostracods, spinicaudatans and a microdrile (Tverdokhlebov, Reference Tverdokhlebov and Morozov1967; Shcherbakov et al. Reference Shcherbakov, Bashkuev, Vasilenko, Karasev, Lukashevich, Tarasenkova, Strelnikova, Felker and Alekseev2019, Reference Shcherbakov, Timm, Tzetlin, Vinn and Zhuravlev2020) – or, in the case of dipnoan fish, as both body and trace fossils (Minikh & Minikh, Reference Minikh and Minikh1997; Sennikov, Reference Sennikov2018). Common spinicaudatants and sparse horseshoe crabs of the Petropavlovka Lagerstätte also typified Mesozoic freshwater communities (Lamsdell, Reference Lamsdell2016; Hethke et al. Reference Hethke, Fürsich, Jiang, Wang, Chellouche and Weeks2019), while remains of diverse temnospondyl amphibians with specific adaptations for feeding on aquatic animals characterize the entire formation (Shishkin et al. Reference Shishkin, Ochev, Tverdokhlebov, Vergay, Gomankov, Kalandadze, Leonova, Lopato, Makarova, Minikh, Molostovskiy, Novikov and Sennikov1995; Novikov, Reference Novikov2018; Sennikov & Novikov, Reference Sennikov and Novikov2018).
As a whole, this assemblage represents a common Early Triassic lacustrine fauna, while terrestrial arthropods, including insects and millipedes, and plants constitute a shore community inhabiting a floodplain environment (Kozur & Weems, Reference Kozur, Weems and Lucas2010; Żyła et al. Reference Żyła, Wegierek, Owocki and Niedźwiedzki2013; Kustatscher et al. Reference Kustatscher, Franz, Heunisch, Reich and Wappler2014; Haig et al. Reference Haig, Martin, Mory, McLoughlin, Backhouse, Berrell, Kear, Hall, Foster, Shi and Bevan2015; Feng et al. Reference Feng, Wei, Guo and Bomfleur2018).
The presence of such a specific palaeocoenosis, in addition to the absence of evaporite minerals, suggests freshwater conditions for the formation of the Lagerstätte, even though most animal groups of the Petropavlovka ecosystem were able to survive and even disperse in brackish basins, such as microdriles (up to saline littoral areas; Brinkhurst, Reference Brinkhurst1971), horseshoe crabs (up to normal marine; Lamsdell, Reference Lamsdell2016), ostracods (up to hypersaline conditions; De Deckker, Reference De Deckker1983; Boomer et al. Reference Boomer, Frenzel and Feike2016) and spinicaudatans (up to 15 g L–1 salinity level; Timms & Richter, Reference Timms and Richter2002). Coeval lungfishes including Ceratodus (Clement & Long, Reference Clement and Long2010; Frederickson & Cifelli, Reference Frederickson and Cifelli2017) and even rhytidosteid temnospondyls – a rare case for amphibians – were able to tolerate saline waters (Jones & Hillman, Reference Jones and Hillman1978; Novikov, Reference Novikov2018). The existence of at least temporal brackish conditions would be a plausible state of the Petropavlovka water body, but other amphibian remains (capitosaurids and brachyopoids) characterizing the formation have never been recorded in assemblages with marine fossils (Novikov, Reference Novikov2018). Finally, the overwhelming majority of the Permian and later dipnoans were restricted to freshwater (Kemp et al. Reference Kemp, Cavin and Guinot2017).
5.b.2. Palaeoecology of the Early Triassic microconchids of the southern Cis-Urals
Two microconchid tubes are attached to a single xiphosuran head shield, but it is impossible to establish if this was a living association, encrustation of an abandoned exuvium, or simply a dead body fragment (Fig. 6a, b). By contrast, aggregative microconchid associations on terrestrial plant fragments consisted of individuals representing different growth stages and restricted to plant stems (Figs 6c, d, 7). Such stems with dense parallel ridges probably represented a relatively firm substrate that was permanently submerged in a lake body and therefore permanently available for a colonization by encrusters (Figs 6c, d, 7a, b, 9). Petropavlovka microconchids belong to the category of planispiral tubes that are completely substrate-cemented. This habit was interpreted as an adaptation for achieving a firm tube attachment under conditions when only a limited hard substrate area was available (Vinn, Reference Vinn2010).

Fig. 9. Reconstruction of an Early Triassic (Olenekian) lacustrine community in the Cis-Urals: microconchid settlements on submerged sphenopsids and a horseshoe crab, benthic ostracods and ‘microdrile’ clitellate settlement, the lungfish Ceratodus on the background (artwork: Andrey Atuchin).
Commonly, microconchid tubes, attached to either plant fragments or animal shells accumulated under freshwater conditions, are poorly preserved (Zatoń & Mazurek, Reference Zatoń and Mazurek2011; Caruso & Tomescu, Reference Caruso and Tomescu2012; Zatoń & Peck, Reference Zatoń and Peck2013). The case of the Lower Devonian Beartooth Butte Formation (Wyoming, USA) is especially typical, as microconchid tubes were repeatedly attached to form aggregations on horizontal lycophyte stems when they were submerged during occasional flood events (Matsunaga & Tomescu, Reference Matsunaga and Tomescu2017). Similar preservation is observed in microconchids from the Petropavlovka Lagerstätte.
Confirming that the water body that supported the Petropavlovka ecosystem was nutrient rich is problematic. We note that dense accumulations of primarily pyrite dodecahedra restricted to attached microconchid tubes, stem veins and rootlets, to which they impart a rusty tint, are common on bedding planes (Fig. 7b–d). As the availability of organic matter that can be metabolized by sulphate-reducing bacteria is one of principal factors of pyrite formation, a high carbon/sulphur ratio might be expected for the appearance of abundant pyrite clusters in a freshwater basin (Berner, Reference Berner1984; Hethke et al. Reference Hethke, Fürsich, Jiang and Klaus2013). In turn, the decomposition of organic matter by sulphate-reducing bacteria favoured low-pH conditions (increased acidity) and would lead to the dissolution of skeletal carbonate and precipitation of early diagenetic pyrite (Butts & Briggs, Reference Butts, Briggs, Allison and Bottjer2011; Fürsich & Pan, Reference Fürsich and Pan2016). With the increased supersaturation of a monosulphide phase, the pyrite crystal habits change from cubic to pyritohedron and other complex crystals, while a wide size range (from 0.5 to 10.0 µm in diameter), including abundant crystals of larger sizes within dominantly dodecahedral pyrite populations, suggests continuous weakly oxygenated lake bottom conditions (Wang et al. Reference Wang, Huang, Wang, Feng and Huang2013; Fürsich & Pan, Reference Fürsich and Pan2016). This sedimentological feature might be indicative of abundant decaying plant and animal remains consumed by benthic bacterial associations at the lake bottom, but not for the redox state of the water column itself. However, a lacustrine palaeocoenosis, consisting mostly of ceratodontids hiding in aestivation burrows, limulids and abundant microconchids that represent the major suspension feeders in the Petropavlovka ecosystem, points to a meromictic eutrophic lake.
5.c. The fresh- and brackish-water microconchid controversy
Fresh- to brackish-water occurrences of microconchids have been documented in the Lower Devonian – Upper Triassic strata (Taylor & Vinn, Reference Taylor and Vinn2006; Caruso & Tomescu, Reference Caruso and Tomescu2012; Zatoń et al. Reference Zatoń, Vinn and Tomescu2012; Zatoń & Peck, Reference Zatoń and Peck2013; Matsunaga & Tomescu, Reference Matsunaga and Tomescu2017). Subsequently, an autochthonous origin for microconchid occurrences in any fresh- and brackish-water continental palaeoenvironments unconnected to the ocean has been disputed by Gierlowski-Kordesch & Cassle (Reference Gierlowski-Kordesch and Cassle2015). These authors explained such occurrences as representing coastal environments, either within a non-marine–marine transition (tidal coast, estuary, delta) or on a distal transition floodplain within a low-gradient coastal area potentially affected by rare storm surges, tsunamis and sea-level oscillations. Indeed, the very presence of aggregative microconchid palaeocommunities in ancient fresh- and brackish-water sites has been questioned. Instead, preservation as single tubes strewn across bedding planes or randomly dispersed through the sedimentary matrix, with only temporary larval settlements on terrestrial plant remains, has been proposed, where larvae brought into restricted fresh- and brackish-water continental environments by sea surges did not mature (Gierlowski-Kordesch & Cassle, Reference Gierlowski-Kordesch and Cassle2015, p. 216).
Zatoń et al. (Reference Zatoń, Wilson and Vinn2016 b) criticized these interpretations, suggesting that the reductionist phoronid (extant lophophorate group) model, used by Gierlowski-Kordesch & Cassle (Reference Gierlowski-Kordesch and Cassle2015) to estimate microconchid salinity tolerance, had no support. Zatoń et al. (Reference Zatoń, Wilson and Vinn2016 b) also pointed out that a number of localities, that lack even poorly preserved marine shells transported by storm surges and tsunamis, contain microconchids including fully developed mature individuals associated only with remains of fresh- and brackish-water organisms, including charophyceans and specialized freshwater bivalves, spinicaudatans and ostracods.
The uppermost Permian and Lower Triassic localities described here indicate that microconchid populations were always aggregative, composed in situ of individuals of a wide age range, and were entirely restricted to firm substrates encrusting fresh or slightly brackish water horseshoe crabs, spinicaudatans, bivalves and submerged plant or algal organs. These localities were not unique for the Permian and Early–Middle Triassic periods. Palaeogeographic reconstructions indicate that microconchid occurrences associated with fresh- and brackish-water animals and plants are present in: the Lower Permian (Asselian) Altenglan Formation of the Saar Nahe Basin in Germany, where microconchids are associated with freshwater stromatolites (Stapf, Reference Stapf1971; Schäfer & Stapf, Reference Schäfer, Stapf, Matter and Tucker1978; Schultze, Reference Schultze2009); the Asselian–Artinskian coal-bearing Upper Sadong Series in South Korea, where these microfossils encrusted land plants (Shikama & Hirano, Reference Shikama and Hirano1969); the uppermost Permian (Changhsingian) floodplain Kul’chumovo Formation containing a rich assemblage of freshwater tetrapods, ostracods and bivalves in the southern Cis-Urals (Tverdokhlebov et al. Reference Tverdokhlebov, Tverdokhlebova, Minikh, Surkov and Benton2005; Kukhtinov, Reference Kukhtinov, Ivanov, Novikov and Yashkov2017); and the Middle Triassic (Anisian–Ladinian) Ouled Chebbi Formation deposited within fresh- to brackish-water environments of Tunisia (Błażejowski et al. Reference Błażejowski, Niedźwiedzki, Boukhalfa and Soussi2017).
In the Ladinian – lower Carnian lacustrine Madygen Lagerstätte of Kyrgyzstan, microconchid aggregations encrusting plant fragments are associated with charophyceans, aquatic liverworts (Ricciaceae), various freshwater bivalves, gastropods, phylactolaemate bryozoans (represented by flotoblasts), spinicaudatans, dipnoans, xenacanthid sharks and basal salamander-like urodeles, as well as several species of schizophorid beetles (Sikstel’, Reference Sikstel’1960; Ivakhnenko, Reference Ivakhnenko1978; Shcherbakov, Reference Shcherbakov2008 b; Moisan et al. Reference Moisan, Voigt, Schneider and Kerp2012; Voigt et al. Reference Voigt, Buchwitz, Fischer, Kogan, Moisan, Schneider, Spindler, Brosig, Preusse, Scholze, Linnemann, Fraser and Sues2017; Schoch et al. Reference Schoch, Werneburg and Voigt2020). The Madygen strata yield oxygen- and strontium-isotope records indicative of freshwater conditions, accumulated in an oxygenated perennial lake located several hundred kilometers away from the nearest marine shoreline within a warm temperate climatic zone (Voigt et al. Reference Voigt, Buchwitz, Fischer, Kogan, Moisan, Schneider, Spindler, Brosig, Preusse, Scholze, Linnemann, Fraser and Sues2017). Another conspicuous Middle Triassic (Anisian) deltaic–lacustrine Lagerstätte is the Grès à Voltzia in the Vosges, northern France, where faunas of a different provenance including a marine-influenced delta were discovered; again, microconchid aggregations are restricted to terrestrial plant fragments, bivalve shells and a fish (chondrichthyan?) egg capsule and associated only with limnomedusae, horseshoe crabs, euthycarcinoids, tadpole shrimps, spinicaudatans, abundant gilled mayfly and aquatic beetle larvae, aquatic insect egg clutches, lingulids and temnospondyl amphibians. This fauna characterizes the fresh- to brackish waters of a deltaic environment with ephemeral temporary channels and ponds (Gall, Reference Gall1971; Gall & Grauvogel-Stamm, Reference Gall and Grauvogel-Stamm2005; Sinitshenkova et al. Reference Sinitshenkova, Marchal-Papier, Grauvogel-Stamm and Gall2005; Ponomarenko & Prokin, Reference Ponomarenko, Prokin, Prokin, Petrov, Zhavoronkova and Tuzovskiy2013). No transported and reworked microconchid tubes of marine origin are observed in other Lower and Middle Triassic probable lacustrine strata, including the Bromsgrave Sandstone Formation of England (Ball, Reference Ball1980) and the Lower Keuper of southern Germany (Kelber, Reference Kelber1987; Kietzke, Reference Kietzke, Lucas and Hunt1989). There the tiny tubeworms always encrusted terrestrial plant fragments, forming dense aggregations of multiple generations. Their settlements preserved in the Lower Keuper are especially remarkable, as microconchids varying in size from 0.35 to 2.25 mm were densely (over 50 individuals per 1 cm2) attached to submerged plant rhizomes (Kelber, Reference Kelber1987).
In summary, Permian and Early–Middle Triassic microconchids constituted an integral part of fresh- and brackish-water lacustrine ecosystems, where they formed dense, encrusting settlements on various firm substrates provided by terrestrial and mostly aquatic plants and animals. No reworked microconchid accumulations or scattered tubes, which could be interpreted as allochthonous remains transported from marine basins, have ever been detected in Permian and Triassic lacustrine strata.
5.d. Microconchids as disaster species
The concept of disaster forms was introduced by Schubert & Bottjer (Reference Schubert and Bottjer1992) for opportunistic generalists, which were the taxa increased dramatically in range and abundance after severe mass extinctions, briefly proliferated during the time of biotic crisis invading vacant ecospace until forced out through competition with specialist taxa, and returned to low level of abundance afterwards. Since that time, this concept was widely used for a number of the earliest Triassic species and palaeocommunities (e.g. Hallam & Wignall, Reference Hallam and Wignall1997; Kershaw et al. Reference Kershaw, Crasquin, Li, Collin, Forel, Mu, Baud, Wang, Xie, Maurer and Guo2012; Benton & Newell, Reference Benton and Newell2014; Song et al. Reference Song, Tong, Wignall, Luo, Tian, Song, Huang and Chu2016).
Microconchids were opportunistic generalists, and colonized any suitable, firm and hard substrate in a given environment (Fraiser, Reference Fraiser2011; He et al. Reference He, Wang, Woods, Li, Yang and Liao2012; Zatoń et al. Reference Zatoń, Vinn and Tomescu2012, Reference Zatoń, Taylor and Vinn2013; Zatoń & Peck, Reference Zatoń and Peck2013; Taylor, Reference Taylor2016). The multiple dense microconchid tube accumulations interlaid with ash beds in the Tunguska Basin indicate that these animals were able to reproduce quickly. Freshwater basins of the Tunguska and Kuznetsk basin were particularly stressful environments undergoing extremely high temperature and redox oscillations during the Permian–Triassic transition.
These tiny encrusters often benefitted from a rich food supply, weak competition from other fouling animals and low predator pressure. Such favourable conditions would have typified, for instance, the Early Triassic Petropavlovka ecosystem. No other encrusters, not even single specimens, were established there. As tiny shelly lophophorates firmly attached to a substrate (where aggregating plants and flexed carapace fragments may also have provided some protection), they were not attractive prey for potential predators of Petropavlovka. These would have included horseshoe crabs, lungfishes and rhytidosteid amphibians. Rhytidosteids probably hunted armoured crustaceans (Sennikov & Novikov, Reference Sennikov and Novikov2018), dipnoans preyed upon various invertebrates, including large shelled individuals (Bemis & Lauder, Reference Bemis and Lauder1986), and limulids fed on larger and “meatier” benthic animals (Błażejowski et al. Reference Błażejowski, Niedźwiedzki, Boukhalfa and Soussi2017).
Moreover, the microconchids were among a tiny handful of marine organisms that survived the Permian–Triassic mass extinction and rapidly spread globally, despite the unstable conditions created by the eruption of the Siberian Traps that probably brought about sulphur pollution, ozone shield deterioration, ocean acidification, oxygen content lowering and enormous coal combustions contributing to severe greenhouse effect and carbon cycle destabilization (Retallack et al. Reference Retallack, Veevers and Morante1996; Hallam & Wignall, Reference Hallam and Wignall1997; Erwin, Reference Erwin2006; Knoll et al. Reference Knoll, Bambach, Payne, Pruss and Fischer2007; Algeo & Twitchett, Reference Algeo and Twitchett2010; Kershaw et al. Reference Kershaw, Crasquin, Li, Collin, Forel, Mu, Baud, Wang, Xie, Maurer and Guo2012; Payne & Clapham, Reference Payne and Clapham2012; Sun et al. Reference Sun, Joachimski, Wignall, Yan, Chen, Jiang and Lai2012; Benton & Newell, Reference Benton and Newell2014; Lau et al. Reference Lau, Maher, Altiner, Kelley, Kump, Lehrmann, Silva-Tamayo, Weaver, Yu and Payne2016; van de Schootbrugge & Wignall, Reference van de Schootbrugge and Wignall2016; Benca et al. Reference Benca, Duijnstee and Looy2018; Wood & Erwin, Reference Wood and Erwin2018; Elkins-Tanton et al. Reference Elkins-Tanton, Grasby, Black, Veselovskiy, Ardakani and Goodarzi2020). In addition to diverse lacustrine environments varying from freshwater to harsh hypersaline basins during Early and early Middle Triassic time, microconchids globally occupied a wide range of marine zones from onshore, tidal to supratidal, shallow waters to a deeper outer shelf under oscillating redox conditions, on both carbonate and siliciclastic substrates. In general, twice as many microconchid occurrences are reported from the Lower Triassic than in the Upper Permian strata (Fig. 10, Table 2).

Fig. 10. Number of occurrences, environmental disparity and ecological diversity of microconchids during Early Permian – Middle Triassic epochs (Table 2). Number of occurrences is equalized with the number of formations yielding microconchids. Environmental disparity is recorded by a number of settings (up to 8) through an environmental profile (freshwater basins, hypersaline basins, marine supratidal, reefs, shallow subtidal, deep subtidal oxic, deep subtidal dysoxic/anoxic); boreal localities are ranked at additional score. The ecological diversity is demonstrated by eight different ecological roles associated with microbialites, sponge reefs, reef-forming, cavity-dwelling and rock-forming detached, and encrusting marine shelly fauna, fresh- to brackish-water shelly fauna and land plants.
Table 2. Permian and Lower–Middle Triassic microconchid palaeogeographical and palaeoenvironmental occurrences

They became ubiquitous members of microbial (thrombolitic and stromatolitic), non-rigid spongal and bivalve reef-building communities (in places, even forming thickets on their own), filling the so-called Early Triassic ‘reef gap’, around the margins of all the main oceanic basins of the time, namely the Panthalassa, the Palaeo- and Neotethys, and the Boreal Ocean. They therefore inhabited different climatic zones from near the equatorial belt to moderately high latitudes (Peryt, Reference Peryt1974; Sano & Nakashima, Reference Sano and Nakashima1997; Pruss et al. Reference Pruss, Payne and Bottjer2007; Nakrem & Ernst, Reference Nakrem, Ernst, Winston, Key and Hageman2008; Hagdorn, Reference Hagdorn2010; Brayard et al. Reference Brayard, Vennin, Olivier, Bylund, Jenks, Stephen, Bucher, Hofmann, Goudemand and Escarguel2011; He et al. Reference He, Wang, Woods, Li, Yang and Liao2012; Foster et al. Reference Foster, Danise, Sedlacek, Price, Hips and Twitchett2015, Reference Foster, Lehrmann, Yu, Ji and Martindale2018; Yang et al. Reference Yang, Chen and Ou2015 a; Zatoń et al. Reference Zatoń, Niedźwiedzki, Blom and Kear2016 a; Adachi et al. Reference Adachi, Asada, Ezaki and Liu2017; Godbold et al. Reference Godbold, Schoepfer, Shen and Henderson2018; Huang et al. Reference Huang, Bond, Wang, Wang, Yi, Yuan, Jia and Su2019; Figs 1, 10; Table 2). In the Tethyan Realm, microconchids became so common that even a stratigraphic unit, named the Spirorbis phlyctaena (in fact, Microconchus phlyctaena; Vinn, Reference Vinn2010) Range Zone was established in the Lower Triassic Series, allowing for correlation of marine strata (Brönnimann & Zaninetti, Reference Brönnimann and Zaninetti1972; Vaslet et al. Reference Vaslet, Le Nindre, Vachard, Broutin, Crasquin-Soleau, Berthelin, Gaillot, Halawani and Al-Husseini2005; Mazaheri Johari & Ghasemi-Nejad, Reference Mazaheri Johari and Ghasemi-Nejad2017). Some marine reef-building microconchids were able to withstand episodic hypersaline and ferruginous anoxic conditions, as suggested for the Neotethys (Heindel et al. Reference Heindel, Foster, Richoz, Birgel, Roden, Baud, Brandner, Krystyn, Mohtat, Koun, Twitchett, Reitner and Peckmann2018; Wood & Erwin, Reference Wood and Erwin2018). Aside from exceptional cases, when microconchids formed shelly packstone to grainstone (Baud et al. Reference Baud, Goudemand, Nützel, Brosse, Frisk, Meier and Bucher2015; Yang et al. Reference Yang, Chen, Wang, Ou, Liao and Mei2015 b), these tiny creatures always encrusted firm substrates. In some localities, they colonized up to 50% of Early Triassic shelly animals, showing preference for the valves of the pterinopectinid bivalve Claraia (Fraiser, Reference Fraiser2011), which was another common opportunistic survivor of the Permian–Triassic mass extinction (Ros-Franch et al. Reference Ros-Franch, Márquez-Aliaga and Damborenea2014; Table 2). In these instances, the density of microconchid encrusting populations reached 30–50 and up to 80 individuals per 1 cm2 (Kelber, Reference Kelber1987; Zatoń et al. Reference Zatoń, Taylor and Vinn2013, Reference Zatoń, Hagdorn and Borszcz2014 b, Reference Zatoń, Niedźwiedzki, Blom and Kear2016 a), while in microbial reefs they comprised up to 3–5% of the framework, a sufficiently high figure for reef-builders (He et al. Reference He, Wang, Woods, Li, Yang and Liao2012; Heindel et al. Reference Heindel, Foster, Richoz, Birgel, Roden, Baud, Brandner, Krystyn, Mohtat, Koun, Twitchett, Reitner and Peckmann2018). Even biostromes (small, 3.5 cm thick and 30 cm in diameter) were built by interlocked microconchid individuals in the Early Triassic Series of East Greenland on their own (Zatoń et al. Reference Zatoń, Niedźwiedzki, Rakociński, Blom and Kear2018).
The rapid appearance and disappearance of ephemeral continental basins as a result of fluctuating sea levels and the beginning of the rifting of Pangaea, as well as an increase in runoff and evaporation (Labat et al. Reference Labat, Goddéris, Probst and Guyot2004) as a result of global warming, would have resulted in greater salinity fluctuations in shallow-water environments that would increase osmoregulation stress (Verschuren et al. Reference Verschuren, Tibby, Sabbe and Roberts2000) and could have produced favourable conditions for the rapid expansion of opportunistic animals such as microconchids. Being resistant to salinity fluctuations, as well as (probably) to redox and temperature fluctuations, these small, lightly calcified tubeworm lophophorates became disaster stress-tolerators at the beginning of a new planetary era.
6. Conclusions
The uppermost Permian and lower Triassic lacustrine strata of the Tunguska and Kuznetsk basins, as well as those of the southern Cis-Urals in Russia, yield rich aquatic faunas, among which tubeworm microconchids were ubiquitous and one of the most abundant groups.
The latest Permian lacustrine faunas of the Tunguska and Kuznetsk basins existed during the initial phase of the Siberian LIP eruptions.
Despite an overall external similarity to tubeworm annelids, microconchids differ significantly from them in their tube microstructure and microsculpture that includes both punctae and pseudopunctae, and which they share with the lophophorates. Microconchids were the main component of the filter-feeding encrusting ecological guild in latest Permian and Early–Middle Triassic freshwater habitats, confirming earlier suggestions regarding their opportunistic nature (Fraiser, Reference Fraiser2011; Zatoń et al. Reference Zatoń, Vinn and Tomescu2012, Reference Zatoń, Taylor and Vinn2013).
During the Early and Middle Triassic epochs, in addition to lacustrine environments varying from fresh and brackish waters to hypersaline basins, microconchids occupied a wide range of marine zones from onshore shallow waters to a deeper outer shelf under oscillating redox conditions, on both carbonate and siliciclastic substrates, and dispersed within different climatic zones from near the equatorial belt to moderately high latitudes.
We infer that microconchids capitalized on the habitat offered by Early and Middle Triassic lakes, where both the competition for substrates and predation pressure were very low in the aftermath of the severe Permian–Triassic extinction event that eliminated heavy calcifiers and grazers.
Acknowledgements
The field work was supported by the Russian Foundation for Basic Research, RFBR projects 10-04-01713 and 16-04-01498. Financial support to OV was provided by Estonian Research Council Project IUT20-34. We are grateful to Elena Lukashevich, Kirill Eskov, Alexey Bashkuev, Dmitry Vasilenko, Eugeny Karasev, Maria Tarasenkova, Olesya Strelnikova and Anastasia Felker (PIN RAS) for their participation in fossil collecting; Sergey Tenishev (Tura) and the late Victor Sivtchikov (Novosibirsk) for help with field work organizing; Gennady Sadovnikov (Russian State Geological Prospecting University, Moscow) and Valentin Tverdokhlebov (Saratov State University, Russia) for information on fossil localities; Vladimir Davydov (Boise State University, USA), Vladimir Silantiev (Kazan Federal University, Russia), Pavel Skutschas (St Petersburg State University, Russia) for valuable comments; Roman Rakitov (PIN RAS) for the superb SEM images; Rachel Wood (University of Edinburgh, UK) for help with English language; and Andrey Atuchin for artistic interpretations of ancient lake communities. We are much obliged to Alexandru Tomescu (Humboldt State University, USA) and Michał Zatoń (University of Silesia in Katowice, Poland) for their significant contribution to the improvement of the manuscript.
Declaration of interest
None.