Introduction
More than two billion people, depending predominantly on starch-rich cereals and tubers as staple food, suffer from iron deficiency-related anaemia and zinc deficiency (WHO, 2002; White and Broadley, Reference White and Broadley2005; Zimmerman and Hurrel, Reference Zimmerman and Hurrel2007) for which there is very limited variability among Triticum durum (Desf.) Husn., Triticum aestivum L. cultivars and landraces (Cakmak et al., Reference Cakmak, Ozkan, Braun, Welch and Romheld2000; Rawat et al., Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009). Bread wheat originated some 10,000 years ago (Dubcovsky and Dvorak, Reference Dubcovsky and Dvorak2007) involving three diploid species through two steps of hybridization and chromosome doubling (Kihara, Reference Kihara1944; McFadden and Sears, Reference McFadden and Sears1946; Feldman et al., Reference Feldman, Liu, Segal, Abbo, Levy and Vega1997), resulting in their immediate isolation from the parental species due to which very limited variability among the progenitor species could get incorporated in the cultivated gene pools of tetraploid and hexaploid wheat. The germplasm of related wild progenitor and non-progenitor Triticum and Aegilops species is a rich reservoir of useful variability for resistance against biotic and abiotic stresses, quality traits, yield and yield components (Damania, Reference Damania1993; Jiang et al., Reference Jiang, Friebe and Gill1994; Friebe et al., Reference Friebe, Jiang, Raupp, McIntosh and Gill1996). Useful variability for several traits has been introgressed into cultivated wheat and exploited commercially (Dvörak, Reference Dvörak1977; Kuraparthy et al., Reference Kuraparthy, Chhuneja, Dhaliwal, Kaur, Bowden and Gill2007; Chhuneja et al., Reference Chhuneja, Kaur, Garg, Ghai, Kaur, Prashar, Bains, Goel, Keller, Dhaliwal and Singh2008). Several diploid and tetraploid wild progenitor species have been found to possess high grain iron and zinc contents (Cakmak et al., Reference Cakmak, Ozkan, Braun, Welch and Romheld2000; Ortiz-Monasterio and Graham, Reference Ortiz-Monasterio and Graham2000; Calderini and Ortiz-Monasterio, Reference Calderini and Ortiz-Monasterio2003a, Reference Calderini and Ortiz-Monasteriob; Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006), which are being used for transfer of the useful variability for biofortification of wheat for high grain iron and zinc contents. Some ‘S’ genome diploid and tetraploid Aegilops species possess variability for two to three fold higher grain iron and zinc contents (Rawat et al., Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009). In addition to direct crosses between wheat cultivars and wild species, numerous synthetic amphiploids have been developed between T. durum or T. aestivum cultivars and the related wild species. The synthetic amphiploids have been used for dissecting alien genomes through the development of alien substitution and addition lines with useful variability for subsequent introgression into elite wheat cultivars using induced homoeologous pairing (Chen et al., Reference Chen, Tsujimoto and Gill1994; Jiang et al., Reference Jiang, Friebe and Gill1994; Aghaee-Sarbarzeh et al., Reference Aghaee-Sarbarzeh, Ferrahi, Singh, Singh, Friebe, Gill and Dhaliwal2002) and molecular cytogenetics (Kuraparthy et al., Reference Kuraparthy, Chhuneja, Dhaliwal, Kaur, Bowden and Gill2007).
This article reports the development and characterization of synthetic amphiploids between bread wheat cultivars and Aegilops kotschyi accessions having higher grain iron and zinc contents than the wheat cultivars.
Materials and methods
Plant material
The plant material consisted of six accessions of Ae. kotschyi viz., pau3774, pau3790, pau14262 (391), pau14264 (393), pau14266 (395) and pau14267 (396) and two T. aestivum genotypes – landrace Chinese Spring (Ph I) with introgressed homoeologous pairing inducing gene (Chen et al., Reference Chen, Tsujimoto and Gill1994), henceforth abbreviated as CS(Ph I) and a bread wheat cultivar WL711 (Table S1, available online only at http://journals.cambridge.org) received from Punjab Agricultural University, Ludhiana. The source and origin of the Ae. kotschyi accessions used in this study are available from one of the authors Dr Kuldeep Singh. The plant material was grown in the experimental field of the Indian Institute of Technology, Roorkee in 2004–2005 as replicated single row of 2 m length with plant to plant distance of 10 cm and row to row spacing of 30 cm with the recommended fertilizers (50:25:25 NPK kg/acre) and irrigation practices as that of wheat. Several F 1 hybrids were produced using wheat as the female parent and the Ae. kotschyi accessions as the male parent (Table S1, available online only at http://journals.cambridge.org). In the following year, the F 1 seeds were sterilized with 1% sodium hypochlorite for 5 min, washed thrice with distilled water and germinated on two layers of sterilized moist filter paper in Petri plates. The chromosomes of the F 1 hybrids were doubled by treating coleoptiles of germinating seeds with 0.25% of colchicine (in 5% DMSO solution) for 5 h. The colchicine-treated seedlings were transplanted in the field. Some F 1 hybrid seeds were also grown in the field without colchicine treatment and crossed with recurrent wheat parents. The colchicine-treated and -untreated plants were grown under similar spacing and cultivation conditions as that of the wheat cultivars.
During flowering, the spikes with anthers dehiscing viable pollen grains and seed set, evidently due to chromosome doubling, were identified and tagged. Seeds (C0 generation of amphiploids) from the doubled sectors of the tagged spikes were harvested carefully before shattering of spikes. The C1 generation of these amphiploids was grown in the field during 2006–2007. Collection of mature spikelets and spikes of the F 1 hybrids and synthetic amphiploids had to be done repeatedly at different intervals over 2–3 weeks because of frequent shattering of spikes. Due to tough glumes and hard threshing in the amphiploids and wild donors, the grains were threshed manually. Mean number of seeds per spike was determined for each amphiploid by taking the average of number of seeds of ten spikes in each replication.
Pollen stainability
Anthers dehiscing pollen grains were collected from five spikes of each replication in the morning, and pollen fertility was determined by staining with iodine potassium iodide solution.
Seed protein electrophoresis
SDS–PAGE of high molecular weight (HMW) glutenin subunits of endosperm proteins of mature and dried seeds of parents and amphiploids was done using 10% acrylamide following the method of Smith and Payne (Reference Smith and Payne1984).
Cytological studies
Spikes of F 1 hybrids and amphiploid plants were fixed for 24 h in Carnoy's solution (ethanol–chloroform–acetic acid; 6:3:1) for meiotic analysis. Spikes were transferred to 70% ethanol after 24 h of fixation. For meiotic study, the anthers were squashed in 2% acetocarmine. Pollen mother cells (PMCs) at meiotic metaphase-I were scored for chromosome number and pairing in all the crosses and synthetic amphiploids.
Micronutrient analysis
Grain analysis
For micronutrient analysis, whole-grain samples of parents and amphiploids were taken at maturity, washed with N/10 HCl to remove contaminating dust if any, and dried in hot air oven at 80°C until constant weight. Grain samples (0.5 g) were digested in a mixture of two parts of concentrated nitric acid and one part perchloric acid as per the procedure described by Zarcinas et al. (Reference Zarcinas, Cartwright and Spencer1987). Digestion was continued till white residue was obtained. Required volume was made after the completion of digestion process and digests were analyzed by Atomic Absorption Spectrophotometer (GBC- Avanta Garde M, Dandenong, Victoria, Australia). Seeds from each accession of wild species and the amphiploids were analyzed as three replicates to minimize the error during analysis.
Flag leaf iron and zinc contents
Flag leaves of WL711, CS(Ph I), Ae. kotschyi parents and amphiploids were analyzed for iron and zinc contents before ear emergence at pre-anthesis stage. The leaves were washed thoroughly with N/10 HCl, dried at 80°C for 8 h in oven prior to digestion. Dried leaf samples were then digested as a minimum of three replications as that for grains. Iron and zinc concentrations in the digests were analyzed by AAS.
Grain ash analysis
One gram dried grains of each of Ae. kotschyi accessions, WL711, CS(Ph I) and the seven amphiploids were cleaned thoroughly and kept for incineration at 600°C for 10 h. The ash was further processed like the grains for AAS analysis.
Results
Plant and spike characteristics and chromosome pairing of F 1 hybrids
The wheat × Ae. kotschyi F 1 hybrids were morphologically intermediate between wheat and Ae. kotschyi parents (Fig. S1; Table S1, available online only at http://journals.cambridge.org). All the F 1 hybrids were completely self sterile and had spelta heads with brittle rachis above the basal spikelet. The hybrids with CS(Ph I) had awnless lemma and glumes, whereas those with WL711 had one glume awn and one lemma awn (Fig. S1, available online only at http://journals.cambridge.org). Chinese Spring has been known to possess awn inhibitor genes on chromosomes 4A and 6B (Sourdille et al., Reference Sourdille, Cadalen, Gay, Gill and Bernard2002).
The rachis of F 1 hybrids disarticulated only above the basal spikelets like that of Ae. kotschyi (Fig. S1, available online only at http://journals.cambridge.org). The details of fertility and chromosome pairing of seven F 1 hybrids between T. aestivum [WL711 or CS(Ph I)] and six accessions of Ae. kotschyi are given in Table 1. There was very limited intergenomic pairing in the F 1 hybrids (Fig. 1) with very high frequency of univalents (25.69–32.74), low frequency of rod bivalents (1.0–4.17) and occasional trivalents (0.09–0.32). One of the F 1 hybrids, CS(Ph I)/Ae. kotschyi 396, showed higher chromosome pairing (25.69 Is, 4.17 IIs and 0.32 IIIs) when compared with other hybrids (Table 1). This may be attributed to induced homoeologous pairing due to Ph I in CS which is epistatic to Ph1, the diploidization gene on the long arm of chromosome 5B (Riley and Chapman, Reference Riley and Chapman1958; Chen et al., Reference Chen, Tsujimoto and Gill1994; Jiang et al., Reference Jiang, Friebe and Gill1994; Aghaee-Sarbarzeh et al., Reference Aghaee-Sarbarzeh, Ferrahi, Singh, Singh, Friebe, Gill and Dhaliwal2002). However, in the other three hybrids of CS(Ph I) with different Ae. kotschyi accessions comparatively less homoeologous chromosome pairing was observed (Table 1). The F 1 WL711/Ae. kotschyi 393 without Ph I also had relatively higher frequency of bivalents (up to 6 II). All the F 1 plants showed very low-pollen stainability (17.6–23.5%), no anther dehiscence and no seed set (Table 1).
Table 1 Chromosome pairing and pollen stainability in Triticum aestivum/Aegilops kotschyi F 1 hybrids


Fig. 1 Chromosome pairing in metaphase-I of wheat/Aegilops kotschyi F 1 hybrids, (a) F 1 CS(Ph I)/Ae. kotschyi 396 (6 II+23 I), (b) CS(Ph I)/Ae. kotschyi 3774 (3 II +29 I), (c) F 1 CS(Ph I)/Ae. kotschyi 393 (1 III +2 II +30 I), (d) F 1 WL711/Ae. kotschyi 393 (1 II +32 I), (e) F 1 WL711/Ae. kotschyi 391 (1 III +3 II +27 I) and (f) F 1 WL711/Ae. kotschyi 3790 (2 II +31 I).
The F 1 hybrid plants treated with colchicine were exactly like F 1 hybrids except for having some doubled sectors or spikes with dehiscing anthers, which could be readily distinguished from non-dehiscent sterile anthers in other spikes. These spikes/sectors with dehiscing anthers had normal seed set, whereas there was no seed set on the otherwise sterile F 1 plants without chromosome doubling. The seeds thus obtained were identified as the potential synthetic amphiploids (C0 generation) for further studies.
Morphology and fertility of the synthetic amphiploids
Comparative morphology of the synthetic amphiploids with the parents showed their intermediate growth habit, tiller number and plant height (Table S1, available online only at http://journals.cambridge.org) like the F 1 hybrids. The amphiploids displayed some of the characteristics of the Ae. kotschyi parent such as spelta head, brittle rachis and red seed colour and other characteristics of the wheat parents such as 1000 grain weight. The number of spikelets per spike exceeded both the parents. Most of the spike characteristics like the number and length of awns of glumes and lemmas were again intermediate to both the parents. Ae. kotschyi accessions had 5–7 glume awns against none and single awn in CS(Ph I ) and WL711, respectively. The glumes of amphiploids with WL711 had single awn, while those with CS(Ph I ) were awnless and had a tooth only. The long lemma awn of WL711 was replaced by small awns in the amphiploids, whereas Ae. kotschyi had two lemma awns (Fig. S1, available online only at http://journals.cambridge.org).
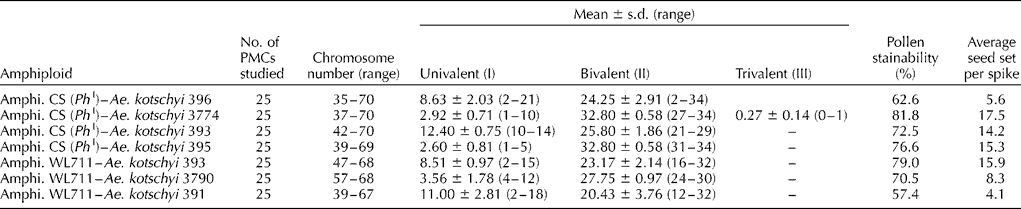
Pollen stainability and seed set in the amphiploids varied within the season. The early flowering spikes had non-dehiscent anthers, low-pollen stainability and less seed set, whereas the late flowering tillers had dehiscing anthers, higher pollen stainability and good seed set. Pollen stainability varied from 62.6 to 81.8% in different amphiploids of CS(Ph I)–Ae. kotschyi accessions, while in amphiploids of WL711–Ae. kotschyi accessions it ranged from 57.4 to 79.0% (Table 2). Variation in the seed set was observed for different combinations of bread wheat lines and Ae. kotschyi accessions. Maximum seed set was observed in the amphiploid CS(Ph I)–Ae. kotschyi 3774 (17.5 seeds/spike) and least in WL 711–Ae. kotschyi 391 (4.1 seeds/spike). Seeds of the amphiploids were longer, red and had 1000 grain weight comparable with those of the wheat parents (Table S1; Fig. S1, available online only at http://journals.cambridge.org).
Table 2 Chromosome number, meiotic pairing and seed set in Triticum aestivum/Aegilops kotschyi synthetic amphiploids (C1)
Chromosome pairing in the synthetic amphiploids
Chromosome number in the amphiploids was highly variable ranging from 35 to 70 chromosomes in CS(Ph I)–Ae. kotschyi 396, 39–69 in CS(Ph I)–Ae. kotschyi 395, 42–70 in CS(Ph I)–Ae. kotschyi 393 and 37–70 in CS(Ph I)–Ae. kotschyi 3774 (Table 2; Fig. 2). There was only a small proportion of PMCs in all the amphiploids with the expected double chromosome number (70) of the F 1 hybrids (35). Comparatively, higher number of bivalents and lower number of univalents in the amphiploids CS(Ph I)–Ae. kotschyi 395 (32.8 II, 2.6 I), CS(Ph I)–Ae. kotschyi 3774 (32.8 II, 2.92 I) and WL711–Ae. kotschyi 393 (23.2 II, 8.5 I) might have resulted in higher seed set (Table 2) in these amphiploids, whereas irregular meiotic behaviour of CS(Ph I)–Ae. kotschyi 396 and WL711–Ae. kotschyi 391 with very wide range of chromosome number, higher frequency of univalents and lower bivalent frequency was associated with low-seed set percentage (4.1 seeds per spike; Table 2).

Fig. 2 Chromosome pairing in wheat–Aegilops kotschyi amphiploids (a) Amphi. CS(Ph I)–Ae. kotschyi 396 (Chr-64, 1 III+28 II+5 I) (b) Amphi. CS(Ph I)–Ae. kotschyi 3774 (Chr-68, 32 II +4 I) (c) Amphi. CS(Ph I)–Ae. kotschyi 393 (Chr-69, 31 II +7 I) (d) Amphi. WL711–Ae. kotschyi 393 (Chr-69, 32 II +5 I), (e) Amphi. WL711–Ae. kotschyi 3790 (Chr-64, 30 II +4 I) and (f) Amphi. WL711–Ae. kotschyi 391 (Chr- 67, 29 II +8 I).
HMW glutenin subunit profiles of amphiploids
The SDS–PAGE profiles of the HMW glutenin subunits of CS(Ph I), Ae. kotschyi accessions and the CS(Ph I)–Ae. kotschyi amphiploids are given in Fig. 3. T. aestivum cultivars PBW343, Kalyan Sona and landrace CS and CS(Ph I) were taken as the control. CS and CS(Ph I) had similar subunit pattern for Glu 1B-controlled 7+8 subunits and Glu 1D-controlled 2+12 subunits of HMW glutenins. All the accessions of Ae. kotschyi (UUSS) expressed 3–5 novel subunits of HMW glutenin subunits. Two of the slowest migrating x subunits had lower electrophoretic mobility than the Glu-D1 subunit 5, while the faster migrating two y subunits were slower than the subunit 7. HMW glutenin subunits of both the wheat and Ae. kotschyi parents were present in all the amphiploids confirming the presence and expression of both the parental genomes (Fig. 3). Similar additive profile of HMW glutenin subunits was observed in the three amphiploids of WL711–Ae. kotschyi.

Fig. 3 HMW glutenin subunit profile of Triticum aestivum cultivars, Aegilops kotschyi accessions and the amphiploids of CS(Ph I) and WL711 with Ae. kotschyi accessions. (a) Lane 1, PBW343; 2, Kalyan Sona; 3, Chinese Spring; 4, Chinese Spring(Ph I); 5, Ae. kotschyi 393; 6, Ae. kotschyi 395; 7, Ae. kotschyi 396; 8, Ae. kotschyi 3774; 9, Amphi. CS(Ph I)–Ae. kotschyi 393; 10, Amphi. CS(Ph I)–Ae. kotschyi 395; 11, Amphi. CS(Ph I)–Ae. kotschyi 396; and 12, Amphi. CS(Ph I)–Ae. kotschyi 3774. (b) Lane 1, WL711; 2, Ae. kotschyi 391; 3, Ae. kotschyi 393; 4, Ae. kotschyi 3790; 5, Amphi. WL711–Ae. kotschyi 391; 6, Amphi. WL711–Ae. kotschyi 393; and 7, Amphi. WL711–Ae. kotschyi 3790.
Grain and flag leaf iron and zinc concentrations of amphiploids
Table 3 shows grain and flag leaf iron and zinc concentrations of the amphiploids along with both the parents viz., WL711, CS(Ph I) and Ae. kotschyi accessions. The micronutrient concentration of Aegilops kotschyi accessions was two to three folds higher when compared with wheat parents. Ae. kotschyi accession 3774 had the highest grain iron (70.8 mg/kg) and zinc concentration (35.7 mg/kg), respectively. The micronutrient concentrations of wheat parents were quite low, iron being 22.8 mg/kg in WL711 and 30.2 mg/kg in CS(Ph I) and zinc 16.6 mg/kg and 18.3 mg/kg in WL711 and CS(Ph I), respectively. The micronutrient concentrations of amphiploids were comparable with those of Ae. kotschyi. The micronutrient content per seed in Ae. kotschyi parents was, however, similar to that of the wheat parents in spite of the fact that they had three times smaller seeds than the wheat cultivars, which could be either attributed to their distinctive genetic system for micronutrient deposition or concentration due to lower harvest index.
Table 3 Whole grain and flag leaf iron and zinc, grain ash iron and zinc concentrations of amphiploids and their parents

Ae. kotschyi also had two to three times higher flag leaf iron and zinc concentrations than the wheat parents, suggesting that their higher grain micronutrient content could be attributed to their distinctive genetic system for deposition rather than to concentration in their smaller seeds. The amphiploids also showed an increase of up to 127% in iron and 92% in zinc in the flag leaves and 2-3 times higher iron and zinc content per seed than the parents due to the inherent distinctive micronutrient deposition system of the Ae. kotschyi parents.
Ash content and ash iron and zinc contents of amphiploids
The grain ash content in Ae. kotschyi was up to 30% higher than that of the wheat cultivars indicating their higher inorganic component, whereas for the wheat–Ae. kotschyi amphiploids it was intermediate between the parental species (Table 3). The grain iron and zinc contents in the amphiploids were more than double of that of the wheat parents, whereas the iron content in the grain ash was 61–85% and zinc was 50–63% higher than that of WL711 cultivar.
Discussion
The wheat/Ae. kotschyi F 1 hybrids as well as the amphiploids were morphologically intermediate between the wheat and Ae. kotschyi parents for plant height, growth habit, tiller numbers per plant, etc. However, other characters like ear shape, glume awns, hard threshing and brittle rachis were more like their Ae. kotschyi parents. The intermediate morphology of the F 1 hybrids and their synthetic amphiploids has been reported in several studies (Sears, Reference Sears1954; Martin and Laguna, Reference Martin and Sanchez-Monge Laguna1982; Sharma et al., Reference Sharma, Aylward and Gill1987; Oliver et al., Reference Oliver, Cai, Xu, Chen and Stack2005). The genes controlling brittle rachis (Br), tenacious glumes (Tg) of Ae. kotschyi appear to be epistatic over the Q locus controlling square head, tough rachis and free threshing in T. aestivum (Endo and Gill, Reference Endo and Gill1996; Li and Gill, Reference Li and Gill2006) as the amphiploids resembled their Ae. kotschyi parents.
All the F 1 hybrids (ABDUSl) had the expected 35 chromosomes (Table 1; Fig. 1) indicating complete parental chromosome complement and chromosome stability. Low to high intergenomic homoeologous chromosome pairing was observed in different F 1 hybrids. High-chromosome pairing observed in F 1 CS(Ph I)/Ae. kotschyi 396 is probably due to the presence of Ph1-inhibitor gene, Ph I, transferred from Aegilops speltoides that is known to induce considerable amount of wheat–alien pairing even in a single dose (Chen et al., Reference Chen, Tsujimoto and Gill1994). Our CS(Ph I) stock seems to be heterogeneous as some other F 1 hybrids with CS(Ph I) had limited pairing. Intermediate homoeologous pairing in hybrids with cultivar WL711 may also be explained due to some pairing promoters in Aegilops species that are known to suppress or enhance pairing in Triticeae (Riley and Chapman, Reference Riley and Chapman1958; Sears, Reference Sears1976; Jauhar, Reference Jauhar2007). Mello-Sampayo (Reference Mello-Sampayo1973) also observed the interaction of pairing promoters that inactivate Ph1 or Ph1-like genes in wheat/Ae. speltoides and wheat/Aegilops longissima hybrids.
The F 1 hybrids had too low-pollen stainability to permit anther dehiscence and hence had no self-seed set. The low- to medium-chromosome pairing permitted some of the paired chromosomes to undergo reduction division and move to anaphase poles before the large number of unpaired univalents align on the metaphase-I plate and divide. Only those paired chromosomes with intact sister chromatids would divide equationally in the second meiotic metaphase, while the univalent chromatids already separated in metaphase-I are expected to move randomly resulting in tetrads with unbalanced chromosome number and micronuclei. However, no fertile first division restitution nucleus was observed as reported for T. durum/Aegilops tauschii and T. durum/Ae. longissima crosses (Matsouka and Nasuda, Reference Matsouka and Nasuda2004; Jauhar, Reference Jauhar2007; Tiwari et al., Reference Tiwari, Rawat, Neelam, Randhawa, Singh, Chhuneja and Dhaliwal2008). Medium to highly fertile synthetic amphiploids (AABBDDUUSlSl) with nearly the expected chromosome number (2n = 10x = 70) were obtained indicating the effectiveness of colchicine treatment for doubling the chromosome number of the F 1 hybrids.
All the amphiploids developed in the present study showed nearly additive parental electrophoretic pattern of HMW glutenin protein subunits (Fig. 3) showing the presence and expression of genomes of both the parents.
Most of the amphiploids having larger grains than the wheat parents, and nearly as high grain iron and zinc and flag leaf iron and zinc concentrations as that of the Ae. kotschyi parent (Table 3), suggest that the higher micronutrient content of Ae. kotschyi as reported earlier (Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Rawat et al., Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009) is due to its distinctive genetic system(s) for uptake, translocation and sequestration in grains rather than due to their smaller grains or lower harvest index (McDonald et al., Reference McDonald, Genc and Graham2008). Higher flag leaf iron and zinc, grain ash and ash micronutrient concentrations in amphiploids with seeds larger than or as large as the wheat cultivars further indicate that Ae. kotschyi possesses genetically distinctive micronutrient uptake, translocation or seed sequestration system(s), which could be introgressed and commercially exploited in elite wheat cultivars. Our inability to record data on grain yield and harvest index in Ae. kotschyi accessions and the synthetic amphiploids due to their shattering and hard threshing and compare the same with wheat in term of micronutrient concentrations continues as a major bottleneck in unequivocal demonstration of Ae. kotschyi possessing superior genetic control for micronutrient biofortification. Ae. kotschyi is still a potential source of useful variability for wheat biofortification for high grain iron and zinc in addition to other progenitor species reported earlier (Calderini and Ortiz-Monasterio, Reference Calderini and Ortiz-Monasterio2003a, Reference Calderini and Ortiz-Monasteriob; White and Broadley, Reference White and Broadley2005; Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006). The work to transfer and dissect useful variability of Ae. kotschyi through recurrent backcrossing and development of alien addition and substitution lines in wheat background is in progress.
Acknowledgements
The help of Department of Biotechnology, Govt. of India for supporting the work through a project, ‘Biofortification of Wheat for enhanced Iron and Zinc content by conventional and molecular breeding’ is gratefully acknowledged. The authors are highly thankful to the Head, Institute Instrumentation Centre, I.I.T. Roorkee and Mr R. Juyal for their help in chemical analysis. Help provided by Mr. Pradeep and Mr. Sunjay Giri in sample preparation is also duly acknowledged.








