INTRODUCTION
The nurse shark, Ginglymostoma cirratum (Bonnaterre, 1788) is a large (at least 300 cm total length (TL)), bottom-dwelling species that inhabits continental and insular shelves along both sides of the tropical and subtropical Atlantic Ocean, and also considered to occur in the eastern Pacific Ocean until 2015 (Compagno, Reference Compagno2001). However, a recent study described the Pacific Ocean population as a distinct species, Ginglymostoma unami (Moral-Flores et al., Reference Moral-Flores, Ramírez-Antonio, Angulo and de León2015) while G. cirratum is currently regarded to be restricted to the Atlantic Ocean (Moral-Flores et al., Reference Moral-Flores, Ramírez-Antonio, Angulo and de León2015).
The nurse shark has been reported as one of the most abundant shark species in the coastal shallow waters off Florida, the Caribbean (Castro, Reference Castro2000; Heithaus et al., Reference Heithaus, Burkholder, Hueter, Heithaus, Pratt and Carrier2007) and north-eastern Brazil (Hazin et al., Reference Hazin, Wanderley and Mattos2000; Castro & Rosa, Reference Castro and Rosa2005; Afonso et al., Reference Afonso, Andrade and Hazin2014). The species is also popular for eco-touristic viewing by divers, particularly off Florida, the Bahamas and the Caribbean, and it is kept for public display in aquariums due to its robustness and ability to survive many years in captivity (Compagno, Reference Compagno2001). Although G. cirratum is commonly found and fished in inshore waters throughout its range, fisheries data on the species is scarce, probably due to the low commercial value of its meat and fins (Castro et al., Reference Castro, Woodley and Brudek1999; Castro, Reference Castro2000), perhaps a reason that the species is listed as ‘Data Deficient’ by the IUCN Red List (Rosa et al., Reference Rosa, Castro, Furtado, Monzini and Grubbs2006). Nevertheless, the western Atlantic population of G. cirratum is regarded as ‘Near Threatened’ (Rosa et al., Reference Rosa, Castro, Furtado, Monzini and Grubbs2006) and ‘Vulnerable’ in Brazil following IUCN criteria, where abundance decline and regional extirpations have been reported (Rosa & Gadig, Reference Rosa, Gadig, Machado, Drumond and Paglia2009).
Despite its distribution in shallow coastal waters and its value to the eco-tourism industry, the current knowledge of G. cirratum ecology is mainly limited to the western North Atlantic Ocean, where most studies have focused on mating behaviour (Klimley, Reference Klimley1980; Carrier et al., Reference Carrier, Pratt and Martin1994; Carrier & Pratt, Reference Carrier and Pratt1998; Pratt & Carrier, Reference Pratt and Carrier2001), growth (Carrier, Reference Carrier1985) and site fidelity and movements (Carrier & Luer, Reference Carrier and Luer1990; Chapman et al., Reference Chapman, Pikitch, Babcock and Shivji2005). A review on G. cirratum biology can be found in Castro (Reference Castro2000).
Data regarding G. cirratum from the South Atlantic Ocean remain scarce, but recent studies have improved the knowledge about population structure and habitat use off north-eastern Brazil (Castro & Rosa, Reference Castro and Rosa2005; Santander-Neto et al., Reference Santander-Neto, Shinozaki-Mendes, Silveira, Juca-Queiroz, Furtunato-Neto and Faria2011; Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013; Afonso et al., Reference Afonso, Andrade and Hazin2014), as well as about the connectivity among western Atlantic populations (Karl et al., Reference Karl, Castro and Garla2011). However, given the current Vulnerable status of G. cirratum off Brazil (Rosa & Gadig, Reference Rosa, Gadig, Machado, Drumond and Paglia2009), further research on the life history of this species is necessary to ensure effective management and give support to future conservation policies. In this sense, we herein report the results from traditional mark-recapture and acoustic telemetry studies aiming to obtain data on G. cirratum in terms of population structure, seasonal patterns of abundance, and description of movement and residence patterns in relation to Marine Protected Areas (MPA) boundaries.
MATERIALS AND METHODS
Study site
The study was conducted in the Fernando de Noronha Archipelago (FEN), an isolated group of volcanic islands located in the western tropical Atlantic, 345 km off the north-eastern coast of Brazil (03o51′S 32o25′W) (Figure 1A). FEN is under the influence of the South Equatorial Current, with annual mean seawater temperature and salinity of 26°C and 36 respectively. About 70% of the main island and the coastal waters spanning from the coastline to the 50 m isobath constitute a MPA (National Marine Park) since 1988 (Maida & Ferreira, Reference Maida and Ferreira1997), where fishing is prohibited, and boat traffic and diving are regulated. The remaining portions of the main island coastline to the 50 m isobath constitute an Environmental Protection Area (EPA) designated for sustainable use (Figure 1B).
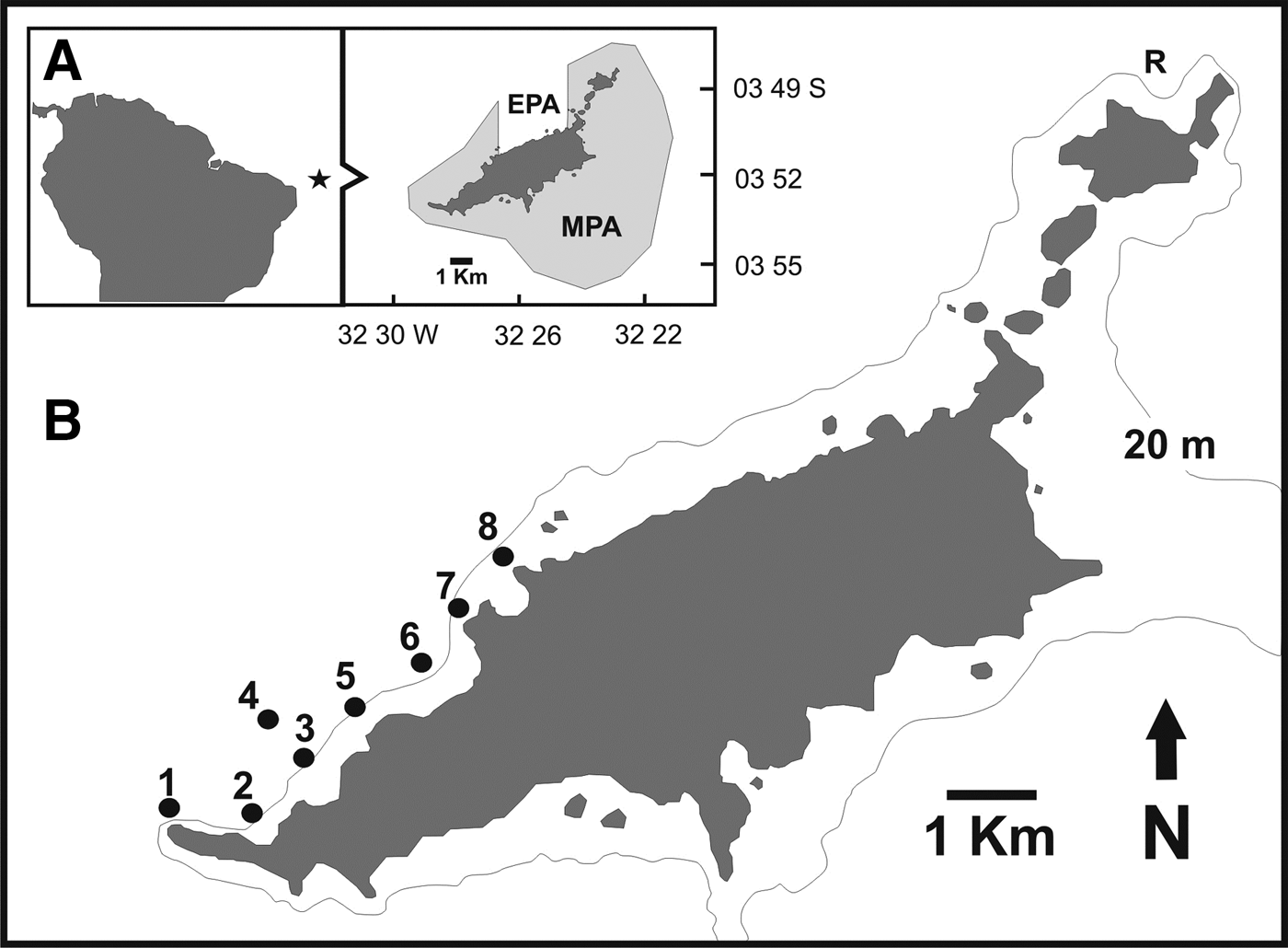
Fig. 1. (A) Fernando de Noronha Archipelago and its sustainable use (EPA) and marine protected (MPA) areas. (B) Location of the automated acoustic telemetry array moored at the lee side of the archipelago from July 2010 to January 2012. Receivers #6 and #8 were deployed on February 2011. R: indicates site of capture of sharks Nos 15 and 19 implanted with transmitters close to Rata Island.
Sampling
Fishing operations were performed in four tagging campaigns, from March to October 2000, in January and July 2001, from February to October 2002, and February 2003 at various locations around FEN, focusing mainly on the leeward side because it provides suitable oceanographic conditions most of the year. Handlines were used with 10/0 or 11/0 barbless circle hooks baited with pieces of locally abundant fishes (e.g. Cephalopholis fulva, Caranx and Carangoides spp.). Between June 2010 and January 2012, additional fishing sets were conducted but were restricted to waters 5–30 m in depth from the south-western portion of FEN's leeward side, which is included in the MPA. In addition, from June 2011 to January 2012, bottom longlines with 10 circle hooks 18/0 spaced every 25 m were deployed at both sides of FEN in depths ranging from 50 to 65 m. Soak times averaged 2.5 h.
The catch per unit of effort for handlines and longlines (CPUE = sharks captured/(number of hooks × time elapsed)) was calculated following Musick et al. (Reference Musick, Musick and Bonfil2005). For longlines the soak time was used as the time of fishing.
Captured sharks were brought alongside the boat, measured for fork length (FL) and total length (TL) to the nearest centimetre and sexed. Juveniles were fitted with numbered nylon-barbed tags (Hallprint, South Australia). Sharks larger than 150 cm TL were tagged with stainless-steel dart-tags (Floytag, USA). All lengths herein reported refer to TL unless when stated otherwise. The approximate life-stage of each shark was defined on basis of external morphology and the previous published data on the size at maturity (Castro, Reference Castro2000). Juveniles were individuals that measured between 60 and 179 cm. Females between 180 and 222 cm TL were regarded as pre-adults and those larger than 223 cm TL as mature adults. Males between 180 and 213 cm TL were regarded as pre-adults and those larger than 214 cm TL, with fully calcified claspers, as adults.
Transmitter attachment and acoustic array
Sharks fitted with acoustic transmitters were caught with handlines from June 2010 to February 2011 at random locations of the south-western portion of leeward side inside the MPA (Figure 1B). After conducting the procedures described in the previous section, all sharks measuring less than 180 cm TL were brought on board and positioned with their ventral side facing upwards inside a PVC trough with a hose placed inside their mouths in order to pump seawater directly to the gills. Two specimens, one measuring 170 cm TL and the other 267 cm TL, were inverted alongside the boat. After periods ranging from 10 to 120 s, the inversion resulted in the onset of tonic immobility (Henningsen, Reference Henningsen1994) allowing for relatively simple surgery for transmitter implantation. Coded acoustic transmitters (model CHP-87-L, 16 mm diameter × 80 mm, frequency 32–83 kHz, burst interval 45–60 s, lifespan 18 months; Sonotronics, USA) were implanted into the abdominal cavity of the animals through a 3–4 cm incision made in the abdominal wall anterior to the origin of the left pelvic fin. The incision was closed with absorbable nylon sutures. The whole procedure usually took 5–10 min. After surgery, sharks were re-inverted alongside the boat until they were able to swim autonomously and then released, typically after 2–5 min. Only sharks cleanly hooked in the mouth with no signs of significant injury were selected for transmitter implantation.
The presence of acoustically tagged sharks was continuously monitored from June 2010 to January 2012 by an overlapping linear array of eight underwater acoustic receivers (SUR-2, Sonotronics) deployed in the area where the sharks were tagged (Figure 1B). Receivers were moored directly to the substrate in depths between 10 to 31 m with shackles and chains and kept vertically stretched by a buoyed line. In all sites receivers were kept from 5 to 10 m above the bottom to inspect as much as possible of the bottom and water column. Preliminary field tests conducted both at night and daylight hours indicated that detection ranges varied from 400 to 500 m, so the receivers were moored ~400 from each other along a 5-km section of the leeward coast included in the MPA. The receivers were retrieved at 6-month intervals for data download and battery refurbishment.
The telemetry study was conducted over a 540 days period during which three temporal gaps occurred at site 3: the first and third gaps encompassed 101 days (between 14 October 2010 and 25 January 2011) and 64 days (11 November 2011 to 15 January 2012), respectively, and were caused by a great number of detections that took up the whole memory capacity of the receivers; the second gap encompassed 154 days (10 February to 15 July 2011) and was caused by malfunction of the receiver. At site 2 there was a gap of 153 days (9 February to 6 July 2011) also due to receiver malfunction. Receivers of both sites were replaced.
Active tracking
One male juvenile nurse shark measuring 158 cm TL was captured with a handline and tracked with active acoustic telemetry to assess short-term, fine-scale movements. Upon capture, the shark was restrained, measured and sexed. A coded ultrasonic continuous transmitter (model CT-82-1-E, 16 mm diameter × 38 mm, lifespan 60 days, Sonotronics, USA) attached to a 3 cm plastic sheep tag (Allflex, USA) with a corrodible wire was fitted to the first dorsal fin of the shark following Cartamil et al. (Reference Cartamil, Vaudo, Lowe, Wetherbee and Holland2003). The handling time for measurement and tag attachment did not exceed 5 min. The shark was tracked for 8 h immediately after release from a 3.5 m skiff equipped with a portable directional hydrophone and receiver (models DH-4 and USR-96, Sonotronics, respectively). The geographic location of the shark was determined by positioning the vessel above the shark, which was verified when the strongest transmitter signal was oriented from underneath the vessel, and recording the position of the vessel every 15 min with a hand-held GPS. The shark was tracked daily for periods ranging from 4 to 11 h during 16 days. All recorded positions were plotted on a map, and the linear distances between successive positions and the activity space used during the tracking period were measured using Google Earth Pro 7.1.
Data analysis
Detections from all receivers were sorted by transmitter, date and receiver to generate a complete monitoring record for each individual. The total number of days over which each shark was detected in the array, the maximum number of consecutive days it was detected, the mean number of daily detections, and detection rates during diurnal and nocturnal periods were also assessed. Detection records were plotted against month to further investigate seasonal patterns of habitat use. The minimum dispersal range of each shark along the array was estimated by measuring the distance between the peripheries of the detection ranges of the two farthest receivers where the shark was detected. The activity space used by each shark within the array was also calculated. Measurements were made using Google Earth Pro 7.1.
Statistical analyses were performed with R software version 3.2.1, adopting a significance level of 5%. Data were log (x) transformed for shark total length and log transformed (x + 1) for CPUE to normalize and homogenize variances. Data normality was verified through Shapiro–Wilk test and data homoscedasticity with Bartlett test. One-way ANOVA were used to verify if shark length differed between seasons, sexes and sampling periods. Kruskal–Wallis tests were used to test for differences of CPUE between seasons, periods and fishing gear.
RESULTS
A total of 93 nurse sharks were captured and tagged during the two studied periods, including 81 juveniles, six pre-adults and six adults. Females ranged from 82 to 240 cm TL (N = 45, mean = 145.58, SD = 36.47) and males from 93 to 265 cm TL (N = 48, mean = 143.96, SD = 33.73) (Figure 2). There were no significant differences in mean TL between sampling periods (F = 0.07, df = 1, P = 0.932), sexes (F = 0.003, df = 1, P = 0.954) or seasons (F = 0.039, df = 1, P = 0.530).
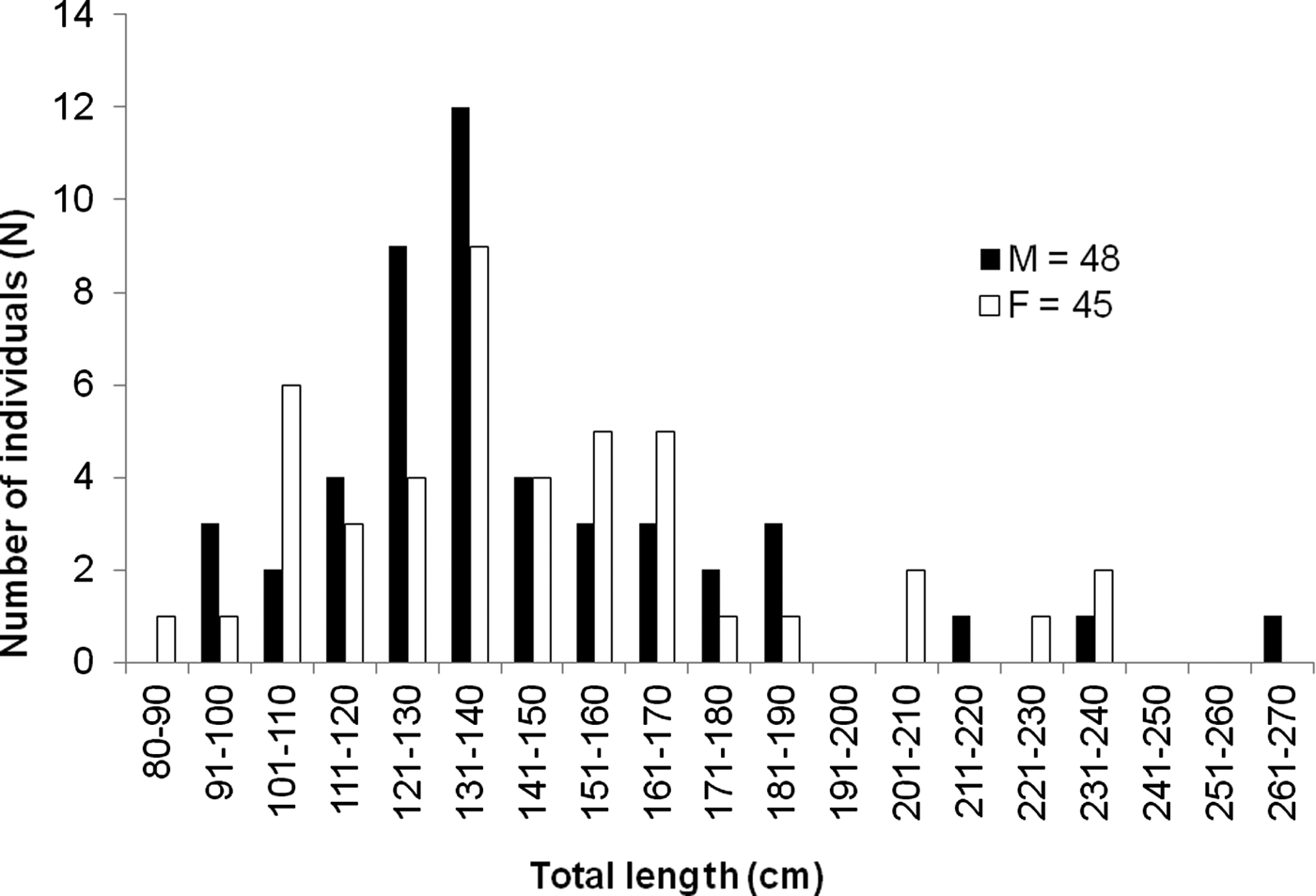
Fig. 2. Length frequency distribution of Ginglymostoma cirratum caught at Fernando de Noronha Archipelago from March 2000 to February 2003 and from July 2010 to January 2012 (N = 93). Solid bars = males; open bars = females.
Handline fishing at 232 sites over 5–30 m depth resulted in the capture of 87 G. cirratum at 44 of the sites (19%), with the CPUE per site ranging from 0 to 0.86 (mean = 0.04, SD = 0.11). There were no significant differences in the mean handline CPUE between sampling periods (Kruskal–Wallis = 1.093, P = 0.295), sexes (Kruskal–Wallis = 0.282, P = 0.594) or seasons (Kruskal–Wallis = 0.202, P = 0.652). Longline fishing effort at 29 sites over 50 m depth resulted in the capture of six nurse sharks at five sites (17.2%), with the CPUE ranging from 0 to 0.1 (mean = 0.01, SD = 0.02).
Fifteen tagged G. cirratum (16% of 93 tagged animals) were recaptured after periods at liberty ranging from 3.5 h to 705 days (mean = 140.9 days, SD = 206.3 days; Figure 3). The distance between tag and recapture locations ranged from 0.07 to 1 km after 3.5 h and 705 days at liberty. One pre-adult female 201 cm TL was resighted during dives in a location distant 3.5 km from the tagging site on six different occasions after 53 to 362 days. Growth rates could be estimated for only four recaptured juveniles (Table 1), indicating a preliminary rate of 10.99 ± 3.56 cm year−1.
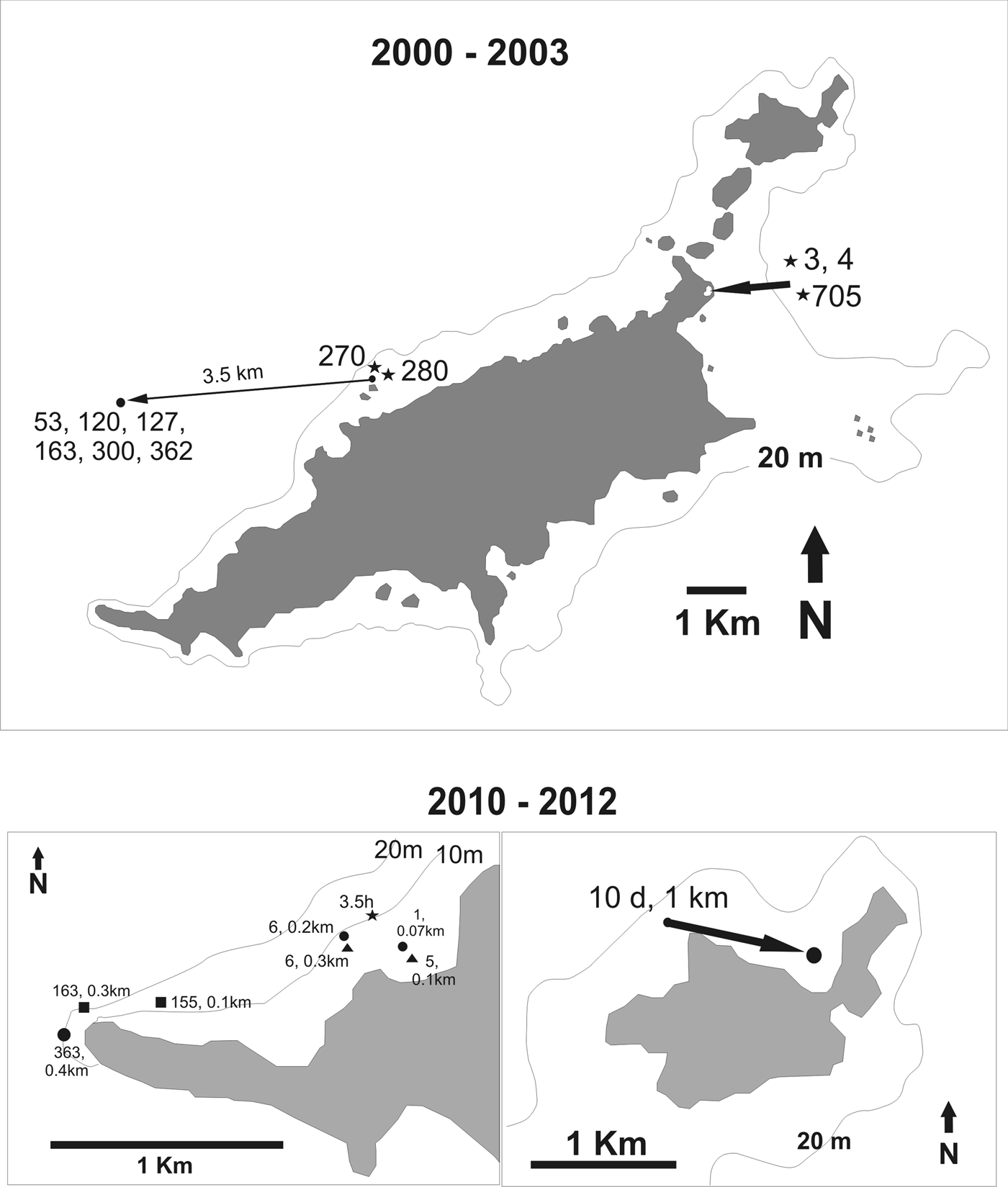
Fig. 3. Recapture locations of Ginglymostoma cirratum at the Fernando de Noronha Archipelago (N = 20). 2000–2003. Stars (⋆) depict sharks that were recaptured at the same location of tagging. For all cases the associated numbers indicate the time, in days, at liberty since tagging and the estimated distance travelled (km). The solid circle (•) indicates multiple underwater sightings of a 201 cm TL female, and the arrow indicates the estimated distance travelled (km) between the tagging and sighting location. 2010–2012: Left panel: star (⋆) indicates the recapture of a shark at the release site 3.5 h after tagging; solid circles (•) indicate the recapture of three distinct individuals, the associated time at liberty and estimated distance travelled; solid triangles (▴) indicate two recaptures of the same individual; solid squares (■) indicate two recaptures of the same individual. Right panel: solid circle (•) indicates the recapture of a shark tagged 10 days before and the arrow indicates the estimated distance travelled between the tagging and recapture location (km).
Table 1. Growth rates of nurse sharks recaptured at Fernando de Noronha Archipelago.

Transmitters were implanted into four female and six male nurse sharks ranging from 107 to 265 cm TL (mean = 154.4 cm TL, SD = 46) (Table 2). Seven sharks were juveniles, and one was a mature male 265 cm TL with calcified claspers with sperm remnants. This adult male (No. 38) and two females (No. 15 and 19) were detected only once by receivers located 4 and 9 km, respectively, from their capture sites and were not included in further analyses.
Table 2. Nurse sharks monitored at Fernando de Noronha Archipelago between July 2010 and January 2012. ID indicates the transmitter number and TL is the total length.

a The number in parentheses indicates the site of capture (see Fig. 1 for location).
b Mean activity space in the array.
Detection records of multiple individuals were concentrated at sites 2, 3, 5 and 6. In contrast, there were no detections from any shark on the receivers placed at the deepest site 4 (31 m) and at the north-eastern extremity of the array (site 8). Shark No. 1 was mainly detected by the receiver located at the site of its capture, while the remaining six sharks (Nos 11, 12, 16, 18 and 39) were detected at up to six neighbouring areas (Figure 4). Minimum linear distances travelled to neighbouring monitoring sites were conservatively estimated at 2.1 to 3.7 km and the maximum number of receivers visited per day was three. Detection records at the sites usually occurred throughout the diel cycle (Table 2).
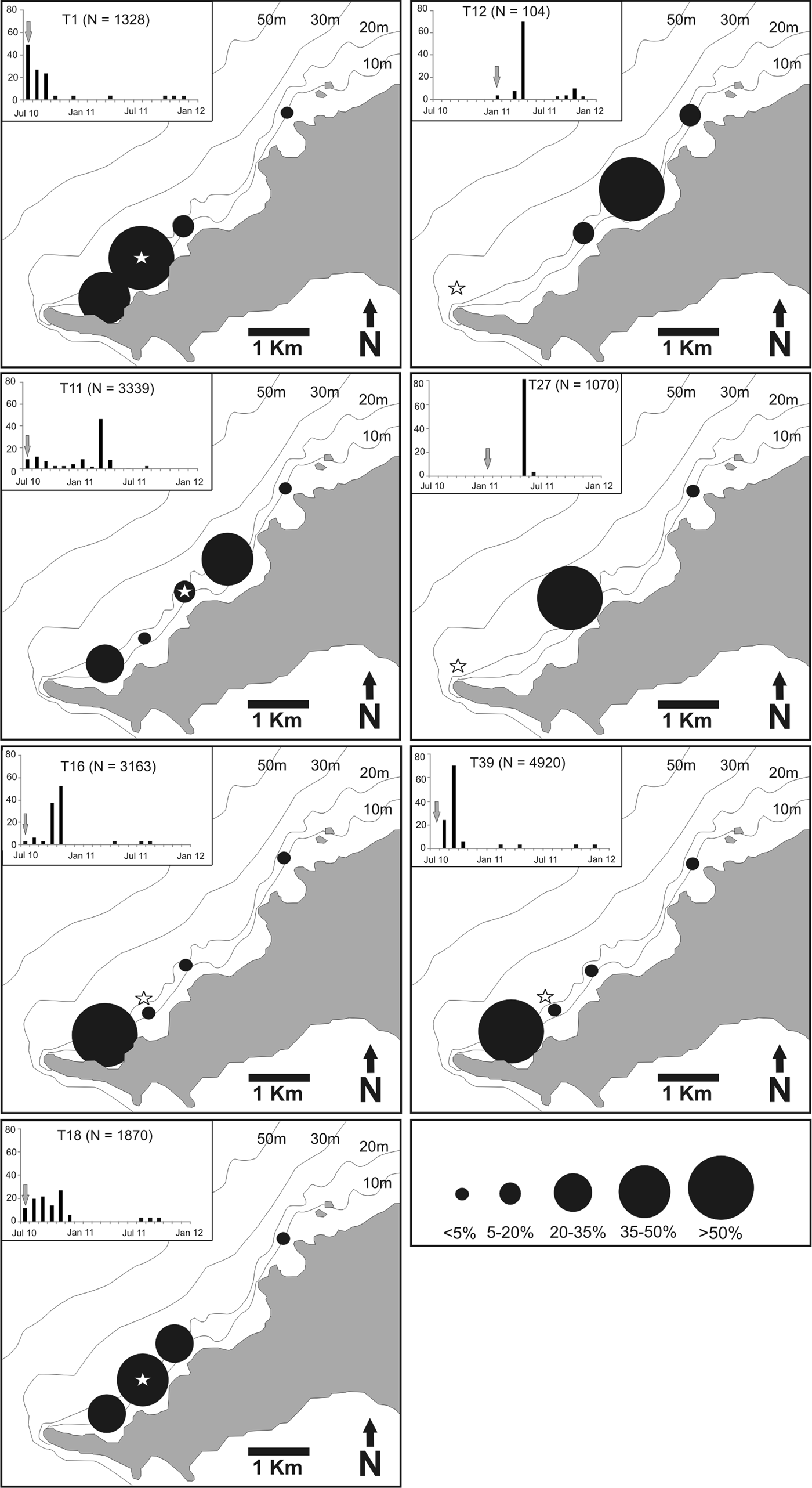
Fig. 4. Distribution, in percentage, of acoustic detections of seven Ginglymostoma cirratum along an array of acoustic receivers deployed at the Fernando de Noronha Archipelago from July 2010 to January 2012. Inset bar graphs indicate the percentage of detections in each month at the receiver array and grey arrows indicate the month of transmitter implantation.
There was evidence of emigration of G. cirratum away from FEN's south-western coastline. Sharks were continuously detected within the receiver array during 3 to 6 months, and then returned after periods ranging from 3 to 8 months later. Figure 5 indicates a bimodal pattern of attendance at this part of the archipelago. The mean period of detection was 66 days and ranged from 13 to 119 days. The mean number of consecutive days a shark was detected by the array was 19, ranging from 2 to 51 days (Table 2). The mean activity spaces used within the array was 1.84 km2, ranging from 1.26 to 2.22 km2 (Table 2).
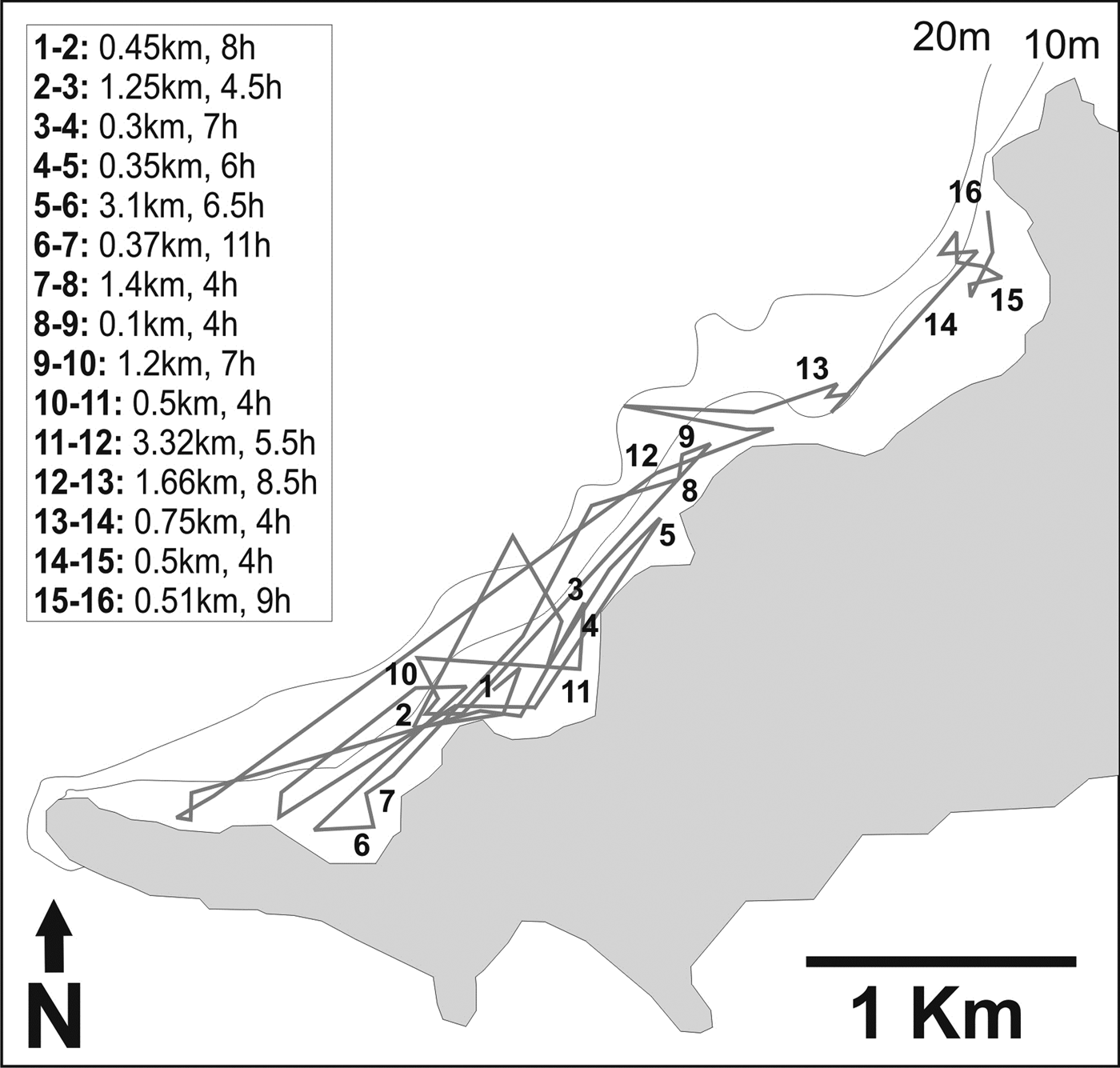
Fig. 5. Active track of one Ginglymostoma cirratum monitored during 16 consecutive days at the lee side of Fernando de Noronha Archipelago in July 2011. The left box indicates the linear distance travelled between successive days and the duration of each. For clarity purposes, only the first localization of each day is provided.
The juvenile male 158 cm TL actively tracked was followed for periods of 4 to 11 h between 18 July and 4 August 2011, in total of 93 h (Figure 5). This shark was relocated daily until tracking sessions had to be prematurely terminated due to the failure of the outboard engine of the skiff. It remained most of the time in waters less than 10 m depth. In most of the tracking sessions the shark exhibited no discernible movement interspersed with slow displacement and occasional longer-range movements of short temporal duration. Those occurred during daytime or night-time. Periods of inactivity lasted from 15 min to 11 h. The activity space used by this shark during the 16-days tracking was 0.84 km2, and the linear distance travelled was 15.81 km. Distances between daily locations ranged from 0.1 to 3.32 km (mean = 1.05 km, SD = 0.99 km).
DISCUSSION
Nurse sharks were captured throughout both sampling periods and seasons, suggesting that the species has a year-round occurrence at the insular shelf of FEN. Results indicate that all life-stages of G. cirratum use the insular shelf of the archipelago. A wide range of sizes was examined, from juveniles to adults. Although not captured, previous investigations have reported the occurrence of neonates in waters up to 15 m deep in distinct years (Garla et al., Reference Garla, Garcia, Lopes and Veras2009, Reference Garla, Garrone-Neto and Gadig2015). Most of the individuals examined were juveniles from 100 to 179 cm TL, captured in depths of 0–30 m. Individuals larger than 200 cm TL can be occasionally observed at this depth range, but were more commonly captured in depths over 25 m, regardless of the fishing gear used.
No significant differences were found between the length frequencies of the two sampling periods, demonstrating a relatively stable population structure during the study. The mean TL of nurse sharks in FEN (144.7 cm) was lower than those reported for Belize (Pikitch et al., Reference Pikitch, Chapman, Babcock and Shivji2005) and other sites of north-eastern Brazil (Castro & Rosa, Reference Castro and Rosa2005; Santander-Neto et al., Reference Santander-Neto, Shinozaki-Mendes, Silveira, Juca-Queiroz, Furtunato-Neto and Faria2011; Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013). This variation in mean TL may be related to distinct growth parameters among populations, but may also reflect use of fishing gear. For example, Pikitch et al. (Reference Pikitch, Chapman, Babcock and Shivji2005) and Ferreira et al. (Reference Ferreira, Afonso, Castilho and Hazin2013) used larger 16/0 and 17/0 circle hooks as standard gear, in contrast to the 11/0 used in most of the sampling sessions in FEN.
No seasonal differences of sex ratio and mean TL were found, indicating that all size-classes can be found year-round in FEN. This lack of seasonal occurrence is similar to the condition observed in Ceará (Santander-Neto et al., Reference Santander-Neto, Shinozaki-Mendes, Silveira, Juca-Queiroz, Furtunato-Neto and Faria2011), but distinct from Recife and Atol das Rocas, where seasonal differences have been reported (Castro & Rosa, Reference Castro and Rosa2005; Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013).
The observed sex ratios of G. cirratum indicated that nearly equal numbers of males and females are present at FEN. The presence of mature individuals of both sexes and observations of reproductive aggregations of up to 15 nurse sharks >2 m TL in a shallow lagoon performing courtship behaviours during July–August 2015 demonstrate that FEN is used as a mating ground (RC Garla, personal communication). In the nearby Atol das Rocas mating activity was observed in May (Castro & Rosa, Reference Castro and Rosa2005). Thus, available data suggest that both the parturition and mating seasons of nurse sharks in oceanic insular areas of the western south Atlantic occur during the austral winter (Castro & Rosa, Reference Castro and Rosa2005; Garla et al., Reference Garla, Garcia, Lopes and Veras2009). This condition is distinct from that observed for sympatric carcharhinid sharks in those areas, whose neonates and adults with fresh mating scars were captured or observed during the austral summer (Freitas et al., Reference Freitas, Rosa, Gruber and Wetherbee2006; Garla et al., Reference Garla, Garcia, Lopes and Veras2009; RCG, personal communication). This pattern of distinct reproduction times of sympatric shark species using the same area has been suggested as a means to reduce interspecific competition and predation mortality during the first few months among species with great discrepancies in their size at birth (Motta et al., Reference Motta, Gadig, Namora and Braga2005). Although this hypothesis needs further investigation, the sizes at birth of the sympatric Negaprion brevirostris and Carcharhinus perezi in FEN are nearly twice that of G. cirratum and the former are born in a later period of the year (Garla, Reference Garla2004), suggesting that this reproductive tactic may occur.
The growth rate determined from recapture data was lower than that previously reported for nurse sharks in the Florida Keys (13.1 ± 9 cm year−1) (Carrier & Luer, Reference Carrier and Luer1990) and Recife (15.77 ± 2.53 cm year−1) (Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013). However, the small sample size precludes further insights on the growth of G. cirratum in FEN and waits additional sampling to provide more accurate estimates.
Most of the tagged sharks were recaptured or resighted in close proximity to the tagging location, suggesting that G. cirratum individuals have restricted movements, as demonstrated by previous studies employing mark-and-recapture techniques (Pratt & Carrier, Reference Pratt and Carrier2001; Pikitch et al., Reference Pikitch, Chapman, Babcock and Shivji2005; Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013). However, when analysing the recaptures together with the automated and active telemetry data, results indicate that nurse sharks exhibited site fidelity but not long-term residency to the array. Seven out of eight sharks fitted with acoustic transmitters repeatedly returned to the monitored area, but remained there for variable periods of time, a condition also observed in Belize and Recife (Chapman et al., Reference Chapman, Pikitch, Babcock and Shivji2005; Pikitch et al., Reference Pikitch, Chapman, Babcock and Shivji2005). Underwater observations and results of the actively tracked individual demonstrate that nurse sharks spend much of their time immobile close to structured bottoms or resting in holes, crevices and caves. This behaviour might result in part of the signals being blocked by solid structures between the transmitter and receiver. However, this would not happen for the entire periods of weeks or months without detections observed in the present study, and likely reflect emigration of the sharks out of the monitored area. All monitored sharks emigrated away from the array at some point, but unlike nurse sharks off Recife (Ferreira et al., Reference Ferreira, Afonso, Castilho and Hazin2013) there was no evidence of synchronized or seasonal migration. Each individual exhibited a distinct behaviour of visitation and emigration away from the array.
Previous studies have shown that G. cirratum can show relatively wide-ranging movements. The longest recorded distance travelled by the species was 541 km in Florida (Kohler & Turner, Reference Kohler and Turner2001), and 29.3 km in Belize, where a juvenile individual almost circumnavigated Glover's Reef Atoll in a 5-month period (Chapman et al., Reference Chapman, Pikitch, Babcock and Shivji2005). This is further supported by the active tracking of a juvenile nurse shark in FEN, which moved 15.81 km along a stretch of the coastline inspected by four receivers of the telemetry array in a 16-day period. Thus, results indicate G. cirratum likely move along wide sections of the insular shelf and use areas outside the protected zones in FEN where they can be exposed to fisheries. In this sense, future research should assess residency and movements over periods longer than 2 years to completely evaluate the conservation benefits of MPAs areas to populations of G. cirratum.
Nonetheless, findings are relevant for a better understanding and have management implications for the species in FEN and at other sites of its distribution. In this sense, MPAs are important to the early life-stages of G. cirratum, such as neonates and small juveniles, but they cannot be the only conservation measure used to protect this species. Despite remaining motionless or exhibiting short range movements for several hours or days, nurse sharks can be relatively wide-ranging (Chapman et al., Reference Chapman, Pikitch, Babcock and Shivji2005) and require different types of protection throughout their life cycle. These should include the use of MPAs, collaborative fisheries monitoring, and fisheries management practices such as the live release of incidentally caught individuals, along with law enforcement.
ACKNOWLEDGEMENTS
Authors are grateful to the Administration of Fernando de Noronha (ADEFN) and the Brazilian Environmental Agency (ICMBio) for research permits (No. 000111/99-36 and No. 12064) and tax exemptions. We thank Dr Ricardo Silva (Depto Química Orgânica, FCFRP-USP) for the statistical support and analysis. We are greatly in debt to the fishing volunteers Pablo Mendonça, Felipe Buloto, Tiego Costa, ‘Franzé’, Shany Nagaoka, Norberto Lopes, Felipe Dorr, Paulo Valobra, the fisheries masters ‘Danda’ and ‘Miguel’, the deckhands ‘Branco’, ‘Nenéu’ and ‘Junior Cenoura’, and also to Mr Alejandro and Mrs Milagros (NGO Karumbé, Uruguay) for lending the active tracking gear.
FINANCIAL SUPPORT
Funding was provided by São Paulo Research Foundation (FAPESP – Grant # 1998/15080-8), Fundação O Boticário de Proteção à Natureza (Grant # 0619-20041), Widlife Trust, PADI AWARE and Fundo Nacional do Meio Ambiente (FNMA/MMA – Grant # 03/2006).









