Introduction
Many organic contaminants undergo long-range transport and can be found at relatively high concentrations in remote environments (Bustnes et al. Reference Bustnes, Tveraa, Varpe, Henden and Skaare2007). Recent studies have demonstrated the presence of organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in the Antarctic marine food web (Schiavone et al. Reference Schiavone, Corsolini, Borghesi and Focardi2009, Taniguchi et al. Reference Taniguchi, Montone, Bícego, Colabuono, Weber and Sericano2009, Corsolini et al. Reference Corsolini, Borghesi, Ademollo and Focardi2011, Cipro et al. Reference Cipro, Colabuono, Taniguchi and Montone2013). Monitoring such contaminants is important to gain a better understanding of the dynamics in polar regions and to assess the impact of these compounds on the environment.
Bird eggs have proven to be particularly useful as bioindicators of organohalogens in aquatic environments and have been used in environmental contamination studies at high latitudes (e.g. Braune et al. Reference Braune, Mallory, Gilchrist, Letcher and Drouillard2007, Schiavone et al. Reference Schiavone, Corsolini, Borghesi and Focardi2009, Vander Pol et al. Reference Vander Pol, Becker, Ellisor, Moors, Pugh and Roseneau2009, Corsolini et al. Reference Corsolini, Borghesi, Ademollo and Focardi2011, Cipro et al. Reference Cipro, Colabuono, Taniguchi and Montone2013). Contaminant concentrations in eggs may also assist in the assessment of hazards faced by adult birds, as the composition of these contaminants directly reflects that in maternal tissues (Russell et al. Reference Russell, Gobas and Haffner1999).
Little data is available regarding contamination in developing embryos, which are often exposed to similar levels of organic contaminants as adults but exhibit greater toxicological sensitivity (Barron et al. Reference Barron, Galbraith and Beltman1995, Russell et al. Reference Russell, Gobas and Haffner1999). Field and experimental studies have shown embryonic exposure during development due to the absorption of contaminants from yolk (Bargar et al. Reference Bargar, Scott and Cobb2001, Zheng et al. Reference Zheng, Luo, Zeng, Wu, Chen and Mai2014). Information on embryonic exposure to contaminants is important both to evaluate toxic effects in early life stages and in making ecological risk assessments.
Ecological patterns may influence the levels of contaminants in birds (Corsolini et al. Reference Corsolini, Borghesi, Ademollo and Focardi2011). Thus, the comparison of species in different trophic positions and with diverse distribution and/or migration patterns is useful in assessing the differences in exposure to contaminants. For example, species that forage or breed in Antarctica in the summer and migrate to lower latitudes may accumulate a greater amount of contaminants if they winter in polluted areas compared to birds that breed on the Antarctic continent or islands and overwinter in the Southern Ocean (Corsolini Reference Corsolini2009).
This study assessed levels of OCPs, PCBs and PBDEs in the eggs of southern giant petrels (Macronectes giganteus (Gmelin)) and chinstrap penguins (Pygoscelis antarcticus (Forster)) breeding in the South Shetland Islands. These birds have distinct distribution ranges and feeding habits. In order to contribute to the scarce data relating to contamination levels in some bird species in the area, organohalogen concentrations are also reported for a small number of eggs from brown skuas (Catharacta antarctica Lesson), kelp gulls (Larus dominicanus (Lichtenstein)) and Antarctic terns (Sterna vittata Gmelin), as well as for embryos of M. giganteus and S. vittata. These data provide a qualitative and quantitative indication of the impact of contaminants in the Antarctic ecosystem.
Material and methods
Sampling
The eggs of five seabird species [C. antarctica (n=2), L. dominicanus (n=1), S. vittata (n=3), M. giganteus (n=8) and P. antarcticus (n=7)] from the South Shetland Islands (62°S, 58°W), Antarctica, were collected during the summers of 2011 and 2012. Sampling was opportunistic and only unhatched or deserted eggs were collected. Egg contents (yolk, albumen and embryos) were stored in glass vials (previously decontaminated at 450°C for 4 hours) and kept frozen at -20°C until analysis. In the case of eggs containing embryos, the residual yolk and albumen were carefully removed, stored and analysed separately from the embryos.
The incubation stage of the embryos was estimated based on Romanoff (Reference Romanoff1960), Hays & LeCroy (Reference Hays and LeCroy1971) and Freeman & Vince (Reference Freeman and Vince1974) using morphological characters and the amount of yolk and albumen in the egg, which are consumed during embryo growth. Albumen is a reserve of protein and water that the embryo does not utilize in the early stages of incubation (Carinci & Manzoli-Guidotti Reference Carinci and Manzoli-Guidotti1968). In later development stages, the albumen and a portion of the yolk are consumed, which is the only source of lipids during embryo growth. Part of the yolk remains after hatching to provide immediate post-hatching energy to the chicks (Romanoff Reference Romanoff1960, Freeman & Vince Reference Freeman and Vince1974).
Chemical analyses
The egg content (yolk and albumen) and whole embryos were homogenized in an Ultra-Turrax apparatus. The analytical procedure was optimized from the method described by MacLeod et al. (Reference MacLeod, Brown, Friedman, Burrows, Maynes, Pearce, Wigren and Bogar1986). Five grams of wet sample were extracted, after the addition of anhydrous Na2SO4, in a Soxhlet apparatus for 8 hours using 80 ml of n-hexane and methylene chloride (1:1, v/v). Before extraction, 2,2’,4,5’,6-pentachlorobiphenyl (PCB 103) and 2,2’,3,3’,4,5,5’,6-octachlorobiphenyl (PCB 198) were added to all samples, blanks and reference material as surrogates for OCPs, PCBs and PBDEs. The extractable lipids were determined by gravimetric method using a 100 µl aliquot. The extracts were cleaned using column chromatography with 8 g of silica and 16 g of alumina, both 5% water deactivated, eluted with 80 ml of methylene chloride. The fraction was further purified by high-performance liquid chromatography using methylene chloride as the eluent, with a flow of 5 ml min-1. The extract was concentrated to a volume of 0.9 ml in hexane. The internal standard 2,4,5,6-tetrachlorometaxylene (TCMX) was added before the gas chromatographic analysis. A procedural blank was included for each set of eight samples.
The OCP identification and quantification analyses were performed using an Agilent Technologies 6890 N gas chromatograph with an electron capture detector (GC-ECD) with a 30 mx0.25 mm i.d. capillary column coated with a 5% phenyl-substituted dimethylpolysiloxane phase (0.25 μm film thickness). Automatic splitless injections of 2 μl were applied and the total purge rate was adjusted to 50 ml min-1. Hydrogen was the carrier gas (constant pressure of 40 kPa at 100ºC) and nitrogen was the make-up gas at a rate of 60 ml min-1. The PCBs and PBDEs were quantitatively analysed using a gas chromatograph (5973N Agilent Technologies) coupled to a mass spectrometer (GC-MS) in the selected ion mode (SIM 70 eV) with a 30 mx0.25 mm i.d. capillary column coated with 5% phenyl-substituted dimethylpolysiloxane phase (0.25 μm film thickness). The volume injected was 1 µl in automatic splitless mode. Helium was used as the carrier gas (constant flow of 1.1 ml min-1).
For the quality assurance/control, the analytical methodology was validated using a standard reference material (SRM) for PCBs, OCPs and PBDEs (SRM 1945; organics in whale blubber, www.nist.gov) purchased from the National Institute of Standards and Technology (NS&T). The recovery of analytes and surrogates in the SRM, spiked blanks and matrices produced satisfactory results within the range accepted by the NS&T (Wade & Cantillo Reference Wade and Cantillo1994). Analytes in laboratory blanks were subtracted from the samples. Method quantification limit (QL) values were (ng g-1 wet weight): <0.11 to 1.27 for OCPs, <0.11 to 2.36 for PCBs, and <0.25 to 1.29 for PBDEs. The quantification of analytes was performed using a nine-level analytical curve and followed the internal standard procedure.
The concentration of organochlorines was expressed on a wet weight basis. Fifty-one PCB congeners (International Union of Pure and Applied Chemistry (IUPAC) # 8, 18, 28, 31, 33, 44, 49, 52, 56, 60, 66, 70, 74, 77, 81, 87, 95, 97, 99, 101, 105, 110, 114, 118, 123, 126, 128, 132, 138, 141, 149, 151, 153, 156, 157, 158, 167, 169, 170, 174, 177, 180, 183, 187, 189, 194, 195, 201, 203, 206 and 209) and seven PBDEs (IUPAC # 28, 47, 100, 99, 154, 153 and 183) were analysed. The OCPs analysed were DDTs (o,p’-DDT, p,p’-DDT, o,p’-DDD, p,p’-DDD, o,p’-DDE and p,p’-DDE), HCHs (α, β-, δ- and γ-isomer), chlordanes (α-, γ-chlordane and oxychlordane), drins (aldrin, isodrin, dieldrin and endrin), heptachlor, heptachlor epoxide A and B, endosulfan I and II, methoxychlor, hexachlorobenzene (HCB), and mirex.
Results
Eggs
Generally, PCBs were predominant in the eggs of C. antarctica, L. dominicanus and M. giganteus (followed by p’p-DDE and HCB). Among OCs, mirex had the highest concentration in some C. antarctica and M. giganteus eggs. In P. antarcticus and S. vittata eggs, HCB was the major compound (Table I). The PBDE congeners were detected only in eggs of C. antarctica (PBDE 47 and 153) and S. vittata (PBDE 47), but at concentrations close to the QL.
Table I Concentration range (ng g-1 wet weight) of organochlorine and brominated contaminants in bird eggs and embryos from Antarctica.

* Eggs containing an embryo.
The eggs of C. antarctica and M. giganteus had the highest concentrations of OCs, which were from one to two orders of magnitude higher than those found in the eggs of P. antarcticus and S. vittata. Intermediate levels of OCs were exhibited by L. dominicanus, lower than C. antarctica and M. giganteus but higher than P. antarcticus and S. vittata (Table I). The only M. giganteus egg that showed low concentrations of OCs (two orders of magnitude lower than the other eggs) contained an embryo in an advanced stage of development.
The PCB profiles in the eggs of C. antarctica, M. giganteus and L. dominicanus were very similar, with a predominance of hexa- and heptachlorobiphenyls and the presence of congeners with eight and nine chlorine atoms. In contrast, in the eggs of P. antarcticus and S. vittata hexachlorobiphenyls were clearly the predominant congeners and PCBs with a lower chlorination number were also detected (Fig. 1).
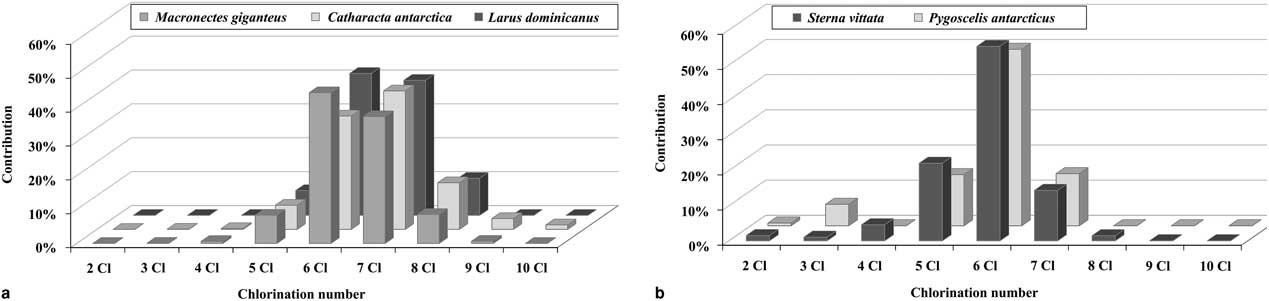
Fig. 1 Contribution of polychlorinated biphenyl (PCB) congeners, according to chlorination number, in the eggs of a. brown skuas (Catharacta antarctica), southern giant petrels (Macronectes giganteus) and kelp gulls (Larus dominicanus), and b. chinstrap penguins (Pygoscelis antarcticus) and Antarctic terns (Sterna vittata).
Embryos
Three out the eight M. giganteus eggs contained embryos. The incubation time of this species is c. 60 days. Based on the presence of few external structures (wings, beak) and a large quantity of albumen and yolk, two embryos were thought to be in the early stages of development. One embryo was in a later development stage, with well-defined external structures (claws, toes, culmen, wings and eyes) and the egg contained only a small portion of yolk and no albumen.
Differences in OC concentrations were up to two orders of magnitude in the M. giganteus embryos (Table I). The embryos in initial growth stage had lower concentrations than their respective eggs (albumen and yolk). The embryo in the more advanced stage of development had the highest OC concentrations and its egg had the lowest concentrations of all OCs detected (Fig. 2).
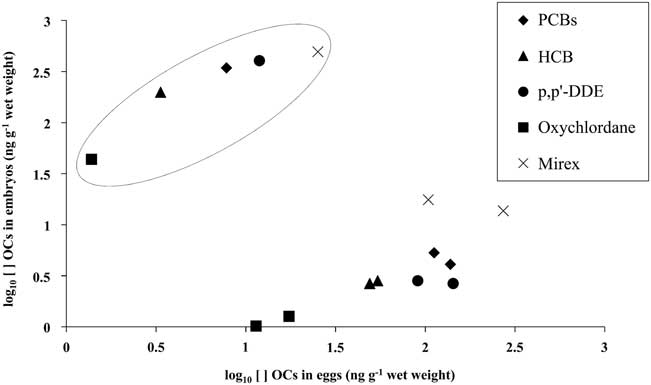
Fig. 2 Concentrations of the major organochlorine (OC) compounds found in the embryos of southern giant petrels (Macronectes giganteus) and their respective eggs. Circled markers correspond to the values detected for the egg/embryo in later development stage, while the remaining markers show the values for the eggs/embryos in earlier development stage.
The incubation time of S. vittata is c. 24 days. An S. vittata embryo found in one of the eggs was estimated to be 9–12 days old, with down just breaking out along dorsal tract and tail, wings, toes and claws visible, but still with a significant amount of yolk and albumen (approximately half of the internal space of the egg). In the S. vittata embryo, PCBs, HCB, p’p-DDE and mirex were detected but at lower concentrations relative to the M. giganteus embryos (Table I).
The S. vittata embryo had a predominance of hexachlorobiphenyls (also seen in the eggs of this species), but also a contribution of tri-, tetra- and pentachlorobiphenyls. Whereas M. giganteus embryos had a predominance of hexa- and heptachlorobiphenyls and the presence of heavier congeners (octa- and nonachlorobiphenyls) (Fig. 3).
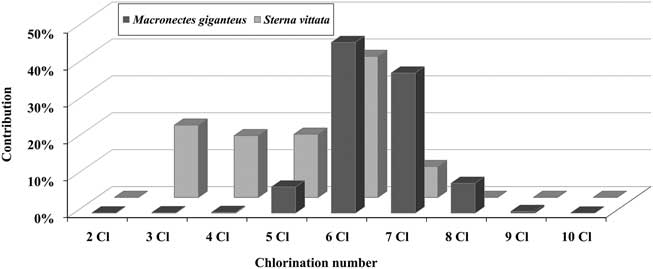
Fig. 3 Contribution of polychlorinated biphenyl (PCB) congeners, according to chlorination number, in the embryos of southern giant petrels (Macronectes giganteus) and Antarctic terns (Sterna vittata).
Discussion
The OC concentrations in the eggs of M. giganteus and P. antarcticus reflect the trophic ecology of these birds and the differences in post-breeding dispersal. Macronectes giganteus is a migratory species and its females have a greater dependence on marine prey, such as fish and cephalopods, although they also feed on carrion of other birds and marine mammals (Hunter Reference Hunter1983, Forero et al. Reference Forero, González-Sólis, Hobson, Donázar, Bertelloti, Blanco and Bortolotti2005). On the other hand, P. antarcticus forages only in the Southern Ocean and feeds mainly on krill and small fish (Volkman et al. Reference Volkman, Presler and Trivelpiece1980), which explains the lower concentrations of contaminants in the eggs of this species.
Despite the small number of samples, the OC levels found in the eggs of the other Antarctic birds included in this study also indicated the influence of trophic status on contaminant patterns (C. antarctica>L. dominicanus>S. vittata). Catharacta antarctica feeds mainly on carrion, eggs and chicks of penguins and Procellariiformes (Pietz Reference Pietz1987, Phillips et al. Reference Phillips, Phalan and Forster2004). Larus dominicanus feeds on a variety of prey, from carrion to amphipods, but the Antarctic limpet (Nacella concinna (Strebel)) is its primary food in the breeding season (Favero et al. Reference Favero, Silva and Ferreyra1997). Sterna vittata represents the lower trophic level among the three species, with a diet similar to P. antarcticus (Volkman et al. Reference Volkman, Presler and Trivelpiece1980, Casaux et al. Reference Casaux, Baroni, Ramón, Favero and Silva2008).
Mirex is one of the most stable and persistent pesticides, which was primarily used as an insecticide in many countries of South America and South Africa (Ritter et al. Reference Ritter, Solomon, Forget, Stemeroff and O’Leary1995). It was banned under the Stockholm Convention on Persistent Organic Pollutants in 2001. During the non-breeding season, M. giganteus and C. antarctica travel to these areas to feed (Del Hoyo et al. Reference Del Hoyo, Elliot and Sargatal1996, Sander et al. Reference Sander, Garcia, Carneiro, Cristofoli and Polito2010). Thus, the higher concentrations of mirex in the eggs of both species are probably associated with their migration habits, additional to biomagnification.
Hexachlorobenzene is a semi-volatile compound that reaches the coldest regions of the planet by long-range atmospheric transport (Simonich & Hites Reference Simonich and Hites1995). An increase in HCB concentrations in recent years is evident through a comparison with data reported in previous studies (Zhang et al. Reference Zhang, Wang, Lu, Zhu, Wu and Vetter2007, Schiavone et al. Reference Schiavone, Corsolini, Borghesi and Focardi2009, Corsolini et al. Reference Corsolini, Borghesi, Ademollo and Focardi2011). In the present investigation, HCB levels were up to one order of magnitude higher relative to levels reported in the eggs of P. antarcticus collected between 2003 and 2005 in the same region (Schiavone et al. Reference Schiavone, Corsolini, Borghesi and Focardi2009). Macronectes giganteus eggs also had higher HCB concentrations than those reported in eggs collected in 2001 and 2002 on the Fildes Peninsula (Zhang et al. Reference Zhang, Wang, Lu, Zhu, Wu and Vetter2007). A similar increase in PCBs and p,p’-DDE has also occurred in the eggs of M. giganteus in comparison to data reported by Zhang et al. (Reference Zhang, Wang, Lu, Zhu, Wu and Vetter2007), whereas P. antarcticus eggs exhibited very similar concentrations to those described by Schiavone et al. (Reference Schiavone, Corsolini, Borghesi and Focardi2009). This could be an indication of a rise in the levels of some contaminants at lower latitudes of the Southern Hemisphere, as well as the Southern Ocean.
Low chlorinated PCBs are expected to reach polar regions faster due their greater volatility in comparison to highly halogenated congeners (Wania & Dugani Reference Wania and Dugani2003). The PCBs with low chlorination levels are reported to be predominant in key species from the base of the Antarctic marine food web, such as silverfish (Pleuragramma antarcticum Boulenger) and krill (Euphasia superba Dana), although heavier PCB congeners have also been found (Corsolini et al. Reference Corsolini, Romeo, Ademollo, Greco and Focardi2002, Cipro et al. Reference Cipro, Taniguchi and Montone2010). Species that feed at lower trophic positions, P. antarcticus and S. vittata, exhibited low chlorinated PCBs (although these compounds were not predominant). Higher chlorinated PCBs were prevalent in the eggs of M. giganteus, C. antarctica and L. dominicanus as a result of bioaccumulation and biomagnification. High chlorinated PCBs are usually predominant in long-living predators that feed at high trophic positions (Corsolini et al. Reference Corsolini, Borghesi, Ademollo and Focardi2011, Cipro et al. Reference Cipro, Colabuono, Taniguchi and Montone2013) due to the easier transformation and elimination of PCB congeners of a low molecular weight, which results in the accumulation of compounds with a greater number of chlorines (Maervoet et al. Reference Maervoet, Chu, De Vos, Covaci, Voorspoels, De Schrijver and Schepens2004).
Generally, PBDEs are found at lower concentrations in comparison to other organic contaminants, such as PCBs and DDTs (Corsolini et al. Reference Corsolini, Covaci, Ademollo, Focardi and Schepens2006, Yogui & Sericano Reference Yogui and Sericano2009). However, PBDE 47, which was detected in the eggs of both S. vittata and C. antarctica, has greater volatility and water solubility and is the most abundant congener in krill and fish in Antarctica (Corsolini et al. Reference Corsolini, Covaci, Ademollo, Focardi and Schepens2006). Similar to some high halogenated PCBs, PBDE 153 is more resistant to biotransformation and tends to accumulate at higher trophic positions, which explains its occurrence only in the eggs of C. antarctica.
The inverse association between concentrations in the residual egg contents and in the embryos of M. giganteus in different development stages may be an indication of the transfer of contaminants during embryo growth. Custer et al. (Reference Custer, Custer and Stromborg1997) reported the transfer of contaminants from yolk to the embryo, but suggest that no metabolic changes appear to occur during embryo growth and lipid mobilization. Yolk is a lipid-rich energy source that remains in the embryo (c. 30%) after hatching (McLaughlin et al. Reference McLaughlin, Marliac, Verrett, Mutchler and Fitzhugh1963) and contains up to 60% of the total concentrations of OCs (Custer et al. Reference Custer, Custer and Stromborg1997). Most of the contaminant load in the egg may be transferred to the embryo, but not readily absorbed and metabolized.
As was observed in the eggs, the PCB profiles in embryos of M. giganteus and S. vittata also reflect the differences associated with trophic status. In comparison to M. giganteus, S. vittata has a lower trophic status and exhibited a predominance of tri- (19.6%), tetra- (16.7%), penta- (17.1%) and hexachlorobiphenyls (38.2%), which accounted for 91.6% of total PCBs, whereas 92.2% of total PCBs in M. giganteus were constituted by hexa- (46.2%), hepta- (37.9%) and octachlorobiphenyls (8.1%).
Conclusions
The present analysis of bird eggs and embryos demonstrates the influence of ecological factors, such as dispersal and diet, on contaminant levels and patterns. These factors should be carefully considered when comparing contamination data between different species and populations. Despite the small number of samples, long-range migratory species, such as M. giganteus and C. antarctica, exhibited contamination from both breeding and migration areas, whereas OCs in resident birds mainly reflected the compounds found at higher concentrations in the Antarctic environment due to atmospheric transport, such as HCB. In agreement with data found in the literature, lower levels of PBDEs were found in comparison to OCs. Comparisons with data from previous studies indicate an increase in concentration of some contaminants, such as HCB, PCBs and p,p’-DDE. The use of eggs (regardless the incubation stage) as bioindicators is an easy, efficient method for the continuous monitoring and evaluation of changes in contaminant concentrations in the Antarctic environment.
Acknowledgement
The authors are thankful to the team at UNISINOS, to the Brazilian Antarctic Program (PROANTAR) and to the National Science and Technology Institute on Antarctic Environmental Research (INCT-APA). We also thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support. The authors would also like to thank the reviewers for their valuable comments.
Author contribution
All authors authorize the publication of the final version of this manuscript. Fernanda I. Colabuono led the study design, chemical analysis, data analysis and manuscript preparation, and also contributed to fieldwork. Satie Taniguchi contributed to chemical analysis and manuscript development. Maria V. Petry led the fieldwork and contributed to the manuscript preparation. Rosalinda C. Montone contributed to data interpretation and manuscript elaboration.







