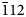Introduction
This paper continues a series of articles on new arsenate minerals from the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N, 160°14′E, 1200 m asl). This active fumarole, discovered by us in July 2012, has already given rise to the new arsenates: yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a); two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b); popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a); structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b); katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a); melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016b); pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017); and arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018a). The general description of this fumarole and data on the distribution of arsenate mineralisation within it are given by Pekov et al. (Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b).
The present paper is devoted to the new mineral arsenatrotitanite, ideally NaTiO(AsO4) (Cyrillic: арсенатротитанит). It is named as an arsenate of sodium (natrium in Latin) and titanium isostructural with titanite. Both the new mineral and its name have been approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2016–015). The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue number 95614.
Occurrence and general appearance
Specimens with the new mineral were collected by us in July 2015 from two pockets in the northern area of the Arsenatnaya fumarole, at the depth of 1–1.5 m under day surface. Temperatures measured using a chromel–alumel thermocouple at the time of collecting in these pockets were 400–420°C. Arsenatrotitanite was deposited directly from the gas phase as a volcanic sublimate or, more likely, formed as a result of the interaction between fumarolic gas and basalt scoria at temperatures not lower than 420–450°C. The basalt scoria seems the most probable source of Ti which has low volatility in volcanic gases, as both thermodynamic calculations (Churakov et al., Reference Churakov, Tkachenko, Korzhinskii, Bocharnikov and Shmulovich2000) and direct measurements carried out for gases present at Tolbachik (Zelenski et al., Reference Zelenski, Malik and Taran2014) show.
Arsenatrotitanite is a minor component of fumarolic encrustations mainly consisting of arsenates, sulfates, oxides, chlorides and silicates. The new mineral is associated closely with orthoclase (As-bearing variety), tenorite, hematite, johillerite, bradaczekite, badalovite (IMA2016–053), calciojohillerite (IMA2016–068), arsmirandite (IMA2014–081), tilasite, svabite, cassiterite, pseudobrookite, rutile, sylvite, halite, aphthitalite, langbeinite and anhydrite.
Arsenatrotitanite occurs as prismatic, tabular or lamellar crystals up to 0.3 mm × 0.8 mm × 2 mm, typically coarse (Fig. 1a) and sometimes divergent. They are separate, or combined in open-work aggregates, or interrupted crusts up to 2 mm × 5 mm in area and up to 0.3 mm thick. Crude acicular crystals up to 0.7 mm long form chaotic open-work aggregates up to 2 mm across in cavities (Fig. 1b). Well-shaped crystals are small, usually not bigger than 0.01 mm, rarely up to 0.05 mm. They are flattened, wedge-shaped, more rarely multifaceted (Figs 2a–c), sometimes skeletal (Fig. 2c). Dendrite-like aggregates are common (Figs 2a and 2d). Arsenatrotitanite overgrows, in close association with johillerite, bradaczekite, tenorite, cassiterite and hematite, incrustations mainly consisting of As-bearing orthoclase that cover basalt scoria altered by fumarolic gas.

Fig. 1. Brownish red to pale pinkish–reddish crystals and their aggregates of arsenatrotitanite with blue johillerite, black tenorite and yellowish fine-grained cassiterite (the latter is visible in (a) on white to pale beige crusts of As-bearing orthoclase). Field of view width is 3.5 mm for both images. Photo: I.V. Pekov & A.V. Kasatkin.

Fig. 2. Crystals and aggregates of arsenatrotitanite. Secondary electron images from scanning electron microscopy.
Physical properties and optical data
Arsenatrotitanite is transparent, brownish red to pale pinkish–reddish with brown hue or, in the thinnest needles, almost colourless. Its streak is white and its lustre is vitreous. The mineral is brittle. Cleavage is perfect on {110}, the fracture is stepped (observed under the microscope). The Mohs’ hardness is ca 5½. Density could not be measured because of the small size of crystals without caverns and the open-work character of aggregates (Fig. 2). Density calculated using its empirical formula is 3.950 g cm−3.
Arsenatrotitanite is optically biaxial (+) with α = 1.825(5), β = 1.847(6), γ = 1.896(6) (589 nm), 2Vmeas = 70(5)° and 2Vcalc = 69°. Other optical data are: dispersion of optical axes is strong, r > v; optical orientation: Y = b; pleochroism is strong: Z (bright pink to, in thicker grains, carmine red) > Y (very pale pinkish to almost colourless) ≥ X (colourless).
Raman spectroscopy
The Raman spectrum of arsenatrotitanite (Fig. 3) was obtained on a randomly oriented crystal using an EnSpectr R532 instrument (Department of Mineralogy, Moscow State University) with a green laser (532 nm) at room temperature. The output power of the laser beam was ~16 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines cm–1 and a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~10 µm. The back-scattered Raman signal was collected with 40x objective; signal acquisition time for a single scan of the spectral range was 1500 ms and the signal was averaged over 10 scans.

Fig. 3. The Raman spectrum of arsenatrotitanite.
The most intense bands with maxima at 878 and 801 cm–1 correspond to As5+–O stretching vibrations of AsO43− anions. Bands with frequencies lower than 700 cm–1 correspond to bending vibrations of AsO4 tetrahedra, Ti4+–O, Fe3+–O and Al–O stretching vibrations and lattice modes. The absence of bands with frequencies higher than 950 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in the mineral.
Chemical composition
The chemical composition of arsenatrotitanite was determined on a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Department of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA, and a 3 µm beam diameter. The following standards were used: albite (Na), wollastonite (Ca), Al2O3 (Al), magnetite (Fe), ilmenite (Ti), SnO2 (Sn), InAs (As) and fluorophlogopite (F).
The chemical composition of arsenatrotitanite (average of 6 spot analyses; wt.%, ranges are in parentheses / standard deviation) is: Na2O 12.26 (11.95–12.84 / 0.30), CaO 3.10 (2.97–3.29 / 0.11), Al2O3 4.39 (3.97–4.55 / 0.22), Fe2O3 9.57 (9.06–10.11 / 0.44), TiO2 17.11 (16.81–17.29 / 0.26), SnO2 1.03 (0.91–1.30 / 0.15), As2O5 50.17 (49.48–51.14 / 0.71), F 3.29 (3.21–3.41 / 0.09), O = F –2.39, total 99.53. Contents of other elements with atomic numbers higher than carbon are below detection limits. Fe is calculated as Fe3+ taking into account strongly oxidising conditions of mineral formation in the Arsenatnaya fumarole: only minerals of trivalent iron are found there (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b).
The empirical formula calculated on the basis of 5 (O + F) atoms per formula unit is Na0.91Ca0.13Al0.20Fe3+0.27Ti0.49Sn0.02As1.00O4.60F0.40 or, after grouping of constituents by structure positions: (Na0.91Ca0.13)Σ1.04(Ti0.49Fe3+0.27Al0.20Sn0.02)Σ0.98(As1.00O4.00)(O0.60F0.40). The simplified, end-member formula is NaTi(AsO4)O, which requires Na2O 13.72, TiO2 35.38, As2O5 50.90, total 100.00 wt%.
X-ray crystallography and crystal structure
Powder XRD data of arsenatrotitanite (Table 1) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and exposure 15 min. Angular resolution of the detector is 0.045 2θ (pixel size 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The parameters of the monoclinic unit cell calculated from the powder data are: a = 6.699(3), b = 8.756(2), c = 7.203(3) Å, β = 114.81(3)° and V = 383.5(3) Å3.
Table 1. Powder XRD data (d in Å) of arsenatrotitanite.

*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
Single-crystal X-ray studies of arsenatrotitanite were carried out using an Xcalibur S diffractometer equipped with a CCD detector. The crystal structure was solved by direct methods and refined with the use of the SHELX-97 software package (Sheldrick, Reference Sheldrick2008) to R = 0.0176. The refined numbers of electrons (e ref) for A (Na scattering curve), M (Ti scattering curve) and (O,F) (O scattering curve) sites were obtained using low-angle reflections and gave the following values: 11.66, 22.22 and 8.88, respectively. The cation distribution [A = Na0.9Ca0.1 (e calc = 11.90), M = Ti0.5Fe0.3Al0.2 (e calc = 21.40) and (O,F) = O0.6F0.4 (e calc = 8.40)] was performed in accordance with both refined numbers of electrons and chemical data. We should note that e calc for the M site could be increased due to the Sn admixture, which was not involved in the refinement because of its minor amount. The unit-cell dimensions and the experimental details are presented in Table 2, atom coordinates and displacement parameters in Table 3, selected interatomic distances in Table 4 and bond-valence calculations in Table 5. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 2. Crystal data, data collection information and structure refinement details for arsenatrotitanite.

Table 3. Сoordinates, site multiplicites (Q), site occupancy factors (s.o.f.), and equivalent and anisotropic displacement parameters (U eq, in Å2) of atoms for arsenatrotitanite.

*Site occupancy factors for the A, M and (O,F) sites were fixed on the last stages of the refinement in accordance to both refined numbers of electrons and chemical data.
Table 4. Selected interatomic distances (Å) in the structure of arsenatrotitanite.

Table 5. Bond-valence calculations for arsenatrotitanite.

Bond-valence parameters are taken from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). All calculations were weighted according to site occupancies given in Table 3.
Arsenatrotitanite is a representative of the well-known titanite structure type. The crystal structure of the new mineral (Fig. 4) contains infinite chains of the MO4(O,F)2 (M = Ti, Fe or Al) octahedra running along the c axis and connected with each other via common vertices. Adjacent chains are linked by isolated AsO4 tetrahedra forming a heteropolyhedral framework with cavities occupied by the A cations (A = Na and Ca) in seven-fold coordination. The location of admixed F in the (O,F) site on the bridge between the M-centred octahedra is confirmed by bond-valence calculations (Table 5).

Fig. 4. The crystal structure of arsenatrotitanite in two projections; M = (Ti, Fe and Al), A = (Na and Ca): see Table 3. The unit cell is outlined.
Discussion
Arsenatrotitanite, ideally NaTiO(AsO4), is isostructural with minerals of the durangite group [isokite CaMg(PO4)F, lacroixite NaAl(PO4)F, panasqueiraite Ca(Mg,Fe)(PO4)(OH,F), durangite NaAl(AsO4)F, maxwellite NaFe3+(AsO4)F, tilasite CaMg(AsO4)F, and kononovite NaMg(SO4)F] and of the titanite group [titanite CaTiO(SiO4), natrotitanite (Na0.5Y0.5)TiO(SiO4), malayaite CaSnO(SiO4), and vanadomalayaite CaVO(SiO4)] (Back, Reference Back2018). Arsenatrotitanite combines chemical features of members of both groups: it is an arsenate, like durangite-group minerals, and, like representatives of the titanite group, contains tetravalent cation (Ti4+) and additional anion O2– as species-defining components. Comparison of the new mineral with closely related species is given in Table 6. We propose placing arsenatrotitanite into the durangite group rather than the titanite group for three reasons: (1) the titanite group traditionally includes only silicates; (2) in the powder XRD data the new mineral is much closer to durangite-group arsenates than to the silicate titanite (Table 5); and (3) arsenatrotitanite contains significant admixtures of Fe, Al, and F and in fact belongs to the solid-solution system with the end-members NaTiO(AsO4) – NaFe3+(AsO4)F (maxwellite) – NaAl(AsO4)F (durangite). This solid-solution system which also involves tilasite CaMg(AsO4)F and kononovite NaMg(SO4)F was discussed, based on data for samples from fumarole deposits, by Pekov et al. (Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b).
Table 6. Comparative data for arsenatrotitanite and closely related minerals.

The end-member arsenatrotitanite can be considered as a natural analogue of synthetic NaTiO(AsO4) reported by Yahia et al. (Reference Yahia, Rodewald and Pöttgen2010).
Arsenatrotitanite has a chemical analogue with K instead of Na, katiarsite KTiO(AsO4), discovered in the same Arsenatnaya fumarole. However, they are quite different in terms of crystal structure: katiarsite is a representative of the KTiO(PO4) (KTP) structure type (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a).
Acknowledgements
We thank Peter Leverett and two anonymous referees for valuable comments. This study was supported by the Russian Foundation for Basic Research, grant no. 17-05-00179. The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.134