Introduction
The species Masonhalea richardsonii (Hook.) Kärnefelt is a unique taxon, which forms a foliose, prostrate, cartilaginous thallus, growing unattached and rolling freely, that is, vagrant, on the tundra, where it is spread primarily by wind (Fig. 1A) (Kärnefelt Reference Kärnefelt1977, Reference Kärnefelt1979; Thomson Reference Thomson1984; Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). The unusual morphology attracted the attention of several lichenologists, resulting in its placement in several other genera, including: Cornicularia, Evernia, Everniopsis, Platysma and Parmelia (Kärnefelt Reference Kärnefelt1977). More recently, this taxon was segregated from Cetraria Ach., and the monotypic genus, Masonhalea Kärnefelt, was established based on several characters, including the presence of large pseudocyphellae, which form irregular patches on the lower surface of the thallus (Kärnefelt Reference Kärnefelt1977). Furthermore, the lateral position of the apothecia in M. richardsonii differs from that of Cetraria, where they appear submarginally on the upper side. In addition, the short cylindrical or bacillariform conidia of M. richardsonii differ from the longer and fusiform, oblong citriform conidia typical of Cetraria s. str. (Thell Reference Thell1995b ). Finally, the secondary chemistry of M. richardsonii is unusual among cetrarioid lichens, being characterized solely by alectoronic acid (Kärnefelt Reference Kärnefelt1977, Reference Kärnefelt1979; Kärnefelt & Thell Reference Kärnefelt and Thell1993). Early molecular studies based on ITS and β-tubulin sequence data, together with morphology, identified M. richardsonii as occupying an isolated position among the core of cetrarioid lichens (Thell et al. Reference Thell, Feuerer, Kärnefelt, Myllys and Stenroos2002a , Reference Thell, Stenroos, Feuerer, Kärnefelt, Myllys and Hyvönen b ; Mattsson & Articus Reference Mattsson and Articus2004). However, subsequent studies that included additional species from the cetrarioid clade (Thell et al. Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009; Nelsen et al. Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) demonstrated a sister group relationship between M. richardsonii and Tuckermannopsis inermis (Nyl.) Kärnefelt.
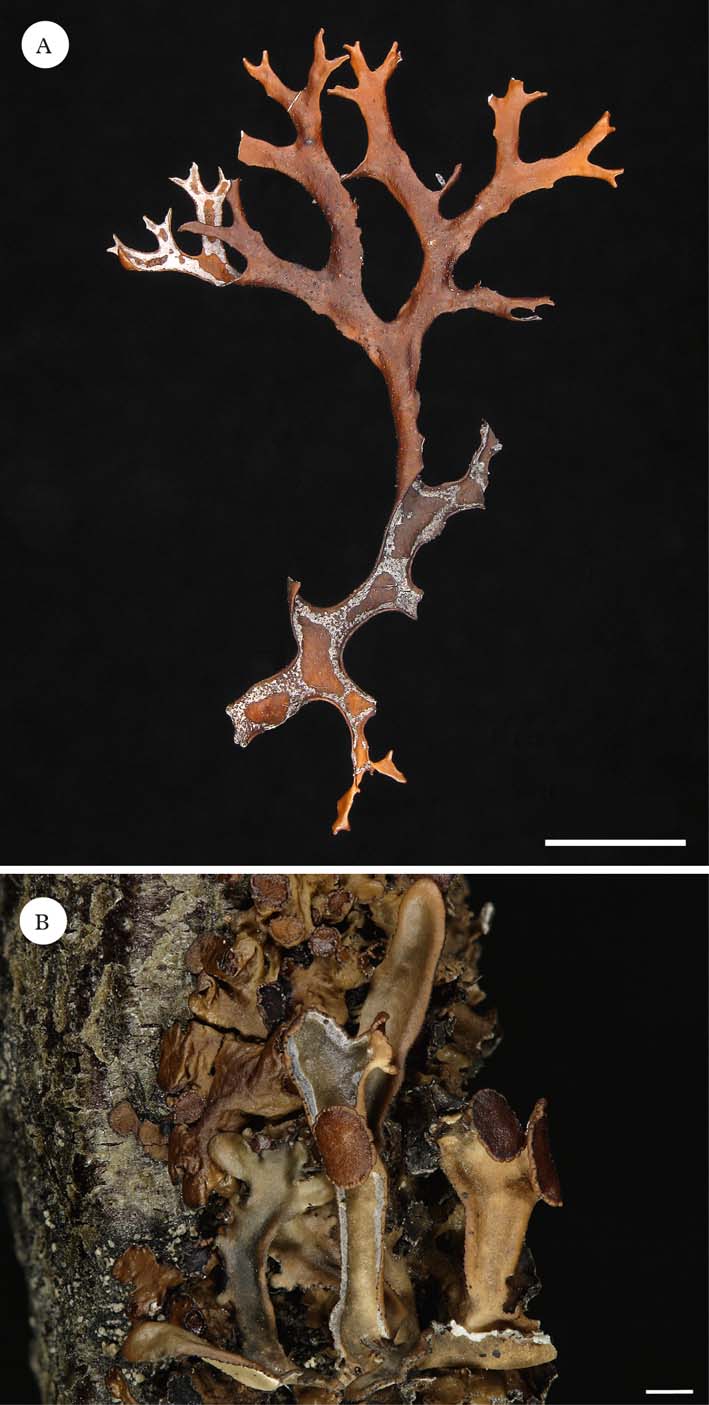
Fig. 1. A, Masonhalea richardsonii, Canada, Northwest Territories, Artillery Lake. J. W. Thomson & J. A. Larsen 92 (LD-1008288); B, Masonhalea inermis, USA, Alaska, Seward Peninsula, Nome area, I. Kärnefelt (LD-1035952).
Recently, the genus Masonhalea was expanded to include the species Masonhalea inermis (Nyl.) Lumbsch et al. (Thell & Moberg Reference Thell and Moberg2011). This species forms an erect, foliose to subfoliose thallus, which primarily occurs on twigs, but occasionally on soil (Krog Reference Krog1973; Kärnefelt Reference Kärnefelt1979; Thomson Reference Thomson1984; Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Thell & Moberg Reference Thell and Moberg2011). The fatty acids, protolichesterinic and lichesterinic acid, are produced in the medulla of M. inermis, both of which occur in a wide range of cetrarioid taxa (Kärnefelt Reference Kärnefelt1979; Kärnefelt & Thell Reference Kärnefelt and Thell1993). Masonhalea inermis (Fig. 1B) had previously been considered a form of Cetraria crispa (Ach.) Nyl., a synonym of C. ericetorum Opiz, where it was classified as Cetraria crispa f. inermis Nyl. Krog (Reference Krog1973) elevated it to the species level as Cetraria inermis (Nyl.) Krog. At this time, Krog (Reference Krog1973) noted the production of lateral apothecia by C. inermis and used this to infer a close relationship with Cetraria subalpina Imshaug, another species which she considered to produce lateral apothecia. However, Kärnefelt (Reference Kärnefelt1979) characterized the apothecia of C. inermis as marginal on lateral branches, a typical position for cetrarioid lichens, and stated that C. inermis and C. subalpina had few characters in common. Later, however, Kärnefelt et al. (Reference Kärnefelt, Mattsson and Thell1993) placed these two species together in Tuckermannopsis, based on the absence of a ring structure in the ascus and the production of subglobose ascospores. Subsequently, Thell et al. (Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009) demonstrated a distant relationship between these two taxa based on molecular sequence data, and both Thell et al. (Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009) and Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) have instead suggested a close relationship between M. inermis (as T. inermis) and M. richardsonii. The studies of Thell et al. (Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009) and Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) both suggested that M. inermis (as T. inermis) was not closely related to the type species of Tuckermannopsis, T. ciliaris (Ach.) Gyelnik. For taxonomy to reflect phylogeny, it became necessary to either describe a new genus for T. inermis or to place it in Masonhalea. This taxon was then transferred to Masonhalea by Lumbsch et al. (Thell & Moberg Reference Thell and Moberg2011); however, co-authors for the new combination were not included in that publication.
Here we highlight anatomical, morphological, chemical and ecological similarities between these two taxa and draw comparisons with variation present in other cetrarioid genera. In addition, we have obtained new DNA sequences from both M. richardsonii and M. inermis and find that the newly obtained sequences validate the originals, thereby supporting the monophyly of M. inermis and M. richardsonii.
Materials and Methods
Characterization of phenotype and habitat
A thorough summary of the morphological, anatomical, chemical, ecological, and biogeographical characteristics of these taxa, and other cetrarioid genera, was compiled. These data were drawn from numerous publications cited throughout the text and were used to compare and contrast the two Masonhalea species, as well as to discuss the variability of these characters in other cetrarioid genera.
Molecular phylogeny
We also sought to verify the apparent sister relationship between M. inermis and M. richardsonii. New ITS sequences were generated for two species: Masonhalea inermis, USA, Alaska, Copter Peak, Plot 4, C. Hampton-Miller 912 (LD), GenBank Acc. No. JQ361046; Masonhalea richardsonii, USA, Alaska, Steese Highway, S. A. Harris 4597 (F), GenBank Acc. No. JQ361047. GenBank accession numbers for other taxa are included in Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ). All DNA extraction, PCR reaction, purification, cycle-sequencing, precipitation and sequencing protocols follow Leavitt et al. (Reference Leavitt, Johnson and St. Clair2011) and Nelsen et al. (Reference Nelsen, Lücking, Umaña, Trest, Will-Wolf, Chaves and Gargas2007, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011b ). New ITS sequences were added to the full alignment (ITS, nuLSU, mtSSU, RPB1) of Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ); sequences were manually aligned, and ambiguous regions removed. A maximum likelihood analysis (ML) and Bayesian analysis were conducted in RAxML 7.2.8 (Stamatakis Reference Stamatakis2006) and MrBayes 3.1.2 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001), respectively. Substitution models and other settings for the analyses followed Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) and were performed using the CIPRES Science Gateway 3.1 (Miller et al. Reference Miller, Pfeiffer and Schwartz2010).
Results
We briefly highlight the similarities and differences between the Masonhalea species, and note the variability of these characters in other cetrarioid genera.
Growth form
One of the most noticeable differences between M. inermis and M. richardsonii is their growth form. Whereas both have foliose thalli with weakly channelled lobes, M. richardsonii produces prostrate, unattached thalli with irregularly to weakly dichotomously branching lobes, and M. inermis is characterized by an erect, foliose thallus that is attached to its substratum and produces unbranched to weakly branched lobes (Krog Reference Krog1973; Kärnefelt Reference Kärnefelt1977, Reference Kärnefelt1979; Thomson Reference Thomson1984; Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). Macroscopic growth form is known to be quite variable within the cetrarioid core. In some genera, such as Cetraria s. str. (Kärnefelt Reference Kärnefelt1979) and Nephromopsis (Randlane et al. Reference Randlane, Thell and Saag1995; Randlane & Saag Reference Randlane and Saag1998), growth form is conserved; in other genera, such as Cetrariella (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992) and Kaernefeltia (Thell & Goward Reference Thell and Goward1996) species vary greatly in their vegetative morphology. Furthermore, the species Cetraria aculeata varies from fruticose to foliose, depending on its habit (attached vs. vagrant) (Pérez-Ortega et al. Reference Pérez-Ortega, Fernández-Mendoza, Raggio, Vivas, Ascaso, Sancho, Printzen and de los Ríos2012). Consequently, macroscopic differences in thallus morphology are known within other genera, and even species within the cetrarioid core.
Upper cortex
The two Masonhalea species also possess different types of upper cortex. Masonhalea inermis produces a single-layered upper cortex composed of paraplectenchyma (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992, Reference Kärnefelt, Mattsson and Thell1993), whereas M. richardsonii has a bi-layered upper cortex, composed of a layer of pachydermatous paraplectenchyma produced above a layer of prosoplectenchyma (Kärnefelt Reference Kärnefelt1977; Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). The production of a bi-layered upper cortex is not restricted to M. richardsonii as several other cetrarioid species are known to produce a bi-layered upper cortex (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992, Reference Kärnefelt, Mattsson and Thell1993). We speculate that the thickened, and dense, bi-layered arrangement of cortical hyphae in M. richardsonii may be related to its vagrant lifestyle. For instance, a thickened cortex in Cetraria aculeata has been viewed as a response to its vagrant lifestyle (Pérez-Ortega et al. Reference Pérez-Ortega, Fernández-Mendoza, Raggio, Vivas, Ascaso, Sancho, Printzen and de los Ríos2012). Although the number of cortical layers is conserved in some cetrarioid genera, such as Allocetraria (Thell et al. Reference Thell, Randlane, Kärnefelt, Gao, Saag, Daniels, Schulz and Peine1995a ), Tuckermannopsis (Kärnefelt & Thell Reference Kärnefelt and Thell2001) and Vulpicida (Mattsson Reference Mattsson1993), it is known to be variable in others. For example, the genus Cetraria s. str. is composed of species with a uni-, bi- or even tri-layered cortex, and the number of cortical layers is even known to be variable within the species C. aculeata (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Pérez-Ortega et al. Reference Pérez-Ortega, Fernández-Mendoza, Raggio, Vivas, Ascaso, Sancho, Printzen and de los Ríos2012).
Pseudocyphellae
Both Masonhalea species bear conspicuous, confluent pseudocyphellae on the lower surface, albeit of different shapes and in different locations. In M. richardsonii they form irregular patches or areas, whereas in M. inermis they appear as a continuous line on a flat border along the margin (Fig. 1A). Some variation in the presence or absence of pseudocyphellae, as well as location, is known among other cetrarioid genera. The genus Cetreliopsis, which appears embedded in a paraphyletic Nephromopsis (supplementary Fig. 1; Thell et al. Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009; Nelsen et al. Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ), forms pseudocyphellae on both surfaces, while Nephromopsis has pseudocyphellae solely on the lower cortex (Randlane et al. Reference Randlane, Thell and Saag1995; Randlane & Saag Reference Randlane and Saag1998). The genus Allocetraria also exhibits variability with A. flavonigrescens lacking pseudocyphellae, and A. ambigua and A. sinensis developing them marginally (Thell et al. Reference Thell, Randlane, Kärnefelt, Gao, Saag, Daniels, Schulz and Peine1995a ). Finally, in the genus Cetraria, pseudocyphellae are situated on the lower cortex, but C. islandica is known to also produce marginal pseudocyphellae.
Ascomata
Both Masonhalea species bear apothecia in a lateral position. This is quite rare among cetrarioid lichens which usually have submarginal apothecia, most commonly laminal on the upper side along the margins, or marginally on the lower side but later turning upwards in nephromoid apothecia (Nephromopsis) (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992), suggesting that the lateral position of the apothecia is a synapomorphy of Masonhalea. The two Masonhalea species differ in the number of exciple layers present in their apothecia (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). Differences in this character are also known to occur within, for example, the genera Cetraria s. str. and Nephromopsis (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Randlane et al. Reference Randlane, Thell and Saag1995; Randlane & Saag Reference Randlane and Saag1998).
Hamathecium, asci and ascospores
The two Masonhalea species form short, straight and nearly unbranched paraphyses, which are thickened towards the tips (Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ). This type of paraphysis is shared with a wide range of other cetrarioid taxa including Allocetraria and Tuckermannopsis (Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ). Both Masonhalea species produce asci of the Lecanora-type (Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ); this ascus type is widespread among cetrarioid lichens, with numerous species also producing the Cetraria-type of ascus, characterized by a large tholus with an amyloid ring (Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ). Masonhalea inermis possesses the Tuckermannopsis-form of the Lecanora-type ascus, which is characterized by globose ascospores arranged uniseriately in a cylindrical ascus with a small tholus, broad axial body and a broad ocular chamber (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ). In contrast, asci of M. richardsonii are categorized as the Nephromopsis-form of the Lecanora-type, containing oblong-obovate ascospores (not arranged uniseriately), which are produced in narrowly clavate asci with a smaller axial body and a conical ocular chamber with a narrow beak (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Thell et al. Reference Thell, Mattsson and Kärnefelt1995b ). Ascospore shape also generally appears conserved among species within individual genera in the cetrarioid core (Mattsson Reference Mattsson1993). However, differences in ascospore shape among closely related cetrarioid species are known to occur in Nephromopsis, where most species have oblong-obovate ascospores, while a small number of taxa, such as N. komarovii and N. nephromoides, have ellipsoid ascospores (Randlane et al. Reference Randlane, Thell and Saag1995; Randlane & Saag Reference Randlane and Saag1998). Furthermore, the genus Cetreliopsis, which is closely related to or part of Nephromopsis (supplementary Fig. 1; Thell et al. Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009; Nelsen et al. Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ), is characterized by ellipsoid ascospores (Randlane et al. Reference Randlane, Thell and Saag1995; Randlane & Saag Reference Randlane and Saag1998). Kärnefelt et al. (Reference Kärnefelt, Mattsson and Thell1992) have suggested that spore arrangement, size of the axial body and ascospore shape are often correlated, though this has not been tested in a quantitative framework. If the evolution of these three characters is indeed correlated, it may not be surprising that M. inermis and M. richardsonii differ in these three characters.
Pycnidia and conidia
Both Masonhalea species are characterized by 1-layered, raised, marginal pycnidia, with a layer of cortical tissue beneath the pycnidial wall (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). Several other cetrarioid genera, such as Cetraria, Nephromopsis and Tuckermannopsis, include species with similar pycnidia (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). However, both Masonhalea species form bacillariform conidia (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1993; Thell Reference Thell1995b ), which are quite rare in the cetrarioid core (Thell Reference Thell1995b ). Together, this combination of pycnidia and conidia appears unique in the cetrarioid core.
Secondary chemistry
Masonhalea richardsonii and M. inermis differ 6in their medullary secondary chemistry, with M. richardsonii producing the depsidone alectoronic acid, and M. inermis developing the fatty acids protolichesterinic and lichesterinic acids (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992). The production of protolichesterinic and lichesterinic acids is quite widespread in the cetrarioid core (Kärnefelt & Thell Reference Kärnefelt and Thell1993). Though less common, alectoronic acid is also known from a range of cetrarioid genera (Kärnefelt & Thell Reference Kärnefelt and Thell1993), including Cetrariella (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Thell Reference Thell1995a ; Kärnefelt & Thell Reference Kärnefelt and Thell2000; Rico et al. Reference Rico, van den Boom and Barrasa2005), Nephromopsis (Randlane et al. Reference Randlane, Thell and Saag1995) and Tuckermannopsis (Kärnefelt & Thell Reference Kärnefelt and Thell2001). Interestingly, the species Cetrariella commixta is known to be variable with respect to the presence or absence of alectoronic acid (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992; Thell Reference Thell1995a ); consequently, the production of alectoronic acid is not constant in the genus Cetrariella (Kärnefelt & Thell Reference Kärnefelt and Thell2000), or even the species C. commixta (Rico et al. Reference Rico, van den Boom and Barrasa2005). Somewhat similarly, the species Nephromopsis pallescens is known to develop either alectoronic acid or protolichesterinic and lichesterinic acids in the medulla, or occasionally, both sets of substances (Randlane et al. Reference Randlane, Thell and Saag1995). Finally, within the genus Tuckermannopsis, T. americana produces alectoronic acid (Thell Reference Thell1998; Kärnefelt & Thell Reference Kärnefelt and Thell2001), while the closely related T. orbata is characterized by protolichesterinic acid (Kärnefelt & Thell Reference Kärnefelt and Thell2001).
Ecology and distribution
Both Masonhalea species share a similar northern Beringian distribution, although the distribution range of M. inermis is smaller, except for an isolated occurrence in northern Norway (Krog Reference Krog1973; Kärnefelt Reference Kärnefelt1977, Reference Kärnefelt1979; Tønsberg & Elvebakk Reference Tønsberg and Elvebakk1993). Despite this similarity, the two species differ ecologically, with M. richardsonii occurring vagrant on soil, and M. inermis instead occurring mostly on twigs, but occasionally also on soil (Krog Reference Krog1973; Kärnefelt Reference Kärnefelt1977, Reference Kärnefelt1979; Thomson Reference Thomson1984; Thell & Moberg Reference Thell and Moberg2011). A number of other cetrarioid genera, such as Allocetraria (Thell et al. Reference Thell1995a ), Arctocetraria (Kärnefelt Reference Kärnefelt1979; Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1993) and Vulpicida (Mattsson Reference Mattsson1993; Mattsson & Lai Reference Mattsson and Lai1993), also include both corticolous and terricolous species or individual species that occur in both habitats.
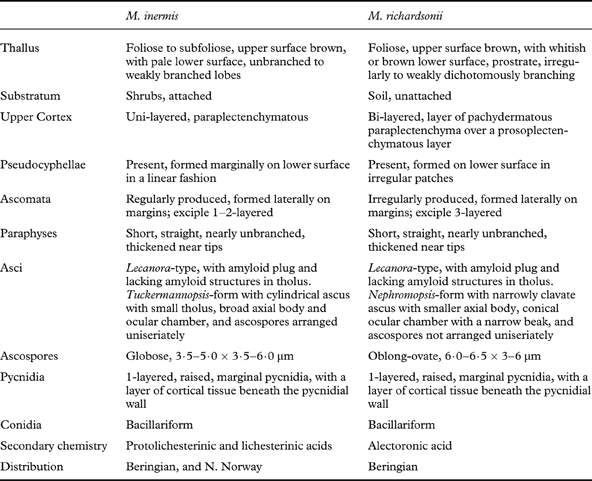
A summary of the morphological, anatomical, chemical and ecological characteristics of the two Masonhalea species is provided in Table 1.
Table 1. Summary of the numerous morphological, anatomical, chemical and ecological characteristics of the two Masonhalea species. Data are derived from literature sources cited throughout the manuscript.
Phylogeny
The final concatenated alignment consisted of 2628 unambiguously aligned characters, and the topology recovered (Appendix Fig. A1) is consistent with that in fig. 2 of Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ). The newly generated ITS sequences are strongly supported as sister to the earlier M. inermis and M. richardsonii sequences, thereby confirming the original sequences. Subsequent sequencing of the mtSSU, nuLSU and RPB1 loci (S. D Leavitt & H. T. Lumbsch, unpublished data) of the recently collected M. inermis specimen produced sequences nearly identical to those used in Thell et al. (Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009) and Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ), thereby confirming their identity. As in Thell et al. (Reference Thell, Högnabba, Elix, Feuerer, Kärnefelt, Myllys, Randlane, Saag, Stenroos and Ahti2009) and Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ), M. inermis is strongly supported as being sister to M. richardsonii (Appendix Fig. A1).
Taxonomy
We previously considered transferring T. inermis to Masonhalea, and indeed proposed this combination in the original Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) submission. Ultimately, we refrained from making this change until our results could be validated with new DNA sequences (Nelsen et al. Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ). However, this change was included and printed in Thell & Moberg (Reference Thell and Moberg2011). Thus, the publication of the name Masonhalea inermis (Nyl.) Lumbsch et al. in Thell & Moberg (Reference Thell and Moberg2011) is the valid combination according to article 33:7 of the International Botanical Code (McNeill et al. Reference McNeill, Barrie, Burdet, Demoulin, Hawksworth, Marhold, Nicolson, Prado, Silva and Skog2006), not Nelsen et al. (Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a ) as printed in Thell & Moberg (Reference Thell and Moberg2011). We correct the authors of this combination below, following a brief summary of the revised circumscription of the genus Masonhalea, which largely adheres to that of Thell & Moberg (Reference Thell and Moberg2011).
Masonhalea Kärnefelt
Bot. Notiser 130: 101–102 (1977);—type: Masonhalea richardsonii (Hook.) Kärnefelt.
Thallus foliose to subfoliose, upper surface brown, lower surface whitish to brown, sometimes prostrate, unbranched to weakly dichotomously branching. Pseudocyphellae present on the lower surface; occurring in patches, or as a continuous line along the margin.
Ascomata zeorine, apothecia formed laterally on margins, asci cylindrical to narrowly clavate with an axial body. Ascospores hyaline, globose to oblong-ovate, 3·5–6·5×3–6 µm. Photobiont trebouxioid.
Pycnidia marginal. Conidia bacillariform.
Chemistry
Alectoronic or lichesterinic and protolichesterinic acids in the medulla.
Substratum
Growing attached on shrubs or unattached on soil.
Distribution
Beringia and northern Norway.
Masonhalea inermis (Nyl.) Lumbsch, M. Nelsen & A. Thell
In Thell & Moberg (eds), Nordic Lichen Flora 4: 67 (2011) as Masonhalea inermis (Nyl.) Lumbsch et al.
Cetraria crispa f. inermis Nyl., Bull. Soc. Linn. Normandie 4, 1: 214 (1887);—Cetraria tenuifolia var. inermis (Nyl.) Räsänen, Kuopion Luonnon Ystäväin Yhdistyksen Julkaisuja, ser. B, 2(6): 23 (1952);—Cetraria inermis (Nyl.) Krog, Bryologist 76: 299 (Reference Krog1973);—Tuckermannopsis inermis (Nyl.) Kärnefelt, Bryologist 96: 403 (1993).
Summary
Macroscopically, M. inermis and M. richardsonii appear quite distinct from one another. However, upon closer examination, several morphological and anatomical similarities can be seen. Furthermore, many of the differences between M. inermis and M. richardsonii appear minor when considered in the context of the vagrant habit of M. richardsonii and when compared with differences included in other genera. Most of the differences observed between these two species exist among species in other cetrarioid core genera. These two species are not found on short branches, suggesting they have been separated for some time, and/or undergone a substantial amount of molecular evolution. Consequently, these two species should be expected to have accumulated a number of differences. They appear to share a unique suite of morphological and anatomical character states among the cetrarioid core, which include the production of lateral apothecia, and bacillariform conidia formed in marginal pycnidia with a layer of cortical tissue beneath the pycnidial wall (Kärnefelt et al. Reference Kärnefelt, Mattsson and Thell1992).
This work resulted from the Encyclopedia of Life (EOL) Parmeliaceae meeting in Chicago, May 2010. We thank Teuvo Ahti, Irwin Brodo and two anonymous reviewers for helpful comments, which improved the manuscript. Matthew Nelsen was supported by the Brown Family Fellowship through the Field Museum. Funding was provided by the University of Chicago (to MPN) and the National Science Foundation (“Hidden diversity in parmelioid lichens”, DEB-0949147 to HTL). All molecular work was performed in the Pritzker Laboratory for Molecular Systematics and Evolution at the Field Museum. S. A. Harris and the ABLS Lichen Exchange are thanked for providing a lichen collection.
Appendix
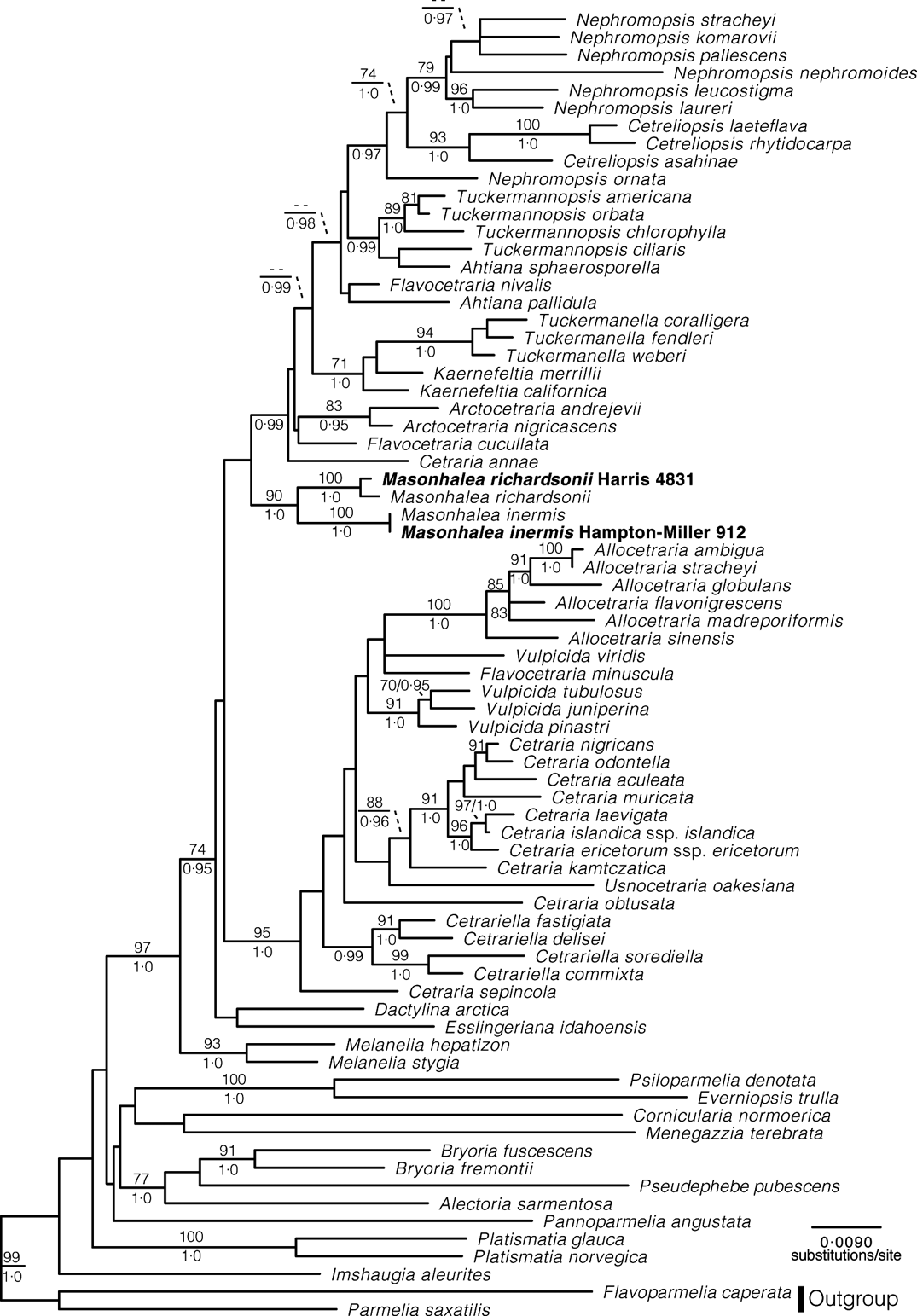
Fig. A1. Maximum likelihood phylogram for the cetrarioid lichens with new ITS sequences for Masonhalea inermis and M. richardsonii added to the full alignment in Nelson et al. Reference Nelsen, Chavez, Sackett-Hermann, Thell, Randlane, Divakar, Rico and Lumbsch2011a. ML bootstrap values ≥70 and Baysian posterior probabilities ≥0·95 are listed either above or below line or left and right of slashes, respectively.





