Introduction
Both Sitobion avenae (Fabricius) (Sa) and Rhopalosiphum padi (Linnaeus) (Rp) are the most important pests, causing damage by direct feeding and as vectors of numerous plant pathogenic viruses and usually coexist on the late period of wheat growth in China. The control of Sa and Rp is pri-marily dependent on the application of insecticides, but the resistance of both aphid species developed slowly in virtue of their short outbreak periods (Chen et al., Reference Chen, Han, Qiao and Qu2007). According to the Chinese cropping system, many natural enemies live through the winter in wheat fields and migrate to cotton or corn fields after the wheat harvest. Therefore, selective insecticides are recommended to conserve natural enemies (Gao et al., Reference Gao, Liu, Zhao and Zheng1991; Chen et al., Reference Chen, Han, Qiao and Qu2007). Pirimicarb is a selective carbamate insecticide and is often applied in aphid management programs (Gao et al., Reference Gao, Liu, Zhao and Zheng1991). Pirimicarb has been used for the control of wheat aphids since the 1990s in China. Difference in the efficacy of pirimicarb between the above two aphid species has been found in wheat field of He'nan (Liu et al., Reference Liu, Ru, Wang and Li2001). Bioassays showed that both aphid species differ in their susceptibility to insecticides. Rp was more susceptible than Sa to most of insecticides used for wheat aphid control.
The objectives of this research were: (i) to evaluate the synergistic effects of PBO, DEF and DEM on differential susceptibilities to pirimicarb; (ii) to determine the activity difference in general esterases and glutathione S-transferases (GST); and (iii) to determine the sensitive difference of acetylcholinesterase (AChE) and carboxylesterase (CarE) to pirimicarb between the two aphid species. These research results are very significant to elucidate differential susceptibility mechanisms of pirimicarb between the two aphid species and to develop an efficient resistance management strategy.
Materials and methods
Insects
Colonies of both aphid species were established from field collections in May 2005 from the same wheat field of the Agricultural Experiment Station, China Agricultural University. They have been maintained in the laboratory without insecticide exposure since May 2005. Two colonies were maintained on wheat seedlings at 18–25°C, a photoperiod of 17:7 h (L:D) and relative humidity 50–70% (Lu & Gao, Reference Lu and Gao2007). Apterous adults were collected in 1.5 ml microcentrifuge tubes and immediately stored at −80°C.
Chemicals
Pirimicarb (95% a.i.) was obtained from Wuxi Ruize Chemical Co. Ltd, China. Piperonyl butoxide (PBO, 98%) and S,S,S-tributylphosphorotrithioate (DEF, 98%) were purchased from Chem Service (West Chester, PA). Acetylthiocholine iodide (ATCh), butyrylthiocholineiodide (BuTCh), acetyl-(β-methyl) thiocholine iodide (MeTCh), propionylthiocholine iodide (PrTCh), bovine serum albumin (BSA), 1-chloro-2,4-dinitrobenzene (CDNB), reduced glutathione (GSH), eserine sulfate, sodium dodecyl sulfate (SDS), α-naphthyl acetate (α-NA), β-naphthyl acetate (β-NA), α-naphthyl caprylate (α-NC), α-naphthyl butyrate (α-NB), ethylenediaminetetraacetic acid (EDTA), fast blue RR salt, 1,2-dichloro-4-nitrobenzene (DCNB), diethyl maleate (DEM), TritionX-100 and 1,5-bis(4-allyldimethyl-ammoniumphenyl)-pentan-3-one dibromide (BW284C51) were purchased from Sigma Chemical Company (St Louis, USA). Coomassie brilliant blue G-250 was purchased from Fluca (Buchs, Switzerland). 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB) and fast blue B salt (O-dianisidine, tetrazotized) were purchased from Fluka Chemical Company (St Quentin, France).
Insecticide bioassays
Insecticide toxicity was assayed using the method of residual film in glass tubes described by Shotkoski et al. (Reference Shotkoski, Mayo and Peters1990) and Shufran et al. (Reference Shufran, Wilde and Sloderbeck1997) with some modifications to the glass tubes (diameter: 2 cm; inner surface: 36 cm2). According to the method of residual film in glass tubes, each insecticide was diluted to the 6–7 required concentration in analytical grade acetone. An aliquot of 200 μl insecticide-acetone solution was applied to every tube and was immediately rotated by using a micro-rotator (American Wheaton Company). Twenty aphids were treated for each concentration with three replications. Controls were used in tubes treated with acetone only. The treated aphids were reared routinely and mortality was checked after three hours, according to the method of Shotkoski et al. (Reference Shotkoski, Mayo and Peters1990) and Shufran et al. (Reference Shufran, Wilde and Sloderbeck1997), with some modifications. Adults failing in exhibiting repetitive (i.e. non-reflex) movement of more than one leg (after gentle prodding, if necessary) were assumed dead (Moores et al., Reference Moores, Gao, Denholm and Devonshire1996).
Synergism bioassays
Toxicity of pirimicarb in the presence and absence of synergists, DEF, PBO and DEM, was evaluated by the bioassay method, as described previously. The maximum sublethal dose and treatment time for synergist were determined according to Liu & Yue (Reference Liu and Yue2000), Scott et al. (Reference Scott, Foroozesh, Hopkins, Alefantis and Alworth2000), Yang et al. (Reference Yang, Zhu, Buschman and Margolies2001) and Mohammadi et al. (Reference Mohammadi, Hejazi, Mohammadi and Rashidi2007). Synergists, DEF, PBO and DEM, were applied at the maximum sublethal dose (final concentrations were 0.039 μg cm−2 for DEF, 0.028 μg cm−2 for PBO and 0.14 μg cm−2 for DEM, respectively) one hour before the insecticide treatment. The synergist alone was used as control. Mortality was recorded after three hours. Synergistic ratios were calculated using the conventional approach of dividing LC50 without the synergist by LC50 with the synergist.
AChE activity and inhibition
Batches of approximate 0.1 g frozen apterous adults were manually homogenized in 1 ml of 0.1 M ice-cold phosphate buffer (pH7.5 containing 0.5% Triton X-100). The procedure was performed on ice. Homogenates were centrifuged at 4°C, 10,000 g (Eppendorf centrifuge 5417R, Germany) for 30 min. The supernatant was used as an enzyme source for measuring the activity of AChE and proteins. Acetylcholinesterase (AChE) activity was measured using the method of Ellman et al. (Reference Ellman, Courtey, Andres and Featherstone1961) with minor modifications by Gao (Reference Gao1987). Briefly, for each reaction, 25 μl substrate (5 mM) and 25 μl enzyme were incubated at 30°C for 15 min. The reaction was stopped by the addition of 900 μl DTNB (0.125 mM) with 40% ethanol, and the optical density (OD) was measured at 412 nm by spectrophotometer (Lambda Bio 40). The control samples contained no enzyme during the incubation. After the addition of the color reagent, appropriate amounts of enzyme solutions were added to the controls. ATCh, PrTCh, MeTCh and BuTCh were used as substrates for AChE activity measurements.
For the AChE sensitivity experiment, pirimicarb was first dissolved in acetone and then diluted into the desired concentrations with phosphate buffer (0.1 M, pH 7.5). An aliquot of 20 μl enzyme was incubated with 5 μl insecticide solution of different concentrations at 30°C for 5 min prior to the addition of the substrate, and the final concentration of acetone was less than 1%. Then, 25 μl ATCh (5 mM) were added to the mixture. The residual AChE activity was determined according to the above method. The value of median inhibition concentration (IC50) for pirimicarb was determined based on log (inhibitor concentration) vs. probit (percentage of inhibition) linear regression.
Purification of AChE from both aphid species was performed by affinity chromatography using procainamide as an affinity ligand (Gao & Zhu, Reference Gao and Zhu2001).
CarE activity and inhibition
To determine carboxylesterase (CarE) activities, batches of approximate 0.1 g frozen apterous adults were manually homogenized in 1 ml ice-cold, 0.04 M phosphate buffer (pH7.0). Homogenates were centrifuged at 4°C, 10,000 g (Eppendorf centrifuge 5417R, Germany) for 30 min, and the supernatants were collected as enzyme sources. CarE activities were measured at 30°C by the method of van Asperen (Reference van Asperen1962) with some modifications using α-NA, α-NB, α-NC and β-NA as substrates. For a separate sample, 1.8 ml homo-genization buffer containing substrate (3×10−4 M) and eserine (3×10−4 M), and 50 μl enzyme diluted from the enzyme preparation with 0.04 M phosphate buffer (pH 7.0) were added to each reaction. The mixture was incubated for 15 min and the enzyme reaction was stopped by the addition of 900 μl fast blue B-SDS solution. The absorbance was determined at 600 nm for α-NA, α-NB and α-NC, and at 555 nm for β-NA using a spectrophotometer (Lambda Bio 40). The optical density (OD) values were converted to the production of naphthol μmol⋅min−1⋅mg−1 protein through naphthol standard curves and protein values.
The α-NA-hydrolyzing esterase activity in individual aphids (110 apterous adults for each species) was determined by the method of Moores et al. (Reference Moores, Gao, Denholm and Devonshire1996), using the microplate assay format with some modifications. Briefly, a single aphid was homogenized in 60 μl ice-cold 0.04 M phosphate buffer (pH 7.0). The homogenates were centrifuged at 10,000 g (Eppendorf centrifuge 5417R, Germany) for 10 min at 4°C. A separate sample containing 50 μl supernatants, 150 μl substrate (α-NA 100 μM) and fast blue RR salt (1.5 mM) mixture filtered prior to use, and 50 μl buffer were added to each well of microplate, the total volume being 250 μl. Reactions were immediately monitored for 5 min with 10 s intervals at 450 nm, 25°C by a Thermomax microplate reader (Tecan Spectra). The activity of esterase was expressed as the slope of linear regressions.
For the sensitivity test, a stock solution of insecticide or synergist was prepared at 10 mM in acetone and diluted to the desired concentrations with phosphate buffer. Enzyme was incubated with the insecticide or synergist at 30°C for 15 min prior to the addition of the substrate, and the final concentration of acetone was less than 1%. A control was included for each experimental run. Each compound was used with at least five concentrations. The value of median inhibition concentration (IC50) was determined based on log (inhibitor concentration) vs. probit (percentage of inhibition) linear regression.
Activity and kinetic analysis of GSTs
Glutathione S-transferase (GST) activity was determined in apterous adults of Sa and Rp using CDNB and DCNB as substrates as described by Habig et al. (Reference Habig, Pabst and Jackoby1974). Batches of approximate 0.1 g frozen apterous adults were homogenized in 1 ml ice-cold, 0.1 M phosphate buffer (pH6.5) containing 1 mM EDTA. Homogenates were centrifuged at 10,000 g (Eppendorf centrifuge 5417R, Germany) for 30 min at 4°C, and the supernatants were collected as enzyme sources. For assay of GST activity, briefly, the assay mixtrue (the total volume was 900 μl) contained 1 mM CDNB or DCNB and 1 mM GSH. The assay was initiated by the addition of 50 μl enzyme for Rp (20 μl for Sa); the absorbance at 340 nm for CDNB or 345 nm for DCNB was monitored for 2 min with 10 s intervals by spectrophotometer (Lambda Bio 40). Controls without enzyme always accompanied each assay. Activity was calculated with an extinction coefficient of 9.6 mM−1cm−1 for CDNB or 8.5 mM−1cm−1 for DCNB. Enzyme activity was expressed as nmol min−1 at 25°C, and the specific activity as nmol⋅min−1⋅mg−1 protein. Michalelis constants (K m) and maximal velocities (V max) were determined by double-reciprocal Lineweaver-Burk plots.
Determination of protein contents
Protein content of the enzyme preparations was determined according to the method of Bradford (Reference Bradford1976) using BSA as standard.
Data analysis
Data of bioassays were analyzed using the SAS-probit program. All statistical tests were performed using a SAS computer program.
Results
Pirimicarb toxicity and synergism bioassay
Pirimicarb was 4.8-fold more toxic to Rp than to Sa. The LC50 values were 0.0052 μg cm−2 for Rp and 0.0250 μg cm−2 for Sa. DEF, an esterase inhibitor, PBO, a cytochrome P450 monooxygenase inhibitor, and DEM, a GST inhibitor, re-duced the toxicity difference between both Rp and Sa, from 4.8-fold to 2.4-, 2.4- and 1.9-fold, respectively (table 1).
Table 1. Comparison of pirimicarb toxicity, with or without synergists, between Rhopalosiphum padi (Rp) and Sitobion avenae (Sa).
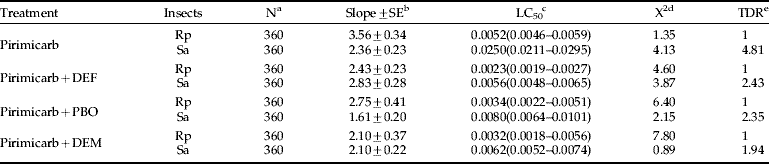
a Number of tested aphids.
b SE=standard error.
c Expressed in μg cm−2; 95% CI of LC50 are given in parenthesis.
d Chi-square testing linearity of dose-mortality responses.
e TDR=the toxicity difference ratio based on LC50 values between Rp and Sa.
AChE characteristics
As the molecular target of carbamate and organophosphate insecticides, the characteristics of crude extract AChE from both Sa and Rp were investigated (table 2). Catalytic activities of AChE toward ATCh, PrTCh, MeTCh and BuTCh were 0.92, 0.70, 0.76 and 1.58 nmol⋅min−1⋅mg−1 protein in Sa, and 1.09, 0.69, 0.81 and 1.19 nmol⋅min−1⋅mg−1 protein in Rp, respectively. There was no significant difference in the catalytic activities of AChE crude extract toward ATCh, PrTCh and MeTCh between Sa and Rp, but the activity difference in BuTCh hydrolysis was observed between both aphid species. However, AChE activities after purification against ATCh, PrTCh, MeTCh and BuTCh were significantly higher in Sa than in Rp (table 3). The sensitivity of AChE from Sa was significantly different from Rp, the median inhibition concentration (IC50 value) on AChE crude extract was 5.37-fold higher for Sa than for Rp (table 4).
Table 2. Comparison of specific activity of crude extract AChE isolated from Rhopalosiphum padi (Rp) and Sitobion avenae (Sa) using ATCh, PrTCh, MeTCh and BuTCh as substratesFootnote a.

a Data are the means of three determinations, means followed by * represent significant difference between Rp and Sa (P<0.05).
Table 3. Comparison of specific activity of the purified AChE isolated from Rhopalosiphum padi (Rp) and Sitobion avenae (Sa) using ATCh, PrTCh, MeTCh and BuTCh as substratesFootnote a.

a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
Table 4. Median inhibition concentration (IC50) of pirimicarb to AChE from Rhopalosiphum padi (Rp) and Sitobion avenae (Sa)Footnote a.
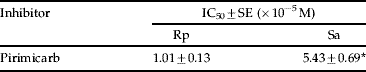
a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
CarE activity and inhibition
The CarE activity was characterized spectrophotometerically against four surrogate ester substrates, α-NA, α-NB, α-NC and β-NA. The Sa exhibited significant higher CarE activities (3.23-, 1.69-, 2.79- and 1.34-fold for α-NA, α-NB, α-NC and β-NA, respectively) than Rp (table 5).
Table 5. Comparison of specific activity of CarE isolated from Rhopalosiphum padi (Rp) and Sitobion avenae (Sa) using α-NA, α-NB, α-NC and β-NA as substratesFootnote a.

a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
The frequency distribution of Rp with respect to the α-NA hydrolyzing esterase activity was significantly different from the distribution of Sa. The esterase activity in Rp ranged from 20 to 100 mOD min−1 aphid, while from 60 to 200 mOD min−1 aphid in Sa (fig. 1).
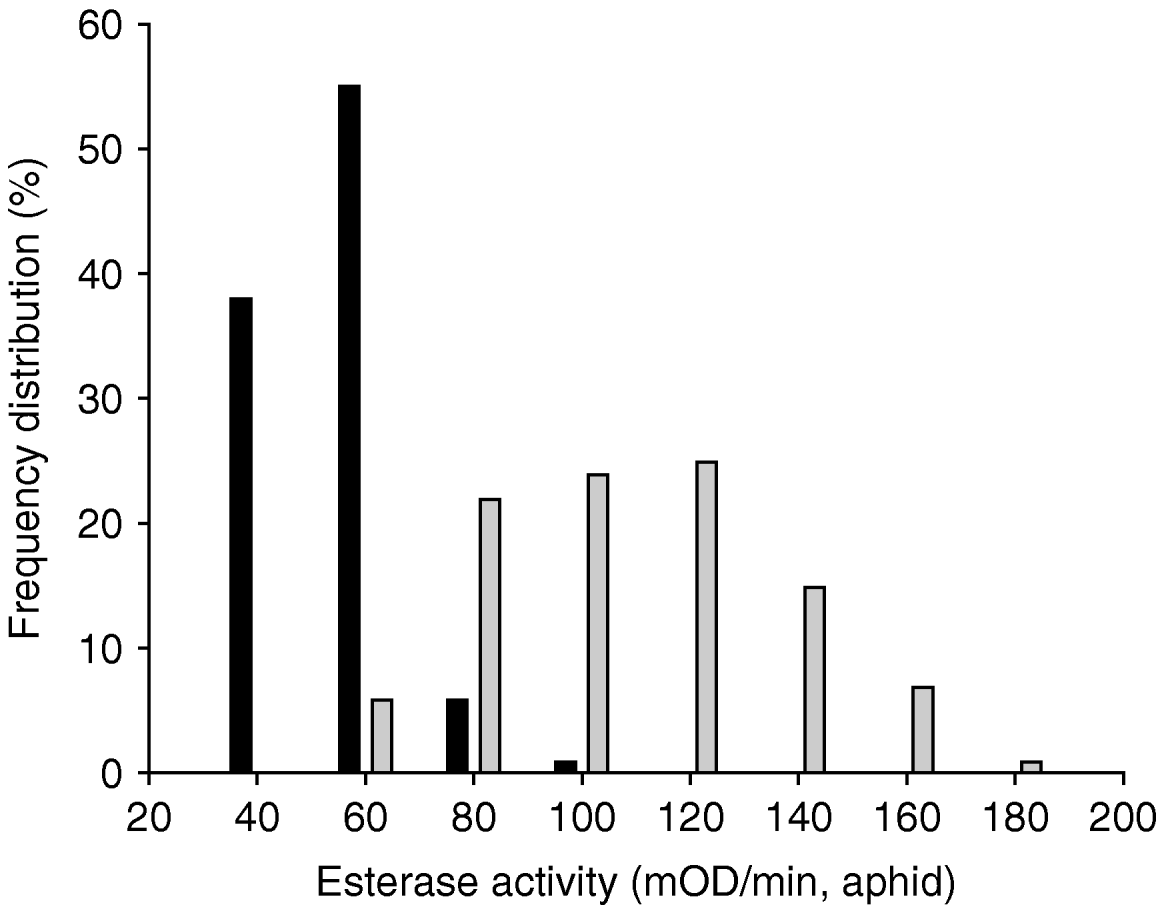
Fig. 1. Frequency distributions of individual esterase activity using α-NA as substrate in Rhopalosiphum padi (Rp) and Sitobion avenae (Sa). The number of tested aphids was 110 aphids for each species (▪, Rp; ![]() , Sa).
, Sa).
The inhibition of pirimicarb and DEF on CarE activity was stronger in Sa than in Rp (table 6).
Table 6. Median inhibition concentration (IC50) of pirimicarb and DEF to CarE in Rhopalosiphum padi (Rp) and Sitobion avenae (Sa)Footnote a.

a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
Kinetic analysis of GSTs
The specific activity of GSTs was significantly higher in Sa than in Rp when CDNB and DCNB were used as substrates (table 7). Kinetic analysis did not indicate statistically significant differences in the K m values but showed a significant difference in the V max values between Sa and Rp (table 8). These results indicated that the detoxification efficiency by GSTs was likely higher in Sa than in Rp.
Table 7. Comparison of specific activity of GSTs between both Rhopalosiphum padi (Rp) and Sitobion avenae (Sa) using CDNB or DCNB as substratesFootnote a.

a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
Table 8. Comparison of kinetic parameters of GSTs isolated from Rhopalosiphum padi (Rp) and Sitobion avenae (Sa) using CDNB and GSH as substratesFootnote a.
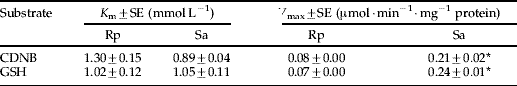
a Data are the mean of three determinations, mean followed by * represent significant difference between Rp and Sa (P<0.05).
Discussion
The toxicity difference of pirimicarb between the two aphid species was observed in wheat field (Liu et al., Reference Liu, Ru, Wang and Li2001). This toxicity difference has affected the field efficacy of pirimicarb in wheat aphid control. After spraying with pirimicarb, the population of Sa was significantly more than that of Rp (unpublished data). Our bioassay results revealed a 4.8-fold difference in LC50 values between Sa and Rp, and the difference in LC50 values between both aphid species was statistically significant based on the criterion of failure of 95% confidence intervals to overlap. Therefore, the alternation or mixture of pirimicarb with other insecticides was considered in wheat aphid management programs because of two aphid species co-infesting the late stage of wheat in China.
Pirimicarb is a fast-acting, selective carbamate aphicide which is active through contact, stomach, fumigant, translaminar and root-systemic routes (Jutsum et al., Reference Jutsum, Franz, Deacon, Payne, Lewis, Paterson, Waage and van Emden1988). As a neurotoxic insecticide, it could inhibit hydrolysis of neurotransmitter acetylcholine (ACh) by AChE, resulting in the disruption of normal nervous system function owing to the accumulation of acetylcholine in the synapse. The selective mechanism of pirimicarb has been reported among different aphid species (Gao & Zheng, Reference Gao and Zheng1989; Gao et al., Reference Gao, Wang and Zheng1990) and between Coccinella septempunctata and Sitobion avenae (Gao et al., Reference Gao, Liu, Zhao and Zheng1991). Usually, the effect of the detoxification enzymes to insecticide toxicity may be revealed primarily by bioassay in the presence and absence of detoxification enzyme inhibitors. DEF and DEM are thought to be inhibitors of esterases and GSTs, respectively. PBO is accepted as an inhibitor of cytochrome P450 monooxygenases, though several reports have exhibited the inhibition of PBO on esterases activity in an opportune time after treatment with PBO (Gunning et al., Reference Gunning, Moores and Devonshire1999; Young et al., Reference Young, Gunning and Moores2005, Reference Young, Gunning and Moores2006; Bingham et al., Reference Bingham, Gunning, Delogu, Borzatta, Field and Moores2008). Treatment with inhibitors of three detoxification enzymes, PBO, DEF and DEM, significantly increased toxicity of pirimicarb against these two wheat aphid species. However, the synergistic extent of three synergists on pirimicarb was approximately 2-fold higher in Sa than in Rp, indicating that esterases, cytochrome P450 monooxygenases and GSTs may contribute to the toxicity difference of pirimicarb between both aphid species. Actually, the CarE activity revealed by four substrates, α-NA, β-NA, α-NC and α-NB, and the GSTs activity conjugating with CDNB or DCNB were significantly higher in Sa than in Rp.
CarE activity and sensitivity to pirimicarb was higher in Sa than in Rp. The higher sensitivity of CarE to pirimicarb in Sa was in favor of protection of AChE from pirimicarb inhibition. This difference in CarE sensitivity between the CarE from Rp and from Sa might partly contribute to the differential susceptibilities of two species of aphids to pirimicarb. Meanwhile, the AChE was more sensitive to pirimicarb from Rp than from Sa. Therefore, the pirimicarb may be more effective for controlling Rp.
The sensitivity difference of molecular targets is one of the most important mechanisms responsible for the toxicity difference of insecticides among different animals. The sensitivity difference of AChE has been demonstrated as an important mechanism for the toxicity difference of organophosphate and carbamate insecticides among various aphids or between lady beetles and aphids (Gao & Zheng, Reference Gao and Zheng1989; Gao et al., Reference Gao, Wang and Zheng1990, Reference Gao, Liu, Zhao and Zheng1991). In our studies, the IC50 of pirimicarb inhibiting AChE from Sa was higher than from Rp. AChE contribution to toxicity differences of pirimicarb may be based on its sensitive difference and activity difference between both aphid species. There was no difference in AChE activity hydrolyzing ATCh, PrTCh and MeTCh in the crude extract between both aphid species. However, the significant difference of AChE activity was observed in the purified AChE. The purified AChE exhibited much lower activity to hydrolyze BuTCh. This is because the enzyme in the crude extracts of AChE (table 2) is complex, including other enzymes, such as esterase, which might hydrolyze BuTCh together with AChE. However, the purified AChE (table 3) can hydrolyze ATCh rapidly, and BuTCh is not the optimal substrate of AChE.
Changes of AChE activity and sensitivity to inhibitor also play an important role in insect resistance to insecticide. Some researchers showed the involvement of a significant increase of AChE activity in resistance mechanisms of greenbug (Schizaphis graminum) (Zhu & He, Reference Zhu and He2000; Zhu et al., Reference Zhu, Gao and Starkey2000; Gao & Zhu, Reference Gao and Zhu2002). Also, some studies on resistance mechanisms in aphids and other pests have suggested the involvement of enhanced AChE levels (Hama et al., Reference Hama, Miyata and Saito1980; Moores et al., Reference Moores, Devine and Devonshire1994; Guedes et al., Reference Guedes, Zhu, Kambhampati and Dover1997; Andrews et al., Reference Andrews, Callaghant, Field, Williamson and Moores2004).
All the data suggested that the detoxification mechanisms (cytochrome P450 monooxygenases, esterases and glutathione S-transferases) and the difference of AChE sensitivity and activity may contribute to the toxicity difference of pirimicarb between Sa and Rp. These results should be helpful in the choice and alteration of insecticides and synergists for controlling wheat aphids.
Acknowledgements
The authors thank Dr Nannan Liu in the Department of Entomology and Plant Pathology, Auburn University for detailed discussion on the revised manuscript. This research was supported by National Basic Research Program of China (Contract No. 2006CB102003), National Key Research Program of China for the Eleventh Five-Years Plan (Contract No. 2006BAD08A03) and the National Natural Science Foundation of China (Contract No. 30530530, 30571232, 30471153, and 30170621).











