INTRODUCTION
Toxoplasma gondii is a globally distributed pathogenic parasite with a heteroxenous life-cycle that infects warm-blooded vertebrates, including humans (Tenter et al. Reference Tenter, Heckeroth and Weiss2000). The parasite undergoes sexual reproduction in the intestines of infected cats, resulting in the shedding of hundreds of millions of environmentally resistant oocysts in the feces (Dubey and Frenkel, Reference Dubey and Frenkel1972; Dubey, Reference Dubey1998). Although felids are the only known definitive hosts, T. gondii infections have been observed in both pelagic and coastal marine mammal species (Dubey et al. Reference Dubey, Zarnke, Thomas, Wong, Van Bonn, Briggs, Davis, Ewing, Mense, Kwok, Romand and Thulliez2003), indicating the presence of land-to-sea pathogen pollution (Conrad et al. Reference Conrad, Miller, Kreuder, James, Mazet, Dabritz, Jessup, Gulland and Grigg2005). Of particular concern are reports documenting T. gondii encephalitis as an important cause of mortality in threatened southern sea otters (Enhydra lutris nereis) (Kreuder et al. Reference Kreuder, Miller, Jessup, Lowenstine, Harris, Ames, Carpenter, Conrad and Mazet2003). Sea otters are vital for maintaining a healthy nearshore ecosystem due to their role as keystone predators (Estes and Palmisano, Reference Estes and Palmisano1974) and can serve as sentinels for marine water contamination (Conrad et al. Reference Conrad, Miller, Kreuder, James, Mazet, Dabritz, Jessup, Gulland and Grigg2005). Because many sea otter prey species are also harvested as seafood for human consumption, the high prevalence of T. gondii in sea otters raises concerns about the risks to human health. Although T. gondii infections are usually asymptomatic in otherwise healthy humans, systemic toxoplasmosis can occur in immunocompromised patients, such as people suffering from acquired immune deficiency syndrome (AIDS), and newly acquired infections in pregnant women may cause abortion, stillbirth or serious birth defects due to congenital toxoplasmosis (Luft et al. Reference Luft, Hafner, Korzun, Leport, Antoniskis, Bosler, Bourland, Uttamchandani, Fuhrer, Jacobson, Morlat, Vilde and Remington1993; Dubey and Jones, Reference Dubey and Jones2008). In addition, immunocompetent adults have been reported to develop ocular diseases such as retinochoroiditis, and latent T. gondii infections may contribute to mental illness (Flegr, Reference Flegr2007; Dubey and Jones, Reference Dubey and Jones2008). Gaining an understanding of transmission processes resulting in the infection of sea otters with zoonotic pathogens is therefore important for both conservation of the species and mitigation of a potential public health threat.
One hypothesis that has been proposed as a possible mechanism for transmission of T. gondii to sea otters is that oocysts, upon being carried into the ocean by fresh-water runoff, are concentrated in filter-feeding shellfish commonly preyed upon by sea otters (Miller et al. Reference Miller, Gardner, Kreuder, Paradies, Worcester, Jessup, Dodd, Harris, Ames, Packham and Conrad2002). Bivalve molluscs have been shown to concentrate T. gondii oocysts from seawater and retain them for at least 3 days in mussels (Arkush et al. Reference Arkush, Miller, Leutenegger, Gardner, Packham, Heckeroth, Tenter, Barr and Conrad2003) and 85 days in oysters (Lindsay et al. Reference Lindsay, Collins, Mitchell, Wetch, Rosypal, Flick, Zajac, Lindquist and Dubey2004) without reducing infectivity. However, epidemiological investigations did not support an association between predation upon bivalves and increased risk of T. gondii exposure in otters (Johnson et al. Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009). Instead, the same study determined that otters with a diet consisting of ⩾10% small marine turban snails (including Promartynia pulligo, Chlorostoma brunnea, Chlorostoma montereyi and Lithopoma undosa) were 12 times more likely to be seropositive for T. gondii than otters that consumed fewer snails, whereas otters with a preference for abalone were significantly less likely to be infected (Johnson et al. Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009). Given that both snails and abalone are herbivorous gastropods, this finding raises the question of why species with such similar diets differ so greatly with respect to their epidemiological association with T. gondii in sea otters.
A prior study performed to evaluate marine snails as potential paratenic hosts for T. gondii showed that snails are capable of concentrating oocysts from seawater by 2–3 orders of magnitude and retaining them for as long as 11 days post-exposure (Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015). However, no such work with abalone has been published to date. As discussed by Johnson et al. (Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009), the observed reduction in exposure risk associated with a preference for abalone could be either the result of a reduced ability of abalone to acquire, concentrate and retain T. gondii oocysts, or a product of the abalone's greater size and the smaller number of individuals therefore needed to satisfy the otter's metabolic needs. The aim of the current study was to examine which of these hypotheses is more plausible by testing whether abalone are physiologically capable of acquiring T. gondii from contaminated seawater, and if they are, to compare T. gondii concentration and retention ability between abalone and small marine snails.
MATERIALS AND METHODS
Study animals
This study was conducted using 15 red abalone (Haliotis rufescens) maintained at the UC Davis Bodega Marine Laboratory. Average animal weight was 31·6 g. This species was selected because it has been shown to be commonly preyed upon by sea otters in resource-rich systems in central California (Wendell, Reference Wendell1994; Tinker et al. Reference Tinker, Bentall and Estes2008), and because it is the most abundant of the abalone species found within the southern sea otter range (Allen et al. Reference Allen, Callaway, Haaker, Kalvass, Karpov, Kashiwada, Lauermann, Moore, O'Leary, O'Reilly, Patyten, Ramsay, Rogers-Bennett, Skeen, Taniguchi, Tillman, Watters and Wine2005).
Toxoplasma gondii surrogate microspheres
To eliminate the biohazardous risk of environmental contamination with T. gondii oocysts at the common working space at Bodega Marine Laboratory, Dragon Green (DG) polystyrene microspheres (10·4 µm) were used as surrogates for T. gondii oocysts. These microspheres were validated as appropriate surrogate particles for T. gondii oocysts due to their similar physical and chemical surface properties (Shapiro et al. Reference Shapiro, Largier, Mazet, Bernt, Ell, Melli and Conrad2009) and have been previously shown to accurately mimic oocyst transport in water and passage behaviour through snails (Shapiro et al. Reference Shapiro, Silver, Largier, Conrad and Mazet2012; Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015). Microspheres were obtained from Bangs Laboratory, Fishers, IN (product number FCO7F/5493).
Concentration and retention of T. gondii surrogates by abalone
Abalone were placed in 4 L tubs, with one control tub (A) containing three abalone and two exposure tubs (B and C) containing six abalone each (Fig. 1). Tubs were filled with continuously aerated filtered (30 µm) seawater and one 5 × 7·5 cm2 kelp (Macrocystis pyrifera) blade per abalone and maintained in a cold (12–15 °C) seawater bath for 72 h to allow for acclimation. During this period, kelp was replaced, seawater changed, and feces collected using a serological pipette twice daily. At the end of the acclimation period, the exposure tubs (B and C) were spiked with T. gondii surrogate microspheres at a final concentration of ten microspheres per mL, while tub A was not spiked and served as a negative control. A fourth tub (designated tub D) containing only kelp and seawater was also spiked with T. gondii surrogate microspheres at a final concentration of ten microspheres per mL to determine surrogate distribution in the tub in the absence of abalone. After a 24-h exposure period, fecal samples were collected from tubs A, B and C, and each abalone was placed in an individual 1 L container after being thoroughly rinsed with filtered seawater to remove any surrogates that may have adhered to the shell or foot of the animal. Each container contained aerated filtered seawater and kelp as a source of food. In addition to feces, water samples (50 mL) were also collected from the top, middle, and bottom of the surrogate control tub (D) at the end of the exposure period to determine surrogate distribution in the tub in the absence of abalone. All remaining kelp fragments were also collected and transported to our laboratory at the School of Veterinary Medicine, UC Davis, for surrogate enumeration as described below.

Fig. 1. A schematic diagram representing experimental setup and sample collection. Abalone were placed in 4 L tubs and allowed to acclimate for 72 h, then exposed to Toxoplasma gondii oocyst surrogates for 24 h. At the end of the exposure period, feces and kelp were collected from tubs A, B and C, and water and kelp were collected from tub D. Abalone were then placed in individual 1 L containers and monitored for 14 days, during which time daily fecal samples were collected from each animal for surrogate enumeration.
For 14 days following the exposure period, remaining kelp fragments were removed, photographed and discarded, and fecal samples were collected from each 1 L container at 24-h intervals. At the same intervals, individual containers were thoroughly rinsed and fresh filtered, aerated seawater and kelp were provided for each animal. To derive data on the amount of kelp consumed by each abalone, kelp was photographed prior to and following each 24-h interval and subsequently analysed using ImageJ (Schneider et al. Reference Schneider, Rasband and Eliceiri2012) to measure the surface areas of each kelp fragment. Aliquots of each fecal sample from days 0, 1, 2 and 3 and complete fecal samples from days 4, 7, 11 and 14 were processed and examined for surrogate enumeration as described below.
Enumeration of T. gondii surrogates in water and fecal samples
Detection of T. gondii surrogate microspheres in water and abalone feces was conducted following previously published protocols (Shapiro et al. Reference Shapiro, Mazet, Schriewer, Wuertz, Fritz, Miller, Largier and Conrad2010a ; Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015). Briefly, water samples were filtered through 5 µm mixed cellulose membranes that were mounted on glass slides. Prepared slides were then examined at 100× using a Zeiss Axioskop epifluorescence microscope equipped with a UVA filter. Fecal samples were centrifuged and the volumes of the resulting fecal pellets were estimated. Fecal pellets were then homogenized with a 20 mL syringe equipped with a blunt-tipped 15-gauge needle, diluted with water to produce a thin slurry, and examined according to the same filtration and microscopy protocols described for water samples.
Enumeration of T. gondii surrogates from kelp surfaces
Kelp fragments from each tub were immersed and agitated in a NaPP/guanidinium/Tween (NGT) solution prepared by adding 17·7 g granular sodium polyphosphate (NaPP) to 1 L boiling deionized water, then adding 9·55 g granular guanidine HCl (0·1 m) and 1 mL Tween 20 (Thermo Fisher Scientific, Waltham, MA) to the cooled NaPP solution. Post-immersion, fragments were scraped on both sides with a plastic spoon and rinsed on each side with 10–12 mL NGT solution. The resulting kelp-washed NGT solution was then diluted with deionized water and examined for T. gondii surrogates according to the filtration and microscopy protocols described above.
Data analysis
Data collected included abalone mass, estimated daily fecal volume, daily surface area of kelp eaten, and daily fecal surrogate counts for each abalone as well as surrogate concentrations for water samples taken from tub D following the 24 h exposure period. Because fecal samples collected on day zero (i.e. immediately following the 24-h exposure period) represent the pooled feces from all animals in each exposure tub, total fecal volume and surrogate counts for these pooled samples were divided by the number of abalone in the tub (n = 3 for tub A; and n = 6 for tubs B and C). Additionally, estimated fecal volume and surrogate counts were used to calculate the concentration of surrogates per mL feces for each abalone. All data was organized using Microsoft Excel and imported into R (version 3.0.3; https:// www.r-project.org) for analysis. The Shapiro–Wilk test was used to assess the normality of the surrogate count and concentration data for each day, and based on the results of this test, the Wilcoxon rank sum test was applied to assess the significance of differences between the median surrogate count and concentration values of control and exposed abalone. The Wilcoxon signed rank test was applied to assess the significance of differences between fecal surrogate concentrations and the nominal ‘environmental’ surrogate concentration of ten surrogates per mL of seawater in the exposure tubs. Cumulative fractions of all surrogates that were recovered over the duration of the study were also calculated for each day.
Finally, to evaluate the role of animal size as a potential ‘risk factor’ for oocyst acquisition and retention, correlation analysis (either Pearson's r or Kendall tau, depending on the normality of the data) was used to test for a relationship between abalone mass and maximum surrogate count, surrogate retention time, fecal volume and amount of kelp eaten. Maximum surrogate count and surrogate retention time were selected as measures of acquisition and retention, while fecal volume and amount of kelp eaten were selected as factors that could potentially influence the number of oocysts an abalone acquires.
Diet-based differences in exposure rates: a feasibility analysis
We conducted a feasibility analysis to further examine the null hypothesis of constant risk of infection: that is, the possibility that differences in seroprevalence between otters specializing on marine snails (high infection rates) and otters specializing on abalone (low infection rates) could be due simply to differences in prey capture rates, rather than differences in the probability that each prey type contains oocysts. Specifically, assuming that the per-item risk of infection (γ) was identical for snails and abalone, we calculated expected infection probabilities for a typical abalone specialist and a typical snail specialist using empirical data on individual prey consumption collected from field studies of tagged sea otters in central California (Oftedal et al. Reference Oftedal, Ralls, Tinker and Green2007; Tinker et al. Reference Tinker, Bentall and Estes2008, Reference Tinker, Guimarães, Novak, Marquitti, Bodkin, Staedler, Bentall and Estes2012, Reference Tinker, Tomoleoni, Weitzman, Staedler, Jessup, Murray, Miller, Burgess, Bowen, Miles, Thometz, Tarjan, Golson, Batac, Dodd, Berberich, Kunz, Bentall, Fujii, Nicholson, Newsome, Melli, LaRoche, MacCormick, Johnson, Henkel, Kreuder-Johnson and Conrad2013). We used available data for 17 adult abalone specialists (N = 9526 dives) and 23 adult snail specialists (N = 10 797 dives) to compute taxa-specific intake rates for each specialist type (±1 standard deviation (s.d.), computed across individual estimated diets), using the Monte Carlo algorithm described in Tinker et al. (Reference Tinker, Guimarães, Novak, Marquitti, Bodkin, Staedler, Bentall and Estes2012). We further assumed the likelihood that an adult otter is infected depends on the cumulative exposure risk over the previous 5-year period (1825 days), and thus we used a hazards model to estimate P i , the probability of infection for specialist type i:
where Ab i represents the mean number of abalone consumed per day by specialist type i and Sn i represents the mean number of snails consumed per day by specialist type i (for simplicity we assume only snails and abalone are infective, and thus ignore all other prey). In equation (1), each prey consumption event represents an additive hazard, and thus P i is simply 1 – the probability of avoiding infection over the previous 5 years. To adjust for differences in absolute consumption rates between otters of different sex and size, we re-scaled consumption rates to the average observed for a 22 kg female sea otter in Monterey or Big Sur (6 kg d−1). At present there are no available data with which to estimate γ, the per-item infection risk; however, because our goal was only to calculate relative (and not absolute) rates of infection under the hypothesis of risk-equivalence, the actual value of γ was unimportant. Accordingly, in order to make our analysis relevant to field data on seroprevalence, we arbitrarily adjusted γ to produce a realized infection rate for snail specialists of 0·85 (corresponding to the value reported for snail specialists by Johnson et al. Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009), and then calculated the corresponding infection rate for abalone specialists. To provide an indication of within-group variability we also report associated s.d. in computed infection rates, based on the variance in individual diets. We compared the results of this feasibility analysis to the empirically derived infection rates for snail and abalone specialists reported by Johnson et al. (Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009).
RESULTS
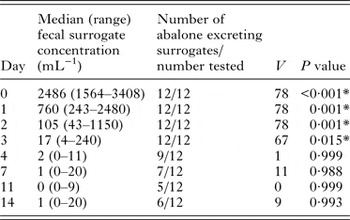
Counts and concentrations of T. gondii surrogate microspheres in fecal samples were found to be non-normally distributed at all-time points except for the count data from days 1 and 3 (Table S1). Therefore, non-parametric tests were applied for further statistical analyses. Fecal surrogate concentrations were significantly greater than the 10 mL−1 ambient exposure concentration through 3 days post-exposure (Table 1), and surrogate counts and concentrations in fecal samples from exposed abalone were significantly greater than in samples from control abalone from 0 to 2 days post-exposure (Table S2). In the first 24 h post-exposure, fecal surrogate concentrations were 2–3 orders of magnitude greater than the ambient concentration of surrogates in seawater during the exposure phase (Fig. 2). Fecal surrogate counts declined rapidly from days 1 to 4, and 99% of all surrogates recovered in fecal samples were detected in samples from this time period. However, six abalone were observed to continue to defecate surrogates through the end of the 14-day post-exposure monitoring period (Table 1).
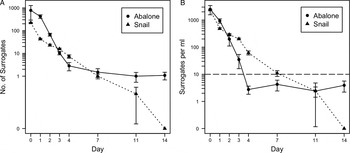
Fig. 2. (A) Mean counts and (B) concentrations of Toxoplasma gondii surrogates detected in fecal samples from abalone and marine snails on days 0, 1, 2, 3, 4, 7, 11 and 14 following exposure to spiked seawater. Error bars represent the s.e. The dashed line represents the baseline ‘environmental’ surrogate concentration (10 mL−1) in the exposure tubs. Snail data were obtained from a previously published investigation (Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015).
Table 1. Results of one-sided Wilcoxon signed rank tests employed to assess the significance of differences between the fecal surrogate concentrations at each time point and 10 mL−1, which was the nominal ambient surrogate concentration of seawater in the exposure tubs
*Statistically significant (P < 0·05).
Unexplained contamination of control abalone occurred approximately 3 days post-exposure, resulting in observation of T. gondii surrogates in fecal samples collected from all three control animals on days 3 and 4. On these days, the median fecal surrogate concentration in control abalone was not significantly greater than the 10 mL−1 nominal exposure concentration (Wilcoxon signed-rank tests, V = 3, P = 0·625; V = 1, P = 0·875). On day 7 and beyond, surrogates were no longer detected in fecal samples from the negative control abalone.
Abalone mass, average feces volume and maximum surrogate counts were found to be normally distributed, while average food intake and surrogate retention time were not (Table S3). Significant correlations were found between abalone mass and mean food intake (Fig. S1A; Kendall tau rank sum correlation, τ = 0·4211, P < 0·05, n = 15), mean fecal productivity (Fig. S1B; Pearson's correlation, r = 0.0.6999, P < 0·01, n = 15), and maximum surrogate count (Fig. S1C; Pearson's correlation, r = 0·8033, P < 0·01, n = 12). However, no correlation was found between abalone mass and retention time (Fig. S1D; Kendall tau rank sum correlation, τ = 0·1551, P = 0·1594, n = 12).
Surrogate concentrations of water samples taken from the top, middle and bottom of the surrogate control tub (D) measured at 2·6, 3·0 and 7·7 mL−1. Assuming even distribution and no loss of microspheres during the spiking procedure, a concentration of approximately ten surrogates per mL should be measured throughout the tub. All observed values were therefore lower than expected. If each concentration value is assumed to be representative of the concentration of one-third of the water column in the tub, then the total number of microspheres suspended in the water column after 24 h is equal to approximately 50% of the total number added. An additional 10 640 surrogate microspheres, representing about 30% of the total number added, were found to have adhered to the surface of kelp fragments (Table 2). The remaining 20% most likely adhered to the walls of the tubs or were lost during the spiking or sample handling procedures. Kelp surface area, number of surrogates and surrogate density per unit area of kelp were all reduced in the presence of abalone (Table 2).
Table 2. Surface area of kelp pieces remaining after the 24-h exposure period, the number and density of Toxoplasma gondii surrogate microspheres adhered to kelp surfaces, and the percentage of total surrogates added that were recovered from kelp surfaces

a Tubs B and C contained abalone as the exposure animals, while tub D served as a surrogate particle control and did not contain abalone. Tub A served as the negative control and therefore no surrogate particles were added to that treatment.
Our analysis of dietary data showed that a typical 22 kg female sea otter specializing on abalone consumed approximately 20 ± 4·6 abalone and 11 ± 32·1 snails per day, while a similar sea otter specializing on snails consumed approximately 0·9 ± 1·6 abalone and 705 ± 251 snails per day. Assuming the per-item risk of infection (γ) was identical for snails and abalone, and arbitrarily setting γ = 1·65E-06, we computed expected infection rates of 0·85 ± 0·099 for snail specialists and 0·09 ± 0·076 for abalone specialists. Estimated infection rates were similar to empirically measured rates of 0·85 for snail specialists and 0·07 for abalone specialists (Johnson et al. Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009).
DISCUSSION
The physiological ability of abalone to ingest, concentrate, and retain T. gondii oocysts from the environment as determined by the present study was similar to that of snails, previously described by Krusor et al. (Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015). Toxoplasma gondii surrogates were detected in abalone feces at significantly higher concentrations than the baseline surrogate concentration of the spiked seawater for three days post-exposure, indicating that abalone are physiologically capable of concentrating oocysts by ingestion. Maximum counts and concentrations of surrogates, which were 2–3 orders of magnitude higher than in the spiked seawater, were observed at 0 and 24 h post-exposure and thereafter declined swiftly through day 4. After day 4, fecal surrogate counts and concentrations did not change significantly as abalone continued to excrete consistently low numbers of surrogates in their feces through the end of the 14-day post-exposure monitoring period. While the infective dose of T. gondii oocysts is not known for sea otters, studies with pigs and rodents indicate that susceptible hosts may become infected following ingestion of a single oocyst (Dubey, Reference Dubey1996; Dubey et al. Reference Dubey, Lunney and Shen1996). Therefore, the low surrogate counts observed at later time points could still be epidemiologically significant.
Based on recently published findings by Krusor et al. (Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015), marine snails and abalone demonstrate similar physiological ability to acquire and retain T. gondii from surrounding seawater (Fig. 2). Both species concentrated surrogates by 2–3 orders of magnitude compared to the concentration of surrogates in exposure water, and snails continued to excrete surrogates for 11 days (Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015), while abalone excreted them for at least 14. Therefore, the observed epidemiologic association between prey specialization on abalone and the reduced risk of T. gondii infection cannot be explained by a reduction in the physiological ability of abalone to concentrate and retain oocysts.
Because the snail study was designed such that six snails were selected for dissection and fecal sample examination each day, the sample size for monitoring fecal excretion of T. gondii was effectively half that of the present study. While unlikely, it is possible that the six snails remaining at day 14 were by chance individuals that had shorter retention times and that the observed difference between snails and abalone is an artefact of the smaller snail population size. In addition, while ingested material should be evident in feces within 24 h based on gut passage time (Garcia-Esquivel and Felbeck, Reference Garcia-Esquivel and Felbeck2009), fecal values of T. gondii surrogates on day 0 should be interpreted with caution in both studies due to the possibility of overestimation arising from accidental collection of non-fecal-associated surrogates from spiked exposure water.
Due to the unexplained contamination of fecal samples from negative control abalone, the data from days 4–14 must be interpreted cautiously, as the possibility of surrogate cross-contamination between animals cannot be ruled out. However, the six abalone that had surrogate-positive fecal samples on day 14 were not spatially clustered, and two of them had consistently excreted surrogates at every time point examined. For these results to be explained by contamination rather than retention of surrogates by the abalone, random, inexplicable contamination events would have had to repeatedly affect the same animals without impacting their close neighbours, which is unlikely. Thus, these results suggest that abalone may be capable of retaining T. gondii oocysts in their digestive tissues for 13 days or more. This retention time is much higher than gut passage times reported for abalone fed glass microbeads, which ranged from 20 to 27·5 h (Garcia-Esquivel and Felbeck, Reference Garcia-Esquivel and Felbeck2009). This observation suggests that oocysts may be selectively retained within the abalone digestive system, extending the parasite retention time and risk of its transmission. To adequately measure oocyst retention time in abalone and correct for issues of contamination, a similar experiment could be performed implementing a longer monitoring period to ensure that all surrogates have been excreted prior to termination of the experiment.
In the surrogate control tub (D) containing kelp but no abalone, the concentration of surrogates was highest near the bottom of the tub, indicating that T. gondii surrogate microspheres tend to sink in the water column in low turbulence seawater. This result is in agreement with the findings of previous studies that examined the behaviour of T. gondii surrogates and oocysts in seawater and supports the hypothesis that oocysts can concentrate in the benthos (Shapiro et al. Reference Shapiro, Largier, Mazet, Bernt, Ell, Melli and Conrad2009, Reference Shapiro, Silver, Largier, Conrad and Mazet2012). After 24 h, an estimated 50% of all surrogates remained suspended in seawater, and an additional 30% had adhered to kelp surfaces at a density of 21 surrogates per cm2. This density was slightly lower in the abalone exposure tubs, most likely because abalone shells provided additional surface area for surrogates to adhere to and because some surrogates had been scraped off and consumed by abalone. Furthermore, abalone size was found to be correlated with mean kelp consumption and maximum fecal surrogate count, indicating that large abalone tended to eat more, which in turn resulted in greater likelihood of ingesting oocysts. These findings support the hypothesis that adherence of oocysts to kelp fronds facilitates ingestion by herbivorous invertebrates (Mazzillo et al. Reference Mazzillo, Shapiro and Silver2013).
Our finding that abalone can indeed serve as mechanical vectors for T. gondii provides indirect support for an alternative explanation for the difference in infection risk observed between snail specialists and abalone specialists: namely, that the former may be at higher risk simply due to differences in the number of prey items consumed. Our feasibility analysis of this hypothesis, informed by extensive data sets on prey consumption rates of snail specialists and abalone specialists, indicated that the discrepancy in reported infection rates (85% of snail specialists vs 7% of abalone specialists; Johnson et al. Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009) can be explained entirely by differences in the number of prey items consumed, even if each individual snail and abalone conferred the same risk of infection. This remarkable outcome reflects the enormous difference in caloric content between a snail (~4·6 kcal) and an abalone (~200 kcal; Oftedal et al. Reference Oftedal, Ralls, Tinker and Green2007), which means that a typical snail specialist will consume over 700 individual snails per day (in addition to other prey) to meet its energetic needs, as compared to an abalone specialist that consumes just 20 abalone. Of course the agreement between our feasibility analysis and empirical data on seroprevalence, while striking, does not provide confirmation for this hypothesis. It does, however, demonstrate that differences in infection risk between otters with different diets do not necessarily imply differences in the mechanical abilities of prey to concentrate and retain oocysts, but rather can occur as a by-product of numerical probabilities.
While the model above suggests that T. gondii exposure in sea otters can be largely influenced by caloric value and hence number of preferred prey that must be eaten, there may be additional factors that contribute to variation in levels of T. gondii contamination among different prey species. For example, one additional factor that may contribute to the probability of abalone vs snails encountering T. gondii under natural conditions is the difference in the feeding behaviour of these marine gastropods. This study focused on determining the physiological capability of abalone to serve as paratenic hosts for T. gondii, and as such the experimental animals were provided with an ample supply of kelp on which to feed. However, wild abalone living within sea otter habitat range are typically found in crevices or other refuges and feed opportunistically on drift algae, or occasionally on fronds of attached macroalgae that sway within their reach (Lowry and Pearse, Reference Lowry and Pearse1973; Tutschulte and Connell, Reference Tutschulte and Connell1988). As a result, the frequency at which they feed – and therefore the likelihood that they will encounter T. gondii oocysts – is governed by chance and the abundance of drift algae. In contrast, marine snails inhabit surfaces of kelp and are therefore able to graze continuously upon fronds or stipes (Watanabe, Reference Watanabe1984). The probability of accidental ingestion of T. gondii oocysts that become associated with kelp surfaces as described by Mazzillo et al. (Reference Mazzillo, Shapiro and Silver2013) is therefore likely to be greater for snails than for abalone that opportunistically consume drifting alga.
If abalone and marine snails tend to occupy distinct sites due to variations in habitat preference, then it is also hypothetically possible that the observed differences in infection risk could be explained by abalone tending to occupy coastal sites receiving less freshwater influence, and therefore with reduced likelihood of exposure to T. gondii oocysts. To our knowledge, there is no data currently available on the prevalence of T. gondii oocyst contamination amongst abalone or snails. However, Johnson et al. (Reference Johnson, Tinker, Estes, Conrad, Staedler, Miller, Jessup and Mazet2009) emphasize that both abalone and snail specialist otters were observed feeding within the identified high-risk coastal segment with no obvious spatial segregation or difference in foraging microhabitats. It therefore seems unlikely that differences in spatial distribution of prey species are responsible for observed variations in infection risk. Still, field studies quantifying the prevalence of T. gondii oocyst contamination amongst different prey species would be useful to elucidate the dynamics of T. gondii oocyst transport in the marine environment. Furthermore, such data could be used as input variables for estimating per-item infection risk used in our feasibility analysis and hence enhance the accuracy of T. gondii infection risk modelling.
To test the hypothesis that abalone and snail contamination with T. gondii is influenced by differential coastal habitat distribution, species population surveys could be performed to assess whether abalone and snails occupy the distinct nearshore sites. If this hypothesis is supported, prey and water samples could be collected from sites representative of typical habitat for each species and tested for T. gondii oocysts. However, one limitation of this approach is that currently, successful detection of T. gondii oocysts in seawater has not been reported, despite our team's effort to do so over duration of 3 years. To test for variations in oocyst exposure due to differences in feeding behaviour, animals could also be collected from a high-risk site occupied by both species. Validation of laboratory methods for detection of T. gondii oocysts in snails have been recently reported (Krusor et al. Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015), though such methods have not been tested to date in abalone. Ideally, samples of invertebrates would be collected during the wet season as well as the dry season to assess seasonal fluctuations in oocyst contamination rate due to rainfall and surface runoff. However, the practicality of conducting such a study is highly challenging. Although Staggs et al. (Reference Staggs, Keely, Ware, Schable, See, Gregorio, Zou, Su, Dubey and Villegas2015) reported T. gondii prevalences of 33 and 54% for small samples of mussels, Miller et al. (Reference Miller, Miller, Conrad, James, Melli, Leutenegger, Dabritz, Packham, Paradies, Harris, Ames, Jessup, Worcester and Grigg2008) detected only one T. gondii-positive mussel out of 1396 tested invertebrates collected from central coastal California, while Krusor et al. (Reference Krusor, Smith, Tinker, Silver, Conrad and Shapiro2015) reported no positives out of 227 sampled marine snails. Low T. gondii contamination prevalences suggest that a sample size of several thousand abalone and snails would be necessary to adequately measure and compare T. gondii contamination in these species, which could be particularly problematic given the protected status of central California abalone populations.
Another factor that may impact the likelihood of abalone acting as paratenic hosts for T. gondii under natural conditions in California is the presence of withering syndrome (WS), an important abalone pathogen in this state. Clinical signs of WS include reduced feeding and withering of the foot muscle, which reduces the animal's ability to attach firmly to the substrate (Moore et al. Reference Moore, Robbins and Friedman2000; Crosson et al. Reference Crosson, Wight, VanBlaricom, Kiryu, Moore and Friedman2014). Reduced feeding would diminish the probability that abalone will obtain kelp and thus ingest T. gondii oocysts, while an inability to firmly attach to substrates would increase its vulnerability to predation, creating a scenario in which abalone that suffer from WS are less likely to contain oocysts and are more likely to be preyed upon by sea otters. While WS-induced mass mortality has only been recorded in warmer waters of southern California, particularly the Channel Islands, clinical disease has been reported as far north as San Francisco, and the infectious agent of WS is distributed from Baja California to Sonoma County, California (Crosson et al. Reference Crosson, Wight, VanBlaricom, Kiryu, Moore and Friedman2014), an area that encompasses the entirety of the southern sea otter's current range.
One limitation in the current study is that due to utilization of T. gondii surrogates to mitigate the biohazard nature of working with viable parasites in large aquaria, the effects of passage through abalone digestive tract on oocyst viability could not be evaluated. It is likely that the robust nature of oocysts renders them resistant to inactivation within abalone, especially as oocysts have been shown to remain infectious after passing through the digestive systems of other marine invertebrates such as mussels and oysters (Arkush et al. Reference Arkush, Miller, Leutenegger, Gardner, Packham, Heckeroth, Tenter, Barr and Conrad2003; Lindsay et al. Reference Lindsay, Collins, Mitchell, Wetch, Rosypal, Flick, Zajac, Lindquist and Dubey2004). However, experiments using viable T. gondii oocysts and mouse bioassays would be needed to definitively evaluate the infectivity of oocysts after passage through the abalone gut.
The ability of abalone to concentrate and retain T. gondii also has important implications for public health, as it demonstrates that this commonly consumed seafood species is capable of harbouring an important zoonotic parasite of humans. Consumption of undercooked meat containing T. gondii tissue cysts has been a major source of human toxoplasmosis in the past, but studies of seroprevalence in livestock have demonstrated that T. gondii infections in meat-producing animals have been declining (Dubey and Jones, Reference Dubey and Jones2008). However, consumption of raw oysters, mussels and clams has been shown to be a significant risk factor for human T. gondii infection (Jones et al. Reference Jones, Dargelas, Roberts, Press, Remington and Montoya2009). If abalone can serve as transport hosts for T. gondii oocysts, then raw abalone could also be an important source of T. gondii infection for humans in spite of its apparent ‘protective’ effect in sea otters, particularly when harvested in large numbers by commercial fisheries.
Considering food web-driven disease transmission mechanisms in the ocean is important for conservation policy and management of both sea otters and abalone. In addition to both being threatened, sea otters and abalone have strong predator–prey interactions. Modelling-based studies have demonstrated that these interactions must be taken into account to set and achieve reasonable recovery goals for both species (Chadès et al. Reference Chadès, Curtis and Martin2012). However, Raimondi et al. (Reference Raimondi, Jurgens and Tinker2015) emphasized the importance of pairing such studies with field-collected data to avoid oversimplifying complex trophic relationships when devising multispecies management plans. The role of abalone in reducing sea otters' risk of exposure to T. gondii adds an additional dimension to their predator–prey interaction and could be incorporated into simulations used to evaluate the effectiveness of wildlife and fisheries management decisions. Because this study indicates that the protective effect of an abalone-rich diet is more likely due to their size and caloric content, rather than a species-specific inability to concentrate T. gondii oocysts, supporting stocks of abalone as well as other large, high-value prey items may reduce the proportion of sea otters relying on low-quality prey animals, such as snails.
Given that even low-risk prey may harbour oocysts, results suggest that focusing on the underlying issue of coastal water pollution is vitally important for sea otter conservation efforts. Wetland vegetation has been shown to remove T. gondii oocysts from runoff water (Hogan et al. Reference Hogan, Daniels, Watson, Oates, Miller, Conrad, Shapiro, Hardin, Dominik, Melli, Jessup and Miller2013; Daniels et al. Reference Daniels, Hogan, Smith, Oates, Miller, Hardin, Shapiro, Huertos, Conrad, Dominik and Watson2014), and coastal development and degradation of estuarine wetlands have been associated with increased transport of T. gondii oocysts from land to sea (Shapiro et al. Reference Shapiro, Conrad, Mazet, Wallender, Miller and Largier2010b ). Management measures aimed at reducing delivery of contaminants such as T. gondii oocysts to the marine environment via overland runoff could therefore include protection of estuarine wetland habitats, as well as encouraging sustainable ‘green’ urban planning in coastal areas that are slated for development (Cook, Reference Cook2007; Shapiro, Reference Shapiro2012). An additional management strategy for reducing environmental contamination with T. gondii could include effective education of cat owners regarding proper fecal waste disposal and improved control of feral cat populations (Shapiro, Reference Shapiro2012). These measures are especially important because the emergence of T. gondii in marine mammals is indicative of broader conservation and public health issues associated with land-to-sea pathogen pollution. Other disease-causing microorganisms, including Sarcocystis neurona – another encephalitis-inducing coccidian parasite – and several zoonotic enteric bacterial pathogens, have recently been observed in sea otters (Miller et al. Reference Miller, Byrne, Jang, Dodd, Dorfmeier, Harris, Ames, Paradies, Worcester, Jessup and Miller2010a , Reference Miller, Conrad, Harris, Hatfield, Langlois, Jessup, Magargal, Packham, Toy-Choutka, Melli, Murray, Gulland and Grigg b ). Findings of this study suggest that continued research into disease transmission processes in the ocean can inform implementation of policies aimed at reducing pollution of marine ecosystems with terrestrial pathogens, protecting the health of nearshore habitats and mitigating potential public health threats.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0031182016001359.
ACKNOWLEDGEMENTS
We would like to thank Dr James Gould for his advising and support. We thank David Dann for his logistical assistance with collection of kelp for study animals. We thank Michelle Staedler, Joe Tomoeoni, Gena Bentall, Jess Fujii, and dozens of other employees and volunteers from the Monterey Bay Aquarium and U.S. Geological Survey for contributing data on sea otter diets and foraging behaviour. Any use of trade, product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. government.
FINANCIAL SUPPORT
Funding for this work was provided by the National Science Foundation (NSF) Ecology of Infectious Disease programme (grant no. OCE-1065990) (to K. S., C. K. and P. A. C.); the Princeton University Office of the Dean of the College (to K. C. S.); U.S. Geological Survey Western Ecological Research Center; and the Department of Ecology and Evolutionary Biology John T. Bonner Senior Thesis Fund (to K. C. S.).







