Introduction
Improvements to the IVM system will benefit from a range of applications, including animal husbandry and medicine, in addition to the potential use of planned in vitro fertilization, including efficient embryo production, cloning, genetic modification and animal models (de Souza-Fabjan et al., Reference de Souza-Fabjan, Panneau, Duffard, Locatelli, de Figueiredo, Freitas and Mermillod2014). Compared with other animals, porcine animals have become the model of choice for human transformation studies due to their similarity in body shape and physiological structure (Cheong et al., Reference Cheong, Kim, Kwak, Jeon and Hyun2015). Although in vivo embryos are better than in vitro embryos, it is more expensive and laborious to produce embryos in vivo. Therefore, the establishment of an in vitro culture system has become a better choice for related research. Evidence suggests that although culture conditions during in vitro embryo production may have a modest effect on the developmental potential of early embryos, the quality of oocytes at the beginning of the process is a key factor in determining the proportion of oocytes to blastocysts (Lonergan and Fair, Reference Lonergan and Fair2016). Many studies have shown that antioxidants, such as melatonin (Miao et al., Reference Miao, Zhou, Bai, Cui, ShiYang, Lu, Zhang, Dai and Xiong2018), glutamine (Gln) (Kim et al., Reference Kim, Koo, Kwon, Kang, Park, Gomez, Atikuzzaman, Jang and Lee2014), vitamin C (Wongsrikeao et al., Reference Wongsrikeao, Nagai, Agung, Taniguchi, Kunishi, Suto and Otoi2007), Ge (Kim et al., Reference Kim, Jeon, Kim, Lee and Hyun2015), etc., can improve the quality embryos by reducing oxidative stress and improving the quality of oocytes.
Kaempferol (3,4′,5,7-tetrahydroxyflavone, KAE) is one of the most common dietary flavonols possessing biological activities, such as anticancer (Qiu et al., Reference Qiu, Lin, Zhu, Zhang, Zeng, Su and Tian2017) anti-inflammatory (Kim et al., Reference Kim, Park, Lee, Yang, Park, Kim, Yi, Baek, Sung, Hossen, Lee, Kim and Cho2015b) and antioxidant activities (Choi, Reference Choi2011). Kaempferol has been reported to exhibit chemopreventive activity in cancer through a variety of mechanisms including the regulation of oxidative stress, inhibition of enzymes that activate carcinogens, modification of signal transduction pathways and interaction with related receptors and proteins (Aiyer et al., Reference Aiyer, Bouker, Cook, Facey, Hu, Schwartz, Shajahan, Hilakivi-Clarke and Clarke2012). Kaempferol upregulates Sirt3 (Yang et al., Reference Yang, Si, Jia, Jian, Yu, Wang and Lin2019), a mitochondrial sirtuin that plays an important role in regulating cellular processes, such as homeostasis, oxidative stress and ageing (Cimen et al., Reference Cimen, Han, Yang, Tong, Koc and Koc2010). Although the antioxidant activity and prooxidant activity of brass compounds have been studied, the antioxidant capacity of kaempferol in porcine oocytes has not been studied (Yang et al., Reference Yang, He, Zhou, Zhao, Shen, Xu and Ni2015).
ROS are a double-edged sword: low levels of ROS are key signalling molecules in many pathophysiological processes and excessive ROS play a key role in destroying cellular components and triggering cell death (Li et al., Reference Li, Zhou, Li, Li, Long, Chen, Zhang, Feng and Li2019). The continuous increase in calcium ions may lead to oxidative stress and cell death (Mata et al., Reference Mata, Marques, Martinez-Burgos, Silveira, Marques, Mesquita, Pariente, Salido and Singh2008). Intracellular calcium overload leads to the accumulation of mitochondrial calcium ions, changes in mitochondrial pH, increases in ROS production, decreases or completely losses in mitochondrial ΔΨm and changes in mitochondrial permeability (Choi, Reference Choi2011). Previous studies have shown synergy between quercetin and kaempferol associated with antioxidant activity and Nrf2-ARE (Saw et al., Reference Saw, Guo, Yang, Paredes-Gonzalez, Ramirez, Pung and Kong2014). The NRF2 pathway can further regulate mitochondrial damage by regulating ROS, thereby affecting mitochondrial apoptosis (Zhang et al., Reference Zhang, Yang, Jiang, Zou, Zeng, Ling, Zhou, Cao and Ao2019). Studies have shown that kaempferol exerts a significant protective effect on cell mitochondrial disorders and the inhibition of ROS production, oxidative stress and mitochondria is the main mechanism of kaempferol protection in cells (Ondricek et al., Reference Ondricek, Kashyap, Thamake and Vishwanatha2012). Therefore, the use of antioxidants may contribute to the resistance of oocytes to oxidative stress during in vitro maturation (Khazaei and Aghaz, Reference Khazaei and Aghaz2017). Although previous studies have reported the biological activity of KAE on a variety of cells, it is not clear whether KAE plays a similar role in the in vitro maturation culture system.
Therefore, in the present study, we investigated the ability of KAE to maintain the quality and promote the subsequent embryonic development in porcine oocytes after parthenogenesis activation. We evaluated the intracellular GSH and ROS levels and mitochondrial membrane potential in oocytes exposed to KAE to gain insight into the effects of KAE on oxidative stress in oocytes.
Materials and methods
Materials
Porcine ovary specimens obtained from a slaughterhouse were placed in sterile saline solution (0.9% sodium chloride, 75 μg/ml penicillin G and 50 μg/ml streptomycin sulfate) and transported back to the laboratory within 2 hrs.
Main reagents and instruments
Tissue culture medium (TCM-199), polyvinyl alcohol (HEPES-TL-PVA), phosphate-buffered saline (PBS), hyaluronidase (Hy), cytochalasin B (CB), Triton X-100 and Hoechst 33342 were purchased from Sigma, a rabbit anti-caspase-3 polyclonal antibody was obtained from Abcam and the DyNAmo SYBR Green qPCR Kit was purchased from Wizbiosolutions. A stereomicroscope was purchased from Olympus, a confocal microscope was obtained from Nikon, a real-time polymerase chain reaction (PCR) instrument was obtained from Agilent and a CO2 incubator was purchased from ESCO.
Collection and in vitro maturation of porcine oocytes
Fresh porcine ovaries were washed two or three times with sterile saline solution (0.9% sodium chloride, 75 μg/ml penicillin G and 50 μg/ml streptomycin sulfate) at 38°C. Cumulus–oocyte complexes (COCs) were aspirated from ovaries with a 10 ml disposable syringe with an 18G needle and placed in a 15 ml centrifuge tube. After precipitation in a water bath at 38°C for 10 min, the supernatant was discarded and HEPES-TL-PVA was added to the pellets. Then, the HEPES-TL-PVA-treated COCs were pipetted into a disposable culture dish and the COCs were examined with a stereomicroscope. The COCs were washed three times with HEPES-buffered medium containing 0.1% (w/v) HEPES-TL-PVA. For each group, 50 COCs were matured in 500 μl of the tissue culture medium TCM-199. To determine the dose effect of KAE on porcine oocytes cultured in vitro, the COCs were cultured with different concentrations (0.1, 1, 10 μM) of KAE under light mineral oil at 38.5°C for 44 h.
Parthenogenetic activation and culture in vitro
The COCs were placed in 1 mg/ml hyaluronidase solution and pipetted repeatedly until the cumulus cells were removed. Enucleated oocytes were parthenogenetically activated twice with pulses at 120 V for 60 µs in 297 mM mannitol solution containing 0.1 mM CaC12, 0.05 mM MgSO4, 0.01% PVA (w/v) and 0.5 mM HEPES (pH 7.2). The activated embryos were cultured in bicarbonate-buffered porcine zygote medium 5 (PZM-5) supplemented with 4 mg/ml bovine serum albumin (BSA) and 7.5 µg/ml cytochalasin B (CB) for 3 h to inhibit pseudosecond polar body extrusion. The oocytes were then thoroughly washed in a 4-well plate with bicarbonate-buffered PZM-5 (day 0) and 4 mg/ml BSA and cultured in an incubator with 5% CO2 at 38.5°C for 7 days.
Immunofluorescence staining
The oocytes were fixed in PBS containing 3.7% paraformaldehyde for 30 min and then permeabilized in 0.5% Triton X-100 for 30 min, followed by blocking the embryos in a blocking buffer (PBS containing 1% BSA) for 1 h. The embryos were incubated with the primary rabbit anti-Caspase-3 antibody at 4°C overnight and then incubated with an Alexa Fluor 488-conjugated secondary antibody. Subsequently, the embryos were blocked again for 1 h and then the DNA was counterstained with Hoechst 33342 (10 μg/ml in PBS) at 25°C. The stained embryos were fixed on a slide and mounted. The slide was imaged using an inverted fluorescence microscope to measure the intensity of the immunofluorescence and data were analysed by ImageJ software.
Determination of reactive oxygen species (ROS) levels in early porcine embryos by quantitative analysis of ROS and GSH
Early embryos were incubated with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Thermo Fisher Scientific, Waltham, USA) for 30 min and then subjected to spectroscopic analysis (green fluorescence, UV filter, 490 nm). Early porcine embryos were incubated with 10 μM cell tracker blue dye 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC) (Thermo Fisher Scientific) for 30 min and analysed by spectroscopic analysis (blue fluorescence, UV Filter, 370 nm). The fluorescence intensity of the embryos was analysed using ImageJ software. Three independent experiments were performed.
Detection of mitochondrial membrane potential in early embryos (ΔΨm)
For the measurement of the ΔΨm potential in cells, the early embryos were compared with JC-1 in a culture dish, covered with mineral oil and placed in an incubator for 10 min. The embryos were washed with PVA–PBS and then placed into the droplets on the dish for 30 min of vital staining. The stained embryos were washed three times with PVA–PBS and placed in PVA–PBS to be observed and imaged under a fluorescence microscope. The ΔΨm potential of the cells was measured by the change in JC-1 emission fluorescence. Accumulation of the ΔΨm potential in the cells was evidenced by the change of JC-1 fluorescence from green to red. The change in the relative ratio of red and green fluorescence reflected the change in membrane potential: a decreased ratio indicated depolarization and an increased ratio indicated hyperpolarization of the membrane.
Real-time reverse transcription polymerase chain reaction
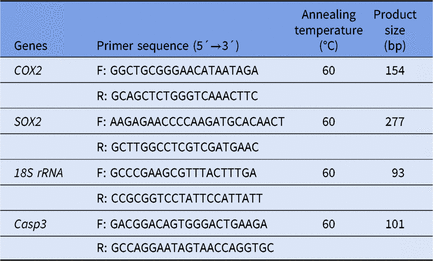
Expression of related genes was detected using real-time fluorescent quantitative PCR. For each group, 20 blastocysts (Day 7) were washed with PBS, rapidly frozen in liquid nitrogen and stored at −80°C for use. The mRNA was extracted using a Dynabeads mRNA kit and reverse transcribed into cDNA using SuperScript reverse transcriptase. The mRNAs were subjected to real-time fluorescence quantitative PCR detection using specific primers (primer sequences are shown in Table 1) a DyNAmo SYBR Green qPCR kit according to the manufacturer’s instructions. The PCR procedure was as follows: 95°C for 3 min, 40 cycles at 95°C for 15 s, 60°C for 30 s and 72°C for 20 s and a final extension at 72°C for 5 min. The target genes were Caspase-3, COX2 and SOX2. The gene encoding glyceraldehyde-3-phosphate (18S rRNA) was used as a reference gene. The primers used to amplify each gene are shown in Table 1. The mRNA quantification data were analysed using the 2−ΔΔCt method (Livak and Schmittgen, Reference Livak and Schmittgen2001). Three separate experiments were carried out with three samples per experiment.
Table 1. Primer sequences for qRT-PCR

Statistical analysis
The results are presented as the means ± standard deviation. Statistical analysis was performed using SPSS software version 22.0 (IBM, IL, USA). The strength of the normal distribution was tested using the Shapiro−Wilk normality test. We used t-tests/analysis of variance (ANOVA)s when the data were normally distributed; otherwise, we used the Mann–Whitney test for data that were not normally distributed. The total numbers (n) of oocytes/embryos used in each group and in the replicates (R) for each experiment are shown in the data column and the legends of each figure, respectively.
Results
Parthenogenetic activation of KAE-treated oocytes increased the rate of blastocyst development
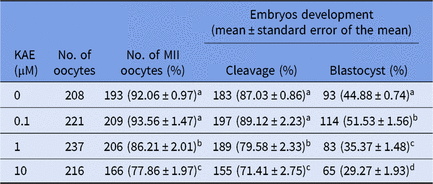
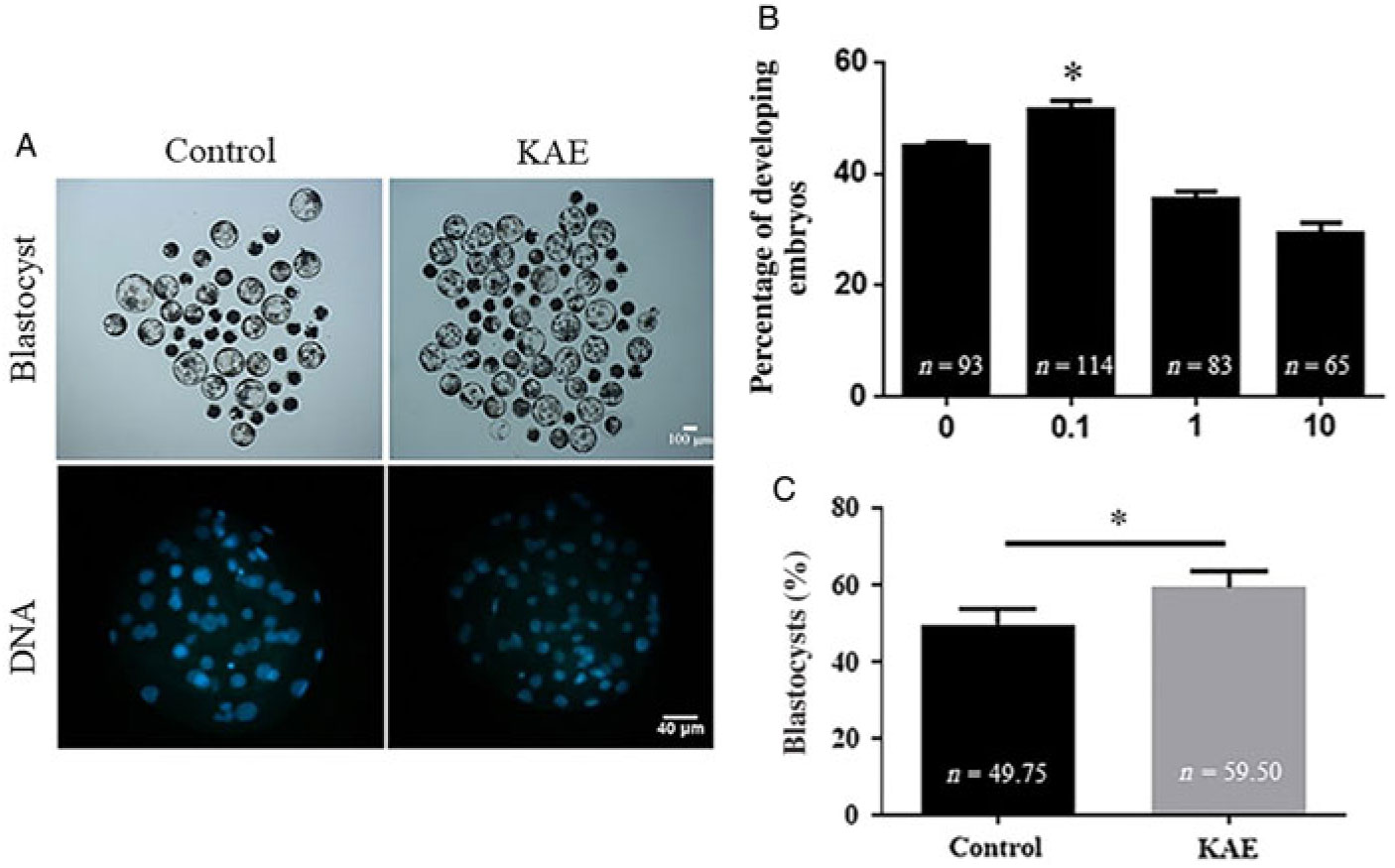
To investigate the effects of different concentrations of KAE on cell maturation rate, division rate and blastocyst formation rate, we used different concentrations of KAE (0, 0.1, 1 and 10 μM) in in vitro culture (IVC) after the parthenogenetic activation of mature oocytes. The results showed that the maturation rate and the cell division rate were not significantly different between the groups (Table 2), however the 0.1 μM KAE group (P < 0.05) showed a significant increase in the blastocyst formation rate compared with that in the other groups (Fig. 1A, B). Moreover, 0.1 μM KAE significantly increased the total cell number in the blastocyst after parthenogenesis activation (Fig. 1A, C). Therefore, we used 0.1 μM KAE for subsequent experiments.
Table 2. Effects of KAE supplements on oocyte maturation, cleavage and blastocyst rates

Note: the same shoulder mark indicates no significant difference between the two groups (P > 0.05); different lowercase letters indicate significant differences (P < 0.05); different uppercase letters indicate extremely significant differences (P < 0.01); the same below. MII, metaphase II.
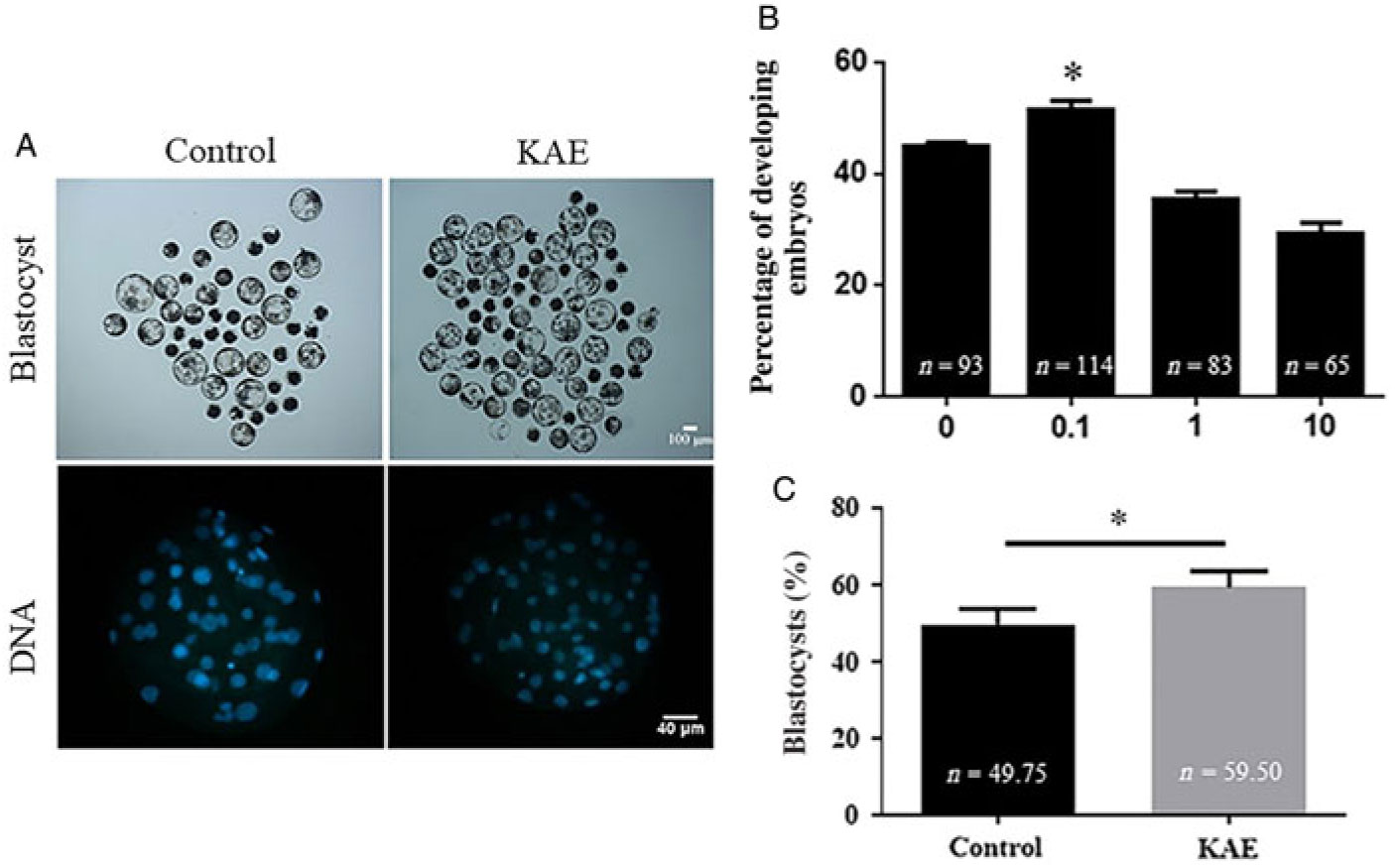
Figure 1. KAE improves embryos development after parthenogenetic activation. (A) Representative pictures of the embryonic development with blastocyst development rate and total cell number of embryos of control and KAE group. (B) Blastocyst formation rate of control and the presence of different concentrations (0, 0.1, 1 and 10 µg/ml) of KAE. R = 7. (C) Total cell number of embryos. R = 5. *P < 0.05 indicates significant differences.
KAE can improve the relative expression of COX2, SOX2 and caspase-3 mRNA in early embryos
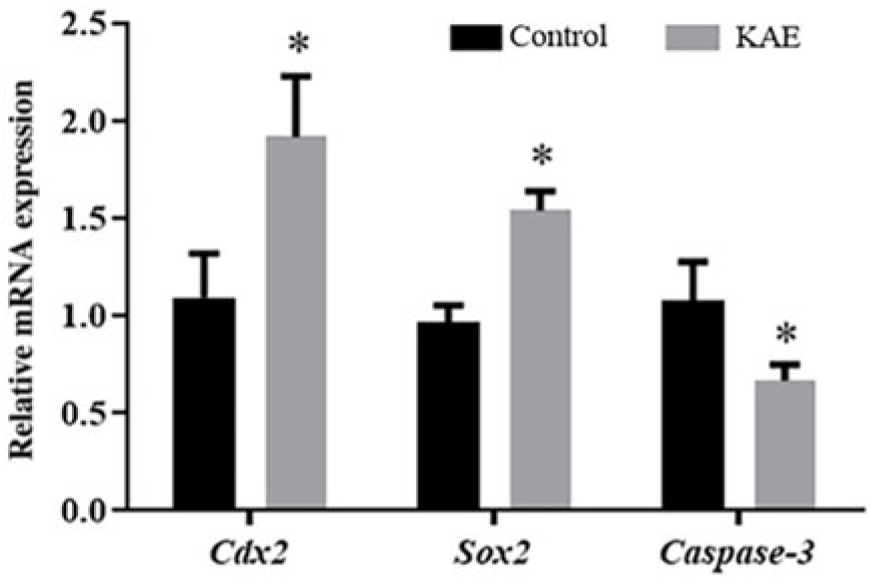
To further investigate the effect of KAE on embryonic development, we examined the effect of 0.1 μM KAE treatment compared with the control on gene expression. The mRNA expression levels of COX2 and SOX2 in the 0.1 µM KAE group were significantly increased (P < 0.05) and the mRNA expression levels of Caspase-3 in the 0.1 µM KAE group were significantly decreased (P < 0.05) (Fig. 2).
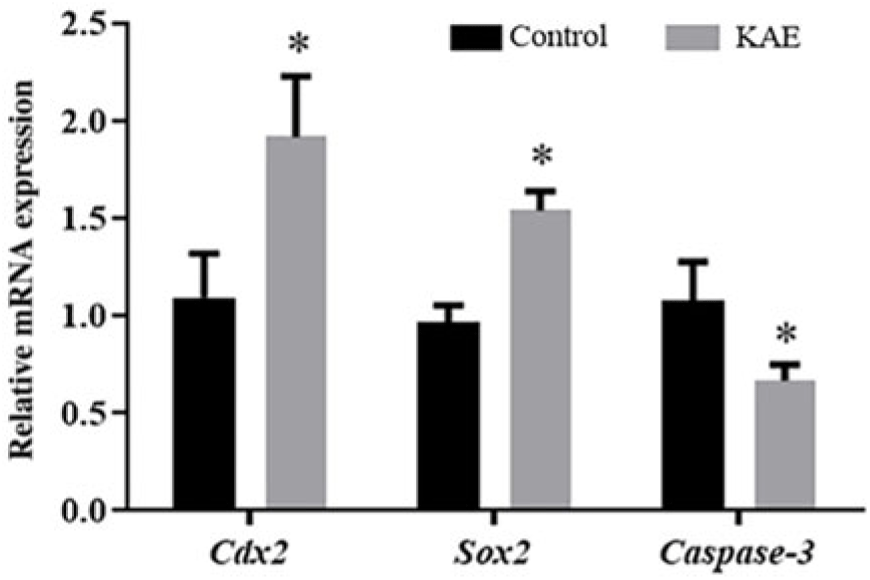
Figure 2. The relative mRNA levels of genes encoding COX2, SOX2 and Caspase-3, as analysed by RT-PCR. R = 3. *P < 0.05 indicates significant differences.
KAE ameliorates oxidative stress in porcine early embryos
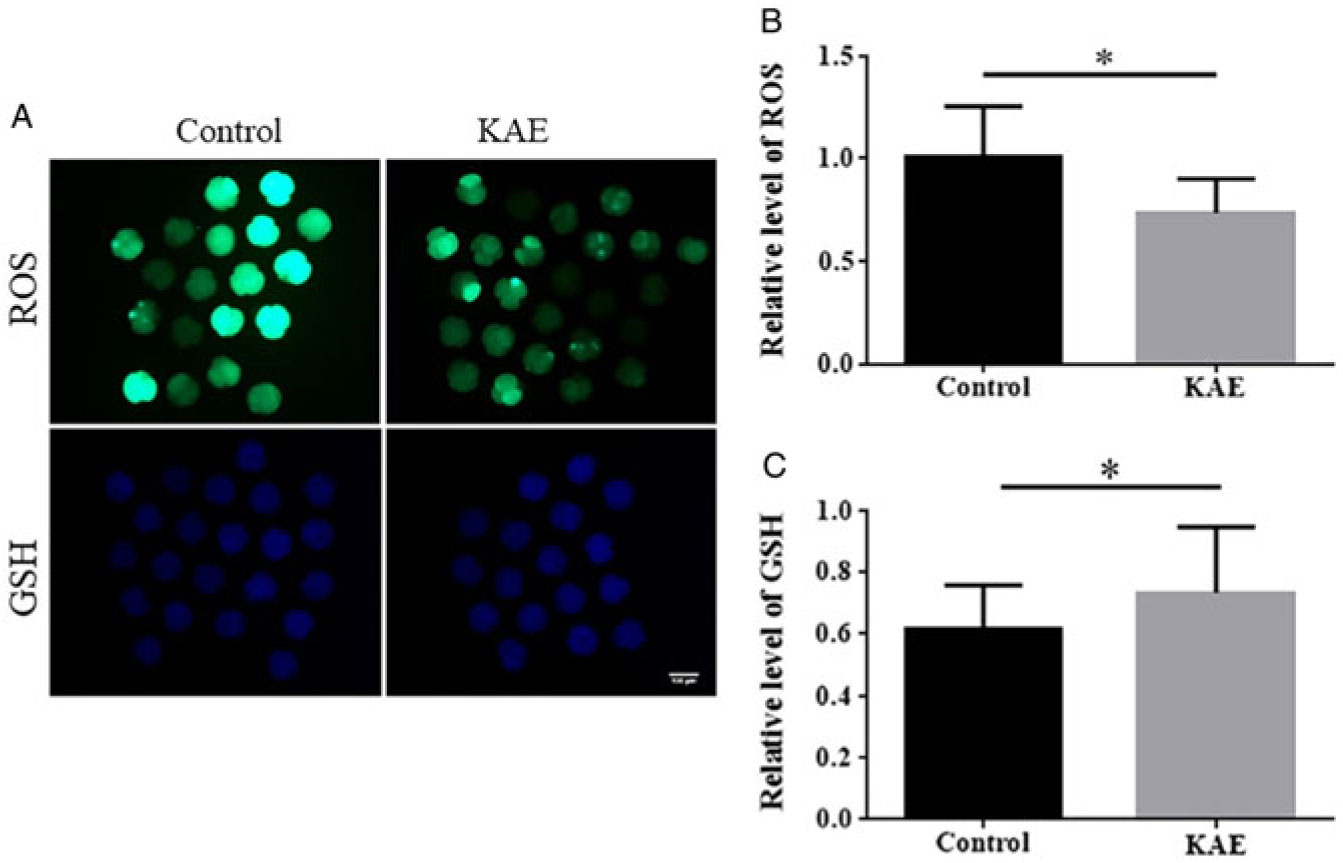
To assess the antioxidant effects of KAE on early embryos, we examined ROS and GSH levels in early embryos from the control and KAE-treated groups (Fig. 3A–C). The results showed that the ROS levels in the control group were significantly higher than those in the KAE-treated group (P < 0.05). Intracellular GSH levels were significantly lower in the control than those in KAE-treated early embryos (P < 0.05).
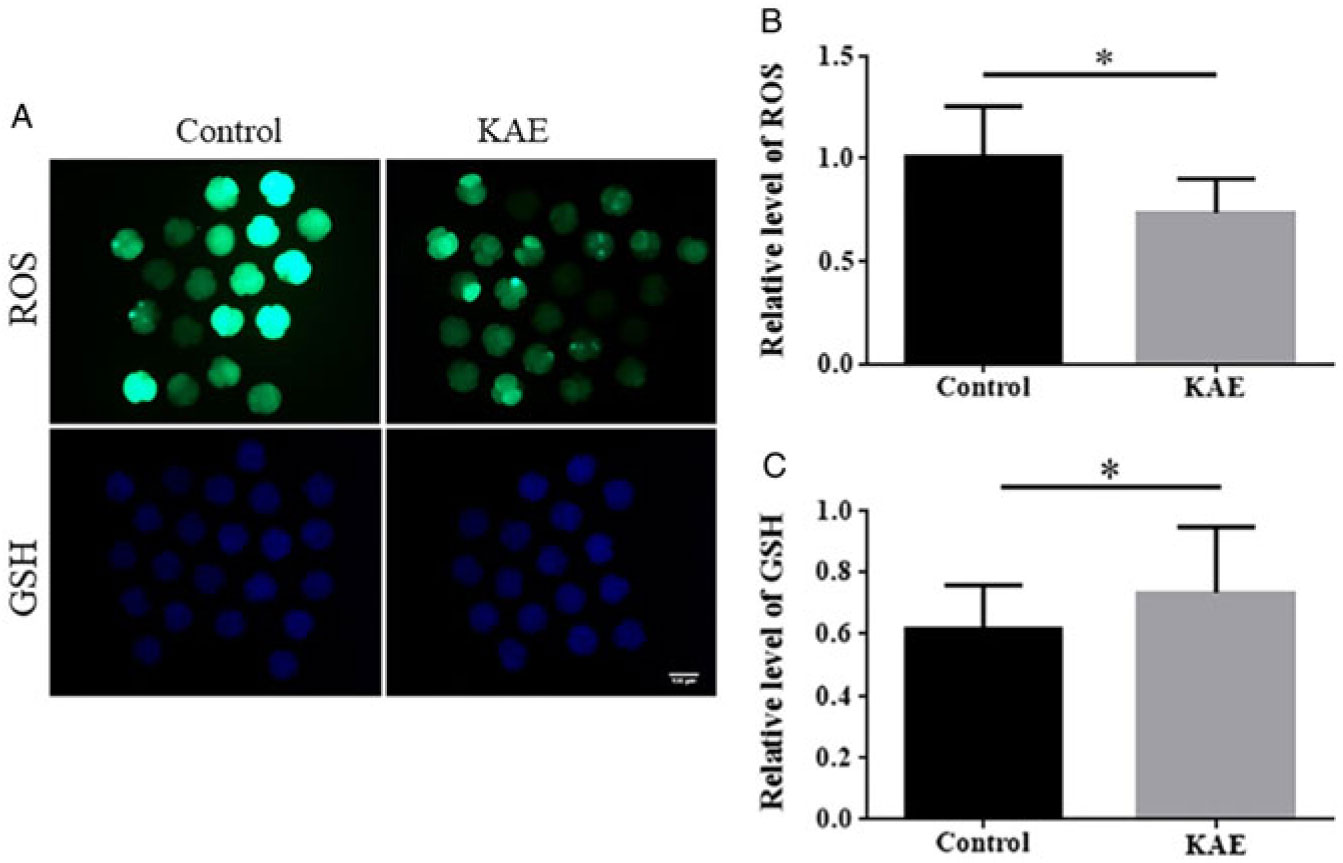
Figure 3. Effects of KAE on ROS and GSH levels in porcine early embryos. (A) Embryos were stained with H2DCFDA to detect the intracellular level of ROS. Embryos were stained with Cell Tracker Blue CMF2HC dye to detect the intracellular level of GSH. (B, C) The relative level of intracellular ROS and GSH in in vitro-matured porcine embryos from the groups (control, KAE). *P < 0.05 indicates significant differences. R = 3.
KAE enhances mitochondrial membrane potential (ΔΨm) in porcine early embryos
Mitochondrial dysfunction is one of the major causes of increased ROS levels and compromises embryo development (Ramalho-Santos et al., Reference Ramalho-Santos, Amaral and Oliveira2008). Therefore, we evaluated the ΔΨm potential status of early embryos by examining the ratio of red/green fluorescence. The ratio of the fluorescence signal was much lower in the control group than that in the KAE-treated group (P < 0.05) (Fig. 4A, B). This observation indicated the remarkable efficacy of KAE in maintaining the ΔΨm potential in porcine early embryos.
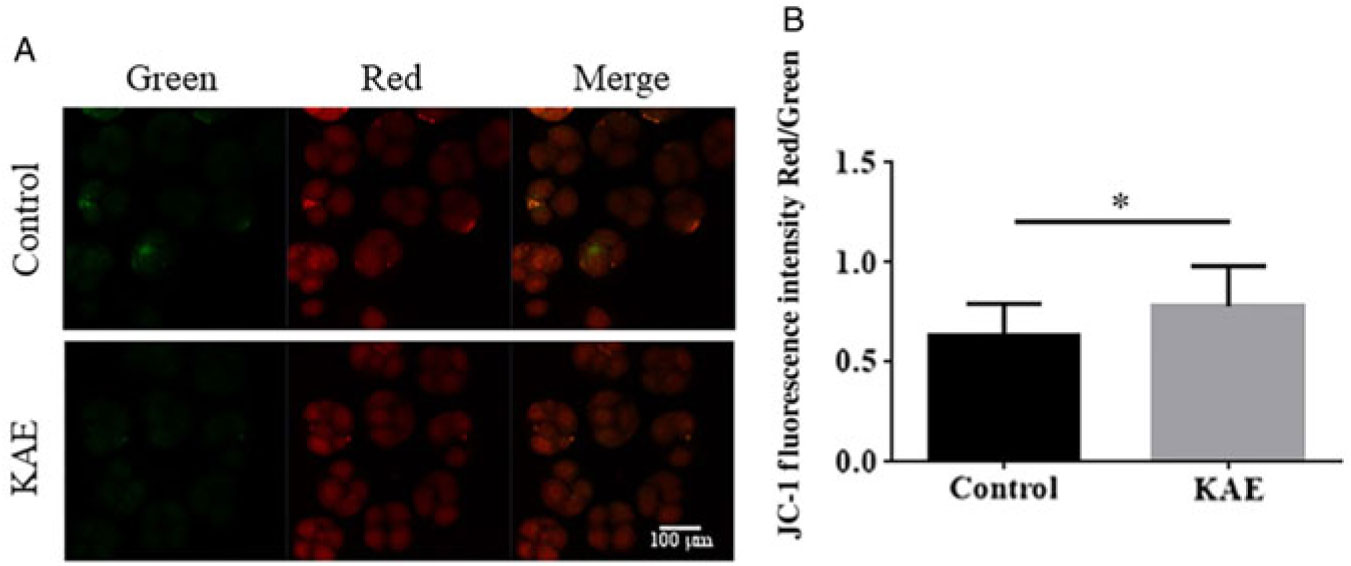
Figure 4. Evaluation of the effect of KAE on mitochondrial membrane potential. (A) 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanineiodide (JC-1) staining of embryos. (B) Fluorescence intensity for JC-1 in embryos. *P < 0.05 indicates significant differences. R = 3.
KAE supplementation decreases caspase-3 activity in blastocysts
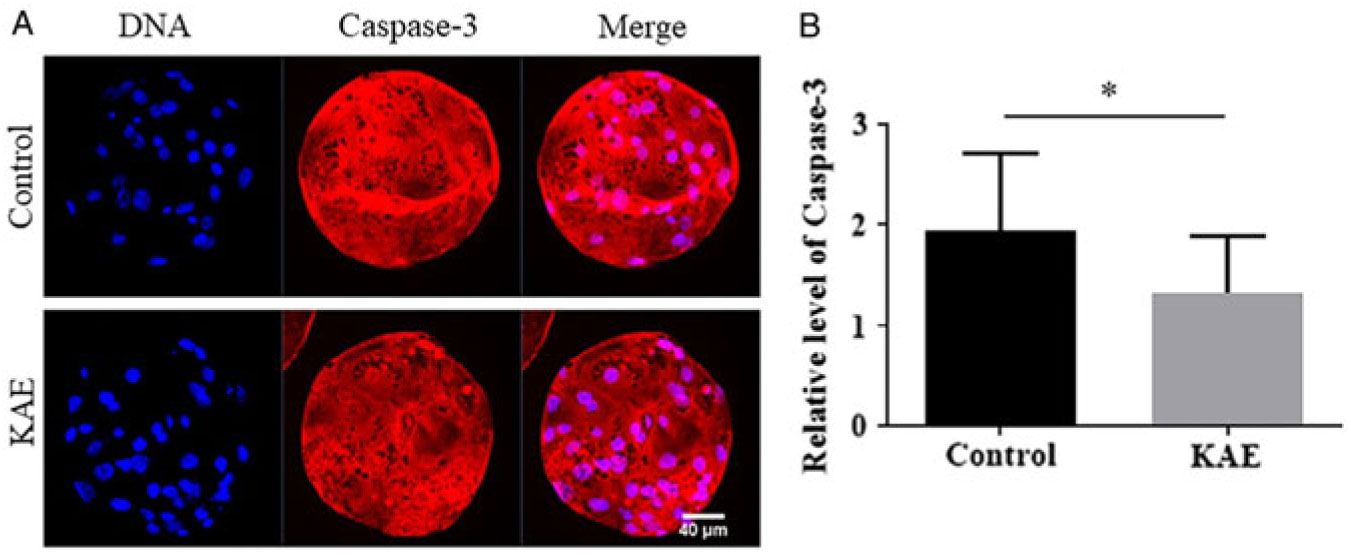
To detect the effect of KAE treatment at the vitro maturation stage of oocytes on the apoptosis of embryonic cells after parthenogenetic activation, we also examined the activity of Caspase-3 in blastocysts (Fig. 5A, B). The results showed that the Caspase-3 activity of the blastocysts in the control group was significantly lower than that in the KAE-treated group (P < 0.05).
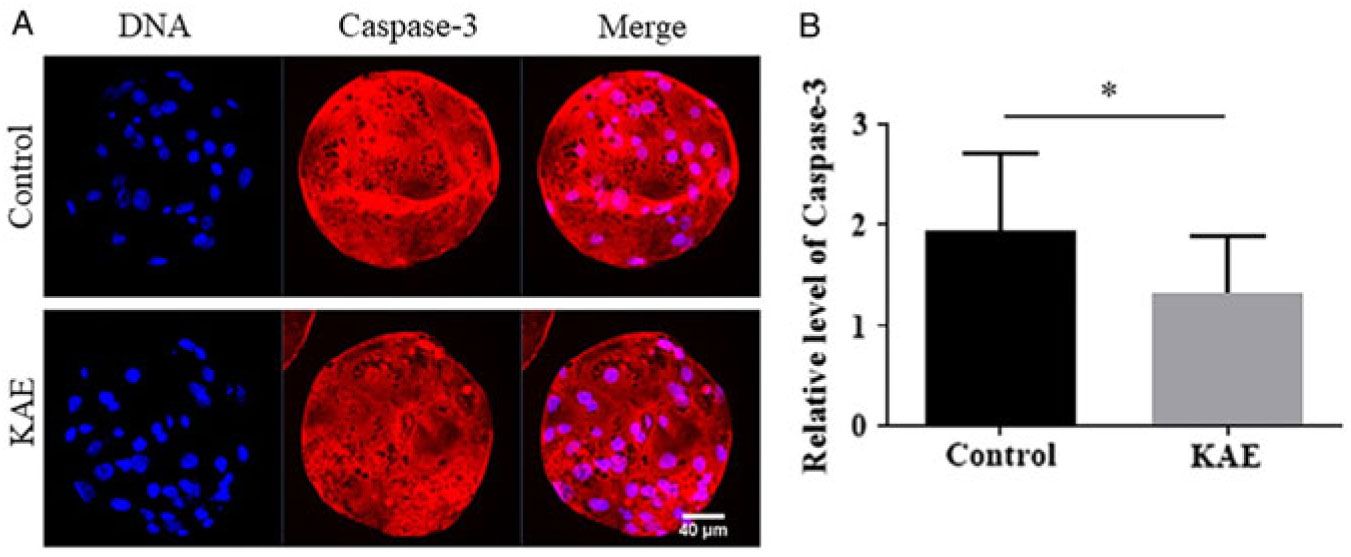
Figure 5. Effect of KAE on the caspase-3 activity of blastocysts. (A) Representative images showing caspase-3 activity in control and KAE-treated blastocysts. (B) Quantified fluorescence intensity for caspase-3 in blastocysts. *P < 0.05 indicates significant differences. R = 3.
Discussion
During these experiments, we examined mature oocytes and found no significant differences. However, it is puzzling that oocytes cultured using KAE exhibited stronger embryonic development. In this study, we used the proportion of developing embryos as a macroscopic indicator of embryo quality assessment. According to our experimental results, 0.1 μM KAE was the optimal concentration for screening. At this concentration, mRNA expression of COX2 and SOX2 in the 0.1 μM KAE treatment group was significantly increased and expression of Caspase-3 mRNA was significantly decreased. Therefore, we speculated that KAE could improve zygotic development after oocyte activation by regulating expression of SOX2, COX2 and Caspase-3. Previous studies have shown that a lack of SOX2 can lead to impaired oocyte maturation, fertilization and early embryonic development (Kim et al., Reference Kim, Park, Lee, Yang, Park, Kim, Yi, Baek, Sung, Hossen, Lee, Kim and Cho2015b). The SOX2 gene is expressed in the inner cell mass of the blastocyst, which is related to the pluripotency of the cell and plays an important role in embryonic development (White et al., Reference White, Angiolini, Alvarez, Kaur, Zhao, Mocskos, Bruno, Bissiere, Levi and Plachta2016). Caspase-3 is required for some typical hallmarks of apoptosis and was indispensable for apoptotic chromatin condensation and DNA fragmentation in all cell types examined (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2017). This finding is consistent with our experimental results and suggested that the reasonable addition of KAE to the medium in vitro not only enhanced the blastocyst rate and the number of blastocysts but also promoted growth and development of early embryos by regulating related growth factors.
Oocyte maturation and oocyte quality are key factors in fertility in all mammals (Gilchrist et al., Reference Gilchrist, Luciano, Richani, Zeng, Wang, Vos, Sugimura, Smitz, Richard and Thompson2016). Oocyte quality is typically affected by the production of oxidative stress (Prasad et al., Reference Prasad, Tiwari, Pandey, Shrivastav and Chaube2016). Previous studies have shown that ROS can target mitochondria, cause oxidative stress and interfere with glutathione metabolism and ROS induction leads to caspase-dependent apoptosis (Wang et al., Reference Wang, Liang, Zhang, Wang, Wan, Tan, Ji and Mao2019). The mitochondrial electron transport chain produces ATP during the maturation process, thereby producing ROS (Sinha et al., Reference Sinha, Das, Pal and Sil2013). When the excess ROS is beyond the physiologically acceptable range, the quality of the oocyte can be reduced (Kala et al., Reference Kala, Shaikh and Nivsarkar2017). With the increase in operation time in vitro, the accumulation of ROS in oocytes was also augmented. However, few studies have examined whether antioxidant-treated oocytes exhibit antioxidant capacities during early embryonic development. Therefore, we tested the ROS and GSH levels in developing embryos. Our results show that KAE treatment significantly decreased the level of ROS and increased the levels of GSH to reduce oxidative stress and improve the quality of oocytes. This finding is similar to other reports showing that KAE can act as an antioxidant to significantly increase GSH levels and inhibit ROS production.
Mitochondria not only control energy metabolism in the body but also regulate apoptosis or necrosis (Wang et al., Reference Wang, Figueiredo-Pereira, Oudot, Vieira and Brenner2017). During the in vitro culture of oocytes, oxidative stress directly affects mitochondrial function (Yao et al., Reference Yao, Jiang, Liang, Shen, Gao, Xu and Kim2018). During early embryo culture, the body needs to provide strict energy in the form of adenosine triphosphate (Spinaci et al., Reference Spinaci, Bucci, Muccilli, Cardullo, Nerozzi and Galeati2019). Previous studies have shown that oocytes with stronger antistress mechanisms are more likely to produce high-quality embryos after fertilization (Chappel, Reference Chappel2013). Studies by Guo et al. (Reference Guo, Liao, Huang, Liu, Yin and He2015) showed that KAE pretreatment attenuated the increase in the ROS levels, as well as the loss of Δψm and the release of cytochrome c (Guo et al., Reference Guo, Liao, Huang, Liu, Yin and He2015). This finding is consistent with our results. The addition of KAE to the in vitro growth environment of porcine oocytes can maintain early embryos with relatively high mitochondrial membrane potential and reduce mitochondrial the energy metabolism caused by oxidative stress.
In addition to inducing mitochondrial function, oxidative stress can also cause DNA damage (Czarny et al., Reference Czarny, Wigner, Galecki and Sliwinski2018). Caspases play important roles in the process of early apoptosis. Caspase-3 is a critical apoptosis protease that functions downstream in the caspase cascade (Wei et al., Reference Wei, Shen, Gong, Deng, Lai and Liang2017). the sequential activation of caspases plays a central role in cell apoptosis. The results of this study showed that KAE treatment reduced the expression of Caspase-3 activity in the control group compared with that in the KAE group. These results showed that KAE can significantly reduce the expression of Caspase-3 and reduce the damage of apoptosis on porcine early embryos.
In conclusion, our research reveals that KAE effectively relieved oxidative stress, decreased early apoptosis levels, maintained mitochondrial membrane potential and remarkably improved the blastocyst rate in porcine animals.
Financial support
Support for this research was provided by the National Key Research and Development Programme (no. 2018YFD0501702) and the Jilin Province Education Department (no. JJKH20191132KJ).
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
Not applicable.