Introduction
Several biotic factors, such as beneficial microorganisms, infection by microbial pathogens or infestation by insect pests (Kessler & Baldwin, Reference Kessler and Baldwin2002; Dicke & Hilker, Reference Dicke and Hilker2003; Pozo et al., Reference Pozo, Van Loon and Pieterse2004), can induce resistance in plants against diseases and insect pests (van der Ent et al., Reference van der Ent, Koornneef, Jurriaan and Pieterse2009; Cohen et al., Reference Cohen, Rubin and Kilfin2010). Induced resistance can also be activated by chemicals such as β-aminobutyric acid (BABA), a non-protein amino acid (Zimmerli et al., Reference Zimmerli, Jakab, Métraux and Mauch-Mani2000). BABA has been documented to induce plant resistance mechanisms in the following host/pest systems: Bremia lactucae, an Oomycete, on lettuce (Cohen et al., Reference Cohen, Rubin and Kilfin2010, Reference Cohen, Rubin and Vaknin2011); Phytophthora infestans on tomatoes (Cohen et al., Reference Cohen, Niderman, Mösinger and Fluhr1994); Acyrthosiphon pisum (Harris), a pea aphid, on legumes (Hodge et al., Reference Hodge, Thompson and Powell2005); Myzus persicae (Sulz.), Brevicoryne brassicae (L.), Trichoplusia ni (Hübner) and Plutella xylostella (L.) on several species in the Brassicaceae (Hodge et al., Reference Hodge, Pope, Holaschke and Powell2006); and Heterodera avenae Woll., Heterodera latipons and Meloidogyne sp., which are plant parasitic nematodes, on wheat and barley (Oka & Cohen, Reference Oka and Cohen2001). In addition, BABA is known to induce resistance against abiotic stresses such as drought, heat, salinity and acid rain (Jakab et al., Reference Jakab, Cottier, Toquin, Rigoli, Zimmerli, Métraux and Mauch-Mani2001; Liu et al., Reference Liu, Jiang, Shi, Chen, Pei and Zheng2011).
BABA induces resistance in plants through a number of physical and biochemical mechanisms (Cohen, Reference Cohen2002). Accumulation of pathogenesis-related (PR) proteins is one of the responses elicited by the application of BABA to plants (Cohen et al., Reference Cohen, Niderman, Mösinger and Fluhr1994; Hwang et al., Reference Hwang, Sunwoo, Kim and Kim1997). Expression of systemic acquired resistance (SAR) in plants as a result of BABA application is related to elevated expression levels of the PR-1, PR-2, and PR-5 genes (Jakab et al., Reference Jakab, Cottier, Toquin, Rigoli, Zimmerli, Métraux and Mauch-Mani2001). BABA-related elevation of PR protein levels can occur in tomato, pepper and tobacco plants in the absence of biotic stress by a plant pathogen (Cohen et al., Reference Cohen, Niderman, Mösinger and Fluhr1994; Hwang et al., Reference Hwang, Sunwoo, Kim and Kim1997), whereas in crucifers, PR protein levels were elevated only after the addition of biotic stress of pathogen infection (Zimmerli et al., Reference Zimmerli, Jakab, Métraux and Mauch-Mani2000, Reference Zimmerli, Métraux and Mauch-Mani2001; Silue et al., Reference Silue, Pajot and Cohen2002). BABA also increases lignin and callose content in foliar tissues and vascular walls, respectively, which can adversely affect feeding by herbivores (Cohen et al., Reference Cohen, Reuveni and Baider1999; Hamiduzzaman et al., Reference Hamiduzzaman, Jakab, Barnavon, Neuhaus and Mauch-Mani2005). Another physical change imposed by BABA is the alteration of nutrient composition and dry-matter content in the host plant (Hodge et al., Reference Hodge, Thompson and Powell2005). However, little is known about the role of BABA on the level of PR proteins and plant nutrients that are known to influence the development of induced resistance against insect pests.
The Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), is one of the most economically important citrus pests, which inflicts both direct and indirect damage to citrus trees (Halbert & Manjunath, Reference Halbert and Manjunath2004; Tiwari et al., Reference Tiwari, Lewis-Rosenblum, Pelz-Stelinski and Stelinski2010). Direct damage by D. citri adults and nymphs includes excessive honeydew production causing growth of sooty mold, notched and curled leaves, and death of terminal growth points (Halbert & Manjunath, Reference Halbert and Manjunath2004). Indirect damage results from the ability of D. citri to transmit Candidatus, Liberibacter asiaticus (Las), the presumed causal agent of huanglongbing (HLB), or greening disease, in citrus (Garnier et al., Reference Garnier, Danel, Bové, Garnsey, Timmer and Dodds1984; Jagoueix et al., Reference Jagoueix, Bové and Garnier1996). Currently, there is no cure for HLB. Management programmes for HLB include aggressive management of D. citri, the use of disease-free planting materials and removal of infected trees (Halbert & Manjunath, Reference Halbert and Manjunath2004).
Insecticides are currently the most effective management tools for D. citri and associated HLB, but D. citri populations are developing resistance to insecticides (Tiwari et al., Reference Tiwari, Mann, Rogers and Stelinski2011a, Reference Tiwari, Pelz-Stelinski and Stelinskib, Reference Tiwari, Gondhalekar, Mann, Scharf and Stelinskic, Reference Tiwari, Stelinski and Rogers2012a, Reference Tiwari, Clayson, Kuhns and Stelinskib). Therefore, it is important to investigate alternative management options that are environmentally friendly and sustainable in the long term. Induced host plant resistance may be an alternative method for managing D. citri. We therefore investigated the role of BABA in inducing resistance against D. citri in citrus. The effects of drench and foliar applications of BABA were measured by the number of eggs, nymphs and adults that were produced on treated plants as compared with untreated plants. In addition, direct exposure bioassays were performed on D. citri adults and nymphs to determine possible direct toxicity of BABA. Levels of various micro- and macronutrients were also compared between control plants and those exposed to BABA. Finally, we investigated whether changes in the expression of the PR-2 gene, an indication of SAR induction, occurred as a result of BABA application alone or in combination with D. citri feeding on plants.
Materials and methods
Plant material
Plants used were 2–3-month-old ‘Swingle citrumelo’ (Citrus paradisi Macf. x Poncirus trifoliata L. Raf.), purchased from a commercial nursery. Plants were potted in 6-inch-diameter plastic pots containing a potting mixture of custom citrus mix (Conrad Fafard, Inc., Agawam, MA, USA). Plants were maintained under greenhouse conditions at 27–28 °C, 60–65% R.H. and a 14:10 (L:D) photoperiod for 2 weeks prior to the onset of experiments.
D. citri culture
The D. citri used in this study were obtained from laboratory colonies continuously reared at the Citrus Research and Education Center, University of Florida, Lake Alfred, FL, USA. The culture was established in 2000 using field populations collected in Polk Co., FL, USA (28.0′N, 81.9′W) prior to the discovery of HLB in the state. It is maintained in a greenhouse at 27–28 °C, 60–65% R.H. and a 14:10 (L:D) photoperiod. D. citri are maintained on sweet orange [Citrus sinensis (L.) Osbeck] plants without exposure to insecticides and are confirmed free of the Las bacterium by using polymerase chain reaction (PCR) methods as described in Tiwari et al. (Reference Tiwari, Lewis-Rosenblum, Pelz-Stelinski and Stelinski2010).
Effect of BABA drench application on developmental stages of D. citri and plant nutrients
The objective of these experiments was to determine whether a soil drench application of BABA (Sigma-Aldrich, Inc., St Louis, MO, USA; purity 97%) affected development of D. citri on citrus. The first experiment was conducted between 6 May 2010 and 3 June 2010. The treatments tested were 25, 50 or 100 mM of BABA dissolved in distilled water compared with a water only control. A second complementary experiment was conducted between 19 August 2010 and 17 September 2010. The treatments compared were 25, 100, 200 or 500 mM of BABA compared with a water control. Each experiment was arranged in a randomized complete block design and there were seven and ten replicates during the first and second experiments, respectively. Each BABA concentration was prepared in distilled water, and 25 ml of each concentration was applied as a drench to each plant. Plants were allowed to prime for 3 days after drenching under controlled greenhouse conditions. Thereafter, five male and five female D. citri adults of similar age were released onto each plant for 5 days. Each plant was covered with a perforated plastic cup to contain the D. citri adults. After 5 days, all of the adults were removed from each plant, and the number of eggs per plant was counted. Subsequently, the numbers of nymphs and adults produced per plant were counted semiweekly. Plants were observed for signs of phytotoxicity throughout the experiment.
At the termination of the second experiment, eight plants that received either 500 mM of BABA or water control were randomly selected for macro- and micronutrient analysis. Plant tissues were washed, air-dried and ground to pass through a 0.38-mm sieve. A plant tissue sample comprising approximately 3 g plant material was shipped to Waters Agricultural Laboratories, Inc. (Camilla, GA, USA) for nutrient analysis. Phosphorus, potassium, calcium, magnesium, sulfur, manganese, iron, copper, zinc, boron, silicon, sodium, molybdenum, nitrate, aluminum and chlorine concentrations were determined by inductively coupled plasma atomic emission spectroscopy.
For each experiment, the total number of eggs, nymphs and adults per plant were analyzed separately by analysis of variance (ANOVA) (PROC GLM) using BABA concentration as the main effect, followed by Fisher's protected LSD mean separation (SAS, 2004). The level of each nutrient was compared between BABA-treated and control plants using ANOVA, followed by Fisher's protected LSD mean separation (SAS, 2004).
Effect of BABA foliar application on developmental stages of D. citri
The objective of this experiment was to examine the effects of foliar applications of BABA on developmental stages of D. citri. The experiment was conducted twice between 8 March 2011 and 22 April 2011, and between 6 May 2011 and 3 June 2011. Each experiment consisted of the following treatments: 5 mM BABA; 625 μl l−1 of imidacloprid (Provado 1.6 F, Bayer CropScience, Research Triangle Park, NC, USA) (positive control); or water (negative control). Each experiment was arranged as a randomized complete block design with ten replicates of each treatment. Imidacloprid was used at the full (625 μl l−1) recommended label rate against D. citri. BABA and imidacloprid were prepared in distilled water. Twenty-five milliliters of each treatment were applied as a foliar application until runoff using a handheld atomizer (The Bottle Crew, West Bloomfield, MI, USA). Plants were allowed to prime for 3 days after treatment application under controlled greenhouse conditions. Thereafter, five male and five female D. citri adults of similar age were released onto each plant and held for 5 days. Each plant was covered with a perforated plastic cup to contain the adults. Thereafter, all of the adults were removed from each plant, and the number of eggs per plant was counted. Subsequently, the numbers of nymphs and adults produced per plant were counted semiweekly. Plants were observed for any signs of phytotoxicity throughout the experiment. Owing to the lack of a significant effect of time on the main response variables, data from both experiments were pooled to analyze the total number of eggs, nymphs and adults per plant using ANOVA, followed by Fisher's protected LSD mean separation (PROC GLM) (SAS, 2004).
D. citri toxicity bioassay
The direct toxicity of four concentrations of BABA (25, 100, 200 and 500 mM) on early (second) and late (fifth) instar nymphs, and adults was determined using a Petri-dish bioassay method (Prabhaker et al., Reference Prabhaker, Castle, Byrne, Henneberry and Toscano2006; Tiwari et al., Reference Tiwari, Pelz-Stelinski and Stelinski2011b) and compared with water alone (negative control) and one-tenth of the recommended label rate of imidacloprid (positive control). The bioassay arena consisted of 60-mm-diameter plastic disposable Petri-dishes (Fisherbrand, Thermo Fisher Scientific, Waltham, MA, USA) containing a 2–3 mm thick solidified bed of 1.5% agar solution. Leaf disks (60 mm diameter) from fresh citrus leaves were excised, dipped for 30 s in BABA solutions made in water and allowed to air dry in a fume hood for 1 h prior to bioassays. For the negative control treatment, leaf disks were dipped in distilled water alone. For the positive control treatment, leaf disks were dipped in an imidacloprid formulation dissolved in distilled water. After 1 h, leaf disks were placed on agar beds and 20–30 nymphs or adults were transferred into each dish using a camel hair brush. The adults were briefly anesthetized with CO2 to facilitate handling and transfer. Petri-dishes were wrapped with parafilm (Pechiney Plastic Packaging, Chicago, IL, USA) to prevent the escape of psyllids. Sealed Petri-dishes with nymphs or adults were transferred into a growth chamber (Percival Scientific, Inc., Perry, IA, USA) set at 25±1 °C, 50±5% RH and 14:10 (L:D) photoperiod. Each BABA concentration was replicated three times (n = 30–45 D. citri per concentration) and the entire experiment was repeated. The mortality of nymphs or adults was assessed 48 h after placement into the growth chamber. Adults found on their side or back that were unable to move when probed with a camel hair brush were considered dead. Nymphs found flaccid, dried, light colored and unable to move when probed with a camel hair brush were considered dead. Percent mortality between treatments was compared separately for each developmental stage using ANOVA (PROC GLM), followed by Fisher's protected LSD mean separation (SAS, 2004).
PR-2 gene (β-1,3-glucanase) expression levels in BABA-treated plants
The expression level of the PR-2 gene was evaluated in citrus after receiving one of the following four treatments: BABA application (500 mM); BABA application (500 mM) on plants exposed to D. citri feeding; plants exposed to D. citri feeding alone; and a control (no exposure of plants to D. citri or BABA). Each treatment was replicated three times. This experiment was conducted using potted ‘Swingle’ plants under controlled conditions on 18 October 2011. Each potted plant was covered with a perforated plastic cup to contain adults on the plants and to prevent external infestation. In the treatment where plants were exposed to D. citri adults, five pairs of similarly aged D. citri adults were released onto each plant. After 5 days, adults were removed from the plants, and 25 ml of 500 mM BABA solution was applied as a drench to each plant receiving treatments of BABA. Control plants or those receiving D. citri alone were treated with 25 ml of water alone. Plants were allowed to prime for 3 days. Thereafter, leaves from each plant were used to evaluate PR-2 gene expression levels. Total RNA was extracted from 200 mg of tissue from each treatment using the RNeasy Mini Kit for plant tissue (Qiagen, MD, USA). The quality and quantity of RNA from each sample was measured on a NanoDrop 1000 Spectrophotometer using the A 260 and the A 260/A 280 ratio to ensure uniform quality and quantity (100 ng μl−1) among all treatments for subsequent real-time reverse transcription PCR (RT–PCR) analysis. One microliter of RNA sample was used for RT–PCR using β-1,3-glucanase primers (Forward = TTCCACTGCCATCGAAACTG; Reverse = TGTAATCTTGTTTAAATGAGCCTCTTG) (Francis et al. Reference Francis, Redondo, Burns and Graham2009). RT–PCR reactions were conducted using a Power SYBR Green Reverse Transcription kit (Applied Biosystems, Foster City, USA) and a temperature cycle consisting of 48 °C for 30 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Three biological replicates were used per treatment.
To compare relative expression of the PR-2 gene among treatments, we used the 2−ΔΔCT method (Livak & Schmittgen, Reference Livak and Schmittgen2001) by normalizing to expression of the plant cytochrome oxidase (COX) gene (Forward = GTATGCCACGTCGCATTCCAGA; Reverse = GCCAAAACTGCTAAGGGCATT) (Li et al. Reference Li, Hartung and Levy2006), followed by normalization to the treatment giving the lowest gene expression. ANOVA was performed to compare the relative expression of the PR-2 gene among plants receiving various treatments (PROC GLM) (SAS, 2004).
Results
Effect of BABA drench applications on developmental stages of D. citri and plant nutrients
In the first experiment, BABA significantly affected the mean numbers of eggs laid (F = 6.76; df = 3, 36; P = 0.0010), nymphs (F = 3.73; df = 3, 36; P = 0.0196) and adults produced (F = 5.39; df = 3, 36; P = 0.0036) per plant (fig. 1a–c). The mean numbers of eggs (fig. 1a), nymphs (fig. 1b) and adults (fig. 1c) on plants receiving 25 mM BABA were significantly reduced by over 50%, 35% and 42%, respectively, compared with control plants. In the second experiment, BABA significantly affected the mean numbers of eggs laid (F = 3.51; df = 4, 30; P = 0.0182), nymphs (F = 3.39; df = 4, 30; P = 0.0212) and adults produced (F = 2.87; df = 4, 30; P = 0.0400) per plant (fig. 2a–c). The mean number of eggs laid per plant was significantly reduced by over 72% on plants receiving 25 mM BABA compared with control plants (fig. 2a). The mean numbers of nymphs and adults were reduced by over 60% and 84% on plants receiving 100 and 200 mM BABA, respectively, when compared with control plants (fig. 2b, c). During both experiments, the control plants had significantly higher numbers of eggs, nymphs and adults per plant when compared with the highest concentrations of BABA tested (figs 1a–c and 2a–c).

Fig. 1. Mean number of D. citri eggs (A), nymphs (B) and adults (C) produced per citrus plant, following drench application of BABA during spring 2010.

Fig. 2. Mean number of D. citri eggs (A), nymphs (B) and adults (C) produced per citrus plant, following drench application of BABA during summer 2010.
Nutrient analysis revealed that levels of iron (F = 9.01; df: 1,14; P = 0.0095), magnesium (F = 5.03; df: 1,14; P = 0.0416), phosphorus (F = 29.07; df: 1,14; P < 0.0001), sodium (F = 13.10; df: 1,14; P = 0.0028), sulfur (F = 7.78; df: 1,14; P = 0.0145) and zinc (F = 12.71; df: 1,14; P = 0.0031) were significantly higher in untreated control plants compared with BABA-treated plants (table 1). No significant difference was found in levels of aluminum, boron, calcium, chloride, copper, manganese, molybdenum, potassium and silica between untreated control and BABA-treated plants (P > 0.05) (table 1).
Table 1. Levels of major macro- and micronutrients for BABA-treated (500 mM) and untreated control citrus plants.

Mean values for each nutrient followed by the same letter within a row are not significantly different (P < 0.05).
Effect of BABA foliar applications on developmental stages of D. citri
Treatments involving foliar applications of BABA, imidacloprid (full recommended rate) and water alone were found to significantly affect the mean numbers of eggs (F = 11.09; df = 2, 57; P < 0.0001), nymphs (F = 12.66; df = 2, 57; P < 0.0001) and adults (F = 15.19; df = 2, 57; P < 0.0001) per plant (fig. 3). A 5 mM BABA alone treatment significantly reduced the number of adults, but not eggs or nymphs when compared with the control treatment. BABA treatments reduced egg, nymph and adult production by 24%, 33% and 60%, respectively, when compared with the control. The imidacloprid positive control reduced egg, nymph and adult production by 96%, 96% and 83%, respectively, when compared with water alone (negative control).

Fig. 3. Mean number of D. citri eggs (A), nymphs (B) and adults (C) produced per citrus plant, following foliar application of BABA. BABA was applied at the concentration of 5 mM and imidacloprid was applied at the recommended label rate of 625 μl l−1.
D. citri toxicity bioassay
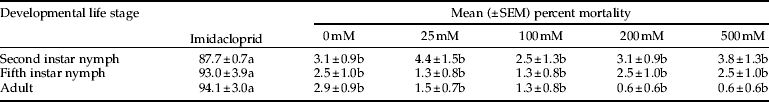
BABA treatments did not cause mortality of D. citri as compared with the control (table 2). Mortality of 2nd instar (F = 362.17; df = 5, 37; P < 0.0001), 5th instar (F = 543.85; df = 5, 37; P < 0.0001) and adult (F = 827.24; df = 5, 37; P < 0.0001). D. citri was significantly greater in treatments receiving imidacloprid (positive control) as compared with the BABA or water (negative control) treatments (table 2).
Table 2. Mean percent mortality of three developmental stages of Asian citrus psyllid, D. citri, following direct contact with BABA or imidacloprid.
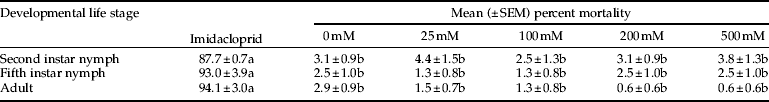
Mean mortality percentage values followed by the same letter within each developmental stage (row) are not significantly different (P < 0.05).
PR-2 gene (β-1,3-glucanase) expression levels in BABA-treated plants
The objective of this experiment was to determine whether exposure of citrus plants to D. citri adult feeding alone or in combination with BABA treatment induces expression of the PR-2 gene (fig. 4). Significant variation in the expression level of the PR-2 gene was observed among treatments (F = 6.84; df = 3, 20; P = 0.0024). Significantly higher expression of PR-2 was observed in plants exposed to 500 mM of BABA in combination with D. citri adult feeding when compared with the control or other treatments tested.

Fig. 4. Relative expression levels of the PR-2 gene in plants exposed to BABA alone (500 mM); BABA (500 mM) and D. citri feeding; D. citri feeding alone; and control (no exposure of plants to D. citri or BABA). C t values obtained from real-time RT–PCR were first normalized to the reference gene, plant COX, followed by normalization to the treatment giving the lowest gene expression using the 2−ΔΔCT method. Values sharing the same letter are not significantly different (P < 0.05; Fisher's protected LSD).
Discussion
The current results demonstrate that treating citrus plants with BABA can negatively impact all three developmental stages of D. citri. During the first experiment, drench applications with as little as 25 mM of BABA significantly reduced the number of eggs, nymphs and adults per plant. However, during the second drench application experiment, 25 mM of BABA significantly reduced the number of eggs, while 100 and 200 mM of BABA reduced the number of adults and nymphs surviving, respectively, compared with the control treatment. Foliar application of BABA was more effective than drench application, with respect to reducing the number of adults with 5 mM of BABA. Our results indicate that the application method affects the impact of BABA on development of D. citri.
The mechanism(s) of BABA-induced resistance in plants against insects are not well understood in general. Therefore, we sought to determine whether BABA was directly toxic to D. citri. Our results proved no direct toxicity of BABA against any of the developmental stages of D. citri tested suggesting an induced resistance response by citrus. The lack of acute toxicity found in this study has also been reported for BABA in other insect species such as aphids, A. pisum and M. persicae (Hodge et al., Reference Hodge, Thompson and Powell2005), and plant pathogenic nematodes, H. avenae, H. lapitons and Meloidogyne sp. (Oka & Cohen, Reference Oka and Cohen2001). The deleterious effects of BABA on development of D. citri might be related to decreased nutritional quality of host plants given significantly lower levels of iron, magnesium, phosphorus, sodium, sulfur and zinc in BABA-treated plants as compared with control plants. BABA-treated Vicia faba L. plants exhibit altered dry-matter content and C, N and H content of leaves, which detrimentally impacts production of A. pisum (Hodge et al., Reference Hodge, Pope, Holaschke and Powell2006). It has been speculated that BABA blocks translocation of nutrients from plant cells to fungal cells, thereby reducing fungal infection (Steiner & Schönbeck, Reference Steiner, Schönbeck, Hartleb, Heitefuss and Hopp1997). Similarly, certain non-protein amino acids cause nitrogen to be stored in a form that is metabolically unavailable to herbivores (Huang et al., Reference Huang, Jander and de Vos2011). The results of this study suggest that lower levels of certain macro- and micronutrients as a result of BABA treatment may be responsible for reduced numbers of D. citri nymphs and adults. Las-infected citrus was characterized by lower levels of nitrogen, phosphorus, sulfur, zinc and iron, which are known to have negative effects on host selection behavior of D. citri (Mann et al., Reference Mann, Ali, Hermann, Tiwari, Pelz-Stelinski, Alborn and Stelinski2012). Several other studies have shown that the growth and development of phytophagous insects depend largely on concentrations of various micronutrients and their relative ratios (Clancy et al., Reference Clancy, Wagner and Tinus1988; Clancy & King, Reference Clancy and King1993; Beanland et al., Reference Beanland, Phelan and Salminen2003). Therefore, it is possible that reduced fitness of D. citri on BABA-treated plants as a result of SAR induction could supplement the need for insecticide application if population levels are sufficiently reduced. It is also possible that this could result in the need for fewer annual insecticide applications. This may be especially important given the recent emergence of insecticide resistance in populations of D. citri in the U.S. (Tiwari et al., 2011a).
Even the highest concentration of BABA (500 mM) tested in the present study caused no phytotoxic effects to citrus plants. The lack of phytotoxicity observed with citrus is similar to that seen with pea (Pisum sativa), broad bean (Vicia faba var. major), runner bean (Phaseolus coccineus), red clover (Trifolium pratense) and alfalfa (Medicago sativa) (Hodge et al., Reference Hodge, Thompson and Powell2005). However, BABA does cause necrosis in tobacco (Cohen & Gisi, Reference Cohen and Gisi1994; Siegrist et al., Reference Siegrist, Orober and Buchenauer2000). The application technique can influence phytotoxicity caused by BABA (Cohen, Reference Cohen1994; Cohen & Gisi, Reference Cohen and Gisi1994). BABA concentrations as low as 1 and 10 mM were found to cause necrotic lesions on tobacco when applied as foliar sprays (Cohen, Reference Cohen1994; Siegrist et al., Reference Siegrist, Orober and Buchenauer2000). However, tomato plants tolerate higher concentrations of BABA when applied as a soil drench (Cohen & Gisi, Reference Cohen and Gisi1994), because they systemically acquire only part of the applied volume. Citrus appears to tolerate BABA applications better than tobacco due to a lack of phytotoxicity after both drench and foliar applications.
Mechanisms for BABA-induced resistance in plants vary among plant families and pest/pathogen types (Cohen, Reference Cohen2002; Marcucci et al., Reference Marcucci, Aleandri, Chilosi and Magro2010). In grapes such as Vitis vinifera, BABA-induced resistance is mediated by the phenylpropanoid and jasmonic acid pathways (Hamiduzzaman et al., Reference Hamiduzzaman, Jakab, Barnavon, Neuhaus and Mauch-Mani2005; Slaughter et al., Reference Slaughter, Hamiduzzaman, Gindro, Neuhaus and Mauch-Mani2008). BABA is also known to induce resistance through enhanced expression of systemically acquired resistance (SAR) genes, which code for pathogenicity-related (PR) proteins (Cohen et al., Reference Cohen, Niderman, Mösinger and Fluhr1994; Hwang et al., Reference Hwang, Sunwoo, Kim and Kim1997; Hamiduzzaman et al., Reference Hamiduzzaman, Jakab, Barnavon, Neuhaus and Mauch-Mani2005). BABA-induced resistance in pepper, Capsicum annuum, occurs through the accumulation of PR proteins, such as β-1,3-glucanase, chitinase isoforms and other salicylic acid-dependent PR proteins (Hwang et al., Reference Hwang, Sunwoo, Kim and Kim1997). BABA-induced resistance is also regulated by the plant hormone, salicylic acid and the defense regulatory protein, NPR1 (Zimmerli et al., Reference Zimmerli, Jakab, Métraux and Mauch-Mani2000; Ton et al., Reference Ton, Jakab, Toquin, Flors, Lavicoli, Maeder, Metraux and Mauch-Mani2005). In citrus, SAR induction was explained by high expression of the PR-2 gene, which in turn relates to a higher resistance of citrus against canker (Francis et al., Reference Francis, Redondo, Burns and Graham2009). The results of the present study show that the PR-2 gene was up-regulated by more than 150-fold in citrus treated with BABA in combination with D. citri adult feeding compared with the control or citrus treated with BABA or D. citri feeding alone. These results suggest that PR proteins, or at least one PR protein in citrus, accumulates as a result of the combined effect of BABA and D. citri feeding. Our results corroborate findings of another study, where green peach aphid, M. persicae, feeding resulted in the activation of defense-related genes, including PR-1 and BGL2 in Arabidopsis (Moran & Thompson, Reference Moran and Thompson2001). Lack of elevated PR-2 gene expression in BABA treatment could have occurred because the concentration of BABA tested may have not been sufficiently high to cause gene expression. Alternatively, the interval between BABA treatment and our assay may have not been sufficiently long to cause PR-2 gene up-regulation. Therefore, optimizing the concentration and priming period of BABA is needed to further elucidate the mechanisms imparting induced resistance in citrus after treatment with BABA. Also, future investigation of the involvement of the jasmonic acid pathway in SAR in citrus is warranted, particularly as pertaining to induced resistance against D. citri.
Our results show that all three developmental stages of D. citri were negatively impacted by BABA through induction of host–plant resistance in citrus. These results suggest an additional viable alternative tool for current D. citri management programmes that heavily rely on insecticides. The synergistic effect of BABA mixed with imidacloprid or other commonly used insecticides requires investigation for the potential use of BABA as a tank-mix with other insecticides for managing D. citri. BABA may also be useful as a tank-mix with other pesticides, given its known synergistic effects with various plant activators and fungicides (Zhang et al., Reference Zhang, Reddy, Kokalis-Burelle, Wells, Nightengale and Kloepper2001; Cohen, Reference Cohen2002). Additional investigations are needed to optimize a cost-effective and efficacious dosage of BABA for management of D. citri under field conditions. The effects of BABA should also be investigated on other insect pests or diseases of citrus and/or other crops. The current results also suggest the potential for investigating other commercially available SAR-inducing products, such as 2,6-dichloroisonicotinic acid, benzothiadiazole, inorganic salts and salicylic acid, which may induce resistance in citrus against insect pests and pathogens. All the above-mentioned products are known to induce resistance in plants against various pests and pathogens (Edreva, Reference Edreva2004).
Acknowledgements
This project was supported by a grant from the USDA and Citrus Research and Development Foundation to L.L.S. We acknowledge A. Hoyte, Y. Cruz-Plemons, D. Diaz, M. Flores and S. Holladay for technical assistance.