Introduction
The biogeography of Southeast Asia is in part defined by the major river systems which run through the area (Meijaard & Groves, Reference Meijaard, Groves, Lehman and Fleagle2006). Thus, species distributions of both freshwater as well as terrestrial animals often end on the banks of the major rivers such as the Mekong or, in the case of some bird species, are confined to the deep river valleys cut by the Brahmaputra, Salween, Mekong and Yangtze (Johansson et al., Reference Johansson, Alström, Olsson, Ericson, Sundberg and Price2007). Both invertebrate species, such as the intermediate snail hosts of the small liver fluke Opisthorchis viverrini sensu lato and O. viverrini sensu stricto (Saijuntha et al., Reference Saijuntha, Sithithaworn, Wongkham, Laha, Pipitgool, Tesana, Chilton, Petney and Andrews2007; Laoprom et al., Reference Laoprom, Saijuntha, Sithithaworn, Wongkham, Laha, Ando, Andrews and Petney2009), and the intermediate hosts of Schistosoma mekongi and S. ovuncatum (Attwood et al., Reference Attwood, Panasoponkul, Upatham, Meng and Southgatem2002, Reference Attwood, Fatih, Campbell and Upatham2008) differ genetically between Thai populations to the west of the Mekong and those from Laos to the east. The Mekong also acts as a dividing line between various mammal species, such as primates (Long et al., Reference Long, Kirkpatrick, Zhongtai and Xiaolin1994; Groves, Reference Groves2007; Roos et al., Reference Roos, Nadler and Walter2008), with species richness being appreciably higher to the east (Meijaard & Groves, Reference Meijaard, Groves, Lehman and Fleagle2006). These findings are consistent with the riverine barrier hypothesis, which states that rivers and their flood plains act as barriers to gene flow between populations (Haffer, Reference Haffer1997; Jalil et al., Reference Jalil, Cable, Sinyor, Lackman-Ancrenaz, Ancrenaz, Bruford and Goossens2008). Relatively little information is available from the Mekong Subregion, however, for terrestrial invertebrates.
Brachytrupes portentosus (Lichtenstein, 1796), the large brown or big-headed field cricket, belongs to the order Orthoptera, family Gryllidae (there are several synonyms, e.g. Tarbinskiellus portentosus (Lichtenstein, 1796) or Acheta portentosa Lichtenstein, 1796). This species spends most of its time up to 30 cm underground with a single specimen per burrow. It feeds on young plants and in some areas can be gathered in thousands at sundown (DeFoliart, Reference DeFoliart2002). It is widely distributed in Southeast Asia where it is important to humans both as a food source (Bristowe, Reference Bristowe1932; DeFoliart, Reference DeFoliart2002), as well as a pest of various agricultural crops, vegetables, lawns and ornamental plants (Chatterjee, Reference Chatterjee1965; Atim et al., Reference Atim, Gopalan, Baharin, Saad and Samuri1992). As a food source, this cricket has a higher economic value than other crickets in Southeast Asia, especially in Thailand, with the price of a hundred live giant crickets being about $US1.5–4.5 (50–150 Thai baht) and, after being cooked (normally by deep frying), up to $US4.5–6.0 (150–200 Thai baht) (Kittibanpacha, unpublished data). It is a valuable protein source, with 100 g dry weight providing 12.8 g protein and energy worth about 113 kcal (Viwatpanich, unpublished data). This is of particular importance as sources of animal protein in this area are limited, with freshwater fish supplying by far most of the requirements for the local population (Sverdrup-Jensen, Reference Sverdrup-Jensen2002).
Previous studies have shown a relatively high genetic variation in other species of field cricket, with genetic differentiation among their populations depending mostly on geographic features, especially among geographical populations of North American field crickets of the genus Gryllus, Hawaiian crickets of the genus Laupala and the Jerusalem cricket of the genus Stenopelmatus endemic to southern California (Huang et al., Reference Huang, Orti, Sutherlin, Duhachek and Zera2000; Parsons & Shaw, Reference Parsons and Shaw2001; Gray et al., Reference Gray, Barnfield, Seifried and Richards2006; Vandergast et al., Reference Vandergast, Bohonak, Weissman and Fisher2007). Moreover, within the singing Orthoptera, many closely related, reproductively isolated species are divergent in ecological and sexual traits yet exhibit little or no morphological divergence (Braswell et al., Reference Braswell, Birge and Howard2006). Such cryptic species pose particular problems for taxonomy. Speciation events can be associated with reduced gene flow caused by physical barriers to migration, along with limited vagility and natal philopatry (Buston et al., Reference Buston, Bogdanowicz, Wong and Harrison2007). There is usually a geographic range within which individuals are more closely related to one another than to those randomly selected from the general population, leading to genetic structuring (Repaci et al., Reference Repaci, Stow and Briscoe2007). The populations of many ground crickets are structured in this way, e.g. sequence divergence in mitochondrial genes between different geographical populations of field crickets, Gryllus spp., sampled from areas of both allopatry and sympatry. The pattern of cytochrome oxidase 1 (CO1) sequence divergence and genetic variation is consistent with allopatric or peripatric speciation in the southeastern and south-central American field cricket (Gray et al., Reference Gray, Barnfield, Seifried and Richards2006). In addition, the divergence of cytochrome b sequence was reported among North American field cricket populations (Huang et al., Reference Huang, Orti, Sutherlin, Duhachek and Zera2000).
Many molecular markers have been used to address the genetic diversity/variation of morphologically or geographically cryptic species. These include allozyme electrophoresis, amplified fragment length polymorphism (AFLPs), restriction fragment length polymorphisms (RFLPs), random amplified polymorphic DNA (RAPDs), mitochondrial DNA (mtDNA) sequence variation and microsatellite markers (e.g. Parsons & Shaw, Reference Parsons and Shaw2001; Dawson et al., Reference Dawson, Bretman and Tregenza2003; Bretman et al., Reference Bretman, Dawson, Horsburgh and Tregenza2008). Allozyme surveys are straightforward and inexpensive and are capable of detecting variation among geographical and/or morphologically distinct populations (Loxdale & Lushai, Reference Loxdale and Lushai1998).
In this study, the genetic variation and geographical relationships among large field cricket populations from Thailand and the Lao People's Democratic Republic (PDR), separated by the Mekong River (on average, about 1 km wide), were investigated by multilocus enzyme electrophoresis (MEE). Three additional ground cricket species, Acheta confirmata Walker, Gryllus bimaculatus de Geer and Gryllus testaceus Walker, were compared as out-groups.
Materials and methods
Sample collection
Two hundred ninety-six adult large brown crickets were collected from five areas in Thailand and three in the Lao PDR (table 1, fig. 1). The specimens were collected by digging them up in grass fields, rice paddies, forests or by buying them at local markets. All crickets came from natural habitats. The other crickets tested, A. confirmata, G. bimaculatus and G. testaceus, were caught in houses in the Maung district, Maha Sarakham Province of Thailand. Species were identified morphologically as described in Triplehorn & Johnson (Reference Triplehorn and Johnson2005). The crickets were transported back to the laboratory alive and, after killing by freezing, the hind legs were removed and immediately stored deep frozen at −80°C. Enzyme homogenates were individually prepared from the muscle of a left hind leg. Femoral muscle was manually ground with an equal volume of lysing solution (100 ml distilled water, 100 μl β-mercaptoethanol, 10 mg NADP) using a glass rod and centrifuged at 10,000 rpm for 20 min at 4°C. The supernatants were stored in capillary tubes as 5 μl aliquots at −20°C until used.

Fig. 1. Map showed the eight different geographical localities of B. portentosus collection. The samples codes as listed in table 1.
Table 1. Geographical locations of B. portentosus collection from Thailand and Lao PDR.

* Sample size.
Multilocus enzyme electrophoresis (MEE)
Multilocus enzyme electrophoresis (MEE) was performed by using cellulose acetate (Cellogel, Milan, Italy) as the support medium. The cellulose acetate gel was cut into an appropriate size (15×30 cm), soaked in 30% methanol and kept at 4°C until used. The cellulose acetate gel was blotted dry between sheets of blotting paper and rapidly transferred to a soaking tank containing approximately 400 ml of the running buffer for at least ten minutes to equilibrate the gel with the buffer. The loaded gel was run at a constant 200 V, running time being adjusted between 120–150 min according to the mobility of each enzyme. Twenty enzymes were examined as follows (abbreviation, Enzyme Commission no.); adenylate kinase (Ak, 2.7.4.3), aldolase (Ald, 4.1.2.13), creatine kinase (Ck, 2.7.3.2), enolase (Enol, 4.2.1.11), fructose-1,6-diphosphatase (Fdp, 3.1.3.11), aspartate amino transferase (Got, 2.6.1.1), glucose-phosphate isomerase (Gpi, 5.3.1.9), alanine amino transferase (Gpt, 2.6.1.2), hexokinase (Hk, 2.7.1.1), isocitrate dehydrogenase (Idh, 1.1.1.42), malate dehydrogenase (Mdh, 1.1.1.37), malic enzyme (Me, 1.1.1.40), mannose-phosphate isomerase (Mpi, 5.3.1.8), peptidase leucine-glycine-glycine (PepB, 3.4.11.4), peptidase phenylalanine-proline (PepD, 3.4.13.9), 6-phosphogluconate dehydrogenase (6Pgd, 1.1.1.44), phosphoglycerate kinase (Pgk, 2.7.2.3), phosphoglucomutase (Pgm, 2.7.5.1), pyruvate kinase (Pk, 2.7.1.40), triose phosphate isomerase (Tpi, 5.3.1.1) and general protein (Gp).
The staining protocol described by Richardson et al. (Reference Richardson, Baverstock and Adams1986) was followed with minor modifications. The stain ingredients commonly used were substrates in solution or dry components, which were weighed prior to preparation of the final stain, staining buffer, stock solution and linking enzymes. The stain solution was thoroughly mixed and spread evenly on the staining plate to the width of the gel by using a disposable plastic bulb-pipette. The gel was removed from the electrophoresis chamber inverted so that the porous, loading surface was in contact with the stain mixture over the surface of the gel. The stain was evenly spread over the gel within five seconds of application. The gel was then left on the stain for 30–60 s with occasional agitation every 10–15 s. The gel was blotted to remove excess stain and quickly transferred, porous side down, onto the plastic sheet. This was wrapped around the gel so that no creases were present to obscure enzyme bands. Wrapping the gel in this manner facilitates handling, whilst allowing the enzyme reaction(s) to be monitored safety. Gels were incubated at 37°C to increase the rate of the enzyme reaction and checked frequently for the appearance of band activity. Highly active enzymes appeared within seconds of stain application, whereas weaker enzymes took up to an hour to stain.
Data analysis
Alleles at each locus were designated by letters of the alphabet, starting with the allele encoding the most anodally migrating allozymes. To examine the genetic differences and relationships between different geographical populations, percent fixed difference (i.e. where a sample did not share any alleles in common with another sample at a particular locus) was calculated and a phenogram constructed based on UPGMA (unweighted pair group method of analysis: Sneath & Sokal, Reference Sneath and Sokal1978) using the GWbasic program to measure the fixed differences between different populations. GENEPOP, version 3.3 (Raymond & Rousset, Reference Raymond and Rousset1995) was used to calculate allele and genotype frequencies for each locus and genetic differentiation among populations (F ST) based on Weir & Cockerham (Reference Weir and Cockerham1984) estimation. The correlation between two matrices of genetic distance (% fixed differences) and geographic distance among B. portentosus populations was performed using a Mantel test (Mantel, Reference Mantel1967) based on a two-tailed test (Pearson) at 95% CI in XLSTAT. The correlation between three matrices of genetic distance vs. geographic distance and river barrier (Mekong River) among B. portentosus populations was performed using a two-tailed partial Mantel test (Quemere et al., Reference Quemere, Crouau-Roy, Rabarivola, Louis and Chikhi2010) with 95% CI using ZT software (Bonnet & Van de Peer, 2002).
Results
Eight geographical samples of B. portentosus showed a relatively high genetic diversity. Allele frequencies at all 23 loci tested in each sample are shown in Appendix 1. Allelic variability within a population of B. portentosus was observed at Enol, Gpt-1, Gpt-2, Me, Mpi, PepB, 6Pgd and Tpi (see Appendix 1). Two populations of B. portentosus from Ubon Ratchathani Province showed allelic variability at five loci (i.e. Enol, Gpt-1, Gpt-2, Mpi and Me), whereas the three populations from Laos showed allelic variability at six loci, three as in Thailand (i.e. Gpt-1, Gpt-2 and Mpi) and three additional ones (i.e. PepB, 6Pgd and Tpi) (Appendix 1). However, there was highly significant differentiation in the pairwise F ST values between eight different geographical populations of B. portentosus. Pairwise F ST values between the populations from Thailand and the populations from Laos varied from 0.899 to 0.971. Pairwise F ST values between five populations from Thailand varied from 0.752 to 0.889, whereas between three populations from Laos varied from 0.518 to 0.861 (Appendix 1).
Of the 23 enzyme loci, fixed differences between different geographical populations of B. portentosus were observed at 12 loci, i.e. Ak, Ald, Ck, Gp, Hk, Idh, Mdh, Me, PepD, Pgk, Pgm and Pk (Appendix 1). Fixed differences were also observed between the populations from Thailand and Lao PDR at eight loci, i.e. Ak, Ald, Ck, Gp, Hk, Idh, Me and Pgk (22–52% fixed difference) (Appendix 1, table 2). A total of 4–26% fixed differences were observed when the allelic patterns among five different geographical populations from Thailand were compared. Fixed differences were observed between the two populations from Ubon Ratchathani Province (UBk and UBm) and the three populations from Maha Sarakham Province (MSm, MSg and MSn) (17–26% fixed difference) at five loci, namely Ck, Gp, Mdh, PepD and Pgm (Appendix 1, table 2). Moreover, comparison among three populations from Maha Sarakham Province revealed that there were fixed difference between the MSn population vs. the MSm and MSg populations at the Ald locus and the MSm population vs. the MSg and MSn populations at Mdh locus. Similarly, it was found that fixed differences occurred between the UBk and the UBm populations from Ubon Ratchathani Province at three loci, i.e. Ck, Gp and PepD, with 13% fixed differences (Appendix 1, table 2). Comparison among the three populations from Lao PDR showed that fixed differences were observed at three loci (PepD, Pgm and Pk) between the two populations from Savannakhet Province (SVg and SVs) and one population from Champasak Province (CSp) at 17–22% fixed differences. In contrast, 4% fixed differences were observed between the SVg and SVs populations, which were differed at Mdh locus (Appendix 1, table 2).
Table 2. Pairwise comparison of the percentage of fixed difference (indicated in bold), F ST values (indicated in italics, lower triangle) and geographical distance (in km, upper triangle) among isolates of B. portentosus collected from different geographical localities.

* Code of eight geographical samples listed in table 1. F ST values compare between all localities were significant different with P-value<0.001.
Two distinct clusters of B. portentosus samples are shown in a phenogram generated from the allelic profile of 23 loci examined (fig. 2). The first cluster contained the five samples from Thailand, which is seen to be genetically different from the cluster comprising the three samples from Laos at 41% fixed differences (fig. 2). The three samples from Maha Sarakham Province (MSg, MSm and MSn) were aligned as a cluster with 4–6.5% fixed differences. The population from Ubon Ratchathani Province, UBk, was clustered with UBm at 13% fixed differences. Moreover, the three samples from Maha Sarakham Province appeared to be genetically different from the two populations from Ubon Ratchathani at 19.5% fixed difference. The cluster of the samples from Laos showed that two populations, SVg and SVs, from Savannakhet Province were clustered at 4% fixed differences. The CSp population from Champasak Province was genetically different from SVg and SVs with 19.5% fixed differences. In addition, B. portentosus was genetically distinct from the other three species of ground cricket tested (i.e. >76% fixed differences). A Mantel test (r=0.515, P-value=0.0061; table 3) supports the notion that the null hypothesis, indicating that the matrices of % fixed difference and Euclidean geographical distance are not correlated, be rejected. A partial Mantel test between ‘geographic distance’ and ‘genetic distance’ after controlling for ‘river barrier’ revealed that r=0.415 (P=0.0275) (table 3). Another partial Mantel test between ‘river barrier’ and ‘genetic distance’ after controlling for ‘geographic distance’ gave r=0.486 (P=0.0007) (table 3). These results suggest that the river is even a more important factor in limiting gene-flow than the geographical distance. Our results also showed that genotypes at all loci deviated from Hardy-Weinberg expectations (HWE) with a highly significant heterozygote deficiency (P<0.001) except for Mpi (P=0.117) and PepB (P=0.289) (Appendix 1).
Table 3. Correlation test between genetic distance vs. geographical distance and river barrier.
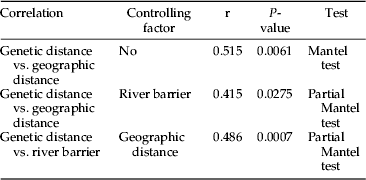

Fig. 2. Phenogram depicts % fixed difference (indicated in each branch) between eight different geographical populations of the field cricket, B. portentosus, from Thailand and Lao PDR and three other species of ground crickets.
Discussion
As with other species belonging to the order Orthoptera (e.g. grasshoppers: Gill, Reference Gill2008; Ademolu et al., Reference Ademolu, Idowu and Olatunde2009; mole crickets: e.g. Nevo et al., Reference Nevo, Beiles, Korol, Ronin, Pavlicek and Hamilton2000; and many field or ground crickets: e.g. Braswell et al., Reference Braswell, Birge and Howard2006), MEE has proven to be an extremely use-efficient tool for examining the genetic variation and relationships between different geographical populations of B. portentosus in Thailand and Lao PDR. In our study, over 23 informative enzyme loci markers were generated with up to 12 loci (52%) being polymorphic among B. portentosus populations. The grouping of B. portentosus samples based on the UPGMA phenogram was in line with the geographic area and distances as recorded, implying that genetic relationships of B. portentosus samples, would depend on geographic features in Thailand and Lao PDR. Moreover, the genetic distance was also concordant with the Euclidean geographical distance between populations compared within Thailand or within Lao PDR, i.e. the samples which were collected between 32–79 km from one another showed 4–13% fixed differences and F ST values of 0.518–0.879, whereas those separated by between 102–236 km showed 17–26% fixed differences and F ST values of 0.830–0.971. Other studies have shown that this is not always the case; thus although genetic difference of cytochrome b sequences of G. bimaculatus De Geer showed a significant correlation with spatial distance between South African and European samples, there was no significant correlation between different geographical populations from within South Africa (Ferreira, Reference Ferreira2006).
The observed deficit of heterozygotes as compared with Hardy-Weinberg expectations could be the result of a variety of factors, such as the Wahlund effect (i.e. due to the mixing of apparently contiguous population samples with different allele frequencies which are in reality separated by geographic barriers), natural selection, phenotypic assortative mating or inbreeding (Karlsson & Mork, Reference Karlsson and Mork1999; Holsinger & Weir, 2009). Which of these acts in the case of B. portentosus cannot be determined from the data available, although the very high F ST levels indicate concomitantly very limited gene flow between the populations sampled (Holsinger & Weir, Reference Holsinger and Wier2009), suggesting that the strong Wahlund effect, selection or inbreeding may be possible. The use of burrows by these crickets may be an indication of limited dispersal, which would be in line with both of these possibilities. Here, more information on the behavior and ecology of B. portentosus is required.
The genetic difference observed between the population samples from Thailand and Lao PDR (average 40% fixed difference) can probably be attributed to the major natural barrier, the Mekong River. The distance between UBk from Thailand and SVs from Laos is only 26 km, but there were 22% fixed differences between populations on either side of the river. Moreover, the correlation between genetic distance was more highly significantly correlated with river barrier than geographic distance. This finding provides strong evidence that the Mekong River obstructs gene flow of this cricket between Thailand and Lao PDR, as is the case for other organisms. The liver fluke, Opisthorchis viverrini, which uses Bithynia snails as first intermediate host and cyprinid fish as a second intermediate host, shows very low levels of gene flow between the samples from Thailand and Lao PDR (Saijuntha et al., Reference Saijuntha, Sithithaworn, Wongkham, Laha, Pipitgool, Tesana, Chilton, Petney and Andrews2007). Gene flow between populations of freshwater fish species, such as cyprinid spp. and catfish, is also interrupted across the Mekong River (So et al., Reference So, Maes and Volckaert2006; Ngamsiri et al., Reference Ngamsiri, Nakajima, Sukmanomon, Sukumasavin, Kamonrat, Na-Nakorn and Taniguchi2007; Hurwood et al., Reference Hurwood, Adamson and Mather2008). This conforms to the riverine barrier hypothesis, which was first suggested by Alfred Russell Wallace to describe the pattern of species distribution in the Amazon area of South America (Wallace, Reference Wallace1853; Haffer, Reference Haffer1997). Over the last few decades, tests of the hypothesis have usually involved taxonomic groups from this area (Gascon et al., Reference Gascon, Lougheed and Bogart1998, Reference Gascon, Malcolm, Patton, da Silva, Bogart, Lougheed, Peres, Neckel and Boag2000; Aleixo, Reference Aleixo2004; Noonan & Wray, Reference Noonan and Wray2006), although some support has also come from Africa (Anthony et al., Reference Anthony, Johnson-Bawe, Jeffery, Clifford, Abernathy, Tutin, Lahm, White, Utley, Wickings and Bruford2007) and islands of Southeast Asia (Jalil et al., Reference Jalil, Cable, Sinyor, Lackman-Ancrenaz, Ancrenaz, Bruford and Goossens2008) for gorillas and orang-utans, respectively.
Nevertheless, the two closest areas in Thailand and the Lao PDR, i.e. UBk and SVs, showed the lowest genetic difference when compared with the other samples from these countries. This is possibly because there was some gene flow between these populations despite the obstruction presented by the Mekong, potentially caused by gene flow between the UBk and SVs populations due to human transport. This human-assisted movement can occur if crickets are brought from the Songkorn district in Laos for sale at local markets in the Khemarat district of Thailand. While all our analyses show substantial genetic differentiation among the B. portentosus samples, a comprehensive assessment of population genetics structure, allele/genotype frequency and gene flow will require larger sample sizes and also more sophisticated approaches, i.e. DNA markers such as microsatellites or mtDNA markers with higher genetic resolving power. A low-frequency of some alleles at some loci tested may have remained undetected in this study due to the comparatively lower resolving power of allozyme markers vs. microsatellites. Moreover, the variation in morphological and biological traits, e.g. flatwing, cercal filiform hairs, body size, genitalia, tegmen length, mesosternum, metasternum, male calling song and seminal protein, which have been reported in other field crickets (Parsons & Shaw, Reference Parsons and Shaw2001; Dangles et al., Reference Dangles, Magal, Pierre, Olivier and Casas2005; Braswell et al., Reference Braswell, Birge and Howard2006; Tinghitella, Reference Tinghitella2008; Cordero et al., Reference Cordero, Llorente, Cordero and Ortego2009), indicates that further work to explore biological differences between geographical populations of B. portentosus is required. One last possibility is certainly that cryptic species may be responsible for the fixed allelic differences observed, but again this requires elucidation using other biological methods such as cross mating experiments.
Acknowledgements
This research was funded by a research grant from Mahasarakham University for the year 2010. We wish to thank the many individuals who help us with sample collection, especially Dr Anchalee Techasen, Ms Kunyarat Duenngai, Ms Sumonta Tepdara, Ms Kanokorn Saimanee, Ms Reamjit Suttisokshueak and Ms Thong Saijuntha. We would also like to thank two referees, Profs H.D. Loxdale, and J. Ortego, for their suggestions which have improved the paper considerably. Prof Ortega kindly suggested and carried out the partial Mantel tests.
Appendix 1
Allele frequency of 23 loci of eight geographical samples of B. portentosus, including Acheta confirmata Walker (Ac), Gryllus bimaculatus de Geer (Gb) and Gryllus testaceus Walker (Gt). All loci deviated from HWE with highly significant heterozygote deficiency at P<0.001, except Mpi (P=0.117) and PepB (P=0.289).

* Sample codes as listed in table 1 and sample size indicated in parenthesis.
** Enzyme band could not be stained.








