INTRODUCTION
Sulphur (S) is an essential element for living organisms, being a component of many proteins, enzymes and cofactors (Solomon et al., Reference Solomon, Lehmann, Zarruk, Dathe, Kinyangy, Liang and Machado2011). In the last few years, S deficiency and increased need for S fertilization are being reported in many parts of the world (Kost et al., Reference Kost, Chen and Dick2008; Scherer, Reference Scherer2009). Intensive land use, decreases in S emissions by power plants (Lehmann et al., Reference Lehmann, Solomon, Zhao and McGrath2008), increasing crop productivity, continuous removal of nutrients by harvests and use of fertilizers with concentrated NPK formulations have made some plants strictly dependent on S fertilization (Kertesz and Mirleau, Reference Kertesz and Mirleau2004). Although many studies with S were published in the 1970s and 1980s, there are still many questions concerning its complex dynamics.
Organic S represents about 90% of the total S pool in Brazilian soils (Neptune et al., Reference Neptune, Tabatabai and Hanway1975) and although not readily plant-available, it can be an important S source in periods of deficiency (De Bona and Monteiro, Reference De Bona and Monteiro2010; Schmidt et al., Reference Schmidt, De Bona, Silveira and Monteiro2012). No-till (NT) cropping systems, widely adopted in the Southern region of Brazil (Derpsh and Friedrich, Reference Derpsch, Friedrich and Joshi2009), are known to enhance soil organic C content and to improve chemical, physical and biological attributes in tropical and subtropical soils (Lal, Reference Lal2006). Thus, these systems may also promote the accumulation and turnover of organic S through increased crop residues and litter decomposition.
Phosphogypsum (PG) is a soil conditioner employed in NT systems with the goal of ameliorating root environment by alleviating aluminum (Al) toxicity and through calcium (Ca) supply (Farina et al., Reference Farina, Channon and Thibaud2000; Sumner, Reference Sumner, Shahandeh, Bouton and Hammel1986; Toma et al., Reference Toma, Sumner, Weeks and Saigusa1999). It is also an important source of S for crops (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a, Reference Caires, Maschietto, Garbuio, Churka and Jorisb), since it provides S in forms readily available for plants, and, when applied in large amounts, it can cause long-term residual effects, mainly in clay soils (Caires et al., Reference Caires, Churka, Garbuio, Ferrari and Morgano2006; Reference Caires, Garbuio, Churka and Joris2011a; Toma et al., Reference Toma, Sumner, Weeks and Saigusa1999), due to retention of S on colloidal surfaces.
Determining availability of S in soil based on extraction methods is very challenging since several forms of S are found in soils (Goh and Pamidi, Reference Goh and Pamidi2003). The available S fractions include readily soluble inorganic sulphate (SO4-S), adsorbed SO4-S and a portion of organic S (C-bonded or ester-S), which can be mineralized during plant growth (Scherer, Reference Scherer2009). The most common methods used to determine available S are based on evaluating soluble + adsorbed S, since the inorganic fraction is the main S pool available in soils with high SO4-S adsorption capacity (Tabatabai, Reference Tabatabai, Tabatabai and Sparks2005), and the methods that evaluate only soluble S are not adequate for soils with high SO4-S adsorption capacity (Ribeiro et al., Reference Ribeiro, Dias, Alvarez-Venegas, Mello and Daniels2001).
Numerous solutions are used in the extraction of available S, such as mixture of salts and weak acids, or extraction by ion exchange resin (Anderson et al., Reference Anderson, Lefroy, Chinoin and Blair1992; Prochnow et al., Reference Prochnow, Boaretto and Vitti1997; Ribeiro et al., Reference Ribeiro, Accioly, Alvarez-Venegas, Braga and Alves1991). Currently, the most common solutions for extracting available S in Brazilian soils are 0.01 M calcium phosphate (Ca(H2PO4)2), initially proposed by Fox et al. (Reference Fox, Olson and Rhoades1964) and the standard extractant adopted in the state of São Paulo; and 0.5 M ammonium acetate (NH4OAc) along with 0.25 M acetic acid (HOAc) (Bardsley and Lancaster, Reference Bardsley, Lancaster and Black1965). The phosphate ion has more affinity for adsorption than the sulphate ion so that Ca(H2PO4)2 solution will extract more of the SO4-S adsorbed to iron and aluminum oxides and hydroxides than the SO4-S adsorbed to soil organic fractions. In contrast, the NH4OAc + HOAc solution will extract soluble and adsorbed S, and a portion of S linked to soil organic matter. This solution has been used because the adsorption of acetate is similar to SO4-S adsorption in soils (Tabatabai, Reference Tabatabai1987).
In Brazilian soils, there are few field studies focusing on the availability of S to plants, especially under NT systems. The objectives of this study were to (i) evaluate the nutrient uptake and S availability in an NT soil to the maize and wheat crops provided by PG applications, and (ii) establish critical levels of S in soil for the crops using Ca(H2PO4)2 and NH4OAc + HOAc extractant solutions. Since NT systems should increase organic S, which is also an S source, in deficient periods, we hypothesized that NH4OAc + HOAc would be a better extractant than Ca(H2PO4)2 for assessing available soil S, especially in surface layers, where the organic matter content is higher than in subsurface layers.
MATERIAL AND METHODS
The experiment was undertaken in Ponta Grossa, Parana state, Brazil (25º08'50'' S, 50º06'20'' W, 819 m a.s.l.) on an Oxisol (clay, kaolinitic, Rhodic Hapludox), previously used as pastureland. The trial area was dominated by the grass Paspalum notatum cultivar Pensacola, and it had no history of liming or fertilizer applications. In 1998, at the beginning of the experiment, soil chemical analyses of the topsoil (0–0.20 m) showed the following results: pH (1:2.5 soil/0.01 M CaCl2 suspension) of 4.6; exchangeable Al, Ca, Mg and K contents of 3, 25, 20 and 3.6 mmolc dm−3 respectively; total acidity at pH 7.0 (H + Al) of 78 mmolc dm−3, P (Mehlich-1) of 0.3 mg dm−3; total organic C (by the Walkley–Black method) of 31 g dm−3 and base saturation of 38%. Particle-size distribution analyses revealed 580, 130 and 290 g kg−1 of clay, silt and sand respectively. A more detailed description of the experiment site can be found in Caires et al. (Reference Caires, Garbuio, Churka and Joris2011a).
Dolomitic lime at a rate of 4.5 t ha−1 was applied on the soil surface in July 1998. The lime rate was calculated to raise the base saturation at the topsoil (0–0.20 m) to 70% (Caires et al., Reference Caires, Alleoni, Cambri and Barth2005). The dolomitic lime used contained 224 g kg−1 Ca, 140 g kg−1 Mg and 89% effective calcium carbonate equivalent. An NT system was established for the grain crop production involving no disturbance to the soil other than the sowing operation.
A randomised complete block design was used, with three replications in a split-plot arrangement. Plot size was 8 × 7 m and subplot size was 4 × 7 m. The treatments consisted of PG application on the soil surface at 0 and 6 t ha−1 in September 2004 to subplots within plots with previously surface-applied PG at 0, 3, 6 and 9 t ha−1 in October 1998. Phosphogypsum, a by-product obtained from a Brazilian phosphate fertilizer industry, contained 235 g kg−1 Ca, 153 g kg−1 S, 3 g kg−1 P and 156 g kg−1 water. The sequence of crops up to 2004 was as follows: soybean (Glycine max (L.) Merr.) in 1998–1999, barley (Hordeum vulgare L.) in 1999, soybean in 1999–2000, wheat (Triticum aestivum L.) in 2000, soybean in 2000–2001, maize (Zea mays L.) in 2001–2002 and soybean in 2002–2003 and 2003–2004. At the time of PG reapplication in 2004, soils had low acidity and non-toxic levels of exchangeable Al3+ as a function of the surface liming performed before the establishment of the experiment in 1998.
In October 2004, maize (hybrid DA 2A120) was sown at a seeding rate of six seeds m−1, and a row spacing of 0.80 m. Fertilizers were applied at the rates of 114 kg ha−1 N (28 kg ha−1 N at planting and 86 kg ha−1 N as urea in top-dressing), 43 kg ha−1 P and 69 kg ha−1 K. In July 2005, wheat (cultivar OR 1) was sown at a seeding rate of 60 seeds m−1, and a row spacing of 0.17 m. Fertilizers were applied at the rates of 124 kg ha−1 N (24 kg ha−1 N at planting and 100 kg ha−1 N as urea in top dressing), 21 kg ha−1 P and 66 kg ha−1 K. These fertilizer rates were applied according to the soil test recommendations for the Parana state (Oliveira, Reference Oliveira2003); in addition, other plant treatments and weed control were performed according to each crop's needs.
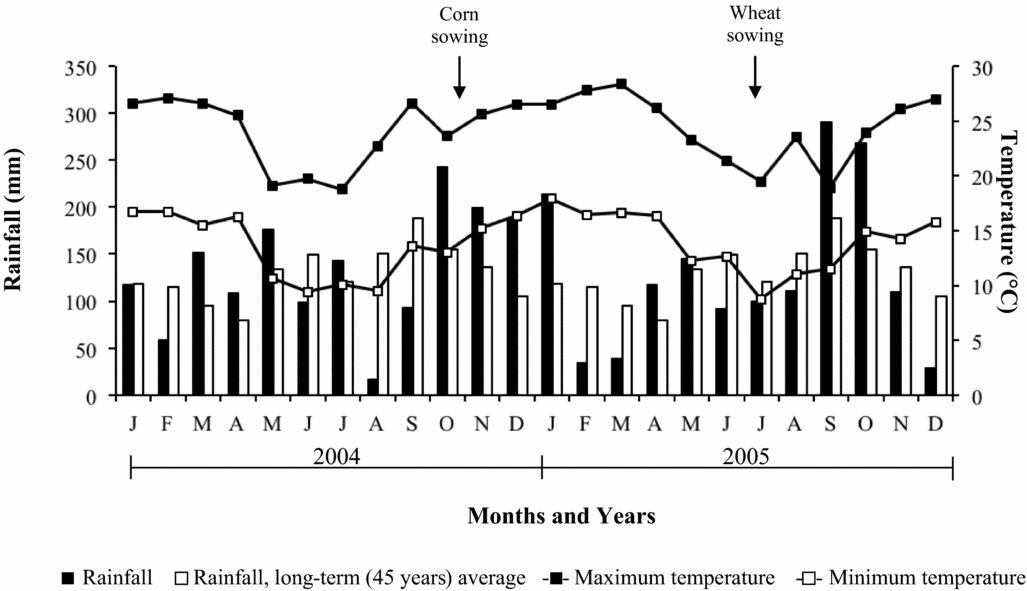
Seasonal rainfall and air temperature data for the duration of the experiment conducted are shown in Figure 1. Throughout the development period of the crops, air temperatures were normal. In the winter of 2005, there was an excessive rainfall, which caused decrease in wheat grain yield (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a).

Figure 1. Monthly rainfall (black areas), minimum (open square) and maximum (closed square) temperatures during the development period of the crops (2004–2005) and the 45-year average monthly rainfall (white areas) in Ponta Grossa, Southern Brazil.
Maize (2004–2005) and wheat (2005) leaf samples (30 leaves per subplot) were collected during the flowering period by taking leaf samples from below and opposite to the ear for maize, and the flag leaf for wheat. The samples were washed in deionized water, dried in a forced-air oven at 60 °C until a constant weight was achieved and subsequently ground. At physiological maturity, aboveground biomass samples of maize (five plants per subplot) and wheat (one linear meter of plants) were also collected to measure total dry matter and S concentration. At the end of the cycle of maturation, crops were manually harvested and grain yields were determined at 130 g kg−1 moisture. Grain S content was also measured. Sulphur uptake and S exports were calculated by multiplying S concentration in aboveground biomass and grains by dry matter and grain yield respectively. The analyses of S in leaf tissues, dry matter and grains were performed using nitric–perchloric acid digestion, and measured using turbidimetry (as BaSO4).
Soil samples were taken in May 2005 and 2006. To obtain a composite sample, 12 soil cores were taken at 0 to 0.05, 0.05 to 0.10 and 0.10 to 0.20-m depths, and five soil cores were sampled at 0.20 to 0.40 and 0.40 to 0.60-m depths in each subplot using a soil probe. Before chemical analysis, soils were air-dried and ground to pass through a 2-mm sieve. Sulphate was extracted with 0.01 M Ca(H2PO4)2 using a 1:2.5 (v/v) soil/solution ratio and later determined turbidimetrically as BaSO4. The soil SO4-S content extracted with 0.5 M NH4OAc along with 0.25 M HOAc was presented by Caires et al. (Reference Caires, Garbuio, Churka and Joris2011a). The extractant 0.01 M Ca(H2PO4)2, initially proposed by Fox et al. (Reference Fox, Olson and Rhoades1964), is indicated for very weathered soils (Tabatabai, Reference Tabatabai1987), since the amount of phosphate present in the solution can replace most of the sulphate adsorbed in these soils, thus extracting the soluble + adsorbed fractions. NH4OAc along with HOAc (Bardsley and Lancaster, Reference Bardsley, Lancaster and Black1965) extracts S that is soluble, adsorbed and part of the easily oxidizable organic fraction. Acetate is similar to sulphate in adsorption characteristics, which makes this solution a good extractor to predict SO4-S availability (Ribeiro et al., Reference Ribeiro, Dias, Alvarez-Venegas, Mello and Daniels2001)
Data were analysed through an analysis of variance and simple regression methods using a randomized complete block design in a split-plot arrangement. The effects of the surface-applied gypsum rates in 1998 were analysed through regression analyses, and the criterion for choosing the model was the magnitude of the determination coefficients significant at p < 0.05. The effects of gypsum application in 2004 were compared using the F-test. Pearson product moment correlations were employed between soil-extracted S and plant S. Critical levels of SO4-S for achieving 90% of relative grain yield were evaluated for both crops by adjusting an inverse first-order equation (ŷ = y0 + a/x) between soil SO4-S levels (at 0–0.20 m) and relative crop yield, using SigmaPlot v11 (Systat Software Inc., Chicago, IL).
RESULTS
Soil-extractable Sulphur
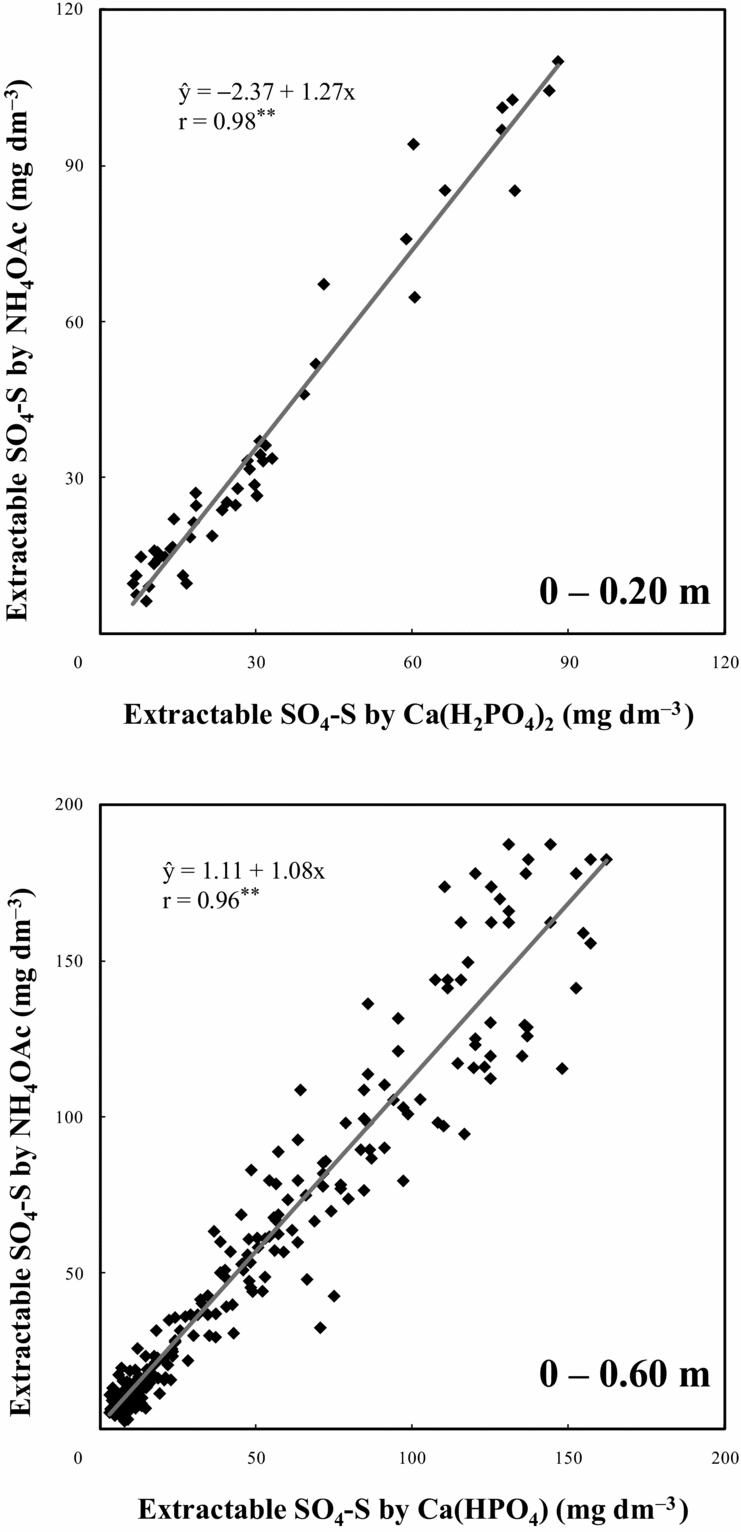
The two extractants were positively correlated in the evaluation of available soil SO4-S (Figure 2) for both topsoil (0–0.20 m, r = 0.98) and all soil profiles (0–0.60 m, r = 0.96). The close relationships between SO4-S extracted by 0.01 M Ca(H2PO4)2 and 0.5 M NH4OAc along with 0.25 M HOAc indicated that both extractants had similar efficacy for determining available SO4-S in an Oxisoil with high clay and organic C contents. Since the slope of the equation was greater than 1, the extractant 0.5 M NH4OAc along with 0.25 M HOAc extracted a little more SO4-S compared with 0.01 M Ca(H2PO4)2, mainly on the soil surface layers, which have higher organic matter content. Since at the deepest soil layers organic matter content is lower and most of the sulphate is adsorbed into Fe- and Al-oxides and hydroxides, differences for both extractants in extracting sulphate in all soil profiles (0–0.60 m) were smaller.

Figure 2. Correlation between chemical extractants for soil SO4-S (0.01 M Ca(H2PO4)2 and 0.5 M NH4OAc in 0.25 M HOAc).**p < 0.01.
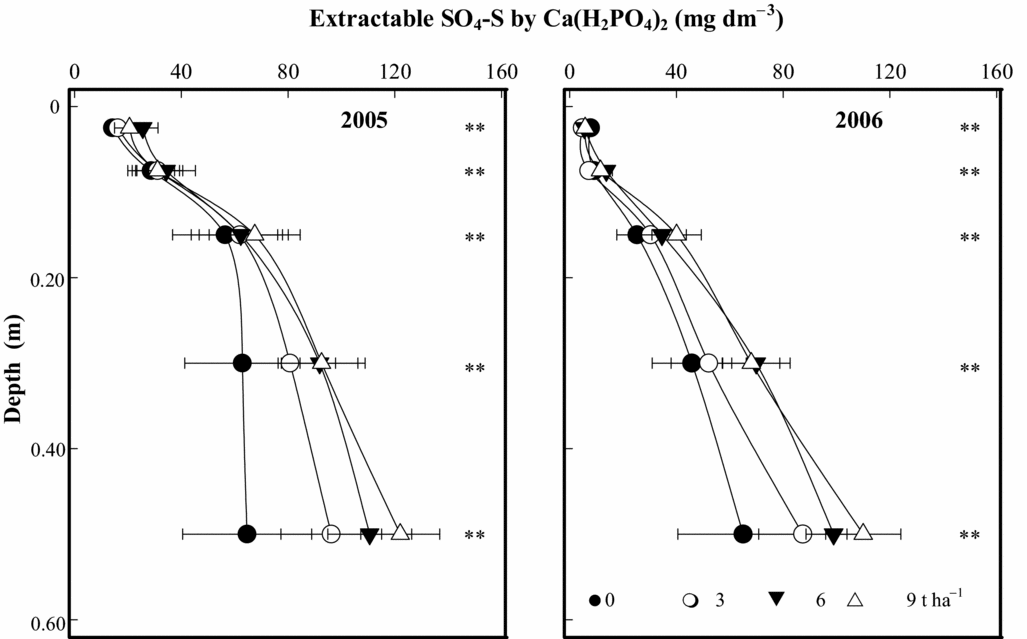
The soil SO4-S contents extracted by 0.01 M Ca(H2PO4)2 have shown residual effects of PG, 6.5 (2005) and 7.5 (2006) years after its application (Figure 3). Comparing this result with the soil SO4-S extracted by 0.5 M NH4OAc along with 0.25 M HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a), we observed that the effects of PG application on extractable SO4-S content were relatively similar with both extractants, and the most pronounced effects occurred on subsoil layers. The soil SO4-S contents at 0.20–0.40 and 0.40–0.60-m depths after PG application at 9 t ha−1 were respectively 90 and 120 mg dm−3 by extraction with 0.01 M Ca(H2PO4)2 (Figure 3) and 130 and 145 mg dm−3 by extraction with 0.5 M NH4OAc + 0.25 M HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a) in 2005, and 70 and 110 mg dm−3 by extraction with 0.01 M Ca(H2PO4)2 (Figure 3) and 70 and 105 mg dm−3 by extraction with 0.5 M NH4OAc + 0.25 M HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a) in 2006.

Figure 3. Extractable SO4-S by 0.01 M Ca(H2PO4)2 at different depths, following phosphogypsum (PG) application on the soil surface in 1998. **p < 0.01 by linear regression.
Results have shown great movement of SO4-S in the soil eight months after PG reapplication (2005), consequently increasing available soil SO4-S levels (Figure 4) in all sampled layers. Higher evidence of SO4-S movement was observed 1.5 years after PG reapplication (2006). Higher SO4-S concentrations, as measured by both extractants, were found in subsoil layers (Figure 4). The soil SO4-S contents at 0.20–0.40 and 0.40–0.60-m depths after PG reapplication at 6 t ha−1 were respectively 120 and 140 mg dm−3 by extraction with 0.01 M Ca(H2PO4)2 (Figure 4) and 165 and 160 mg dm−3 by extraction with 0.5 M NH4OAc + 0.25 M HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a) in 2005, and 85 and 125 mg dm−3 by extraction with 0.01 M Ca(H2PO4)2 (Figure 4) and 90 and 115 mg dm−3 by extraction with 0.5 M NH4OAc + 0.25 M HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a) in 2006. The higher affinity for anion phosphate, lower soil acidity and higher cation exchange capacity on the soil surface layers might explain the lower SO4-S contents on the soil surface. Of the total SO4-S provided by the reapplication of 6 t ha−1 of PG (480 mg dm−3), 37% was recovered (180 mg dm−3) by extraction with Ca(H2PO4)2 and 56% (270 mg dm−3) with NH4OAc + HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a) at 0 to 0.60-m depth. Only a small fraction of the total applied SO4-S was taken up by the crops or lost by erosion through runoff, indicating substantial movement to deeper layers beyond the depth of 0.60 m.
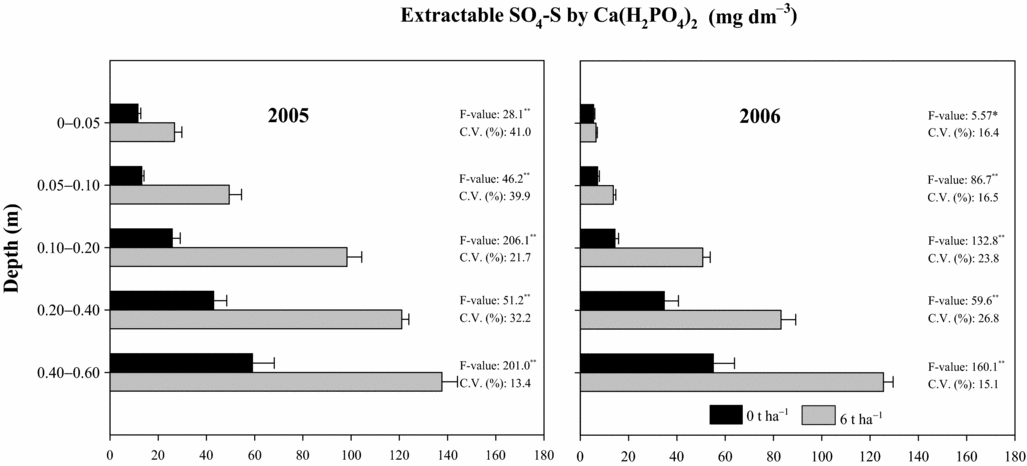
Figure 4. Extractable SO4-S by 0.01 M Ca(H2PO4)2 in different soil depths following phosphogypsum (PG) reapplication on the soil surface in 2004. *p < 0.05 and **p < 0.01.
Effects of Sulphur on maize and wheat plants
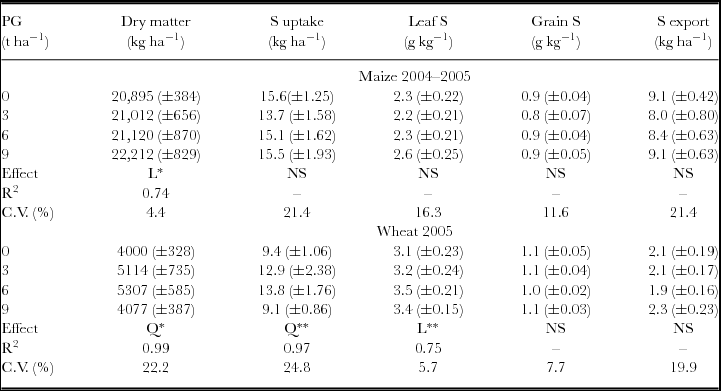
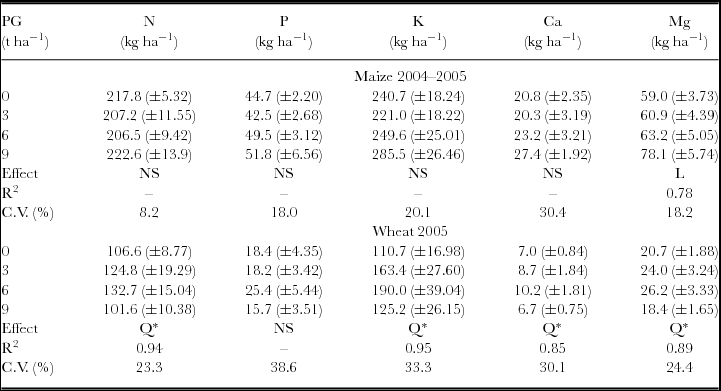
The attributes related to maize and wheat nutrition and aboveground biomass were not influenced by the interaction between the surface-applied PG rates in 1998 and 2004. Maize total aboveground biomass, evaluated at physiological maturity, was affected by PG treatments with linear response. Wheat aboveground biomass (ŷ, in kg ha−1) increased with PG rates (x, in t ha−1) (ŷ = 3975.14 + 600.18x – 65.12x2, R2 = 0.99). The maximum wheat dry mass production was obtained with the estimated rate of 4.6 t ha−1 of PG. Besides increasing biomass production, wheat plants also showed increased accumulation of S in aboveground biomass and leaves, even after 7.5 years of PG application (Table 1). Other plant nutrients accumulated on the aboveground biomass, such as Mg in the maize plants and N, K, Ca and Mg in the wheat plants, were also influenced by PG application (Table 2).
Table 1. Dry matter and S contents in maize and wheat plants following phosphogypsum (PG) application on the soil surface in 1998.

NS: non-significant; *p < 0.05 and **p < 0.01; L: significant by linear regression; Q: significant by quadratic regression.
Standard errors (n = 6) are given in parentheses.
S uptake and S exports were calculated by multiplying S concentration in aboveground biomass and grains by dry matter and grain yield respectively.
Table 2. Uptake of macronutrients in maize and wheat plants following phosphogypsum (PG) application on the soil surface in 1998.

NS: non-significant; *p < 0.05 and **p < 0.01; L: significant by linear regression; Q: significant by quadratic regression.
Standard errors (n = 6) are given in parentheses.
Nutrient uptake was calculated by multiplying the concentration in aboveground biomass by dry matter.
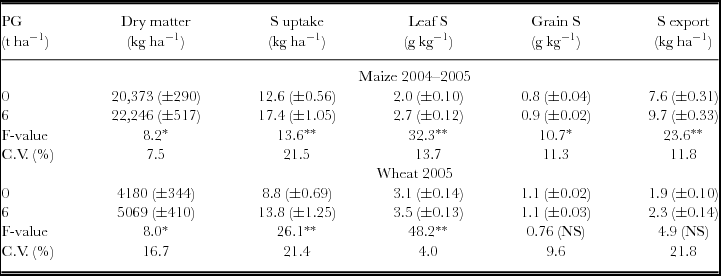
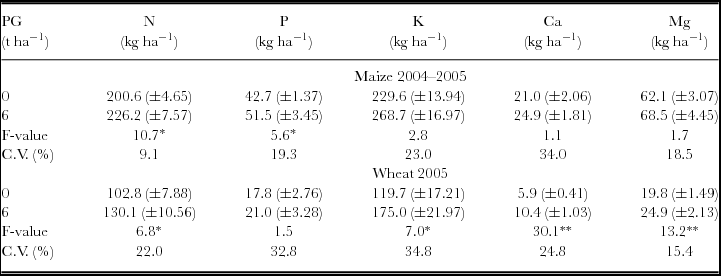
Reapplication of PG in 2004 increased total aboveground biomass, S uptake, leaf S content and S exports by the maize crop (Table 3). Wheat harvests in 2005 were also increased by PG reapplication with positive responses on aboveground biomass production, S uptake and leaf S content (Table 3). Reapplication of PG increased the uptake of N and P on maize plants, and that of N, K, Ca and Mg on wheat plants (Table 4).
Table 3. Dry matter and S content in maize and wheat plants after phosphogypsum (PG) reapplication on the soil surface in 2004.

NS: non-significant; *p < 0.05 and **p < 0.01.
Standard errors (n = 12) are given in parentheses.
S uptake and S exports were calculated by multiplying S concentration in aboveground biomass and grains by dry matter and grain yield respectively.
Table 4. Uptake of macronutrients in maize and wheat plants after phosphogypsum (PG) reapplication on the soil surface in 2004.

*p < 0.05 and **p < 0.01.
Standard errors (n = 12) are given in parentheses.
Nutrient uptake was calculated by multiplying the concentration in aboveground biomass by dry matter.
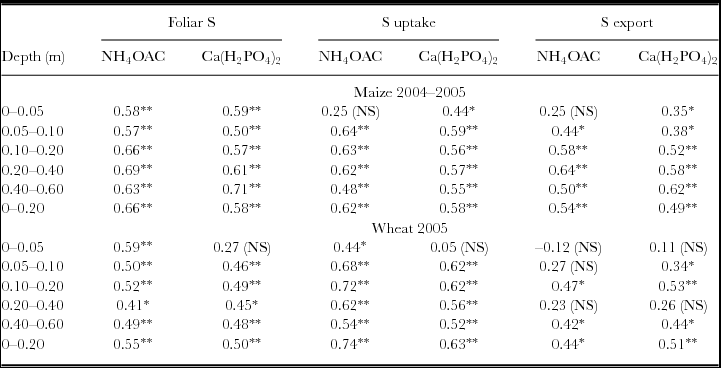
Sulphur uptake by plants and S concentration of maize and wheat leaves were more sensitive indicators of increase of S in the soil than was the S exported by grains (Table 5). Correlating the available soil S-SO4 content with plant parameters, we observed that both extractants were very similar (Table 5).
Table 5. Correlation coefficients (Pearson) between soil S extracted by Ca(H2PO4)2 and NH4OAc and foliar S, S uptake by maize and wheat plants and S exported by crop harvest (n = 24).

NS: non-significant; *p < 0.05 and **p < 0.01.
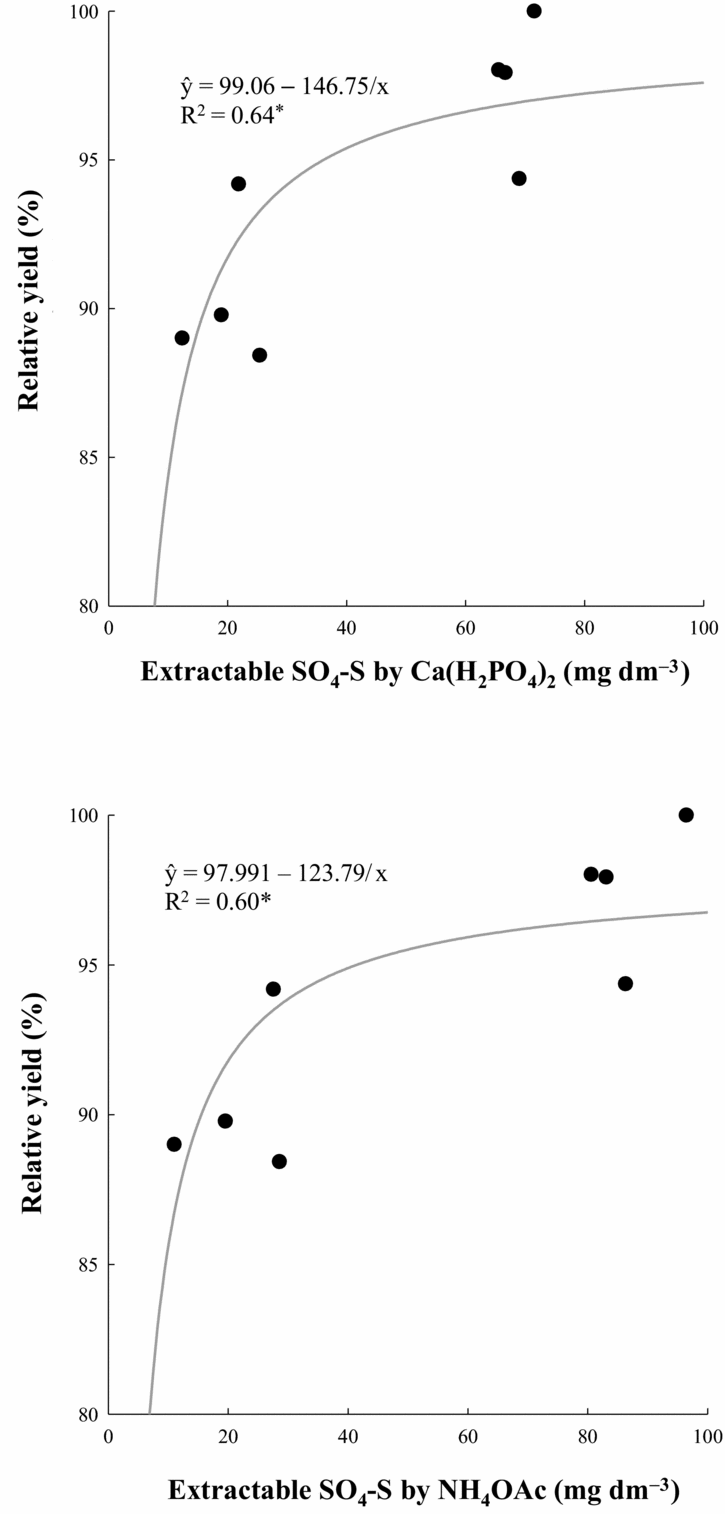
The critical level of SO4-S for achieving 90% of relative grain yield was evaluated for both crops, but results were significant only for the maize crop. The obtained results were very similar for both extractants (Figure 5), estimated as 16.2 mg dm−3 by 0.01 M Ca(H2PO4)2 and 15.5 mg dm−3 by 0.5 M NH4OAc along with 0.25 M HOAc.

Figure 5. Relations between maize relative yield in 2004–2005 and soil SO4-S content extracted by 0.01 M Ca(H2PO4)2 and 0.5 M NH4OAc in 0.25 M HOAc. *p < 0.05.
DISCUSSION
Soil-extractable Sulphur
The solutions of NH4OAc + HOAc and Ca(H2PO4)2 employed in our study extract soluble and adsorbed SO4-S (Bardsley and Lancaster, Reference Bardsley, Lancaster and Black1965; Fox et al., Reference Fox, Olson and Rhoades1964). The phosphate ion has more affinity for adsorption than the sulphate ion so that Ca(H2PO4)2 solution extracts more of the SO4-S adsorbed to Fe- and Al-oxides and hydroxides than the SO4-S adsorbed to soil organic fractions. In contrast, the NH4OAc + HOAc solution extracts soluble and adsorbed S and a portion of S linked to soil organic matter. In our study, both extractants had similar efficacy for determining available SO4-S in an Oxisoil with high clay and organic C contents (Figure 2). Because of high organic matter content, especially in topsoil (0–0.20 m), the extractant 0.5 M NH4OAc along with 0.25 M HOAc extracted a little more SO4-S compared with 0.01 M Ca(H2PO4)2. Ribeiro et al. (Reference Ribeiro, Dias, Alvarez-Venegas, Mello and Daniels2001) observed that either NH4OAc or Ca(H2PO4)2 was efficient as soil SO4-S extractant, showing high correlation with S uptake by sorghum plants. However, in soils with a low SO4-S adsorption capacity, the more efficient extractant was 0.01 M CaCl2, showing that S availability in these soils was mainly driven by the mineralization of organic forms (Ribeiro et al., Reference Ribeiro, Dias, Alvarez-Venegas, Mello and Daniels2001).
Sulphate adsorption capacity has been positively related to Fe- and Al-oxide contents (Alves and Lavorentti, Reference Alves and Lavorentti2004; Chao et al., Reference Chao, Harward and Fang1964), and negatively related to pH level (Zhang and Yu, Reference Zhang, Yu and Yu1997) as well as to organic matter content (Couto et al., Reference Couto, Lathell and Bouldin1979). Residual effects of PG application on soil SO4-S availability were found in some long-term studies performed in soils with a pH value of around 4.0 in subsurface layers (Farina et al., Reference Farina, Channon and Thibaud2000; Toma et al., Reference Toma, Sumner, Weeks and Saigusa1999). In our study, soil pH in CaCl2 was 5.0 and ΔpH had negative values (varying from –0.9 to –0.6) in all soil profiles (0–0.60 m). Because of low acidity and high soil organic C contents determined by the Walkley–Black method, whose levels ranged from 27 g dm−3 (0–0.05 m) to 17 g dm−3 (0.40–0.60 m), the dominance of negative electric charges should have favoured the SO4-S movement from PG application (Figure 3) and re-application (Figure 4) to even deeper soil layers. However, even on an Oxisol with a high density of electrical charges due to high organic C contents throughout the soil profile, a considerable amount of PG sulphate remained in the subsoil for several years, which is relevant information for diagnosing availability of S in soils.
Effects of Sulphur on maize and wheat plants
By the time of cropping, maize and wheat crops showed no visual signs of S deficiency. Even so, application of PG in 1998 increased leaf S content and total S uptake by the wheat crop in 2005 (Table 1). In addition, reapplication of PG in 2004 also increased leaf S content and total S uptake in both maize (2004–2005) and wheat (2005) crops (Table 3). Although grasses in general require less S compared with legumes or brassicas (Dick et al., Reference Dick, Kost, Chen and Jez2008), beneficial effects of the application of various S sources on S nutrition of grasses have been reported in the literature. Bullock and Goodroad (Reference Bullock and Goodroad1989) observed an increase in S content in maize leaves and grain due to S application as ammonium sulphate or elemental S. An increase in maize grain S content was also observed by Ferreira et al. (Reference Ferreira, Araújo, Pereira and Cardoso2001) and Chen et al. (Reference Chen, Kost and Dick2008) with the application of ammonium sulphate and S from flue-gas desulphurisation respectively. In a greenhouse study using isotopic labeling S, Silva et al. (Reference Silva, Alvarez Venegas, Ruiz and Sant'Anna2003) found that corn and soybean showed different behaviours in S absorption and redistribution. In spite of having larger root absorption, corn retained great part of that S in the root, while soybean absorbed considerably less, but presented greater translocation efficiency. This result may also help explain the fact that maize more often responds to S application than soybean. Another reason for the greater response of grasses to S additions could be a function of plant breeding, which provided more productive, and also more N demanding, varieties.
The interaction between N × S is reported in the literature, since both nutrients are the components of amino acids and essential for protein synthesis (Epstein and Bloom, 2006). Therefore, plant S requirement is closely related to N nutrition (Tables 2 and 4) (Chen et al., Reference Chen, Kost and Dick2008). Nitrogen and S interaction was reported on aboveground dry mass production of Brachiaria brizantha, cv. Marandu, in a greenhouse trial (De Bona and Monteiro, Reference De Bona and Monteiro2010), on Brachiaria decumbens in field trial (Schmidt et al., Reference Schmidt, De Bona, Silveira and Monteiro2012) and on maize (Chen et al., Reference Chen, Kost and Dick2008) and wheat (Salvagiotti et al., Reference Salvagiotti, Castellarín, Miralles and Pedrol2009) grain yield under field conditions. These studies have shown that S nutrition has promoted N uptake and increased N use efficiency by plants, which can reduce production costs and degradation of water quality associated with oversupply of N (Chen et al., Reference Chen, Kost and Dick2008; Salvagiotti et al., Reference Salvagiotti, Castellarín, Miralles and Pedrol2009).
When studying soil extractants, the best method is the one that provides better correlation to plant uptake. Cui et al. (Reference Cui, Wang, Wang, Xing, Chen, Haneklaus, Fleckenstein and Schnug2006) evaluated different extractants in China pastures, and observed high correlation coefficients between soil S and S absorbed by the crop, especially with 0.01 M Ca(H2PO4)2 as extractant. However, the authors pointed out that in their study the correlation coefficients between soil S and leaf S were unsatisfactory for field experimentation, which did not occur in our study.
Many solutions can be used as SO4-S extractants in Brazilian soils to obtain the available contents, but the most frequently used are Ca(H2PO4)2 and NH4OAc along with HOAc (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a; Prochnow et al., Reference Prochnow, Boaretto and Vitti1997; Ribeiro et al., Reference Ribeiro, Dias, Alvarez-Venegas, Mello and Daniels2001). Since the choice of an extractant is a function of its physical and chemical characteristics (Anderson et al., Reference Anderson, Lefroy, Chinoin and Blair1992; Blair et al., Reference Blair, Chinoin, Lefroy, Anderson and Crocker1991) and its correlation to the plant, in our study the extractant Ca(H2PO4)2 could be replaced by NH4OAc + HOAc, which is cheaper and subjected to less interference in quality depending on solubility. Correlations were positive in almost all soil layers studied. Moreover, the 0–0.20-m depth has been shown to be adequate for diagnosing available SO4-S content in the maize and wheat crops cultivated under NT. Prochnow and Boaretto (Reference Prochnow and Boaretto1995), studying available S in sicilian lemon, observed that S absorption occurs mainly in the 0–0.20-m depth, indicating that this depth must be considered to evaluate the available soil SO4-S content. Besides, sampling at just 0.20-m depth has practical advantages for farmers, and therefore increases the possibility of adopting methods of S diagnosis, which may increase overall S use efficiency.
A possible involvement in acid–subsoil amelioration and/or cationic balance on crop response to PG apart from S supply is difficult to exclude entirely in our study. In addition, PG contained P (3 g kg−1) in its composition. Increasing the surface-applied PG rate in 1998 linearly increased the uptake of Mg by maize and raised the uptake of N, K, Ca and Mg by wheat (Table 2). Besides, PG reapplication in 2004 increased the uptake of N and P by maize, and the uptake of N, K, Ca and Mg by wheat (Table 4). The displacement of other cations from the exchange complex is the main effect of PG applications to soils. Therefore, Mg and K leaching has often been observed in the studies involving PG applications (Caires et al., Reference Caires, Maschietto, Garbuio, Churka and Joris2011b; Farina et al., Reference Farina, Channon and Thibaud2000; Oliveira and Pavan, Reference Oliveira and Pavan1996). The leaching of exchangeable Mg after PG application can be beneficial for plant nutrition, increasing the uptake of Ca and K (Tables 2 and 4), especially when the soil has high Mg levels and a low Ca/Mg ratio in the most superficial layers (Caires et al., Reference Caires, Kusman, Barth, Garbuio and Padilha2004, Reference Caires, Maschietto, Garbuio, Churka and Joris2011b), as observed in our study. Increase in Mg uptake by maize and wheat crops as a result of PG application in 1998 (Table 2) and by the wheat crop after PG reapplication in 2004 (Table 4) was certainly due to gains in the aboveground biomass of plants promoted by PG applications (Tables 1 and 3). Although PG is also effective at improving P plant nutrition under NT systems (Caires et al., Reference Caires, Blum, Barth, Garbuio and Kusman2003; Reference Caires, Maschietto, Garbuio, Churka and Joris2011b), the P uptake only increased in the maize crop after PG reapplication in 2004 (Table 4).
Despite complex changes in soil chemical properties and plant nutrition as a result of PG application, because wheat and corn grain yields were positively related only to the Ca and S leaf contents, it was considered more probable that yield benefits imparted by PG resulted from increased Ca and SO4–S availability to cereal crops (Caires et al., Reference Caires, Garbuio, Churka and Joris2011a). In an attempt to isolate the effect of soil SO4-S availability from PG to cereal crops, the results showed that the critical levels of SO4-S in the topsoil (0–0.20 m) for the NT maize crop were 16.2 mg dm−3 by 0.01 M Ca(H2PO4)2, and 15.5 mg dm−3 by 0.5 M NH4OAc along with 0.25 M HOAc (Figure 5). Since root length of the maize and wheat crops under NT is concentrated at 65–80% in the topsoil (0–0.20 m) (Caires et al., Reference Caires, Barth, Garbuio and Kusman2002a, Reference Caires, Feldhaus, Barth and Garbuiob, Reference Caires, Garbuio, Churka, Barth and Corrêa2008), this soil layer was demonstrated to be sufficient to determine available SO4-S content to maize and wheat crops in this cropping system. Since closed relationships were obtained, these critical levels of SO4-S may be useful for NT farming because many doubts remained concerning the establishment of critical levels of SO4-S in most soils of the world. For tropical soils under conventional tillage systems, an SO4-S concentration of 10 mg dm−3 extracted with NH4OAc or NH4OAc + HOAc has been proposed as a critical level (Kang and Osiname, Reference Kang and Osiname1976; Vitti, Reference Vitti, Büll and Rosolem1989). An increase in required SO4-S levels is expected nowadays, especially for the maize crop, which has experienced a twofold increase in yield over the last two decades (IBGE, 2012; Wallington et al., Reference Wallington, Anderson, Mueller, Morris and Ginder2012) due to improved combined agronomic practices such as breeding and higher inputs of fertilizers, mainly N, thus exporting more nutrients and requiring higher S replenishment than before.
Acknowledgements
This research was supported by Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES). We also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing a research fellowship to E. F. Caires. Special acknowledgements to Jenniffer Blesh, who carefully revised this manuscript and improved the English language.