Introduction
The phylum Rotifera is one of the most common, abundant and widespread groups of microscopic animals in any continental aquatic environment (Fontaneto & De Smet Reference Fontaneto, De Smet and Schmidt-Rhaesa2015, Sa-ardrit et al. Reference Sa-ardrit, Pholpunthin and Segers2013). Rotifers are aquatic organisms, but they are also able to withstand periods without water such as drought and frost due to their desiccation capabilities (Ricci Reference Ricci2001). The two main groups of rotifers have differential adaptations to desiccation. For the Monogononta, generally the adult individuals die in the absence of water (García-Roger et al. Reference García-Roger, Serra and Carmona2014), but the product of sexual reproduction, called resting egg, survives (Pourriot & Snell Reference Pourriot and Snell1983); for the Bdelloidea newborn, young stages and adult can survive, but for most species eggs are not desiccation-tolerant (Kaczmarek et al. Reference Kaczmarek, Roszkowska, Fontaneto, Jezierska, Pietrzak, Wieczorek, Poprawa, Kosicki, Karachitos and Kmita2019, Ricci Reference Ricci1987, Reference Ricci and Dallai1992). Such different desiccation strategies, quicker for bdelloids and requiring more time for monogononts, have the consequence that bdelloids are better adapted to highly ephemeral waters and limno-terrestrial habitats such as mosses, lichens and soil (Donner Reference Donner1965, Ricci Reference Ricci1987, Reference Ricci2001), whereas monogononts are more common and abundant in permanent water bodies and ephemeral water bodies that do not dry out too rapidly (Wallace et al. Reference Wallace, Snell, Ricci, Nogrady and Dumont2006).
Most ecological studies on rotifers in aquatic habitats deal with monogonont rotifers, especially in the plankton (Herzig Reference Herzig1987). The group Monogononta represents about 75% of the species diversity of the phylum, whereas Bdelloidea, with less than 500 known species (Segers Reference Segers2007), is a group of rotifers that is rarely considered in ecological studies of aquatic habitats, and mostly in limno-terrestrial habitats (Fontaneto et al. Reference Fontaneto, Ficetola, Ambrosini and Ricci2006). In addition, identifying most bdelloid species can be reliably done only from living and active organisms (Wallace et al. Reference Wallace, Snell, Ricci, Nogrady and Dumont2006). Moreover, the only available complete identification key was written in German (Donner Reference Donner1965), limiting its general use.
Here, we deal with an unusual survey, given that we focus on the genus Rotaria in several aquatic habitat types across one tropical country, Thailand, trying to disentangle the environmental correlates of biodiversity. The genus Rotaria is a common group of mostly freshwater taxa, with about 25 species. Many Rotaria species, as well as other rotifer species (Fontaneto et al. Reference Fontaneto, Barbosa, Segers and Pautasso2012), are well known from temperate and very little known from tropical areas. Thailand is potentially one of the best known areas for rotifers in tropical South-east Asia (Sa-ardrit et al. Reference Sa-ardrit, Pholpunthin and Segers2013), and previous studies on Rotaria revealed eight species, including one new to science and up to now still known only from Thailand (Jaturapruek et al. Reference Jaturapruek, Fontaneto, Meksuwan, Pholpunthin and Maiphae2018).
Most of the data on the environmental correlates of the occurrence of species of Rotaria come from the temperate zones (Bērziņš & Pejler Reference Bērziņš and Pejler1987, Reference Bērziņš and Pejler1989; Pejler Reference Pejler1995, Pejler & Bērziņš Reference Pejler and Bērziņš1993). Such a bias is common, not only in Rotaria, but also for rotifers in general (Fontaneto et al. Reference Fontaneto, Barbosa, Segers and Pautasso2012, Reference Fontaneto, Iakovenko and De Smet2015; Obertegger et al. Reference Obertegger, Thaler and Flaim2010). The present study aims to address the knowledge gap in tropical bdelloids living in aquatic habitats by analysing which environmental parameters may drive species diversity in hundreds of sampled communities across Thailand.
Materials and methods
Description of the study site
Thailand is a tropical country located in the middle of mainland South-east Asia between latitude 5°37′–20°27′N and longitude 97°22–105°37′E (Setapan Reference Setapan1999). The geography of this country is diverse with high mountains, several watersheds, marine and freshwater habitats. Thus, a number of species are expected, given the heterogeneous ecosystems.
Aquatic habitats were chosen for this study because most Rotaria have been recorded in aquatic habitats around the world (Donner Reference Donner1965). Rotaria were collected throughout Thailand covering six regions: North (N), Northeast (NE), West (W), Central (C), East (E) and South (S). Different type of water bodies, such as swamps, marshes, peat-swamps, ponds, rice fields, canals and rivers were analysed, in order to include the largest possible diversity of Rotaria.
Habitats were categorized in 15 types by their characteristics (Keddy Reference Keddy2010). A ‘swamp’ was defined as a wetland that is forested and occurs close to large rivers or lakes where it is critically dependent on natural water level fluctuations; some swamps were covered by aquatic vegetation, and were called ‘vegetative swamp’ or ‘algae swamp’ depending on the type of aquatic vegetation. A ‘marsh’ was defined as a wetland dominated by herbaceous vegetation such as grasses, rushes or reeds rather than woody plant species and often found at the edge of lakes and streams. A ‘peat swamp’ was defined as a wetland where waterlogged soil prevents dead leaves and wood to fully decompose; over time, this formed a thick layer of acidic peat. A ‘pond’ was defined as an area of standing water, either natural or artificial, which is smaller than a ‘lake’ (less than 80 000 m2), and could be covered by vegetation (‘vegetative pond’). A ‘rice field’ was defined as a flooded parcel used for agricultural rice crops. A canal was defined as a waterway channel or artificial waterway of running (‘canal RW’) and standing water (‘canal SW’) smaller and shallower than a river; some standing water canals were covered by algae (‘algae canal SW’) or by vegetation (‘vegetative canal’). A ‘river’ was defined as a natural flowing watercourse, usually freshwater, flowing towards an ocean, sea, lake or another river. A ‘reservoir’ was defined as a man-made body of standing water.
Sampling, sorting and examination
A total of 390 samples was taken by qualitative method among aquatic plants and water bodies with a plankton net of 20 μm mesh size. All samples were maintained alive in bottles without chemical preservation and observed in the laboratory within a few hours for sorting and identification. Sampling was conducted from May 2015 to June 2019. Some samples were taken from the same water body, but from different sites, different seasons and/or different habitats to cover their microhabitats as completely as possible. Several environmental measurements were taken in the field, including water temperature (ºC), conductivity (μs cm−1), total dissolved solids (mg L−1), salinity (ppt), dissolved oxygen (mg L−1) and pH, using a calibrated water analysis checker (YSI EXO1 Multiparameter Sonde and YSI EXO Handheld Display 599150). Coordinates (latitude and longitude in WGS84 system) and elevation were obtained by GPS tracker (Garmin eTrex Summit). The detailed habitat information and sampling dates for each locality are listed in Supplementary Table S1 and Figure 1.

Figure 1. Map of sampling sites in Thailand. The north direction and a geographic scale in kilometres are reported. Code numbers are explained in Supplementary Table S1.
The active animals were sorted with a pipette under a stereomicroscope (Olympus SZ51) at magnifications up to 80×. The taxonomically relevant morphological characteristics, such as shape and length of whole body, rostrum, trunk, foot, spurs and toes were observed under compound light microscope (Olympus CH-2) at magnification up to 400×. The identification of species of Rotaria was performed following Donner (Reference Donner1965), Ricci & Melone (Reference Ricci and Melone2000) and the recent update of Jaturapruek et al. (Reference Jaturapruek, Fontaneto, Meksuwan, Pholpunthin and Maiphae2018).
Species richness
Species richness of the genus Rotaria was counted as the total number of species in the genus for each sample. We also estimated how much of the potential diversity for the country was actually observed by using extrapolations from species accumulation curve. First-order Chao (Chao 1), first-order jackknife (jackknife 1), and bootstrap estimates were calculated to obtain a potential estimated number of species (Colwell & Coddington Reference Colwell and Coddington1994). The analyses were performed in R v3.3.3 (R Core Team 2017) using the package vegan v2.4-5 (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2017).
We searched for potential environmental drivers of species richness. First we analysed which explanatory variables could affect the occurrence of the genus, using presence/absence data as a response variable in a generalized linear model (GLM) with binomial error structure; then we performed a similar analysis with species richness as a response variable in a GLM with Poisson error structure. As explanatory variables we included: a set of measurements obtained directly in the field, namely habitat type, water temperature, conductivity, salinity, total dissolved solids (TDS), dissolved oxygen (DO), pH, a set of measurements of bioclimatic variables, namely actual evapotranspiration (AET), precipitation, mean air temperature (MAT) and solar radiation (SRAD). The bioclimatic variables were obtained from the WorldClim2 database with average data from the period 1970–2000 (Fick & Hijmans Reference Fick and Hijmans2017), extracted using the geographic coordinates of the sites, from raster layers of 30 seconds resolution (~1 km2). Bioclimatic data were gathered, handled and averaged by year using the R packages raster v2.6-7 (Hijmans Reference Hijmans2017) and rgdal v1.2-16 (Bivand et al. Reference Bivand, Keitt and Rowlingson2017).
Before performing any analysis, we checked for multicollinearity between explanatory variables, using the logarithmic transformation for three of them (conductivity, salinity and TDS). Three variables revealed Pearson’s r correlation values higher than 0.6: one of them, conductivity, was retained, whereas the other two, salinity and TDS, were removed from the models. Model fit was assessed by visual inspection of normality of histograms of residuals and plots of residual versus fitted values, normal Q-Q, scale-location, and residual versus leverage values (Crawley Reference Crawley2012). All analyses were performed in R. The outputs of GLMs are reported as Analysis of Deviance Tables, using the package car v2.1.6 (Fox & Sanford Reference Fox and Sanford2011).
Species composition
Differences in species composition were assessed using a Jaccard index of community dissimilarity (Wolda Reference Wolda1981). In order to test for the effect of limnological and bioclimatic variables on differences in species composition, a Permutational Analysis of Variance (PERMANOVA) was performed using the same explanatory variables used in the analyses of species richness. Such analysis was performed with the function adonis in the R package vegan. To visualize the relationship between species composition and habitats, a Two-Way Cluster Analysis (TWCA) was performed using PC-ORD program version 7 (McCune & Mefford Reference McCune and Mefford2016). However, as this approach can deal only with data where the genus was present, three habitat types including lake, reservoir and vegetative canal were excluded from this analysis.
Results
Species richness
A total of nine species, namely Rotaria macrura (Ehrenberg, 1832), R. megarostris Jaturapruek, Fontaneto, Meksuwan, Pholpunthin & Maiphae, Reference Jaturapruek, Fontaneto, Meksuwan, Pholpunthin and Maiphae2018, R. mento (Anderson, 1889), R. neptunia (Ehrenberg, 1830), R. neptunoida Harring, 1913, R. ovata (Anderson, 1889), R. rotatoria (Pallas, 1766), R. tardigrada (Ehrenberg, 1830), and a yet undescribed species, here called Rotaria sp., were identified in this study (Table 1). The highest number of species was recorded from Na Tub vegetative swamp, Songkhla (sample number 390 in Supplementary Table S1), with six species, followed by the vegetative swamp opposite dorm 13, Kasetsart University, Bangkok (sample number 286), with five species. Rotaria neptunia was the most common species (found in 76 of the 390 samples), followed by R. rotatoria (62 samples); R. macrura and Rotaria sp. were rare species, Rotaria sp. occurring in only one sample (Table 1).
Table 1. Occurrence of bdelloid rotifers of the genus Rotaria in the 390 samples from Thailand from this study. The number identifying each sampling site refers to Figure 1 and Supplementary Table S1. * New record for Thailand and new to Oriental region.

One of the nine species, R. macrura, was a new record for Thailand and new to the oriental region. R. macrura was recorded in two parts of Thailand: KM. 62 Chiang Muan district vegetative swamp, Phayao (sample number 17) and NP12 vegetative swamp, Nakhon Pathom in different seasons (sample number 264 and 266). Most of the species have a wide range of distribution in the country: R. megarostris, R. mento, R. neptunia, R. ovata and R. tardigrada were found in every part of Thailand; R. neptunoida and R. rotatoria were reported in five parts, except in the eastern part of the country, whereas Rotaria sp. was recorded only in one specimen and restricted to a standing water canal opposite the Faculty of Engineering, Kasetsart University (sample number 273).
The distribution of the nine species in the 390 samples provided estimates of total species for the genus in Thailand to range from 9.0 ± 0.5 (Chao 1 estimator ± standard error), to 9.4 ± 0.5 (bootstrap) and 9.9 ± 0.99 (jackknife 1).
The occurrence of the genus Rotaria in the 390 samples in Thailand was significantly related to dissolved oxygen (Table 2), even if this variable had an adjusted R2 of only 2.3%: on average, samples without Rotaria had higher oxygen levels than the ones where Rotaria was present (Figure 2). Although pH did not significantly affect the presence of Rotaria, we noted that Rotaria was absent at sites with high pH, which were also the ones at high values of dissolved oxygen. Habitat type significantly affected occurrence of Rotaria as a genus (Table 2), which were absent from lakes, reservoirs and vegetative canals. None of the other variables revealed any significant effect at the genus level (Table 2).
Table 2. Results of the Generalized Linear Model with binomial error structure to explain the presence/absence of the genus Rotaria in the 390 samples from Thailand. The output of an Analysis of Deviance Table is reported, with Likelihood Ratio Chi-squared test (LR), degrees of freedom (df), P values (P) and adjusted R2 values.


Figure 2. Histograms of the frequency distribution of the values of dissolved oxygen in sampling sites where Rotaria were absent or present. Rotaria had a tendency to disappear at higher values of dissolved oxygen.
Species richness of Rotaria, including only the 155 samples where the genus was present, was not affected by any of the limnological or bioclimatic variables that we analysed (Supplementary Table S2), even if differences in habitat type explained 4.4% of the variability and actual evapotranspiration 2.3%.
Species composition
The only significant variable explaining differences in species composition was habitat type (Table 3), with 13.3% of explained variance. None of the other limnological and bioclimatic variables reached 1.5% of explained variance (Table 3). The most dissimilar habitat from the others was river (Figure 3), whereas no clear further subdivision in groups could be highlighted (Figure 3).
Table 3. Output of the permutational analysis of variance to explain differences in species composition (Jaccard index) in relationship to limnological and bioclimatic variables. R2 values and P values are reported.

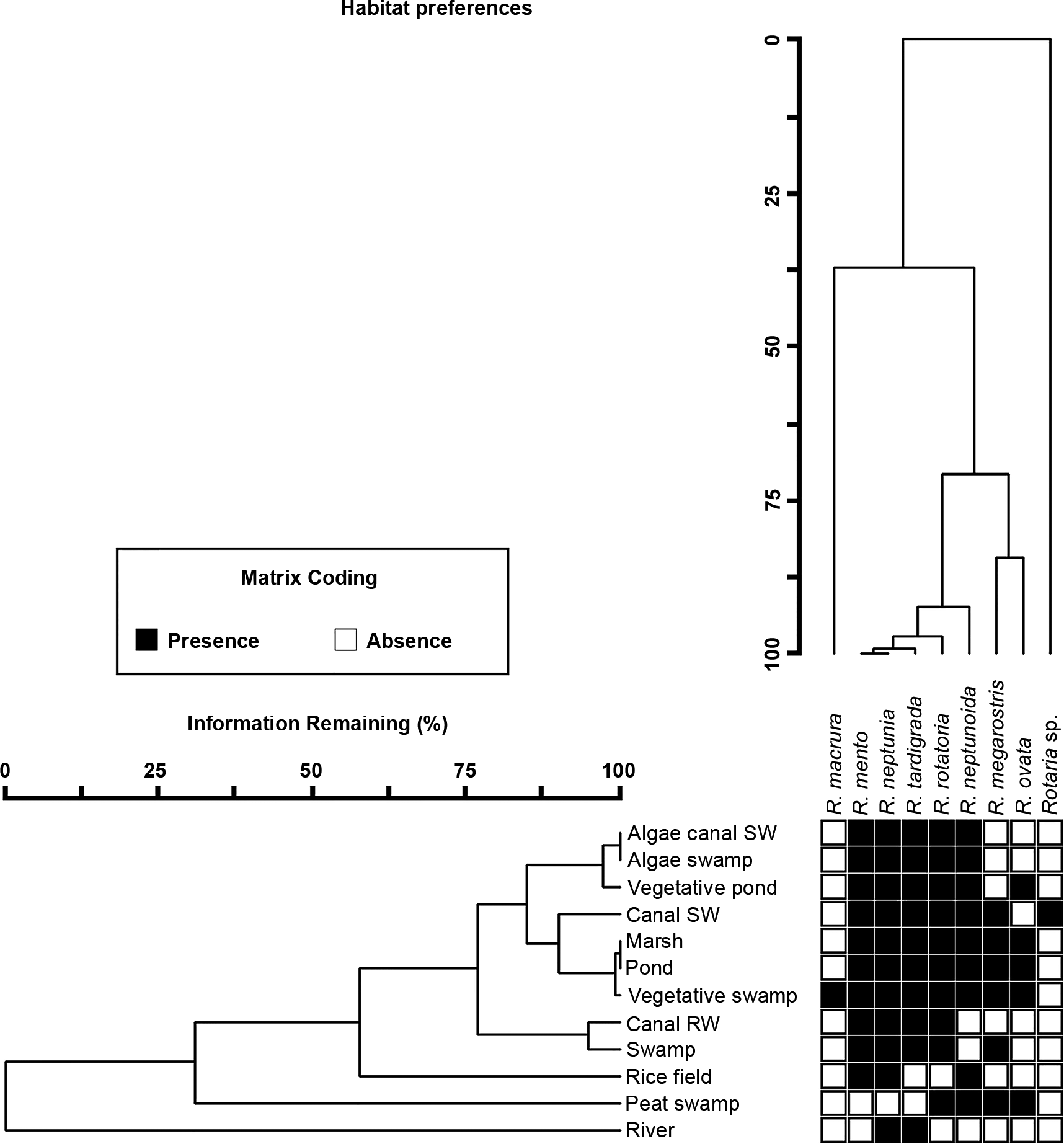
Figure 3. Habitat preferences of Rotaria reported as a two-way cluster plot with species and habitats ordered according to their similarity in species occurrence.
Discussion
Most species of the genus Rotaria found in this study were already known in Thailand, except for R. macrura, which was encountered for the first time in South-east Asia after it was recorded from Korea (Song & Lee Reference Song and Lee2019), extending its distribution in the tropical region. One other species, Rotaria sp., was found only once and there are some characters that make this species different from any other previously known species. However, the detailed characters were insufficient to clarify its status. The species diversity of Rotaria in Thailand increased from eight (Jaturapruek et al. Reference Jaturapruek, Fontaneto, Meksuwan, Pholpunthin and Maiphae2018) to 10 species. This confirmed that the effect of sampling effort still exists on faunistic studies in rotifers, even in relatively well known areas (Fontaneto et al. Reference Fontaneto, Barbosa, Segers and Pautasso2012, Jaturapruek et al. Reference Jaturapruek, Fontaneto, Meksuwan, Pholpunthin and Maiphae2018). However, the nine species found in our survey matched the expected richness by the estimators, which yielded an estimate of 9–10 species. Such estimates indicate that our sample collections seem to cover the actual diversity of this genus in Thailand for the habitat types we sampled, making our dataset reliable for ecological inference.
The limnological factors that significantly affected the occurrence of the genus Rotaria, expressed as presence/absence, were dissolved oxygen and habitat type (Table 2). Individuals of Rotaria tended to be present at lower values of dissolved oxygen at 0.2–13 (mg L−1), most preferred sites at ~4.5–6.8 mg L−1 (Figure 2). A lack of effect of other limnological and bioclimatic variables, confirmed for the occurrence of the genus and for species richness, might be the actual pattern. One explanation for the absence of effects on species richness could be related to compensatory effects, with different species displacing each other under different environmental regimes (Fischer et al. Reference Fischer, Frost and Ives2001). Another explanation could be that other unmeasured limnological variables could be more essential in driving species diversity in Rotaria, for example chlorophyll, as algae are known as the preferential food for some species of Rotaria (Donner Reference Donner1965).
Differences in species composition of Rotaria seem to be related to habitat types (Table 3). Although bdelloid rotifers, including Rotaria, are small, disperse easily, and are able to withstand harsh conditions, allowing most species to become nearly cosmopolitan (Fontaneto Reference Fontaneto2019), habitat filtering still exists (Fontaneto et al. Reference Fontaneto, Westberg and Hortal2011). Habitat type, with all the inherent ecological differences between habitats, is indeed a strong selecting factor not only in bdelloid rotifers (Fenchel & Finlay Reference Fenchel and Finlay2004, Fontaneto et al. Reference Fontaneto, Westberg and Hortal2011), but also in other freshwater organisms (e.g. Bohonak & Jenkins Reference Bohonak and Jenkins2003, Cohen & Shurin Reference Cohen and Shurin2003). In the case of Rotaria in Thailand, species composition between rivers and other habitats was highly different; moreover, more Rotaria species live in vegetated than in non-vegetated habitats (GLM: z = 4.4, P <0.001). The existence of vegetation in water bodies brings more microhabitats (Maiphae et al. Reference Maiphae, Pholpunthin and Dumont2005), increasing complexity of aquatic ecosystems (Kuczyńska-Kippen Reference Kuczyńska-Kippen2018). The importance of vegetation may be linked to the fact that only three species, namely R. neptunia, R. rotatoria and R. tardigrada, occurred in both standing and running water. If we assume that they occur in the littoral vegetated areas also of open running waters, their behavioural traits may explain their occurrence. Rotaria neptunia and R. rotatoria are considered good swimmers (Kuczyńska-Kippen Reference Kuczyńska-Kippen2018, Ricci & Melone Reference Ricci and Melone2000) and move frequently also in the open plankton, even if they were never found in lakes in our survey, where the distance to the littoral habitats is excessive for them. On the other hand, R. tardigrada commonly attaches to the substrate but has been reported also from open waters (Koste & Shiel Reference Koste and Shiel1986). Thus, even a small propensity for swimming in this species could have allowed it to be found in running waters, displaced from aquatic plants along the canal and river while swimming. In addition, we note that from the only 97 samples where we measured chlorophyll a content, R. neptunia was mostly recorded from meso-eutrophic habitats (chlorophyll a at 3.9–24.1 μg L−1) (Supplementary Table S1). Our results support the suggestions from previous studies, reporting R. neptunia from eutrophic habitats (Sládeček Reference Sládeček1983).
Overall, our research enhanced knowledge of bdelloid rotifers of the genus Rotaria in a tropical region by increasing the species diversity from eight to 10 species, of which one was a new record for Thailand and another one was a yet unidentified species. Such diversity could reliably represent the actual number of species for this genus in Thailand. In addition, we also tested the effect of limnological and bioclimatic variables on species richness and species composition. The results supported previous suggestions that parameters such as habitat type, potentially related to the level of habitat complexity, play a major role in driving species diversity of bdelloid species (Fontaneto et al. Reference Fontaneto, Ficetola, Ambrosini and Ricci2006, Reference Fontaneto, Westberg and Hortal2011; Kuczyńska-Kippen Reference Kuczyńska-Kippen2018).
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S0266467421000018
Acknowledgements
We are grateful to Thanida Saetang, Natthida Jantawong and Watcharapong Boonnam for help in field sampling throughout Thailand.
Financial support
This research was supported by Science Achievement Scholarship of Thailand (SAST), Thailand Research Fund (RSA6080032), The Graduate School and Department of Zoology, Faculty of Science, Kasetsart University.
Ethical statement
The present study was approved by the ethics committee of Kasetsart University (approval no. ACKU 59-SCI-004) for collecting Rotaria specimens.









