INTRODUCTION
Siphonophores are hydrozoans characterized by having complex colonies, comprising functional and specialized polymorphic units (zooids) that perform different tasks, including feeding and defence (gastrozooids), and reproduction (gonophores). The general body plan of siphonophores varies between taxonomic levels, and colonies range in size from a few centimetres to tens of metres (Mapstone, Reference Mapstone2014). Some siphonophores display diel vertical migration, whilst others do not (Pugh, Reference Pugh1984; Mackie et al., Reference Mackie, Pugh and Purcell1988; Mapstone, Reference Mapstone2014). They play an important role in the ecology of marine systems: they reproduce quickly (Mackie et al., Reference Mackie, Pugh and Purcell1988); they can be voracious predators of zooplankton and fish larvae (Alvariño, Reference Alvariño1985; Purcell, Reference Purcell1985; Arai, Reference Arai1988; Kršinić & Njire, Reference Kršinić and Njire2001; Mills, Reference Mills2001); they compete with fish for food (Arai, Reference Arai1988; Buecher, Reference Buecher1999; Thibault-Botha et al., Reference Thibault-Botha, Santo and Neauport2013); and they are distributed throughout the water column, particularly in the deep sea (Silguero & Robison, Reference Silguero and Robison2000). When abundant, they can impact economic activities such as tourism and marine aquaculture (Fosså et al., Reference Fosså, Flood, Olsen and Jensen2003; Baxter et al., Reference Baxter, Rodger, McAllen and Doyle2011; Licandro et al., Reference Licandro, Souissi, Ibanez and Carré2012).
In the Pacific Ocean, there are many studies on the composition and distribution of siphonophores in temperate waters (Palma, Reference Palma1973; Reference Palma1999; Pagès et al., Reference Pagès, Gili and Bouillon1990; Gasca & Suárez, Reference Gasca and Suárez1992a, Reference Gasca and Suárezb; Palma & Rosales, Reference Palma and Rosales1995; Gasca, Reference Gasca2002; Palma & Apablaza, Reference Palma and Apablaza2004; Palma & Silva, Reference Palma and Silva2004; Apablaza & Palma, Reference Apablaza and Palma2006; Palma et al., Reference Palma, Apablaza and Soto2007; Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Alba, Alatorre and Flores-Coto2007, Reference Sanvicente-Añorve, Alba, Flores-Coto and Castillo-Rivera2009; Lo et al., Reference Lo, Kang and Hsieh2012; Reference Lo, Yu and Hsieh2013, Reference Lo, Yu and Hsieh2014; Gamero-Mora et al., Reference Gamero-Mora, Ceballos-Corona, Gasca and Morales-Blake2015), but comparatively few from equatorial and tropical waters. Bigelow (Reference Bigelow1911) identified 40 siphonophores from samples collected during the Albatross expedition in October 1904 to March 1905, but few samples were collected from the Eastern Tropical Pacific. Alvariño (Reference Alvariño1971) also documented siphonophore species from the tropical-equatorial Eastern Pacific Ocean collected during two expeditions outside of the Colombian marine territory – Shellback (May–August 1952) and Capricorn (November 1952–February 1953). Andrade (Reference Andrade2012) identified 15 species around Santa Clara Island (Ecuador) from hyponeustonic samples collected in September and November 2007, with Muggiaea atlantica and Chelophyes appendiculata being the dominant species at flood tide. The only work on the composition, distribution and abundance of siphonophores from neritic equatorial waters and from the Colombian Pacific Ocean is unpublished research from the thesis of Cely & Chiquillo (Reference Cely and Chiquillo1993). They found 29 species, with Chelophyes appendiculata, Diphyes dispar and Abylopsis eschscholtzii dominant. This latter study was based on oblique samples collected in a single cruise conducted in waters adjacent to the coast in February–March 1991.
By comparison with the rest of the planktonic environment, the hyponeuston is poorly known and yet it provides an important habitat for early life history stages of fishes (Banse, Reference Banse1964; Ahlstrom & Stevens, Reference Ahlstrom and Stevens1976; Jeong et al., Reference Jeong, Suh, Lee and Soh2014), whose abundances may be impacted by siphonophores (Purcell, Reference Purcell1985). Here we provide new insights into the poorly known hyponeuston of the tropics, which are some of the most biodiverse areas of the world (Roy et al., Reference Roy, Jablonski, Valentine and Rosenberg1998; Boltovskoy, Reference Boltovskoy1999; Harris et al., Reference Harris, Wiebe, Lenz, Skjoldal and Huntley2000; Macpherson, Reference Macpherson2002; Pierrot-Bults & Angel, Reference Pierrot-Bults and Angel2013). We investigate siphonophore diversity within the hyponeuston for two major orders of siphonophores (Calycophorans and Physonects) and its relation to environmental variables. Samples were collected from coastal and oceanic waters of the tropical Colombian Pacific Ocean (CPO) over five cruises conducted over four consecutive years.
MATERIALS AND METHODS
Study region
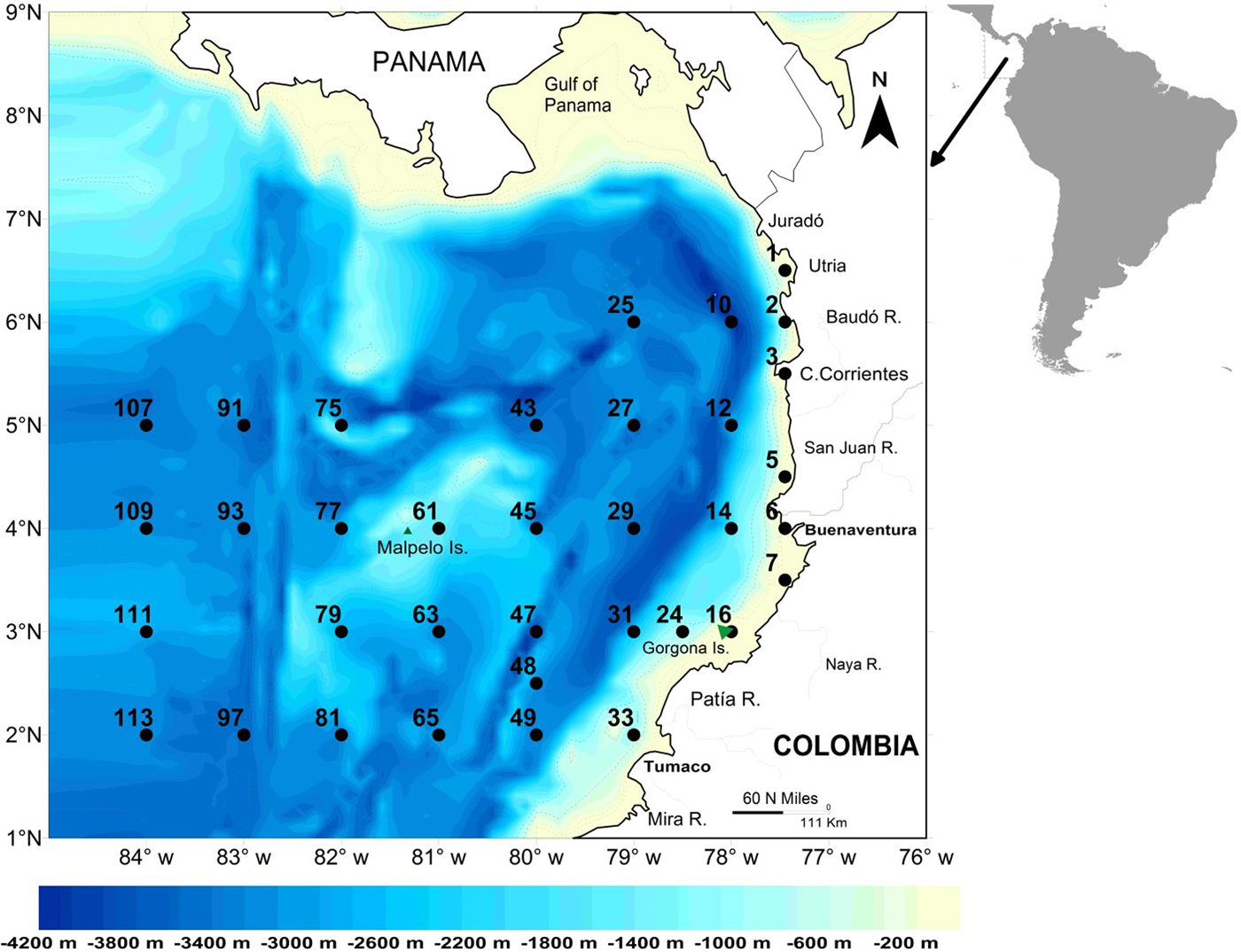
The study area (339,000 km2) was the CPO and part of the Panamá Bight (CCCP, 2002) (Figure 1). In this region, trade winds are strongest from December to March (Rodríguez-Rubio & Wolfgang, Reference Rodríguez-Rubio and Wolfgang2003), so all cruises were conducted outside peak upwelling season. The development of the Colombian Current (in neritic waters) (CCCP, 2002; Martínez et al., Reference Martínez-Aguilar, Giraldo López and Rodríguez-Rubio2007) and the presence of oceanic upwelling occur as a result of the presence of the NE trade winds in the CPO from November–March. These oceanographic features decline once the trade winds lose intensity toward the second half of the year (Wyrtki, Reference Wyrtki1966; Rodríguez-Rubio & Wolfgang, Reference Rodríguez-Rubio and Wolfgang2003) when our study was conducted.
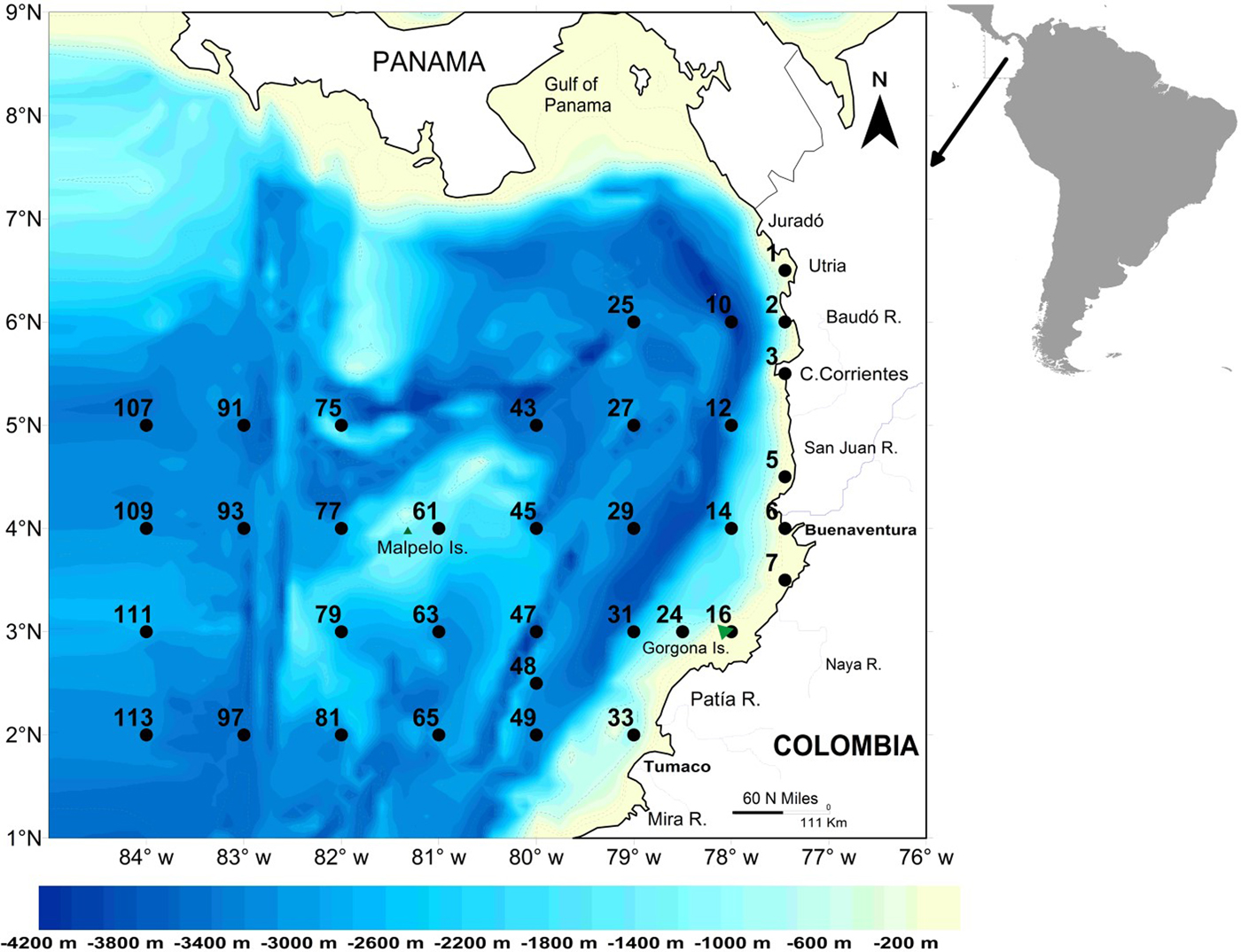
Fig. 1. Map of the Colombian Pacific Basin showing the bathymetry and location of the sampling stations.
Sampling
Five cruises were conducted on board the Colombian Army research platforms ARC-Malpelo and ARC-Gorgona on 23 June–12 July 2001 (denoted Jun–Jul 2001), 27 August–15 September 2001 (Aug–Sep 2001), 3–22 September 2002 (Sep 2002), 2–21 September 2003 (Sep 2003), and 16 September–8 October 2004 (Sep–Oct 2004). Biological samples and environmental data were collected between 77–84°W and 2–6°N on a fixed grid of 35 stations, although not all stations were sampled during each cruise because of severe weather conditions and technical issues (Figure 2). Stations were 30 nautical miles apart in coastal waters and 60 nautical miles apart in oceanic areas. Samples were designated as being day (6 am–6 pm) or night according to the local time of arrival at the site. Two stations (49 and 111) were sampled more than once on the cruise conducted in Jun–Jul 2001.
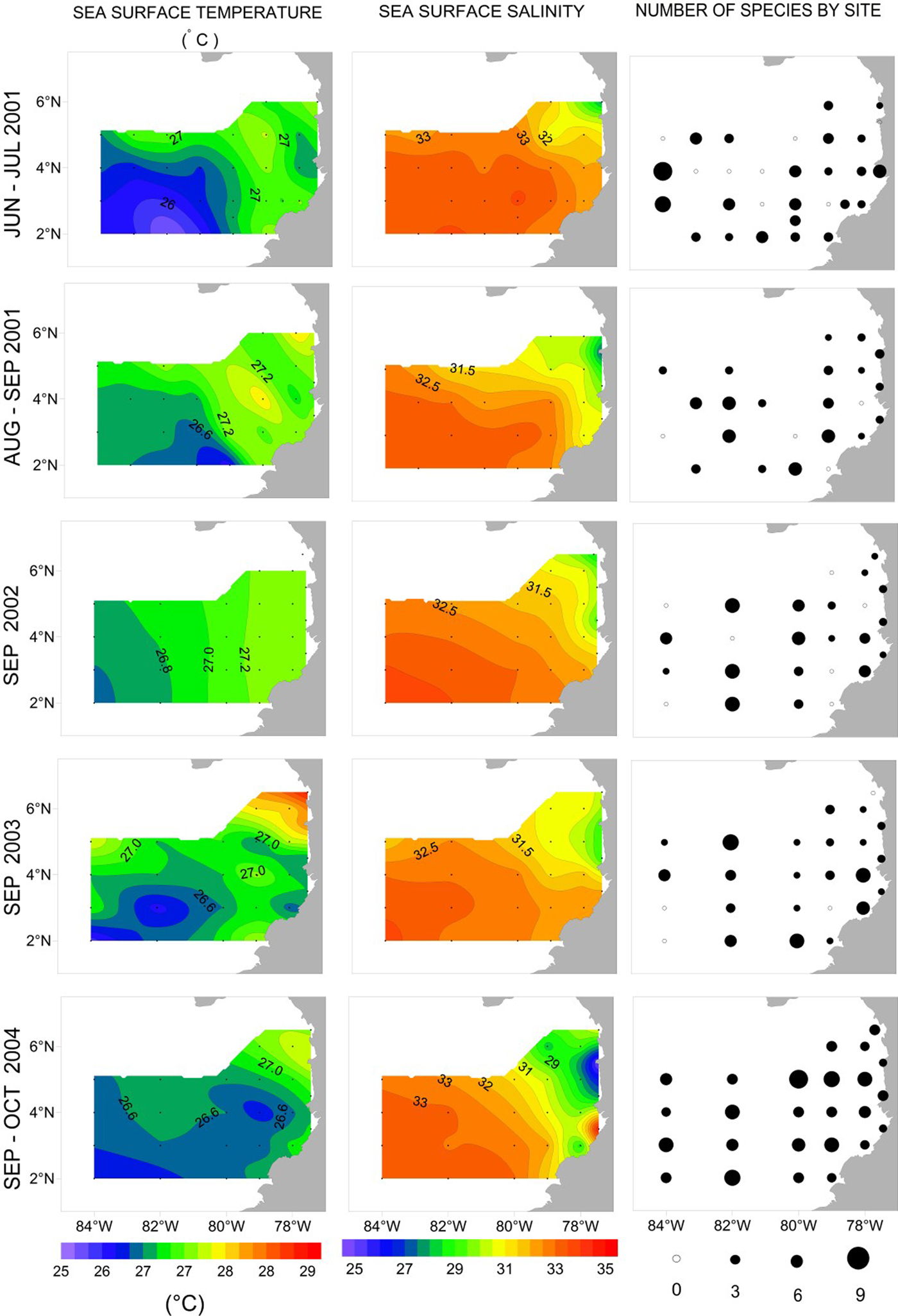
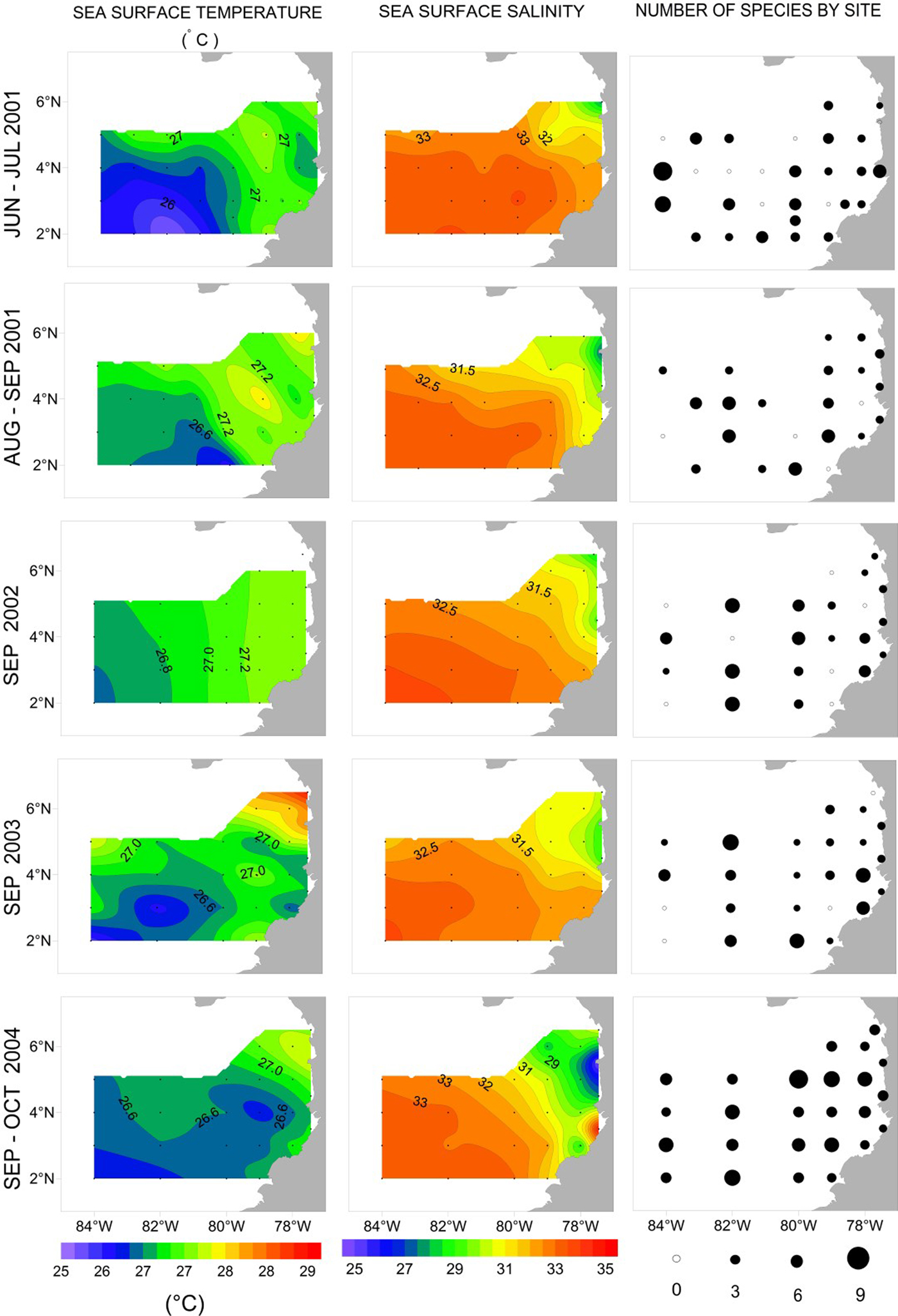
Fig. 2. Contour maps of temperature and salinity, and number of species of siphonophores found by site in the CPO during June–July 2001, August–September 2001, September 2002, September 2003 and September–October 2004.
At each station, sea surface temperature was measured with a reversing thermometer because it could be deployed close to the sea surface. Salinity was measured with a CTD (SeaBird electronics Series SEACAT) and the first reliable data were at 4 m depth because of ship movement and waves. Samples of surface water were collected at each station with a 5 l Niskin bottle, and a 500 ml subsample of this was stored at −20 °C for later analysis of nutrient concentration (ammonia, nitrite, nitrate, phosphate and silicate), following protocols of Garay et al. (Reference Garay, Panizzo, Ramírez and Sánchez1993).
To investigate the siphonophore community in the hyponeuston, we collected zooplankton samples in the first 0–3 m of water. Plankton samples were collected using a circular net of 0.5 m diameter and 363 µm mesh size, following a standardized circular trawling trajectory for 10 minutes. Samples were fixed and preserved in 5% buffered formalin.
In the laboratory, all siphonophores were identified from the samples using compound (Carl Zeiss S-1000 and Leica Zoom 2000) and dissecting microscopes (Carl Zeiss-Axiostar). Species were identified following Pagès et al. (Reference Pagès, Gili and Bouillon1992) and Palma (Reference Palma1973) on the basis of whole organisms (Cystonectae) or portions of colonies, nectophores, eudoxids, gonophores and bracts (Physonectae and Calycophorae). Confirmation of the species was completed in 2005 following direct communication with the late Dr Francesc Pagès.
Statistical analyses
Most of our analyses are based on presence:absence of siphonophores as we could not estimate abundance robustly on all cruises because the flowmeter did not always work properly. For the two cruises with flowmeter data, estimates of siphonophore abundance for each species were obtained by summing the number of polygastric and reproductive stages. For Calycophorae, this was the greater of the number of anterior or posterior nectophores (or gonophores/bracts for eudoxids). For Physonectae, counts were based on pneumatophores; multiple bracts and nectophores without a pneumatophore were considered to originate from a single individual. We assessed how representative the presence:absence data were by correlating the frequency of occurrence of each species (based on presence:absence data) against abundance for cruises in Sep 2002 and Sep–Oct 2004.
To assess whether there might be bottom-up or top-down control between siphonophores and their prey, we obtained data on the abundance of copepods and fish eggs from unpublished work from the same net samples as the siphonophores. We assumed that bottom-up control would manifest as a positive relationship between siphonophore abundance (response) and copepods or fish eggs (predictors), and top-down control as a negative relationship between siphonophores and copepods or fish eggs (Richardson & Schoeman, Reference Richardson and Schoeman2004). Visual assessment of diagnostic plots showed that log10 (x + 1) transformation of both the response and predictors improved normality and homogeneity of variance assumptions. Data on fish egg and larval abundance were only available for the two cruises when flowmeter data were available: Sep 2002 and Sep–Oct 2004. Abundances were converted to densities (per 1000 m3), with a knowledge of the fraction of the sample examined (most commonly 1/8) and the volume of water filtered by the nets estimated using a flowmeter. Linear models were constructed in R and visualized using the effects package (Fox, Reference Fox2003).
To map the data for each cruise, we constructed contour plots of sea surface temperature and salinity using kriging in Surfer® 8.04 (Golden software, LLC, 2003). To assess the variation of environmental variables, we built a linear model with Temperature and Salinity (untransformed) as response variables and included two factors, Region (Coastal (<600 m) or Oceanic) and Cruise (the five surveys), and their interaction.
Multivariate analyses
To examine the response of the siphonophore community to environmental conditions, we performed non-metric Multidimensional Scaling (nMDS) analysis (Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014). nMDS is an ordination technique that seeks to preserve the rank dissimilarities between objects (here samples) based on a suite of variables (here species). On the 2-D ordinations, the closer two samples are, the more similar are their communities. We used the Bray–Curtis dissimilarity measure because it is robust to joint absences, so samples do not appear closer on the ordination because they have no species in common (Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014). We performed 1000 iterations using the metaMDS function in the R package vegan (Oksanen, Reference Oksanen2011, Reference Oksanen2017). We related the nMDS ordination to Region, Cruise, SST and Salinity; nutrient concentrations and chl-a were excluded because there were too many missing data. To aid interpretation, we included vector plots of environmental variables, species scores, and 95% confidence limits of centroids for Regions (Coastal vs Oceanic) and Time of Day (Day vs Night) on the ordination. To assess significant differences between groups and for continuous predictors, we performed a Permutational Multivariate Analysis of Variance (ADONIS) (Anderson, Reference Anderson2001). This gives the significance and the proportion of variance in the multivariate data (siphonophore communities) explained individually by each of the environmental predictors, added sequentially. To identify siphonophore species that were driving community differences, we used the Dufrene–Legendre Indicator Species Analysis (Dufrene & Legendre, Reference Dufrene and Legendre1997) in the R package labdsv. We used 0.05 as the level of significance in all statistical tests, unless otherwise specified.
RESULTS
Environmental conditions
There were relatively small differences in temperature in the tropical CPO during the study (Figure 2). Mean SST was 26.9 °C (±0.57 °C, standard deviation) with a range of 3.5 °C, with coolest temperatures (25.5 °C) in Jun–Jul 2001 and Sep–Oct 2004 in offshore waters to the south-east of the CPO. Waters with temperature >27 °C were located between 80°W and the coast in Jun–Jul 2001, Aug–Sep 2001 and Sep 2002, and to the north-east of the CPO in Sep 2003 and Sep–Oct 2004.
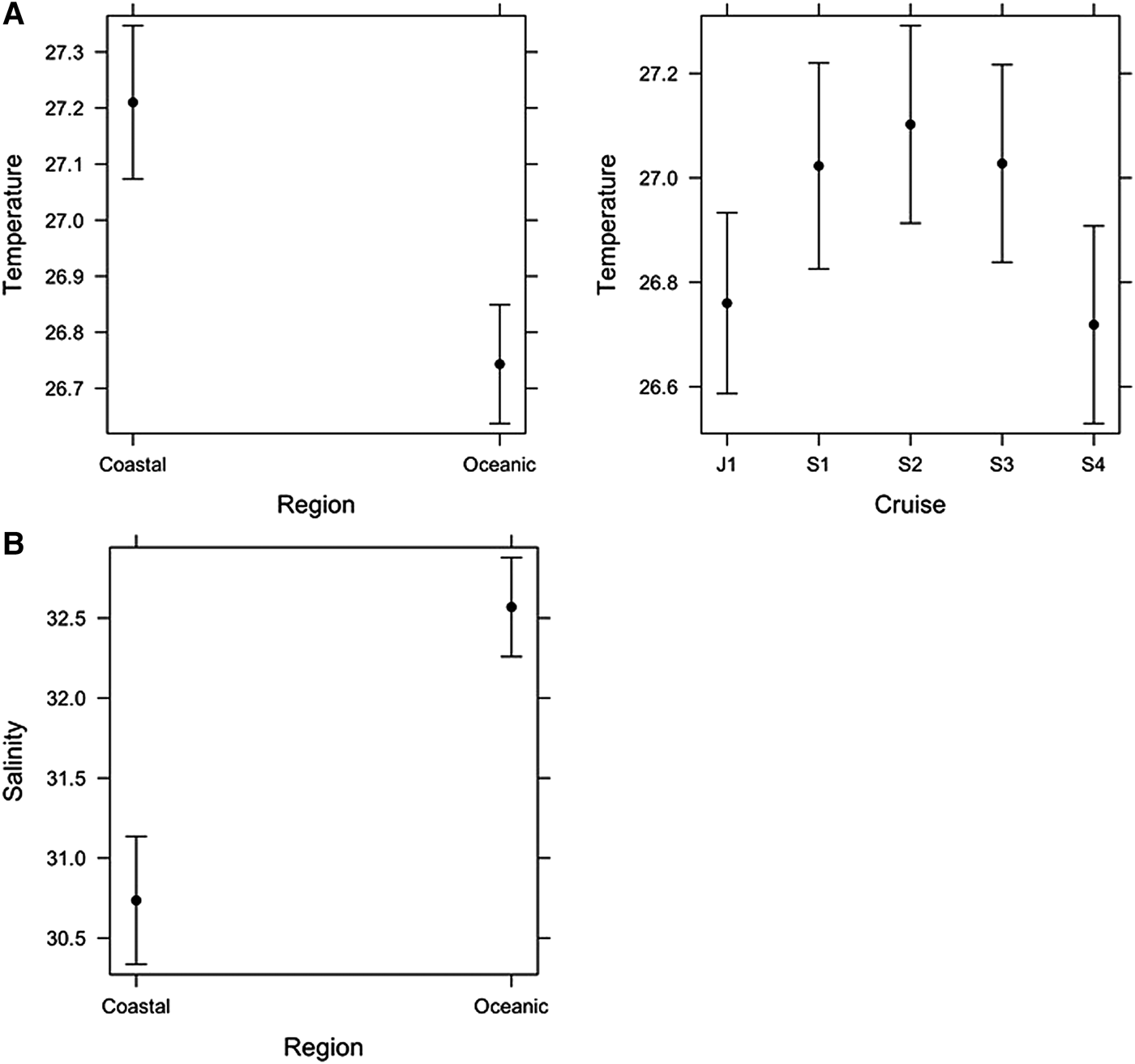
The linear model for SST showed that there was no significant interaction between Region and Cruise, suggesting the temperature difference between Regions (Coastal and Oceanic) was similar among Cruises. Coastal waters were significantly warmer (27.2 ± 0.07 °C, mean ± standard error) than Oceanic (26.7 ± 0.06 °C) waters, although the mean difference was only 0.5 °C (Figure 3A). Temperature also varied significantly among Cruises (Figure 3A): Jun–Jul 2001 (26.7 ± 0.1 °C), Aug–Sep 2001 (27.0 ± 0.1 °C), Sep 2002 (27.1 ± 0.1 °C), Sep 2003 (27.0 ± 0.1 °C) and Sep–Oct 2004 (26.7 ± 0.1 °C), but the effect size (i.e. maximum mean difference among cruises) was only 0.4 °C.
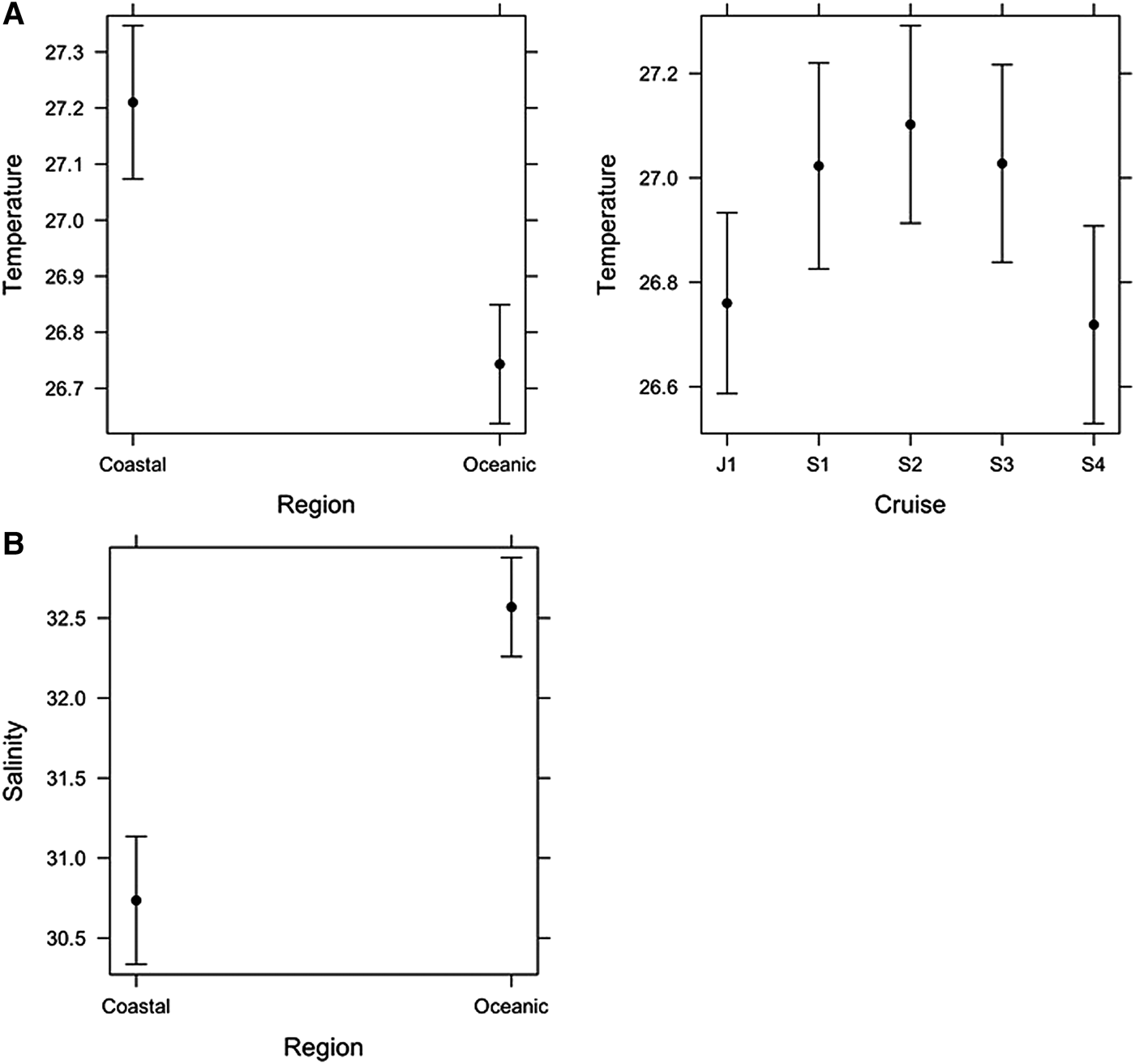
Fig. 3. Final linear models of (A) Temperature and (B) Salinity. Means ± confidence intervals are shown.
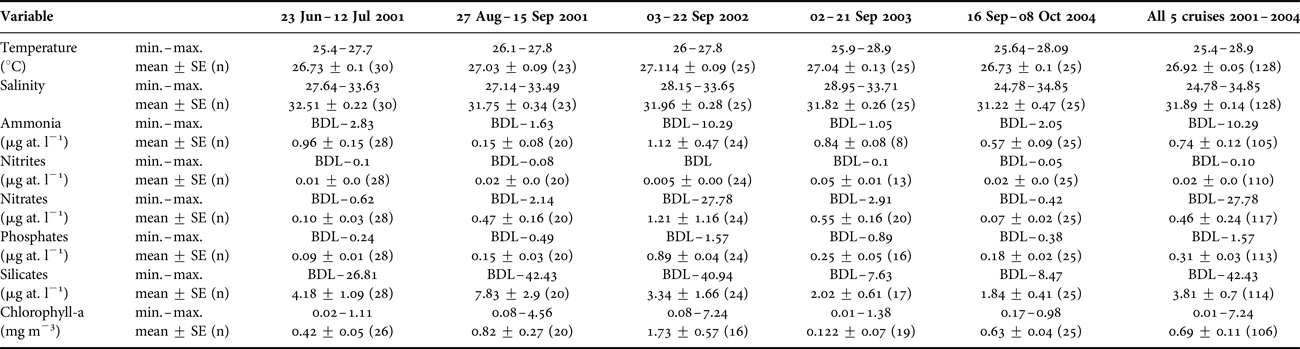
There was a relatively large range in salinity (10.07) among sites during the study (Figure 2). Low salinities were often found in the north-east section of the CPO and extended away from the coast. The lowest salinity (24.78) was recorded during Sep–Oct 2004 in the area off Cabo Corrientes, where a number of large rivers emerge. In general, concentrations of nutrients were often below detection levels, except for highest values near the San Juan River mouth in Sep 2002 (NH4 10.29 µg at. l−1, NO3 27.8 µg at. l−1, PO4 1.6 µg at. l−1 and SiO4 40.9 µg at. l−1; Table 1). Mean chlorophyll-a concentration was relatively high at times in coastal waters, up to 7.24 mg m−3, but low offshore (Table 1).
Table 1. Summary of environmental variables by cruise.

SE, Standard Error; BDL, below detection level.
The linear model for Salinity showed there was no significant interaction between Region and Cruise, suggesting the salinity difference between Regions (Coastal and Oceanic) was similar among Cruises. The main effect of Cruise was not significant, but Region was significant (Figure 3B). Coastal waters were significantly less saline (30.7 ± 0.3) than Oceanic waters (32.6 ± 0.1).
Siphophonore species present
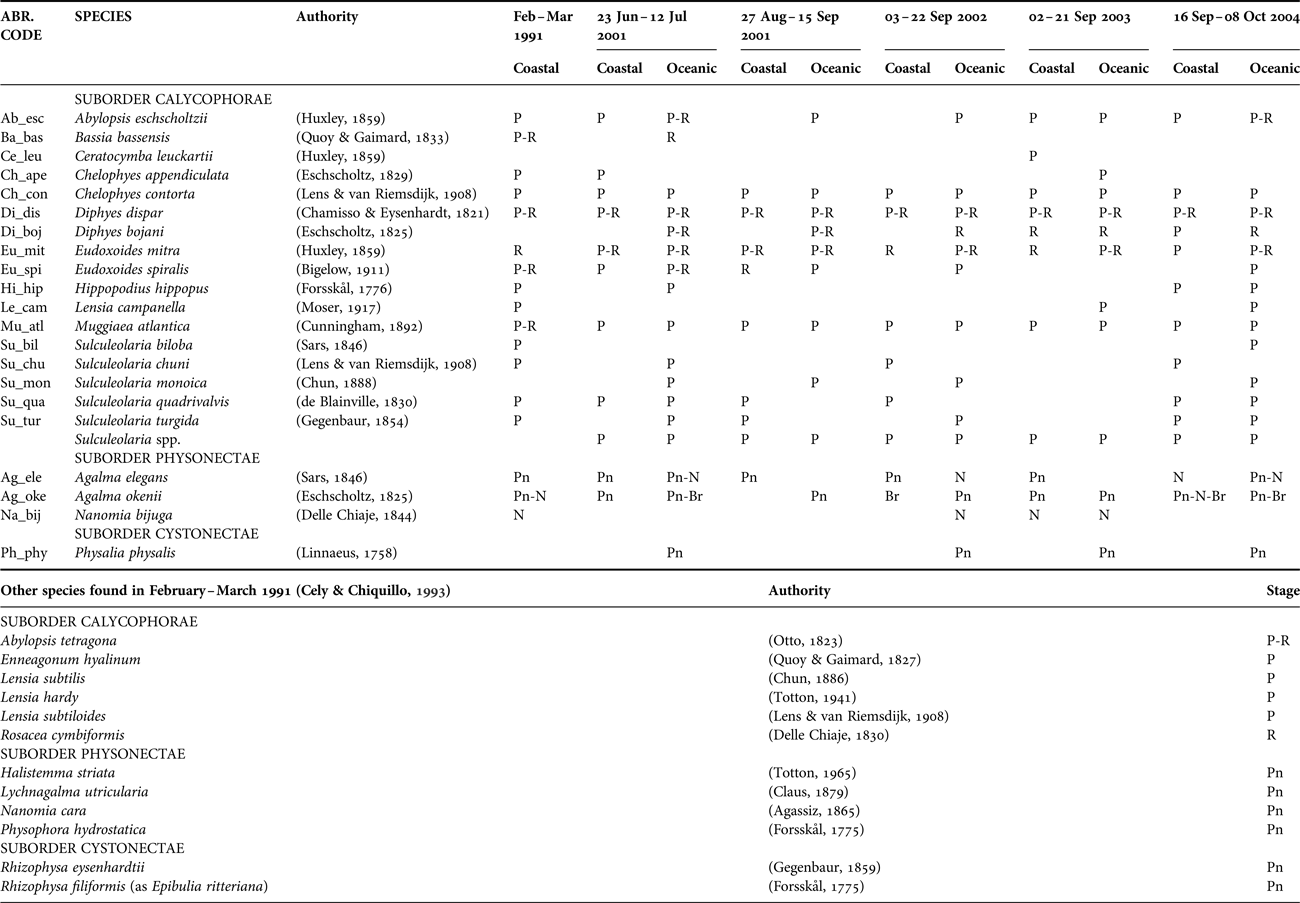
A total of 21 species of siphonophores were recorded from surface waters of the CPO (Table 2). Nectophores and associated pneumatophores, and bracts were recorded for two agalmatids (Agalma okenii and Agalma elegans) and polygastric and reproductive stages for five calycophorans (Abylopsis eschscholtzii, Diphyes dispar, Diphyes bojani, Eudoxoides mitra and Eudoxoides spiralis). Of these, only the eudoxid stages of D. bojani were collected in Sep 2002 and Sep 2003, and only the polygastric stages of E. spiralis were recorded in oceanic waters in Aug–Sep 2001, Sep 2002 and Sep–Oct 2004. Bassia bassensis was identified only once from a single gonophore in Jun–Jul 2001, whilst the remaining 11 species of Calycophorae were identified from polygastric stages (mainly the anterior nectophore), and Nanomia bijuga from nectophores.
Table 2. Siphonophore species identified from the samples collected in the CPO 2001–2004, and in February–March 1991 (Cely & Chiquillo, Reference Cely and Chiquillo1993).
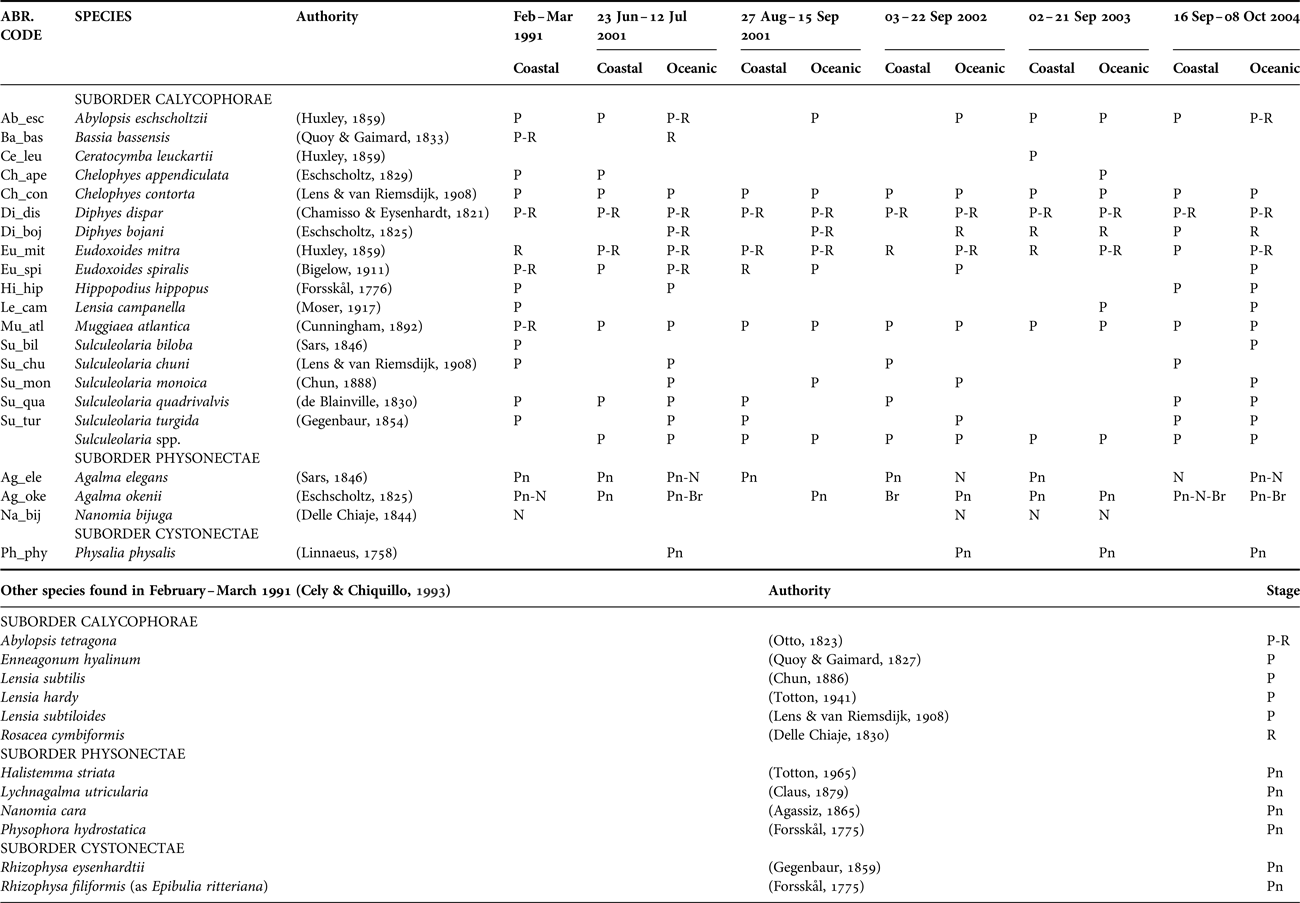
The presence of each species is shown based on the type of morphological stage found in the samples. For calycophorans: polygastric (P) and/or reproductive (R). For physonects: pneumatophore (Pn) and nectophore(s) (N). For cystonects: pneumatophore (Pn).
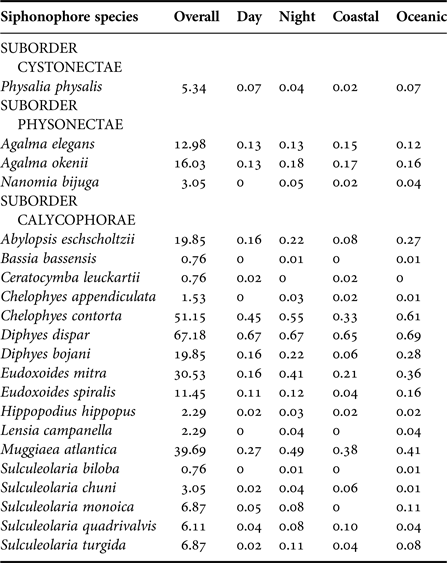
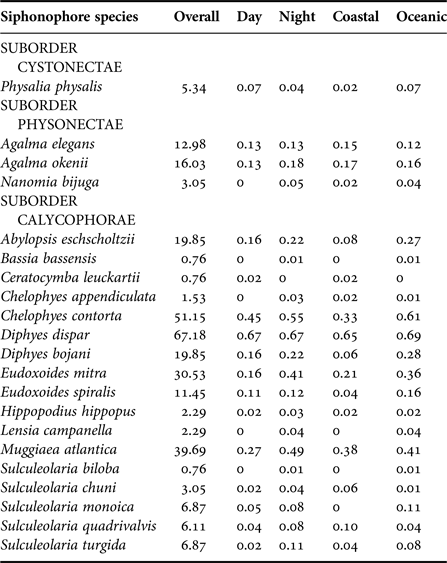
The eight most common species, in decreasing order of importance (from presence:absence data), were D. dispar (67.2%), Chelophyes contorta (51.1%), Muggiaea atlantica (39.7%), E. mitra (30.5%), A. eschscholtzii (19.8%), D. bojani (19.8%), A. okenii (16.0%) and A. elegans (13.0%) and were found in all five surveys. Eudoxoides spiralis, Sulculeolaria monoica, Sulculeolaria quadrivalvis and Sulculeolaria turgida were absent in Sep 2003, and Physalia physalis was absent in Aug–Sep 2001 (Table 2). Bassia bassensis, Ceratocymba leuckartii, Chelophyes appendiculata, Hippopodus hippopus, Lensia campanella, N. bijuga, Sulculeolaria biloba and Sulculeolaria chuni were recorded sporadically.
Siphonophore richness
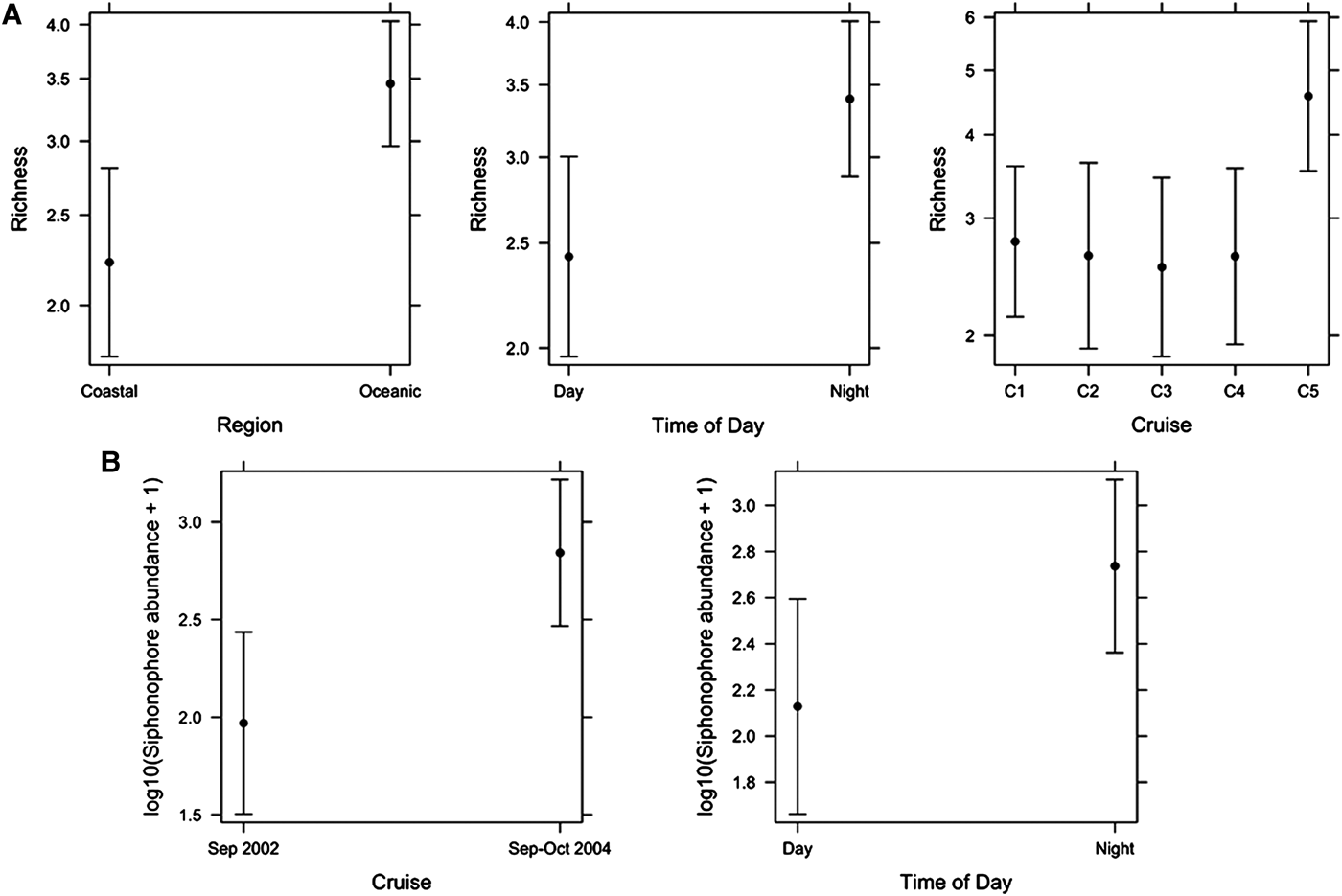
The species richness of siphonophores varied spatially and temporally. In oceanic waters there were up to nine species in a sample and in coastal waters up to seven species in a sample (Figure 2). There was higher richness in coastal waters off Buenaventura in Jun–Jul 2001 and Sep 2003 (Figure 2). There appeared to be higher siphonophore species richness in Sep–Oct 2004 than in other cruises. In the final linear model of siphonophore Richness (response) and Region, Time of Day and Cruise (predictors), only the main effects were significant (pseudo r 2 = 16.2%, Figure 4A). Richness was significantly higher in Oceanic (3.5 ± 0.08) than Coastal waters (2.2 ± 0.12). Richness was also significantly higher at Night (3.4 ± 0.08) than during the Day (2.4 ± 0.11). Richness varied significantly among cruises, with significantly higher Richness in Sep–Oct 2004 (4.6 ± 0.13) than Jun–Jul 2001 (2.8 ± 0.13), Aug–Sep 2001 (2.6 ± 0.16), Sep 2002 (2.5 ± 0.16) or Sep 2003 (2.6 ± 0.15).
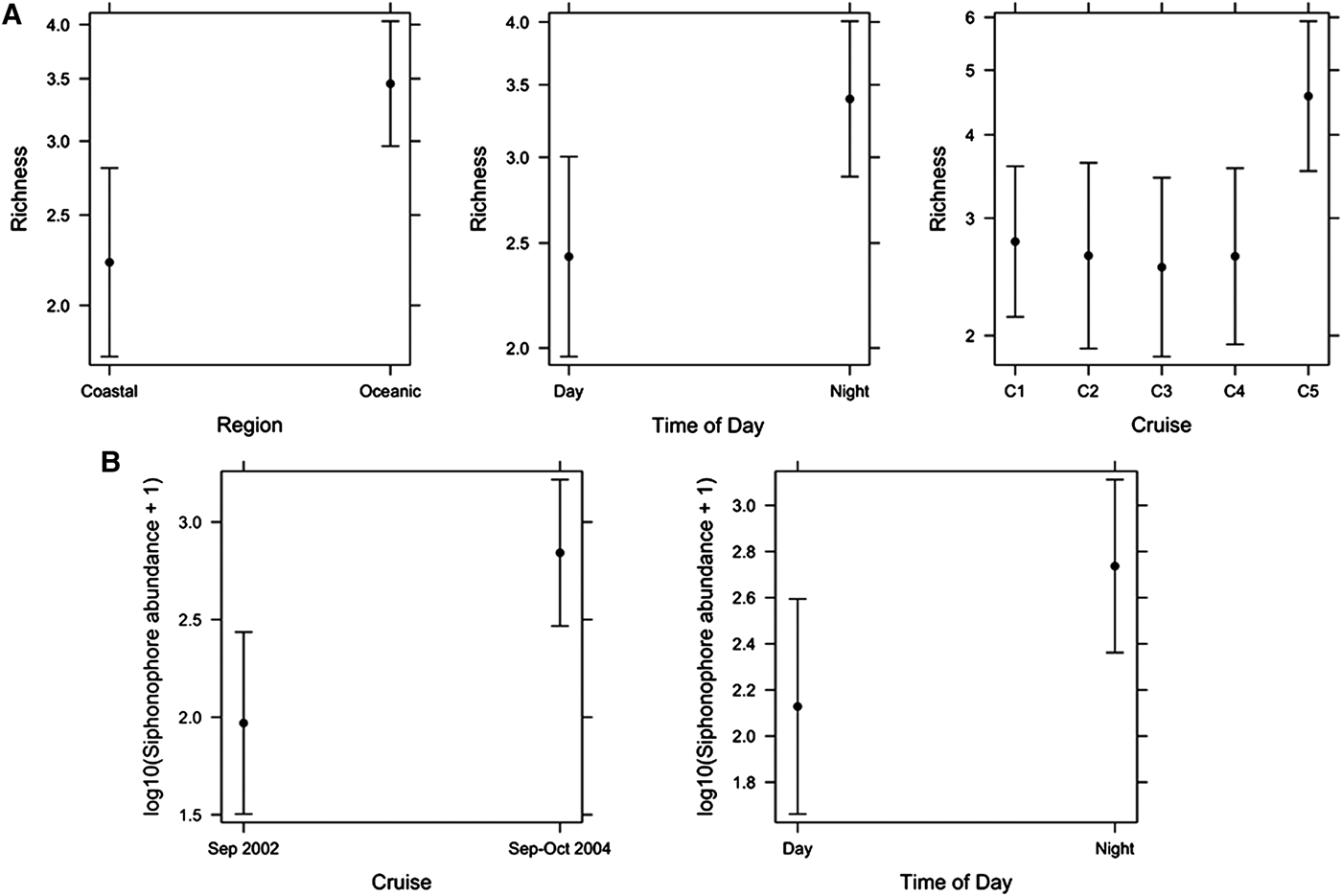
Fig. 4. Final linear models of (A) Siphonophore richness and (B) Siphonophore abundance for Sep 2002 and Sep–Oct 2004. Means ± confidence intervals are shown.
Siphonophore communities
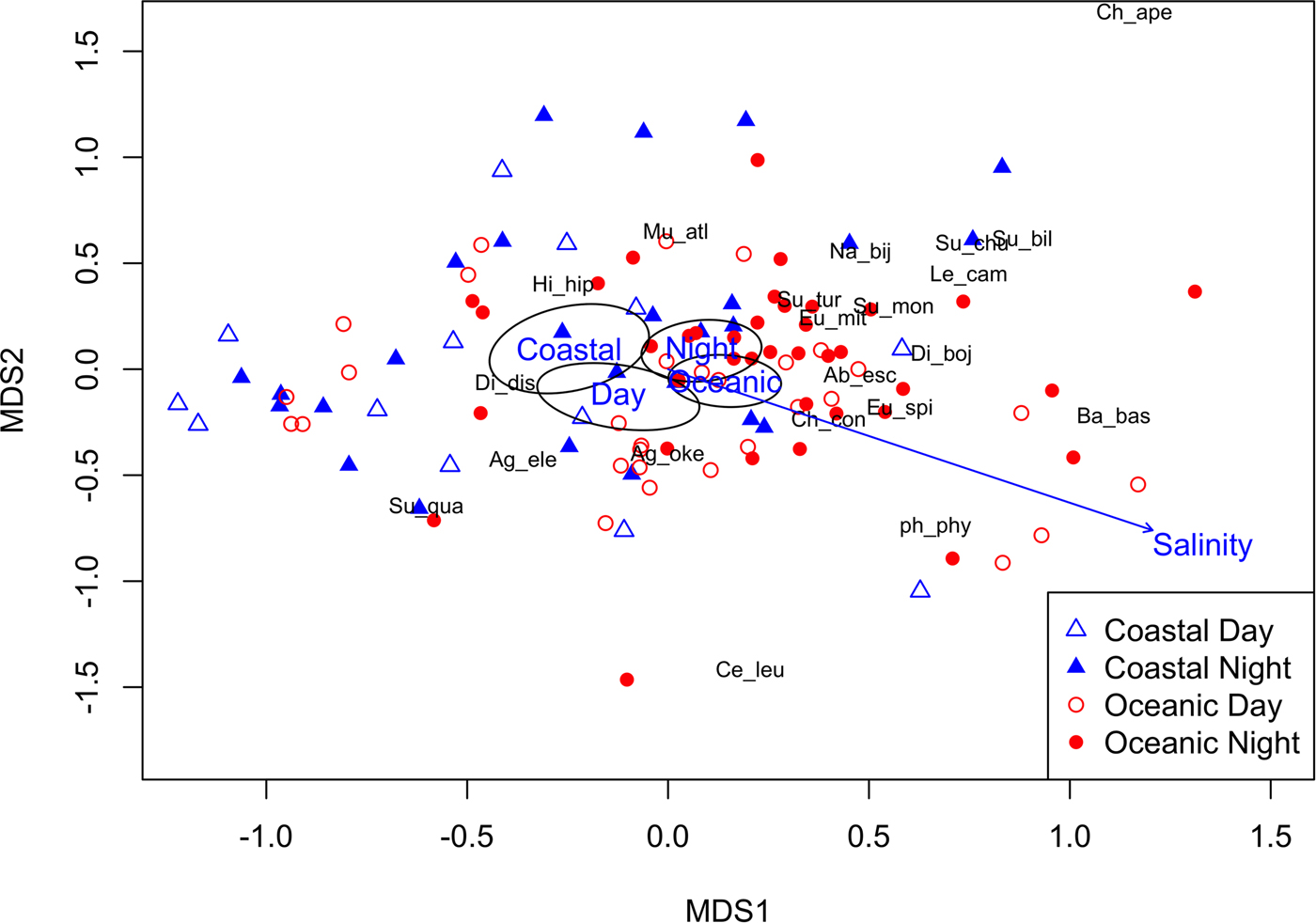
The nMDS ordination shows that there is a modest amount of structuring of the siphonophore community in the CPO (Figure 5). The Adonis model explained 10.8% of the variation in siphonophore communities, with Region (5.0%), Time of Day (3.7%) and Salinity (2.1%) all significant, and Cruise and Temperature not significant. Centroids for the Coastal vs Oceanic effect are close together, but their 95% confidence intervals do not overlap, suggesting that species toward the left of the ordination tend to be more Coastal and those toward the right tend to be more Oceanic. The Indicator Species Analysis showed that the species most different between Coastal and Oceanic samples were C. contorta (IV = 0.48, P = 0.02, Relative frequency of 41.0% Coastal vs 75.0% Oceanic), E. mitra (IV = 0.28, P = 0.092, 25.6% Coastal vs 44.1% Oceanic), D. bojani (IV = 0.28, P = 0.01, 7.7% Coastal vs 33.4% Oceanic), A. eschscholtzii (IV = 0.25, P = 0.01, 10.3% Coastal vs 32.4% Oceanic), E. spiralis (IV = 0.15, P = 0.07, 5.1% Coastal vs 19.1% Oceanic) and S. monoica (IV = 0.13, P = 0.03, 0% Coastal vs 13.2% Oceanic).
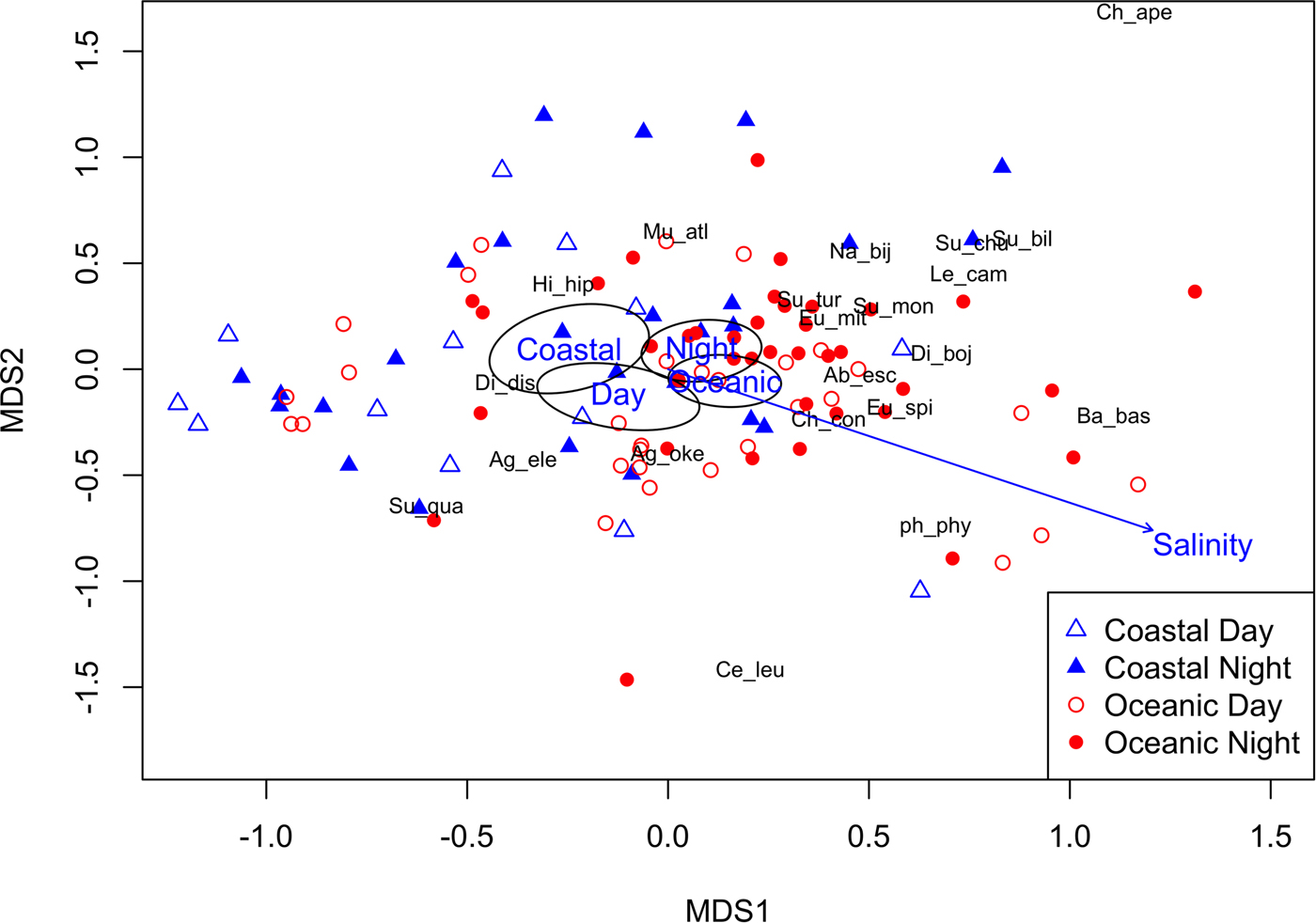
Fig. 5. A nMDS of the 21 siphonophore species, with species scores included. Environmental predictors are also plotted from the final Adonis model: Region (Coastal and Oceanic) and Time of Day (Day and Night) centroids and 95% confidence intervals superimposed, and the environmental vector Salinity.
The centroids for Day vs Night are separated (although still relatively close together), implying some species are different during the Day and Night. Species that were most different between Day and Night were M. atlantica (IV = 0.36, P = 0.07, 35.9% Day vs 57.8% Night), E. mitra (IV = 0.34, P = 0.01, 20.9% Day vs 48.4% Night) and S. turgida (IV = 0.11, P = 0.08, 2.3% Day vs 12.5% Night) (see also Table 3), suggesting they vertically migrate.
Table 3. Observation of species (%) during the entire study, discriminated by day and night and by coastal and oceanic areas of the CPO.
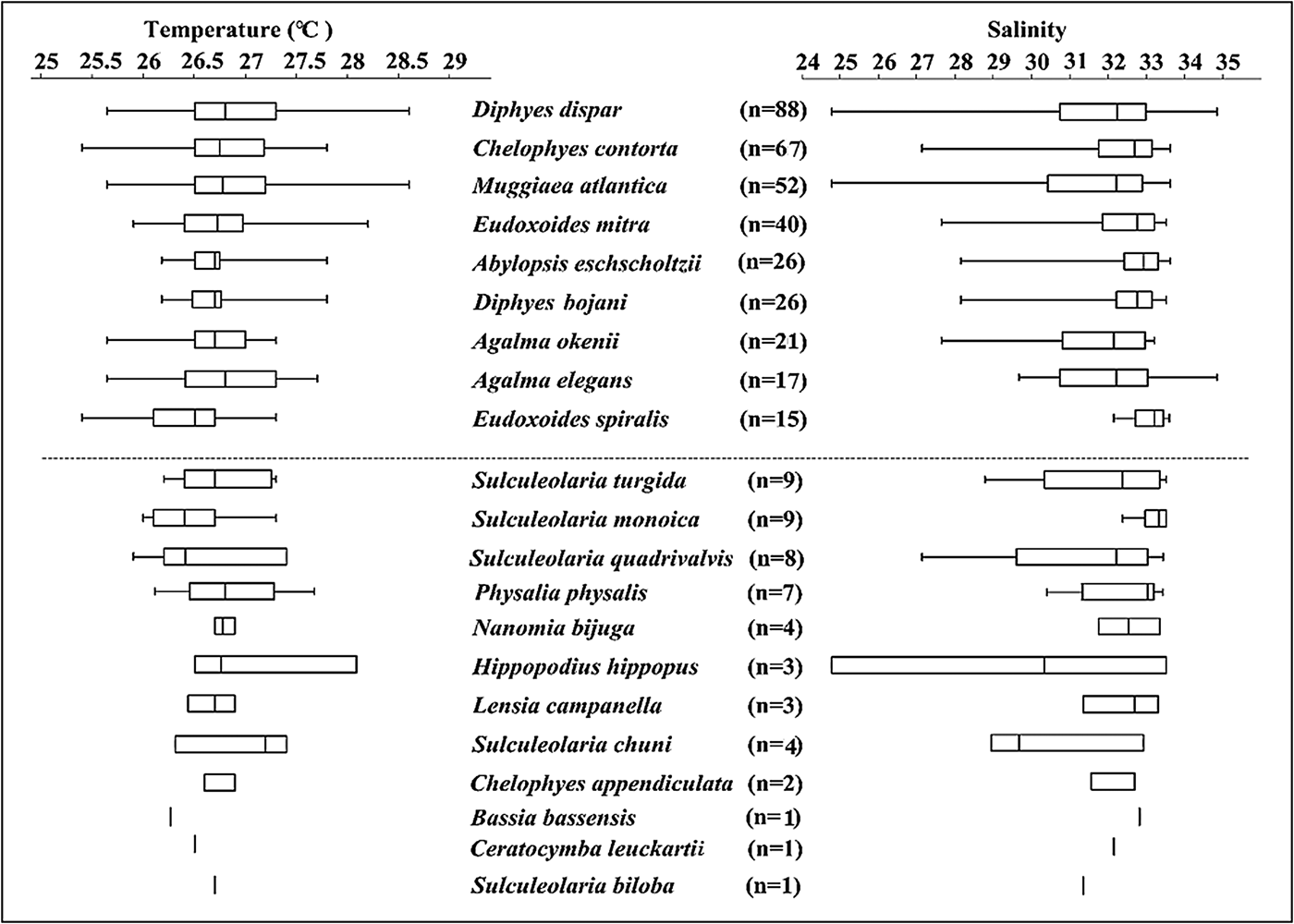
The gradient of Salinity points toward the bottom right in the ordination, indicating B. bassensis, E. spiralis, D. bojani, C. contorta and A. eschscholtzii preferred more saline water, whereas H. hippopus and D. dispar preferred less saline waters. Siphonophore species were most common in waters with a salinity of 31–33.5 (Figure 6), and this could simply reflect that there was more water of this salinity in the region (Figure 2). Interestingly, D. dispar and M. atlantica were found in water with salinity as low as 24.7 (Figure 6). Two rare species (S. chuni and H. hippopus) were also found at lower salinities (29 and 24.8, respectively).
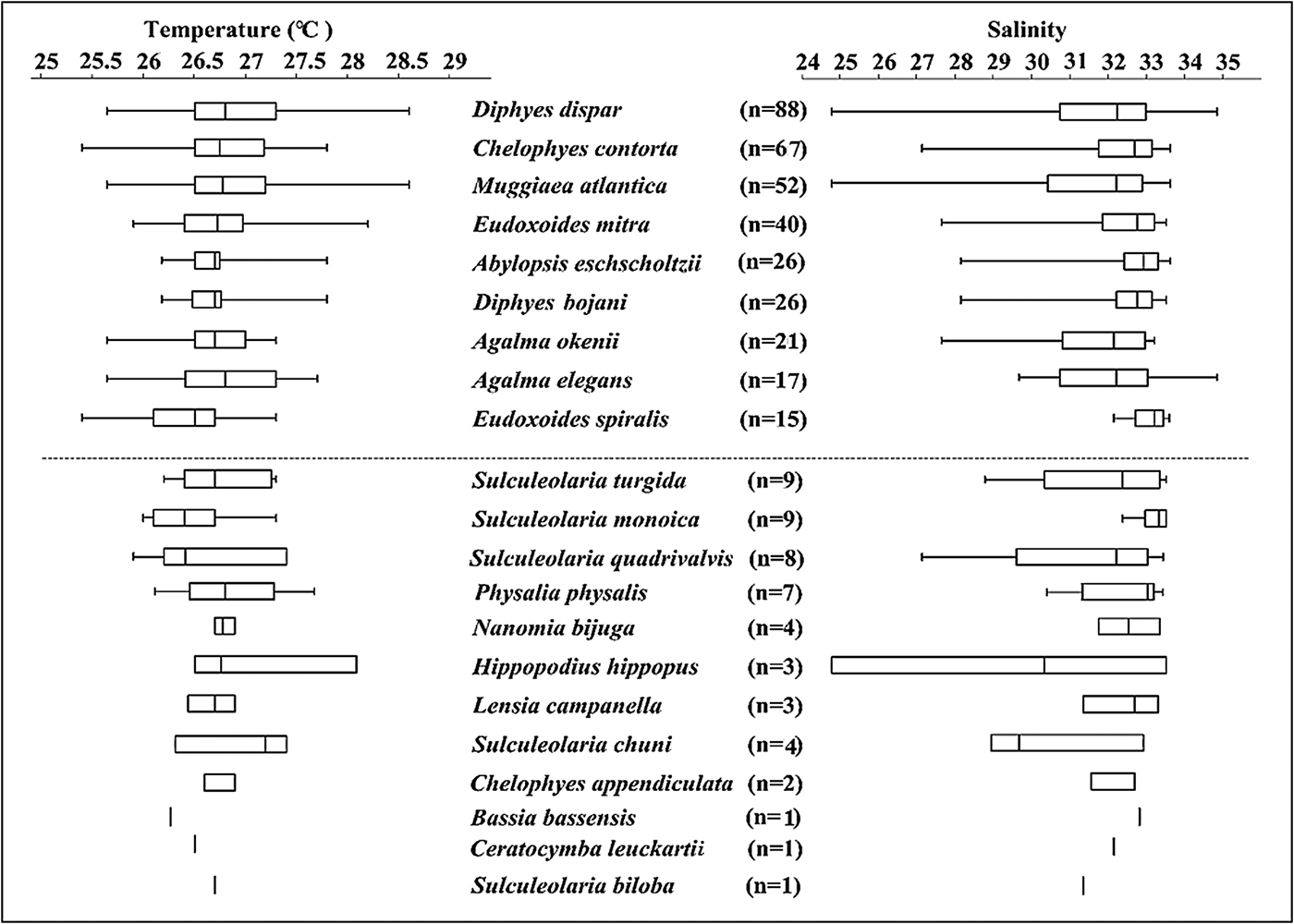
Fig. 6. Box and whisker plots of the occurrence of each siphonophore species identified during the five surveys in the Colombian Pacific Ocean in relation to temperature and salinity. The vertical bar is the median, the box is the 25th and 75th percentiles, and the whiskers represent the range. n is the number of stations where each species was present during the study.
Temperature was not significant in the Adonis model. Siphonophore species were most common in waters of 26–27.5 °C (Figure 6), and this could also simply reflect that there was more water of this temperature in the region (Figure 2). Common species such as D. dispar and M. atlantica were ubiquitous throughout the study area (25.6–28.6 °C) waters (Figure 6).
Siphonophore abundances
For the two cruises where we had abundance data, there was a significant positive correlation between species mean abundance and relative frequency (r = 0.90, N = 40, P = 0.00001), providing support for the use of presence:absence data as a measure of relative abundance. Mean densities (per 1000 m3) indicate that the most abundant species were D. dispar (mean = 447.6 ± 201.2, standard error), E. mitra (213.6 ± 85.5), C. contorta (189.7 ± 46) and M. atlantica (130.0 ± 39.7) (Table 4). Physonectae were less numerous, and of the two species of Agalma identified, A. okenii (53.3 ± 29.9) were slightly more numerous than A. elegans (40.1 ± 24.3). Densities of the reproductive stages of D. dispar were almost twice as high as those of the polygastric stages; 4.6 times in E. mitra, and almost 35 times more in D. bojani.
Table 4. Abundance of siphonophores species found in the Colombian Pacific Ocean on September 2002 and Sep–Oct 2004 and associated environmental data.

The linear model of Siphonophore abundance (response) showed that Cruise (P = 0.0004) and Time of Day (P = 0.05) were significant (r 2 = 33.4%), but Region, Temperature and Salinity were not significant (Figure 4B). Siphonophore abundance was greater in Sep–Oct 2004 than in Sep 2002, and was greater at Night than during the Day.
For the two cruises with abundance data, there was a significant positive correlation between siphonophore abundance and both total copepod abundance (r = 0.64, N = 40, P = 0.00001) and total fish eggs (r = 0.58, N = 40, P = 0.00001), suggesting that there could be bottom-up control of siphonophore numbers.
DISCUSSION
Our study provides new insights into the ecology of siphonophores in the hyponeuston of the Equatorial Pacific Ocean. We show that there is a moderate structuring of the siphonophore community by environmental conditions in the CPO. This structuring is a result of the Coastal-Oceanic effect, reflected in the gradient of salinity from inshore to offshore, and also the greater siphonophore species richness during the night. The effect of temperature did not play a significant role in structuring siphonophore communities in the CPO, even though there is a temperature gradient present during each cruise (warmer inshore), perhaps because of the narrow range of temperature observed during the study (3.5 °C). Our analysis suggests potential bottom-up control of the total number of siphonophores, as their density is related positively to the density of fish eggs and copepods in Sep 2002 and Sep–Oct 2004.
This is the first study to describe the surface-dwelling siphonophores from both oceanic and coastal regions of the CPO. Of the 21 species of siphonophores recorded, 17 were also recorded in the unpublished study of Cely & Chiquillo (Reference Cely and Chiquillo1993) from the coastal area of the CPO. Otherwise the species observed here from the CPO have all been recorded previously in the Pacific Ocean (Alvariño, Reference Alvariño1971; Pagès et al., Reference Pagès, Gili and Bouillon1990). Species such as A. eschscholtzii, Abylopsis tetragona, B. bassensis, C. appendiculata, C. contorta, E. spiralis, M. atlantica, S. chuni and S. quadrivalvis are widely distributed in tropical waters of the Eastern Pacific (Gasca & Suárez, Reference Gasca and Suárez1992a, Reference Gasca and Suárezb) and the Caribbean (Gasca, Reference Gasca1999), further west in the tropical Pacific (Pagès et al., Reference Pagès, Gili and Bouillon1990; Lo et al., Reference Lo, Kang and Hsieh2012, Reference Lo, Yu and Hsieh2014; Hsieh et al., Reference Hsieh, Yu and Lo2013) as well as cooler Chilean (Palma, Reference Palma1999; Palma & Silva, Reference Palma and Silva2004) and Peruvian (Ayón et al., Reference Ayón, Criales-Hernandez, Schwamborn and Hirche2008) waters. Most species of siphonophore have a near global distribution (Mackie et al., Reference Mackie, Pugh and Purcell1988), with all of the species found here reported from either the Atlantic (Pugh, Reference Pugh and Boltovskoy1999) or the Indian oceans (Daniel, Reference Daniel1974).
All species found in our study are epipelagic (Mackie et al., Reference Mackie, Pugh and Purcell1988; Mapstone, Reference Mapstone2014) and perform vertical migration (Pugh, Reference Pugh1984; Mackie et al., Reference Mackie, Pugh and Purcell1988), which explains the higher richness we found at night. Consistent with the idea that there is a peak in siphonophore richness in subtropical/tropical waters (Mackie et al., Reference Mackie, Pugh and Purcell1988; Boltovskoy, Reference Boltovskoy1999; Macpherson, Reference Macpherson2002; Pierrot-Bults & Angel, Reference Pierrot-Bults and Angel2013), we found more species (21 species) than in temperate waters such as off Chile (16 species, Palma, Reference Palma1999) and in the Mediterranean Sea (12 species, Thibault-Botha et al., Reference Thibault-Botha, Santo and Neauport2013).
Our study provides several other interesting findings that we will describe in more detail.
Calycophorans dominate the hyponeuston
Our samples were dominated by species of Calycophorae, both in terms of abundance (the seven most abundant species were all calycophorans) and richness, with six times as many species present as Physonectae. Only two species of physonects were commonly encountered (Agalma okenii and A. elegans), with a third (Nanomia bijuga) present in only four samples. Although calycophorans are usually more common than the more fragile physonects in plankton samples (Pugh, Reference Pugh1974; Gasca & Suárez, Reference Gasca and Suárez1992a, Reference Gasca and Suárezb; Mapstone, Reference Mapstone2014), this effect could have been exaggerated because many physonect species are generally found deeper in the water column than calycophorans (Robison et al., Reference Robison, Reisenbichler, Sherlock, Silguero and Chavez1998; Silguero & Robison, Reference Silguero and Robison2000). Similarly, no physonectids were found in other eastern Pacific surface waters surrounding Easter Island (Palma, Reference Palma1999) and Santa Clara Island in Ecuador (Andrade, Reference Andrade2012). Studies on siphonophores from other tropical, subtropical and temperate regions all indicate a predominance of calycophorans in the hyponeuston (Pagès et al., Reference Pagès, Gili and Bouillon1990; Andrade, Reference Andrade2012; Thibault-Botha et al., Reference Thibault-Botha, Santo and Neauport2013; Jeong et al., Reference Jeong, Suh, Lee and Soh2014).
Why are there fewer than expected siphonophore species in our study?
The number of species found in our study (21 species) is lower than other tropical studies in the eastern Pacific by Cely & Chiquillo (Reference Cely and Chiquillo1993) or Gasca & Suárez (Reference Gasca and Suárez1992a) (29 species in both studies), both of which collected fewer samples than our study. Here are two potential explanations.
First, both Cely & Chiquillo (Reference Cely and Chiquillo1993) and Gasca & Suárez (Reference Gasca and Suárez1992a) sampled throughout the water column and it is generally accepted that the number of siphonophore species in the hyponeuston is less than the number in the rest of the water column (Hempel & Weikert, Reference Hempel and Weikert1972; Pagès et al., Reference Pagès, Gili and Bouillon1990; Andrade, Reference Andrade2012). This is partly because physonects are relatively rare in the hyponeuston (Robison et al., Reference Robison, Reisenbichler, Sherlock, Silguero and Chavez1998; Silguero & Robison, Reference Silguero and Robison2000); we only recorded three species, although other factors might be at play.
The second potential explanation is that the Cely & Chiquillo (Reference Cely and Chiquillo1993) and Gasca & Suárez (Reference Gasca and Suárez1992a) studies both included upwelling conditions, whereas there was no upwelling during our study. There is generally greater richness of siphonophore species in upwelled water, such as off the Peruvian coast (Ayón et al., Reference Ayón, Criales-Hernandez, Schwamborn and Hirche2008), the Californian coast (Gasca & Suárez, Reference Gasca and Suárez1992b), the Dome of Costa Rica (Gasca & Suárez, Reference Gasca and Suárez1992a), the Benguela off South Africa (Thiriot, Reference Thiriot, Boje and Tomczak1978; Gibbons & Thibault-Botha, Reference Gibbons and Thibault-Botha2002; Thibault-Botha & Gibbons, Reference Thibault-Botha and Gibbons2005). During the study by Cely & Chiquillo (Reference Cely and Chiquillo1993) in the CPO, the combination of north-east trade winds (Rodríguez-Rubio & Wolfgang, Reference Rodríguez-Rubio and Wolfgang2003) and the development of the Colombian current (CCCP, 2002) led to active upwelling. Further, we found only one species of Lensia (L. campanella was found only three times), whereas Cely & Chiquillo (Reference Cely and Chiquillo1993) found three Lensia species (L. subtilis, L. hardy, L. subtiloides) during the upwelling season (Dec–Mar) in the CPO. Other studies in colder waters, such as Thibault-Botha et al. (Reference Thibault-Botha, Santo and Neauport2013) from the Bay of Marseilles, have found more (four) Lensia species. Lensia are often considered to be restricted to cooler waters (Mackie et al., Reference Mackie, Pugh and Purcell1988; Mapstone, Reference Mapstone2014). In tropical waters, Lensia might be more abundant in colder waters below the thermocline, and only reach the surface during active upwelling.
Potential allopatric relationship between Chelophyes appendiculata and Chelophyes contorta
It was surprising that C. appendiculata was rare in our study and that C. contorta was much more common, opposite to the findings of Andrade (Reference Andrade2012) and of Cely & Chiquillo (Reference Cely and Chiquillo1993). This difference can be explained by the suggested allopatric relationship in the distribution of C. appendiculata and C. contorta, with C. appendiculata replaced by C. contorta in warmer water (Alvariño, Reference Alvariño1971). Mean sea temperatures were considerably cooler in the study by Andrade (Reference Andrade2012) (22.8 °C) than the present study (26.9 °C), favouring C. appendiculata over C. contorta. Similarly, sea temperatures were also likely to be cooler in the study by Cely & Chiquillo (Reference Cely and Chiquillo1993) because the CPO in Feb–Mar is influenced by trade winds (Wyrtki, Reference Wyrtki1966) that stimulate oceanic upwelling (Rodríguez-Rubio & Wolfgang, Reference Rodríguez-Rubio and Wolfgang2003) and lead to cooler waters, again favouring C. appendiculata over C. contorta.
Warmer upper thermal limit for Muggiaea atlantica
Although M. atlantica is a widely distributed species in warm and cold temperate waters (Alvariño, Reference Alvariño1971; Mapstone, Reference Mapstone2014) and is probably the best-studied siphonophore (Thibault-Botha et al., Reference Thibault-Botha, Lutjeharms and Gibbons2004; Batistić et al., Reference Batistić, Lučić, Carić, Garić, Licandro and Jasprica2013; Blackett et al., Reference Blackett, Licandro, Coombs and Lucas2014, Reference Blackett, Lucas, Harmer and Licandro2015), our data indicate that M. atlantica can be found in temperatures up to 28.6 °C, far beyond the upper critical limit (24 °C) proposed by Batistić et al. (Reference Batistić, Lučić, Carić, Garić, Licandro and Jasprica2013). This could suggest some form of local population adaptation, perhaps with regard to eudoxid production.
Whilst M. atlantica has a wide distribution in tropical shelf waters of Colombia (Cely & Chiquillo, Reference Cely and Chiquillo1993) and Panamá (Alvariño, Reference Alvariño1971, Reference Alvariño1974), it is also common in the Humboldt Current (Bigelow, Reference Bigelow1911; Santander et al., Reference Santander, Luyo, Carrasco, Véliz and de Castillo1981) and in neritic and oceanic waters off Chile (Palma & Silva, Reference Palma and Silva2004; Pavez et al., Reference Pavez, Landaeta, Castro and Schneider2010). Indeed, Palma & Silva (Reference Palma and Silva2004) extend the distribution of this species to sub-Antarctic latitudes of Chile, where they found it at a range of temperatures from 4–13 °C. Temperatures >10 °C seem to stimulate eudoxid production for this species, as has been found by Blackett et al. (Reference Blackett, Licandro, Coombs and Lucas2014) in an extensive study in the Western English Channel. However, in warmer waters M. atlantica typically is replaced by Muggiaea kochii (Will, 1844) (Russell, Reference Russell1934; Alvariño, Reference Alvariño1971; Carré & Carré, Reference Carré and Carré1991), but this species was not observed in our study even though Alvariño (Reference Alvariño1974) recorded M. kochii in the Pacific waters of Panamá.
Lower salinity tolerances for some species
Siphonophores are an exclusively marine group and it is presumed that they have an aversion to low salinity water. Sanvicente-Añorve et al. (Reference Sanvicente-Añorve, Alba, Alatorre and Flores-Coto2007) investigated siphonophores in coastal waters of the Caribbean and extended the salinity tolerance of D. bojani, E. mitra, D. dispar and M. atlantica down to 30.7, but there was no water below this salinity in their study. Blackett et al. (Reference Blackett, Lucas, Harmer and Licandro2015) further extended the salinity tolerance of M. atlantica down to 28.5. We found C. contorta, D. bojani and E. mitra down to a salinity of ~27.5, and D. dispar and M. atlantica down to 24.7. These species thus have a lower salinity tolerance than previously recorded.
Reproductive and polygastric stages of calycophorans
We found that three dominant species of siphonophore (D. dispar, D. bojani and E. mitra) had more reproductive than polygastric stages, which suggests their populations are growing rapidly. For example, the number of reproductive stages of D. dispar were almost twice the number of polygastric stages in Sep 2002 and Sep–Oct 2004. Favourable environmental conditions for siphonophores stimulate the production of a high number of reproductive stages (Gasca, Reference Gasca1999). Interestingly, only polygastric stages of M. atlantica were found. The possibility that the reproductive stage of M. atlantica in tropical waters might be found in deeper waters where the temperature is more appropriate for its production warrants further study.
Siphonophore abundance is related to their prey abundance
We found siphonophore abundance was positively related to the abundance of copepods and fish eggs during Sep 2002 and Sep–Oct 2004. The top 13 most abundant species (D. dispar, E. mitra, C. contorta, M. atlantica, A. okenii, A. elegans, D. bojani, S. turgida, A. eschscholtzii, E. spiralis, L. campanella, S. quadrivalvis and S. monoica) all have positive correlations with both copepod abundance and fish eggs. These positive correlations imply bottom-up control of siphonophores by their common prey (Richardson & Schoeman, Reference Richardson and Schoeman2004). There is growing evidence that the abundance of siphonophores is related to their prey. In Mexican Caribbean waters, Sanvicente-Añorve et al. (Reference Sanvicente-Añorve, Alba, Alatorre and Flores-Coto2007) found a significant relationship between the community of siphonophores and the zooplankton wet biomass. In waters of the Mediterranean Sea, Fernández de Puelles et al. (Reference Fernández de Puelles, Alemany and Jansá2007) found a positive correlation between the abundance of Muggiaea, Lensia, Eudoxoides and Abylopsis, and abundance of copepods. In subtropical waters of Taiwan, Lo et al. (Reference Lo, Yu and Hsieh2014) suggested that the abundance and distribution of siphonophores are related to zooplankton biomass.
CONCLUSION
This study provides new insights into the poorly known hyponeuston of the tropics. The moderate structuring of the siphonophore community found in our study is likely to be related to the gradient of salinity from inshore to offshore in waters from the CPO in the non-upwelling season. However, further research is required to determine the effect of environmental variables on the diversity, distribution and abundance of siphonophore species from the Equatorial Tropical Pacific during upwelling. A more comprehensive study of this group of cnidarians during upwelling season also might help to clarify the finding from our study related to the trophic relation between the siphonophores community and their most common prey (copepods, fish eggs) in the hyponeuston and in the water column from coastal and oceanic regions of the CPO.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315417002065.
ACKNOWLEDGEMENTS
We thank the Vicerrectoría de investigaciones of the Universidad Nueva Granada and the Centro Control Contaminación del Pacífico CCCP-DIMAR, for supporting the research on zooplankton from the Colombian Pacific Ocean. We also want to thank the crew of the Colombian Research Platforms (ARC-Malpelo and ARC-Providencia) for their invaluable help in collecting samples and environmental information in the field. Thanks to Eddie Farid (Gorgona drifter), Gloria Giraldo from the Library of the Universidad de Bogotá Jorge Tadeo Lozano for bibliographic support, Andrea Uribe-Palomino for her collaboration gathering data in Colombia and Frank Coman for helping to review the drafts of the manuscript. The authors would like to dedicate this article to the memory of Dr Francesc Pagès and his work on siphonophores around the globe. The authors thank the three anonymous reviewers for their time and constructive comments that contributed to improvement of the manuscript.
FINANCIAL SUPPORT
This study is part of the project CIAS 2004–016, entitled: ‘Variación espacio temporal del zooplancton durante los cruceros de julio y septiembre de 2005 de la serie ERFEN en el océano Pacífico colombiano'. This project was financially supported by the Vicerrectoría de Investigaciones from the Universidad Militar Nueva Granada – Colombia.