INTRODUCTION
This article is not a comprehensive review, but a brief personal account of the development of helminth biochemistry over the last 40 years, concentrating on the areas in which I have been directly involved. Consequently many important developments in helminth biochemistry are not mentioned, but are covered elsewhere in this volume. Research, of course, is not carried out in isolation, there are always others working on the same or related topics and there is a constant interchange of ideas. Unfortunately there is not room to properly acknowledge all those who have contributed to the developments I am going to describe, but extensive references to early work can be found in von Brand (Reference von Brand1966, Reference von Brand and Van den Bossche1972) and Barrett (Reference Barrett1981) and I have also tried to include review articles where possible.
Standard biochemistry text books rarely make any mention of parasitic helminths and their unique metabolism. Helminth biochemistry, like mammalian biochemistry, grew out of physiology and in the early 1970s a significant number of biochemical papers still dealt essentially with physiology. In particular, the effects of physical factors such as pO2, pCO2, temperature, osmotic pressure and ionizing radiation on oxygen uptake and the effects of external substrates and inhibitors were being investigated (Barrett, Reference Barrett1968, Reference Barrett1969, among others). However, at that time, relatively few species of parasite had been studied and most of the enzyme publications involved histochemistry. Attempts to culture adult helminth parasites in vitro and tegumental transport in tapeworms and flukes were also major research interests throughout the 1960s and 1970s (see for example, Pappas et al. Reference Pappas, Uglem and Read1973; Ash and Read, Reference Ash and Read1975; Smyth and Davies, Reference Smyth and Davies1975; Esch and Smyth, Reference Esch and Smyth1976).
Progress in helminth biochemistry has always been dependent on progress in other fields, and has been marked by sudden bursts of activity in selected areas that then decline. The development in the 1960s of spectrophotometric methods for enzyme assays, coupled with the availability of 14C-labelled substrates at last enabled helminth pathways to start to be unravelled. Similarly, advances in analytical techniques allowed more accurate identification of end products. Differences in metabolism between parasites and their hosts are of course potential sites for chemotherapy, whilst differences between parasites and their free-living relatives can give an insight into the molecular basis of parasitism. Similarly, the presence of unusual organic acids in, for example, parasite excretory products, or the isolation of novel lipids or amino acids, or the identification of unusual nucleic acid modifications are good indicators of the presence of novel pathways.
CATABOLIC PATHWAYS
The production of organic acids by helminths was first reported as long ago as 1850; however, it was not until 1950, when Epps et al. (Reference Epps, Weiner and Beuding1950) showed that organic acids were produced by axenic Ascaris, that it was fully accepted that these compounds were produced by the parasite and not by contaminating bacteria. By the end of the 1970s the major pathways of energy metabolism in helminths had been worked out. It was clear that adult, parasitic helminths had an absolute dependency on carbohydrate, either in the form of glycogen or glucose, as their sole energy source. In adult helminths there was no evidence for beta-oxidation of fatty acids, no significant catabolism of amino acids, no evidence for the co-fermentation of amino acids and carbohydrate or for the co-fermentation of fatty acids and carbohydrate (Barrett, Reference Barrett1984).
An interesting variant of the usual TCA cycle was found in developing Ascaris lumbricoides eggs. During the development of Ascaris eggs, careful analytical studies had shown that there was an apparent net conversion of lipid to carbohydrate (Passey and Fairbairn, Reference Passey and Fairbairn1957). The net conversion of lipid to carbohydrate requires a special anapleuric cycle called the glyoxalate cycle, this consists of 2 enzymes, isocitrate lyase and malate synthase which effectively short circuit the TCA cycle. The glyoxalate cycle was well known in plants and micro-organisms, but had not been shown to occur in animals. We were able to show that not only was there an active glyoxalate cycle in Ascaris eggs, but its peak activity corresponded to the peak conversion of lipid to carbohydrate (Barrett et al. Reference Barrett, Ward and Fairbairn1970a). Subsequently, the glyoxalate cycle has been demonstrated in a range of other nematodes (mostly free-living or plant parasitic), but here the cycle seems primarily concerned not with the conversion of lipid to carbohydrate, but with glyconeogenesis from ethanol (Butterworth and Barrett, Reference Butterworth and Barrett1985).
Characteristically, helminths have been shown to breakdown carbohydrate to reduced organic acids or more rarely alcohols that are then excreted, the pathways involved being essentially anaerobic. On the basis of their end products, parasitic helminths were divided into 2 main groups. First, those which relied essentially on glycolysis and excreted lactate or some other derivative of pyruvate and secondly, those which fixed carbon dioxide and had what may be described as a partial, reversed TCA cycle, the initial end products of metabolism being succinate and pyruvate (Fig. 1). The latter usually being further metabolized to short chain fatty acids. As more species of parasite were investigated it became clear that these 2 types of metabolism are just convenient divisions and in reality there is a continuous spectrum between them (Barrett, Reference Barrett1984).

Fig. 1. Carbohydrate catabolism in adult Ascaris lumbricoides. Glycogen is broken down by the normal glycolytic sequence as far as phosphoenolpyruvate. Carbon dioxide fixation takes place and phosphoenolpyruvate is converted to oxaloacetate by a cytoplasmic phosphoenolpyruvate carboxykinase and the oxaloacetate is then reduced to malate by a cytoplasmic malate dehydrogenase. Malate is transported into the mitochondrion where a dismutation takes place, part is oxidatively decarboxylated to pyruvate and part is reduced to succinate. Pyruvate and succinate are then the starting point for branched chain fatty acid synthesis (after Barrett, Reference Barrett1984).
METABOLIC END PRODUCTS
Two common end products of carbohydrate breakdown in helminths are succinate and actetate (particularly in digeneans and cestodes). The pathways involved in succinate and acetate production are similar to those in mammals, at least as far as succinylCoA and acetylCoA production. But the cleavage of these two potential high-energy compounds involves not hydrolases, but CoA transferases (Köhler et al. Reference Köhler, Bryant and Behm1978). This conserves energy and effectively increases the yield of ATP per mol of glucose catabolized. Subsequently it was shown by members of Saz's group that CoA transferases have a prominent role in the synthesis of branched chain fatty acids in Ascaris muscle, with a consequent increase in ATP production (Komuniecki et al. Reference Komuniecki, Komuniecki and Saz1981; Pietrazack and Saz, Reference Pietrazack and Saz1981).
The production of alcohols rather than acids by some helminth parasites presented some interesting biochemical problems. In yeast, pyruvate is converted to acetaldehyde by means of a soluble pyruvate decarboxylase and there is an NAD-linked alcohol dehydrogenase. Helminths have alcohol dehydrogenases, although in many cases they are NADP-linked, not NAD-linked (Körting and Fairbairn, Reference Körting and Fairbairn1972). However, helminths have no detectable pyruvate decarboxylase, instead acetaldehyde production seems to be a partial reaction of the pyruvate decarboxylase complex (Barrett and Butterworth, Reference Barrett and Butterworth1984).
The production of propanol by parasites such as Haemonchus contortus presents even more of a problem. SuccinylCoA from the TCA cycle is converted to methylmalonylCoA, which in turn is decarboxylated to propionylCoA. Removal of the CoA group by either hydrolysis or CoA transferase yields propionic acid. However, from the energetic point of view the direct reduction of propionic acid to propanol is extremely unfavourable. Instead, H. contortus contains an NAD-linked CoA reductase capable of reducing propionylCoA to propanol, a reaction more usually associated with bacteria (Barrett et al. Reference Barrett, Behm, Kolhagen and Bryant1987).
Another ‘bacterial type’ enzyme found in parasitic helminths is nucleoside diphosphate kinase. This enzyme occurs in particularly high levels in the cytoplasm of those helminths that have a partial reverse TCA cycle (Barrett, Reference Barrett1973). These are parasites, such as adult Ascaris, in which phosphoenolpyruvate is converted to oxaloacetate via phosphoenolpyruvate carboxykinase (PEPcarboxykinase). This enzyme is usually either GDP or IDP linked (not ADP linked as in mammals) and nucleoside diphosphate kinase transfers high energy phosphate from the resulting GTP or ITP to ADP.
MISSING PATHWAYS
Perhaps as intriguing as the presence of unusual enzyme systems in parasites was the discovery of the apparent absence, at least in adult helminths, of what might be considered to be certain key pathways (although quite often metabolic pathways which are absent from the adult parasite are active in the larval or free-living stages). Amongst the ‘missing’ systems are phosphagens (Rogers and Lazarus, Reference Rogers and Lazarus1949). Phosphotransferases transfer high-energy phosphate bonds between a nucleoside triphosphate and a phosphagen such as arginine or creatine. Although their role is controversial, phosphagen phosphates probably act as short-term energy stores, particularly during muscle contraction. Nematodes, like other invertebrates, contain arginine phosphate and have the corresponding arginine phosphotransferase (Wickramasinghe et al. Reference Wickramasinghe, Uda, Nagataki, Yatawara, Rajapakse, Watanabe, Suzuki and Agatsuma2007). However, adult cestodes and digeneans appear to have no phosphagens although phosphotransferases may again be present (Barrett and Lloyd, Reference Barrett and Lloyd1981).
Another apparent anomaly is the glyoxalase system. This consists of 2 enzymes, glyoxalase 1 and glyoxalase 2 which together catalyse the conversion of methylglyoxal to D-lactate. The physiological role of these enzymes is not clear, but is probably related to the control of intracellular methylglyoxal levels in relation to regulating cell division. Nematodes have a normal glyoxalase system, but again in adult cestodes and digeneans there is no glyoxalase 1, although glyoxalase 2 is present and active (Brophy et al. Reference Brophy, Crowley and Barrett1990).
The demonstration of the enzymes of the beta-oxidation sequence in a number of adult helminth parasites posed a different question. Although adult helminth parasites such as Fasciola hepatica, A. lumbricoides and Hymenolepis diminuta cannot catabolize long chain fatty acids by beta-oxidation, they appear to have all the enzymes necessary (Ward and Fairbairn, Reference Ward and Fairbairn1970; Barrett and Körting, Reference Barrett and Körting1976). One possibility is that these parasites have adapted old pathways for new uses, and in the case of beta-oxidation this might be fatty acid chain lengthening by a malonylCoA independent pathway.
SYNTHETIC PATHWAYS
Most studies on metabolic pathways in helminths have concentrated on the pathways involved in energy metabolism, and for 2 reasons. First, inhibition of pathways involved in energy metabolism is likely to be rapidly fatal and, therefore, a potential target for chemotherapy, and secondly catabolic enzymes usually have relatively high specific activities making their assay much easier. The high activity of catabolic enzymes perhaps providing a buffer for fluctuating energy demands. Inhibition of synthetic pathways, on the other hand, is unlikely to lead to the rapid death of the parasite (protozoa with their rapid division rates are an exception). Also synthetic enzymes often have very low specific activities and are usually under tight metabolic control making their assay more difficult.
Much of the comparative biochemistry of synthetic pathways in parasitic helminths is concerned with pathways which are either missing or have been down regulated. Parasitic helminths, for example, seem unable to synthesize sterols de novo (although they may be able to take up plant sterols from the host intestinal contents and modify them) nor can they synthesize long chain fatty acids de novo (Meyer et al. Reference Meyer, Meyer and Bueding1970; Barrett et al. Reference Barrett, Cain and Fairbairn1970b). Helminths also seem to have a reduced ability to synthesize and interconvert amino acids (Barrett, Reference Barrett1991). There are other examples where synthetic pathways seem to be missing or inactive, for example the synthesis of haem and purines. Helminths can synthesize pyrimidines de novo, but salvage pathways seem to be more important (Wong and Yeung, Reference Wong and Yeung1981).
There are, however, some unusual synthetic enzymes in parasitic helminths, including cystathionine beta-synthase in nematodes. In vertebrates the main physiological function of this enzyme is the synthesis of cystathionine from serine and homocysteine, and this is the rate-limiting step in the conversion of methionine to cysteine. However, the enzyme from nematodes also catalyses a reaction between cysteine and an hydroxythiol (such as mercaptoethanol) to give a thioether plus hydrogen sulphide. This activity was found to occur in almost every nematode species investigated (free-living and parasitic) and the activity was often present as a series of isoenzymes (Walker and Barrett, Reference Barrett1991). There is also some experimental evidence that intact nematodes can catalyse the production of thioethers when exposed to hydroxythiol compounds, but the physiological role of this strange enzyme activity remains a mystery (Walker et al. Reference Walker, Barrett and Thong1992).
METABOLIC CONTROL
The main pathways of carbohydrate catabolism were largely worked out using crude homogenates. In the mid 1970s attention turned to how these pathways were controlled using purified or semi-purified enzymes. In general, helminth and mammalian enzymes were found to be modulated by a similar range of effectors, although there are minor differences (Stone and Mansour, Reference Stone and Mansour1967; Behm and Bryant, Reference Behm and Bryant1975; Mied and Bueding, Reference Mied and Bueding1979; McManus and Smyth, Reference McManus and Smyth1982; Starling et al. Reference Starling, Allen, Kaeini, Payne, Blytt, Hofer and Harris1982, among others).
In many helminths, phosphoenolpyruvate occupies a central position in energy metabolism (a position analogous to that of acetylCoA in mammals). Phosphoenolpyruvate in helminths can either be metabolized to pyruvate via pyruvate kinase and hence to lactate, acetate or ethanol or else via phosphoenolpyruvate carboxykinase to succinate and so eventually to propionate, propanol or short chain fatty acids. In general there is a correlation between the major end products of carbohydrate metabolism in helminths and the ratio of pyruvate kinase activity to phosphoenolpyruvate carboxykinase activity. Parasites which rely primarily on glycolysis have ratios in the region of 2–10, whilst helminths which fix carbon dioxide have ratios of the order of 0·1–0·05 (Bryant, Reference Bryant1978). However, the pattern of end-product production is labile and many studies showed that the relative proportions of succinate, acetate and lactate excreted by parasites such as F. hepatica or H. diminuta depended on the physiological conditions; more lactate being produced under aerobic conditions but more acetate under anaerobic conditions (Bryant, Reference Bryant1978; Barrett, Reference Barrett1984). The relative flux through the two pathways is also influenced by the presence of exogenous substrates and by physiological modulators such as serotonin (Mansour, Reference Mansour1962).
The logical way for the phosphoenolpyruvate branch point to be controlled would be to have reciprocal modulation. Thus the activators of pyruvate kinase would act as inhibitors for phosphoenolpyruvate carboxykinase and vice versa. However, although a whole range of activators and inhibitors have been described for pyruvate kinase, there are no known physiological modulators for phosphoenolpyruvate carboxykinase (Prichard, Reference Prichard1976; Bryant, Reference Bryant1978; Barrett, Reference Barrett1981; Lloyd and Barrett, Reference Lloyd and Barrett1983). However, metabolic control theory provides another explanation for the control of the branch point (Heinrich et al. Reference Heinrich, Rapoport and Rapoport1977; Kacser and Burns, Reference Kacser and Burns1979; Barrett, Reference Barrett1988). Control theory suggests that metabolic control is, in fact, distributed throughout the whole pathway and not just concentrated in one ‘rate-limiting’ enzyme. So, for example, the flux through the pyruvate kinase branch is influenced, not only by the enzymes in that branch of the pathway, but also by the activities of enzymes in the other branch of the pathway and also by the enzymes in the main glycolytic sequence.
The effects of the different enzymes on the branch point can be quantified by calculating the flux control coefficients that in turn can be estimated from the simultaneous measurement of metabolite levels and flux rates under different physiological conditions. When this analysis was applied to H. diminuta it showed that an increase in pyruvate kinase activity exerted a strong negative effect on the flux through the phosphoenolpyruvate kinase branch (Precious and Barrett, Reference Precious and Barrett1993; Barrett and Precious, Reference Barrett and Precious1995). This is because of the differences in the Km of the two enzymes for phosphoenopyruvate. Pyruvate kinase has a low Km and is saturated with substrate under physiological conditions, whilst phosphoenolpyruvate carboxykinase has a relatively high Km and is only partially saturated with substrate. So small changes in the steady state levels of phosphoenolpyruvate in the tissues have a marked effect on the flux through phosphoenolpyruvate carboxykinase, but no effect on the flux through pyruvate kinase. So in parasites that have a phosphoenolpyruvate branch point, it is not in fact necessary to have reciprocal effectors, the flux through pyruvate kinase is regulated by enzyme activators and inhibitors, whilst the flux through phosphoenolpyruvate carboxykinase is controlled by small changes in intracellular phosphoenolpyruvate levels.
DETOXIFICATION PATHWAYS
As well as anabolic and catabolic pathways, cells contain other enzyme systems. Of particular relevance to parasites are the enzymes involved in detoxification reactions and their possible role in drug resistance. In mammals, toxic compounds are metabolized in 3 stages (Table 1). In phase 1 reactive groups such as hydroxyl groups, amino groups or sulphydryl groups are introduced into the molecule. In phase 2, the activated molecule is conjugated with a low molecular compound such as glutathione prior to phase 3 where the conjugated molecule may be excreted, sequestered or further metabolized. In most organisms the principal phase 1 reactions are oxidative and are catalysed by cytochrome P450 mono-oxygenases. In contrast, phase 1 in helminths relies primarily on reductive or hydrolytic enzymes and there are few or no oxidative phase 1 reactions (Munir and Barrett, Reference Munir and Barrett1985; Precious and Barrett, Reference Precious and Barrett1989). Phase 2 metabolism in helminths seems similarly limited. Mammals can conjugate xenobiotics with a wide range of compounds including amino acids and sugars, as well as glutathione and inorganic ions. In contrast, the principal and probably sole conjugation reaction in helminths is with glutathione, a reaction catalysed by the multi-functional enzyme glutathione transferase (Brophy and Barrett, Reference Brophy and Barrett1990a).
Table 1. The three phases of detoxification
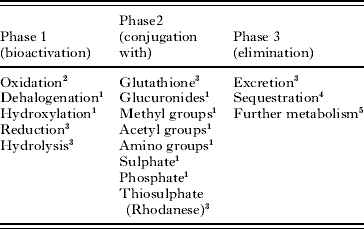
1 Not demonstrated in helminths.
2 Low activity detected in helminths, possibly associated with the synthesis of prostaglandins
3 Demonstrated in helminths.
4 High levels of binding proteins found in helminths, but detoxification role not established.
5 Low activities of cysteine conjugate beta-lyase found in helminths (from Barrett, Reference Barrett1997).
To date, 7 species-independent classes of glutathione transferases have been proposed (Alpha, Mu, Pi, Theta, Sigma, Zeta and Omega). However, studies have shown that helminth glutathione transferases do not fall clearly into these classes. Cestode glutathione transferases are most similar to the Mu class. In Schistosomes, Sm28 also has an overall homology with the Mu class, but Sj26 shows a mixture of Mu and Alpha features (Barrett, Reference Barrett1995). In nematodes, where a more detailed survey has been carried out, it has been found that the majority of nematode glutathione transferases belong to 1 of 2 new nematode-specific classes (Van Rossum et al. Reference Van Rossum, Jefferies, Rijsewijk, La Course, Teesdale-Spittle, Barrett, Tait and Brophy2004; Schuller et al. Reference Schuller, Liu, Kriksunov, Campbell, Barrett, Brophy and Hao2005). In addition, nematodes also have Alpha and Pi type isoenzymes.
Like their mammalian counterparts, helminth glutathione transferases bind a wide range of ligands including haem, unsaturated fatty acids and bile salts. In a number of helminths (for example cestodes and acanthocephalans) bile salts have a role as highly specific environmental triggers and it is possible that glutathione transferases are acting as intracellular receptors. A number of anthelmintics, especially those containing a phenolic ring can also bind. These ligands are not conjugated with glutathione, but are bound reversibly to the protein suggesting there is probably a separate binding site distinct from the active site (Walker et al. Reference Walker, Crowley, Moreman and Barrett1993).
Although glutathione transferases react with a wide range of exogenous chemicals, it is not certain what their natural intracellular targets might be. One group of compounds with which helminth glutathione transferases show high activity are long-chain, unsaturated aldehydes such as trans-2-nonenal and trans-2-octenal and their hydroxyl-derivatives such as 9-hydroxynonenol. These aldehydes are extremely reactive and cytotoxic, and are produced from the breakdown of lipid peroxides. Lipid peroxides are formed by a chain reaction between unsaturated fatty acids in the cell membranes and reactive oxygen intermediates such as superoxide or hydroxyl radicals (Brophy and Barrett, Reference Brophy and Barrett1990b). So glutathione transferase forms part of the parasite's anti-oxidant defence system. Helminths are, of course, exposed to damage by oxygen radicals as part of the effector arm of the hosts' immune response.
Conjugation with glutathione does 2 things, it increases water solubility of the compound and, for most chemicals, it decreases chemical reactivity. However, glutathione conjugates are potent inhibitors of glutathione dependent enzymes including glutathione transferase itself and glutathione reductase. In mammals glutathione conjugates are removed from cells by an ATP-dependent pump, in addition mammals are also able to metabolize glutathione conjugates to other derivatives such as mercapturic acid. Only low activities of cysteine conjugate beta-lyase have been detected in helminths (Adcock et al. Reference Adcock, Brophy, Teesdale-Spittle and Buckberry2000), but a glutathione conjugate pump with many of the characteristics of the mammalian pump has been found in cestodes (Barrett, Reference Barrett1997).
BINDING PROTEINS
An alternative to metabolism or excretion for xenobiotic compounds is sequestration. In mammals there are a variety of lipid-binding proteins: they are all low molecular weight, cytoplasmic proteins, but with differing PIs, binding affinities and relative abundances. Proteins similar to mammalian lipid-binding proteins have been found in nematodes, cestodes and digeneans. In addition, 2 other types of lipid-binding protein have been found in parasitic helminths, the first are the polyprotein allergens found in nematodes that have been extensively studied by Kennedy and colleagues (Kennedy, Reference Kennedy2000). The other is a group of unusual, polymeric, hydrophobic binding proteins found in cestodes (Barrett et al. Reference Barrett, Saghir, Timanova, Clarke and Brophy1997). These are small, 8–10 kDa proteins, which form oligomers in solutions, and unlike mammalian lipid binding proteins, which have a beta-barrel structure, these tapeworm proteins are composed of 4 alpha-helices arranged in a bunch, like the fingers of a hand.
The lipid-binding protein from M. expansa binds both saturated and unsaturated long-chain fatty acids (but not their CoA esters). It also binds steroids and retinol as well as a number of anthelmintics, the latter binding with KDs in the micromolar range: the structural requirement for binding being a planar molecule with a significant hydrophobic region and a charged carboxylate or hydroxyl head group.
Binding proteins can mediate a wide variety of intracellular processes, including regulating gene action and intracellular transport as well as the protection of intracellular components from toxic compounds. They could, in theory, aid the intracellular transport of anthelmintics, facilitating for example the movement of anthelmintics from the cell surface to intracellular organelles. Ligands bound to binding proteins may also have a kinetic advantage as substrates for other enzyme systems compared with unbound substrates and so be preferentially metabolized. This again could be important in determining the fate and site of action of protein-bound anthelmintics and could have a role in resistance (Barrett and Saghir, Reference Barrett and Saghir1999).
GENOMICS, PROTEOMICS AND METABOLOMICS
The last few years have seen tremendous advances in experimental techniques, especially in genomics, proteomics and metabolomics. These techniques are now enabling us to go back and look at problems which hitherto had seemed impossible to investigate experimentally.
Genome sequencing promised a new paradigm in which drug and vaccine targets might be rapidly identified from the genome. To date, only 3 parasitic helminth genomes have been fully sequenced (Brugia malayi, Schistosoma manson and S. japonicum) although several more are at various stages of completion and there are a number of extensive EST databases available (www.sanger.ac.uk/Projects/Helminths). As the sequencing projects are completed the data will allow detailed comparisons between species and higher taxa and lead to the identification of conserved and divergent gene families as well as regulatory regions. However, many of the genes so far identified in parasites do not have homologues in commonly studied model organisms and lack an obvious cellular function. The free-living nematode Caenorhabditis elegans has been extensively used as a model system for parasitic nematodes (unfortunately there is nothing similar for digeneans or cestodes). Large-scale RNAi screens in C. elegans have greatly added to our knowledge of gene function and this can be exploited. However, regardless of its phylogenetic position, C. elegans still represents a heterologous model for parasitic worms. For the majority of parasites the tools required for functional genomics have not yet been developed and although there are several examples of successful application of RNAi techniques to helminths (e.g. Boyle et al. Reference Boyle, Wu, Shoemaker and Yoshino2003; Britton and Murray Reference Britton and Murray2006; McGonigle et al. Reference McGonigle, Mousley, Marks, Brennan, Dalton, Spithill, Day and Maule2008), in other cases it has proved impossible to use RNAi (Viney and Thompson, Reference Viney and Thompson2008; Lender et al. Reference Lender, Doligalska, Lucius and Hartmann2008). Techniques for over expression and mutant recovery in helminths are currently lacking.
An approximate definition of proteomics is the large scale or systematic characterization of the proteins present in a single cell or tissue. So proteomics differs from the conventional reductionist scientific investigations that dominated the 1970s and 80s which typically focused on a single gene or protein. It is now possible, on a 2D polyacrylamide gel, to resolve up to 2000 proteins and, if there is a protein database for the organism, to identify the different spots by mass spectroscopy. As well as the relative amounts of protein, proteomics can reveal the extent and nature of any protein modification, protein/protein interactions and by suitable pulse incubation with labelled substrates it is also possible to estimate rates of protein turnover. Unlike the genome, which is the same in most cells, the proteome is a dynamic entity, constantly changing in response to internal and external conditions, so there really is no such thing as a single representative proteome. However, proteomics does allow us to address such questions as how do changes in the proteome correlate with biological functions such as drug resistance or host specificity.
There have been 2 slightly different approaches to proteomics in helminths. The first, ‘expression proteomics’ concentrates on looking at global proteomes, usually from parasites under different physiological conditions. For example, Heligmosomoides bakeri from fast and slow responder mice (Morgan et al. Reference Morgan, La Course, Rushbrook, Greetham, Hamilton, Barrett, Bailey and Brophy2006) or the effects of anthelmintic exposure on Ascaris larvae (Islam et al. Reference Islam, Miyoshi, Yamada, Alim, Huang, Motobu and Tsuji2006). Alternatively subproteomes have been studied either using specific tissues e.g. the schistosome tegument (Braschi et al. Reference Braschi, Curwen, Ashton, Verjowski-Almeida and Wilson2006) or concentrating on changes in specific enzymes or proteins, for example glutathione transferases (Chemale et al. Reference Chemale, Morphew, Moxon, Morrasuti, LaCourse, Barrett, Johnston and Brophy2006). Protein spots that are up or down regulated can then be further characterized. The other approach, ‘functional proteomics’ focuses on identifying the role of individual proteins and their interactions with other proteins, nucleic acids or low molecular weight ligands. Protein-protein interactions play a central role in the structural and functional organization of cells. Disruption of protein-protein interactions is frequently associated with disease, and modulation of protein-protein interactions is increasingly seen as a target for chemotherapy. Benzimidazole drugs, for example, are known to disrupt protein interactions by binding to tubulin and drug resistance is correlated with specific mutations in the tubulin molecule (Prichard, Reference Prichard2001).
Proteomic studies have shown that many helminth proteins are post-translationally modified. With over 400 kinds of different protein modifications known from the literature, it is estimated that any one protein has on average 10 post-translationally modified forms. Such a high degree of modification suggests important regulatory functions, but these have yet to be studied in detail in helminths. Several of the newer proteomic techniques such as activity-based protein profiling and isotope-coded affinity tags are still essentially at the proof of principal stage and it remains to be seen how soon these techniques can be applied to parasite problems.
Whilst proteomics is the global analysis of proteins, metabolomics is the global analysis of the low molecular weight metabolites in the tissue or cell. In theory the advantage of metabolomic analysis is that the biochemical consequences of, for example, mutations, environmental change or drug treatment can be viewed directly. This could help with understanding how drugs work and interact and with the development of new drugs. High throughput metabolomic techniques, like genomics, can generate vast amounts of data. Data interpretation, however, can be difficult, especially when whole organisms are being used. Different tissues (and even different cell types) can have different metabolite profiles and cellular compartmentation means that metabolites are not evenly distributed throughout the cell (Nicholson et al. Reference Nicholson, Holmes, Lindon and Wilson2004). In the case of parasites there is the added complication of the host contribution. At the moment, the emphasis in metabolomics is in biomarker discovery (metabonomics) rather than fundamental biochemistry (Wang et al. Reference Wang, Utzinger, Xiao, Xue, Nicholson, Tanner, Singer and Holmes2006).
FUTURE DEVELOPMENTS
In general the biochemistry of helminths has lagged behind that of parasitic protozoa. In part this is probably due to our inability to culture adult helminths or their cell lines in vitro for any length of time. Since the early 1970s the major pathways of energy metabolism in adult parasitic helminths have been worked out in some detail. The mode of action of most anthelmintics is now known, but in most cases the mechanism of drug resistance remains elusive and this is likely to remain a major area of research in the future. The overall synthetic capabilities of adult helminths are now reasonably well known, but synthetic enzymes have been relatively little studied. Extensive changes take place in energy metabolism between the different life-cycle stages of helminths. Whether there are similar major changes in synthetic pathways is not known, although this may become clearer from micro-array studies which show that different groups of genes are being transcribed at different life-cycle stages (Vermeire et al. Reference Vermeire, Taft, Hoffmann, Fitzpatrick and Yoshino2006). However, genes may be transcribed ahead of the environmental event and only translated after transition. The discovery of the role of small RNAs (20–30 nucleotide long pieces) in developmental processes may give new impetus to this area (Grosshans and Filipowicz, Reference Grosshans and Filipowicz2008).
There are many fascinating aspects to helminth life cycles that have yet to be addressed. For example: what is the basis of host/site specificity, in parasites which undergo tissue migration what biochemical cues do they use to navigate through the body and how do they subvert/avoid the hosts' immune response? Biochemistry may be well placed to tackle some of these problems in the coming decade.
High throughput methods such as proteomics, metabolomics, micro-arrays and genome sequencing are generating data faster than we can analyse it. In order to find associations hidden across different kinds of data in different databases developing methods for enabling disparate sorts of data to be integrated will become a priority (Barrett et al. Reference Barrett, Brophy and Hamilton2005). Ideally this would involve the use of a common ontology (a controlled, structured vocabulary). Whilst this may be feasible in the future there remains the question of how to incorporate older data into the new database structure? Advances in data mining unstructured text may provide the way forward in the future (Buckingham, Reference Buckingham2004).
I should like to thank all my students, post-docs and numerous colleagues and collaborators with whom I have worked over the past 40 years, and the support of the various funding bodies that have allowed me to undertake my research.




